First Utilization of Magnetically-Assisted Photocatalytic Iron Oxide-TiO2 Nanocomposites for the Degradation of the Problematic Antibiotic Ciprofloxacin in an Aqueous Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
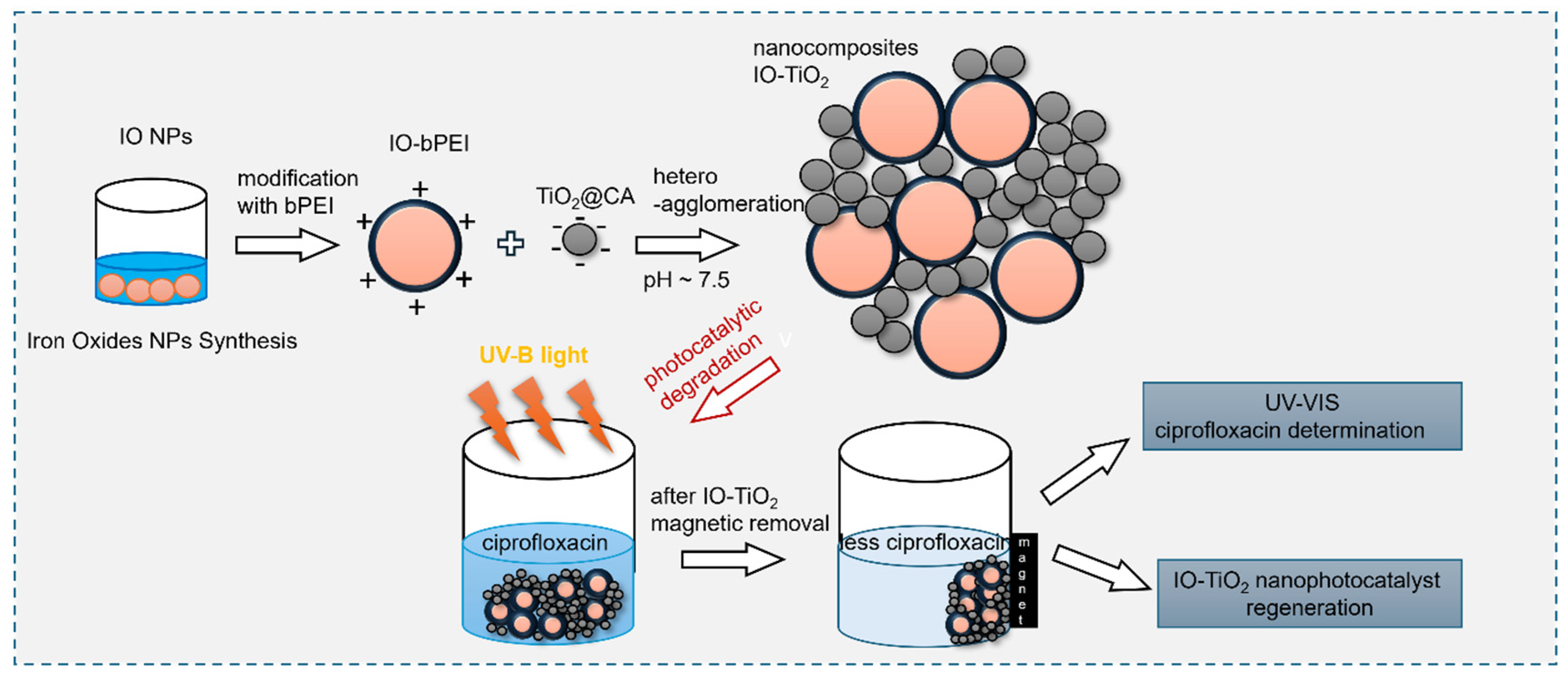
2.2. Synthesis and Functionalization of Magnetic Iron Oxide Nanoparticles with Branched Polyethylene Imine (IO-bPEI)
2.3. Preparation of IO-TiO2 Photocatalytic Nanocomposites
2.4. Characterization of Photocatalytic Nanocomposite
2.4.1. pH Titration Curves
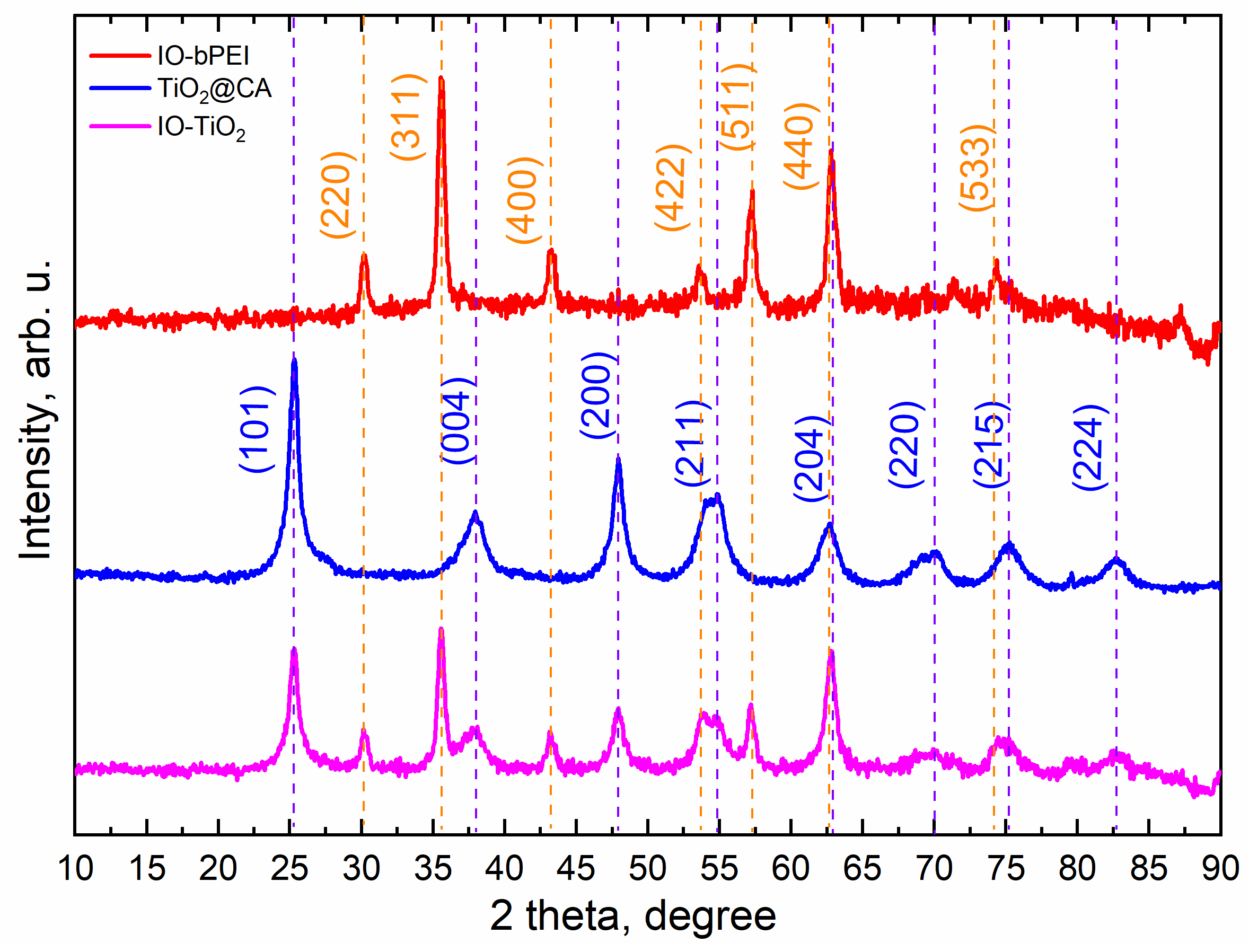
2.4.2. X-ray Powder Diffraction (XRD)
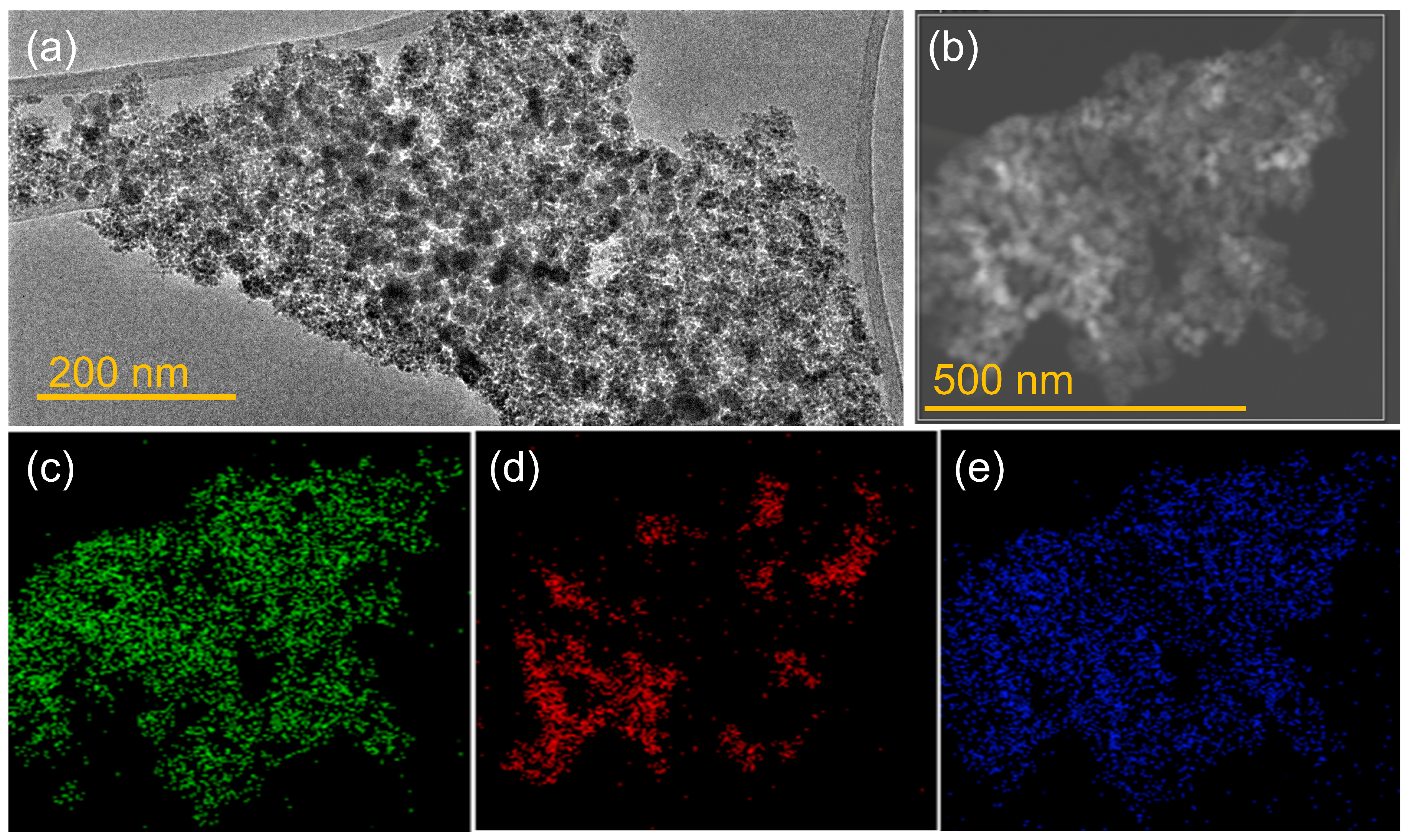
2.4.3. Transmission Electron Microscopy (TEM)
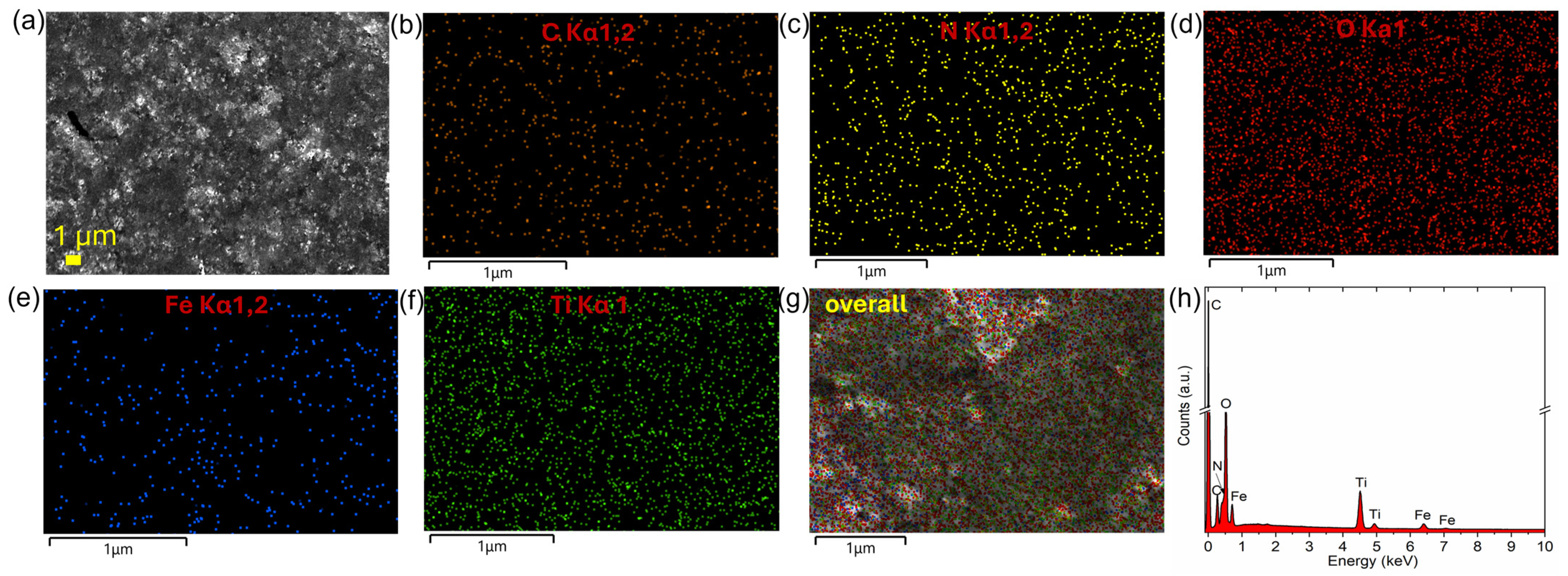
2.4.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDXS) Mapping
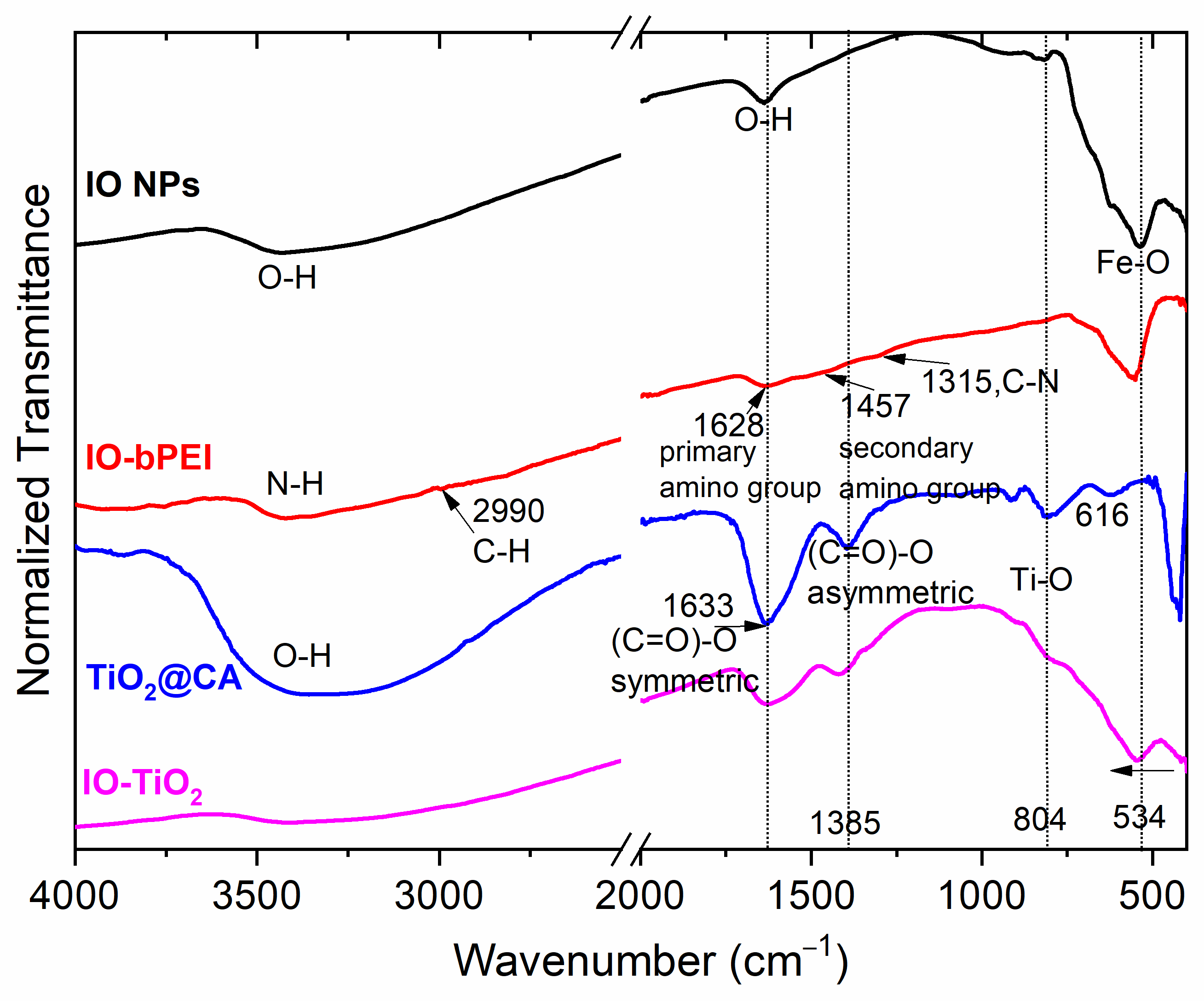
2.4.5. ATR-FTIR
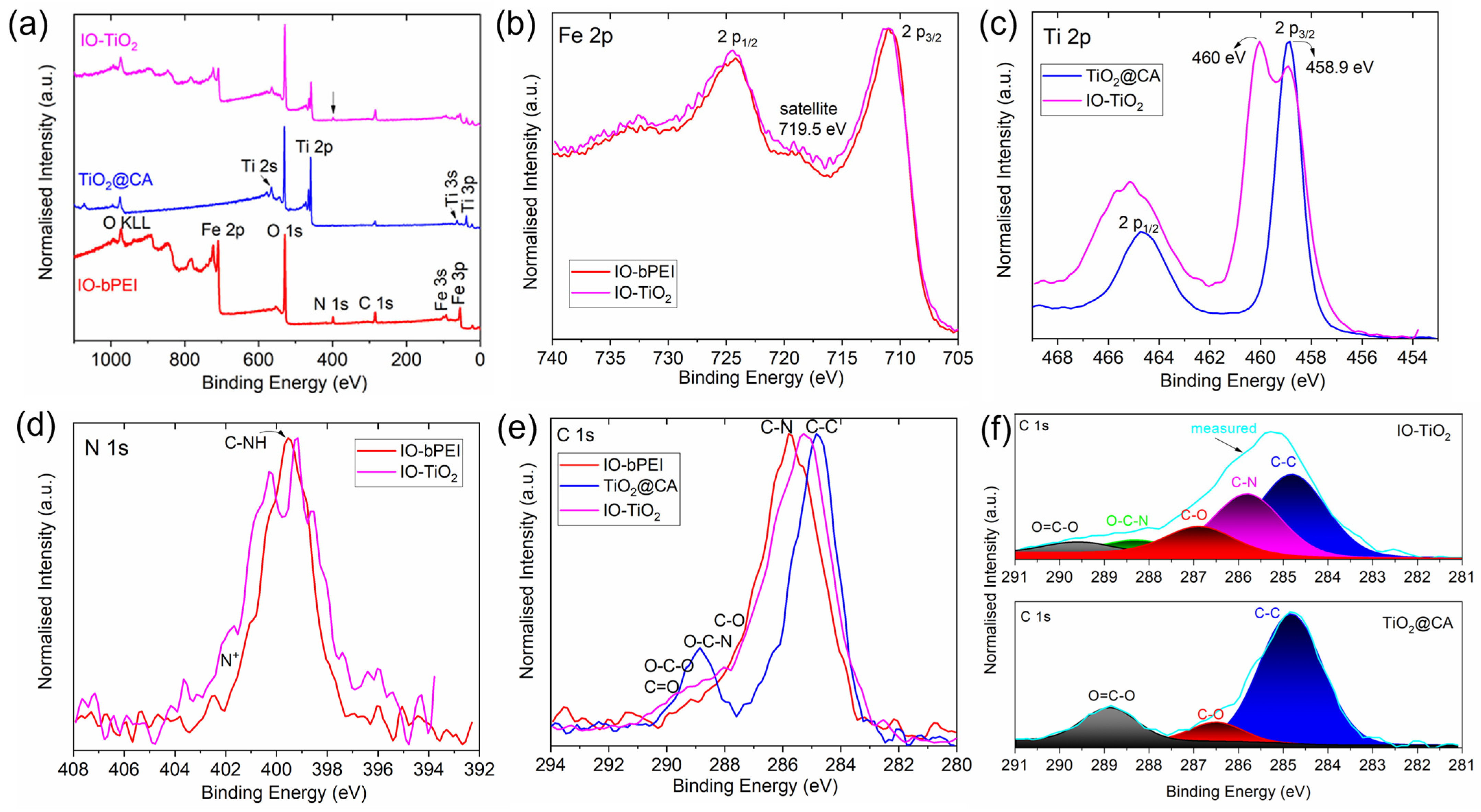
2.4.6. X-ray Photoelectron Spectroscopy (XPS)
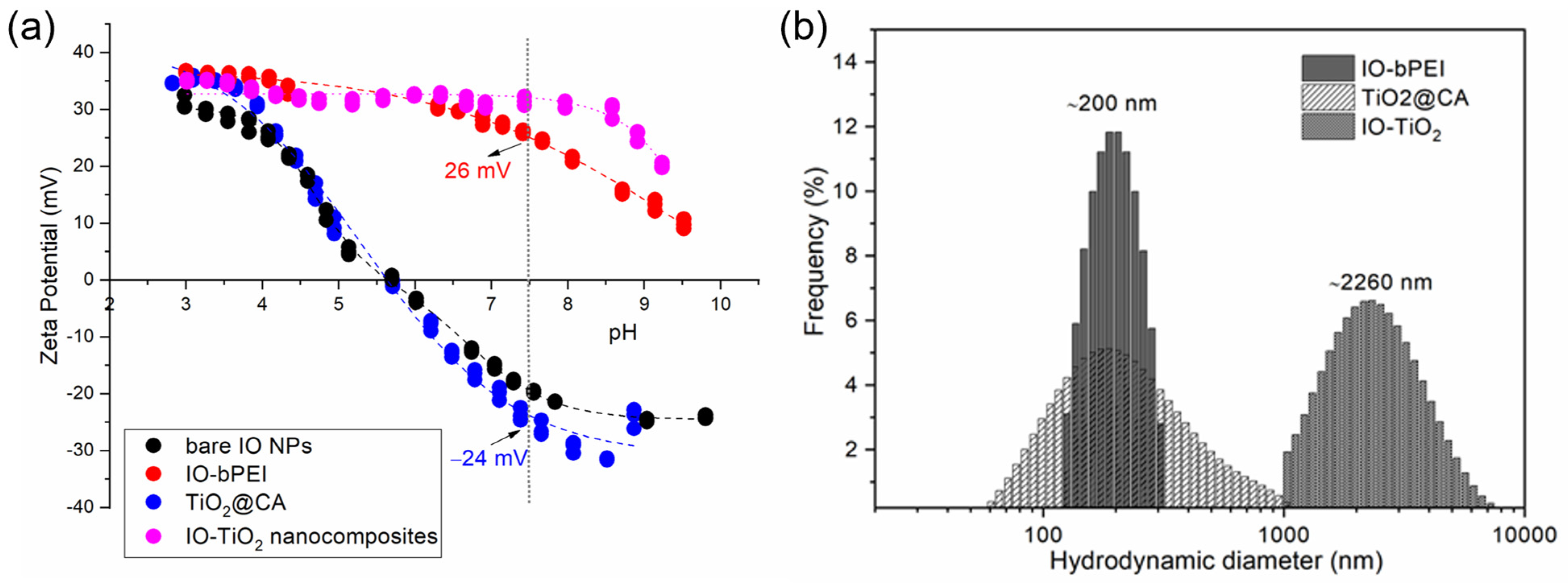
2.4.7. Zeta Potential and Hydrodynamic Diameter
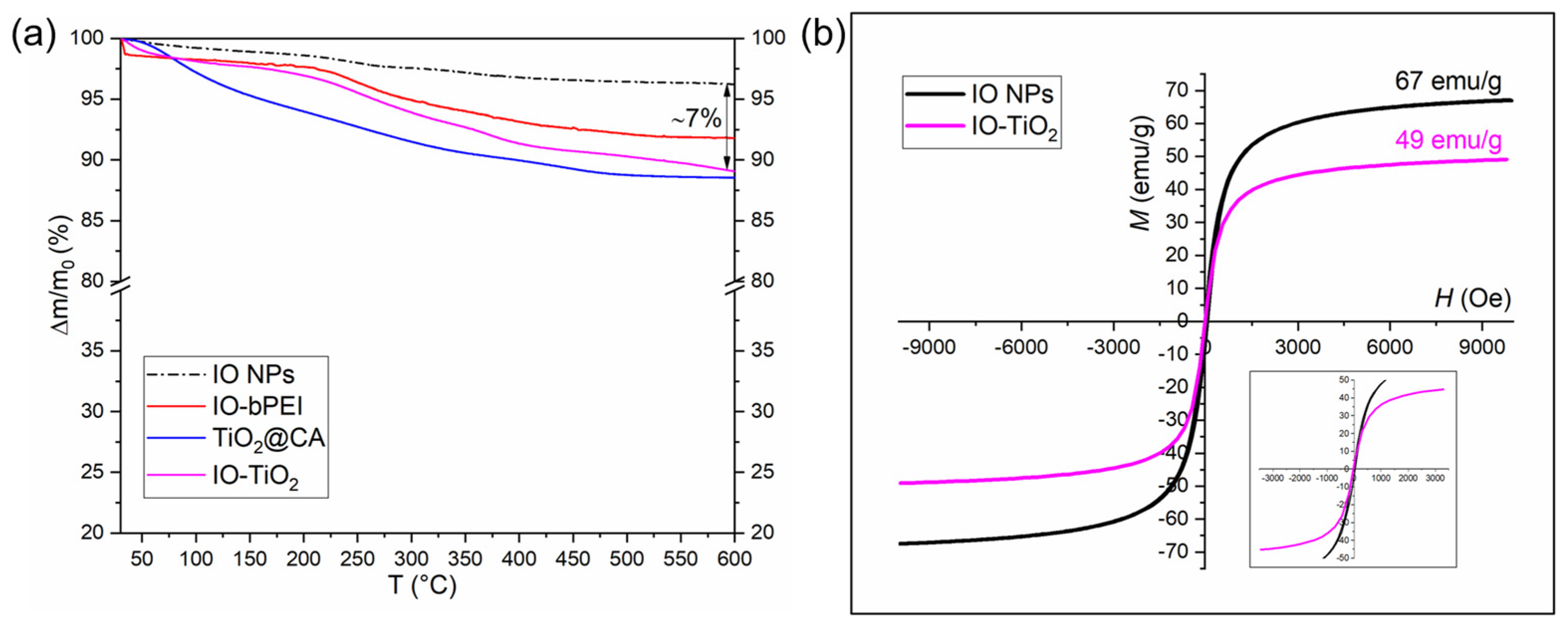
2.4.8. Thermogravimetric Analysis and Magnetic Properties
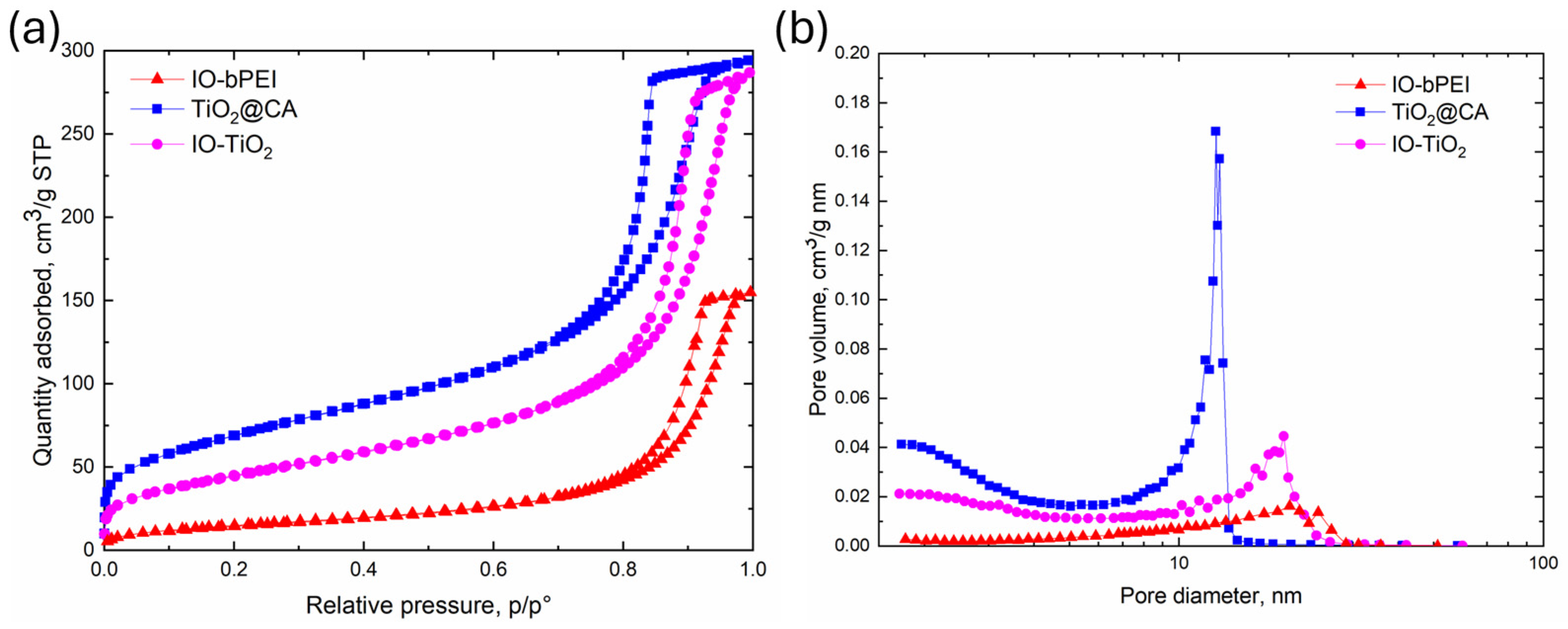
2.4.9. Specific Surface Area, Pore Volume, and Average Pore Size of the Materials
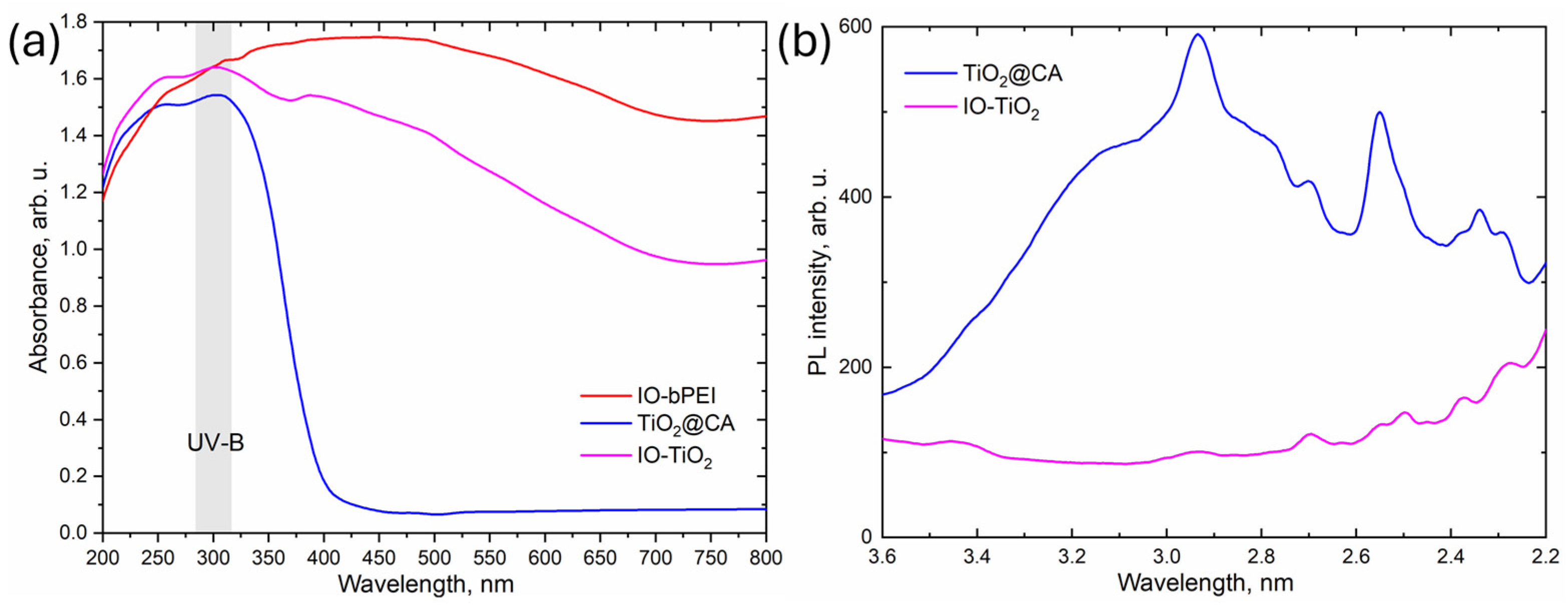
2.4.10. UV-Vis Diffuse Reflectance (UV-Vis-DR) and Solid Photoluminescence (PL) Spectra
2.5. Exploitation of Photocatalytic Performance towards Ciprofloxacin
2.6. Statistical Analysis
3. Results and Discussion
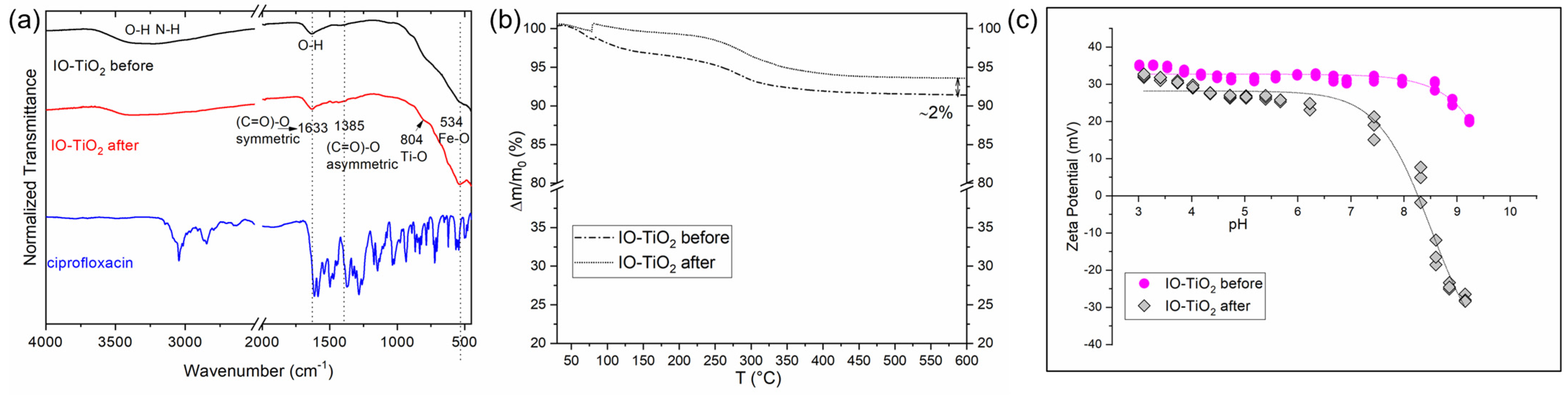
3.1. Characterization of Designed Photocatalytic Nanocomposite
3.2. Photocatalytic Degradation of Ciprofloxacin
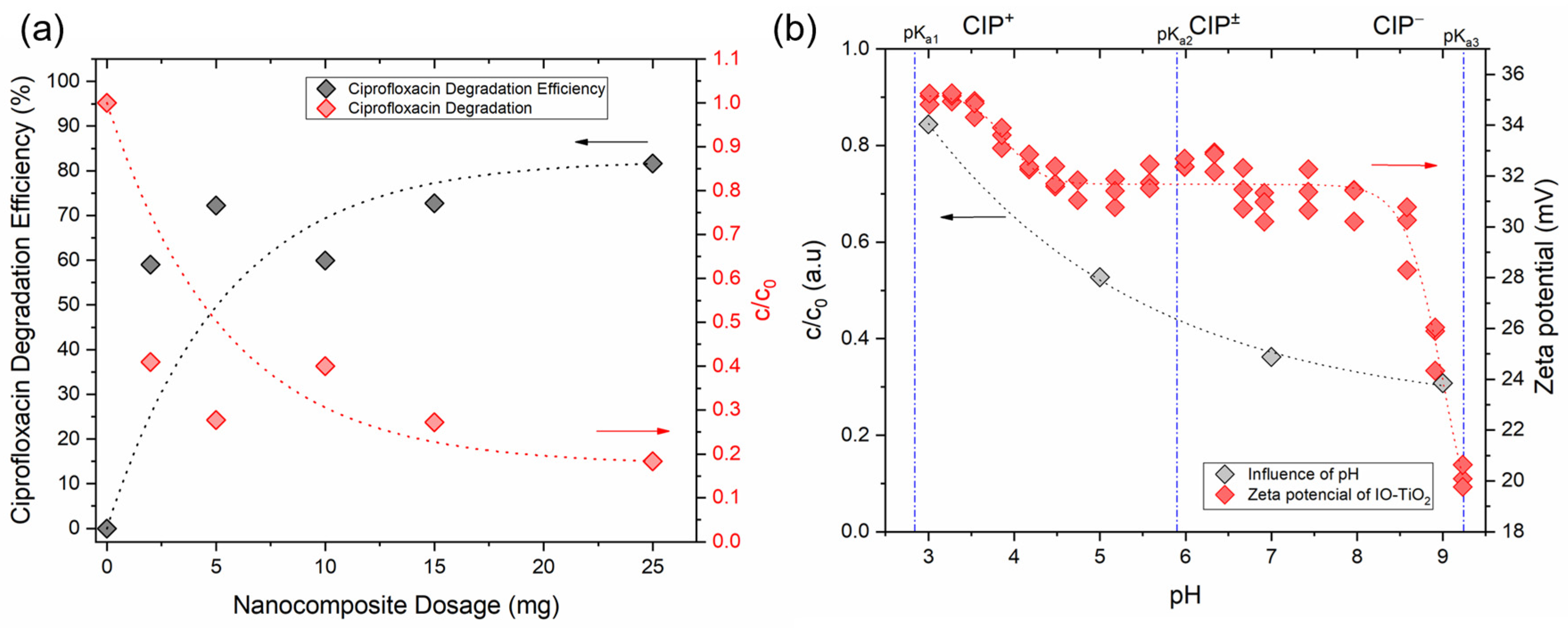
3.2.1. Influence of Nanocomposite Dosage
3.2.2. Influence of pH
3.2.3. Effect of Nanocatalyst Type and Influence of Time on Ciprofloxacin Degradation
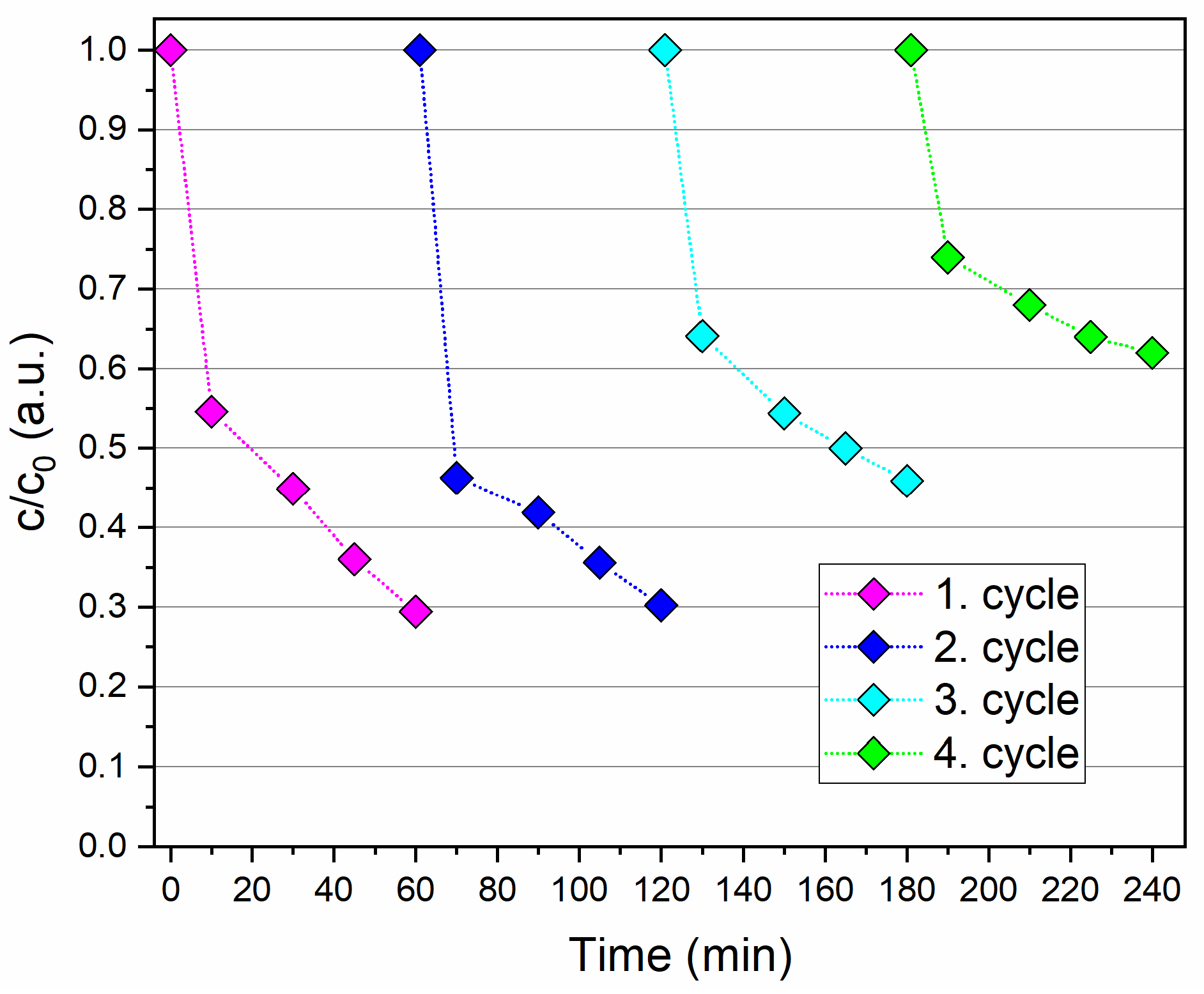
3.2.4. Reusability and Stability Evaluation of the Nanocatalyst
3.3. Plausible Degradation Mechanism
- (i)
- Transfer of ciprofloxacin from the aqueous dispersion to the surface of the IO-TiO2 nanocomposite.
- (ii)
- Adsorption of ciprofloxacin onto IO-TiO2.
- (iii)
- Photocatalytic oxidation and reduction of ciprofloxacin by reactive species as described in Equations (3)–(7).
- (iv)
- Removal of ciprofloxacin degradation products from the IO-TiO2 surface.
- (v)
- Transport of these degradation products from the IO-TiO2 surface back into the aqueous dispersion.
- (vi)
- Magnetically assisted removal of IO-TiO2 after the photocatalytic process.
- (vii)
- Reuse of IO-TiO2 for another photocatalytic process.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, R.; Sharma, S. Role of alternatives to antibiotics in mitigating the antimicrobial resistance crisis. Indian J. Med. Res. 2022, 156, 517–520. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Data on Antimicrobial Resistance (AMR): Use of Antibiotics in the EU Decreases but More Needs to be Done; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Setiawan, A.; Widodo, A.D.W.; Endraswari, P.D. Comparison of ciprofloxacin, cotrimoxazole, and doxycycline on Klebsiella pneumoniae: Time-kill curve analysis. Ann. Med. Surg. 2022, 84, 104841. [Google Scholar] [CrossRef]
- Shariati, A.; Arshadi, M.; Khosrojerdi, M.A.; Abedinzadeh, M.; Ganjalishahi, M.; Maleki, A.; Heidary, M.; Khoshnood, S. The resistance mechanisms of bacteria against ciprofloxacin and new approaches for enhancing the efficacy of this antibiotic. Front. Public Health 2022, 10, 1025633. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, J.; Zeng, J.; Li, J.; Liu, Y.; Sun, X.; Xu, L.; Li, L. Complete degradation and detoxification of ciprofloxacin by a micro-/nanostructured biogenic mn oxide composite from a highly active Mn2+-oxidizing pseudomonas strain. Nanomaterials 2021, 11, 1660. [Google Scholar] [CrossRef]
- Patrolecco, L.; Rauseo, J.; Ademollo, N.; Grenni, P.; Cardoni, M.; Levantesi, C.; Luprano, M.L.; Caracciolo, A.B. Persistence of the antibiotic sulfamethoxazole in river water alone or in the co-presence of ciprofloxacin. Sci. Total Environ. 2018, 640–641, 1438–1446. [Google Scholar] [CrossRef]
- Ory, J.; Bricheux, G.; Togola, A.; Bonnet, J.L.; Donnadieu-Bernard, F.; Nakusi, L.; Forestier, C.; Traore, O. Ciprofloxacin residue and antibiotic-resistant biofilm bacteria in hospital effluent. Environ. Pollut. 2016, 214, 635–645. [Google Scholar] [CrossRef]
- Monahan, C.; Nag, R.; Morris, D.; Cummins, E. Antibiotic residues in the aquatic environment–current perspective and risk considerations. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2021, 56, 733–751. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Chang, H.; Wei, W.; Yu, H.; Chen, Z.; Cheng, X.; Chen, D.; Jin, Y.; Han, D.; Xu, W. Highly Effective Removal of Ciprofloxacin Antibiotic from Water by Magnetic Metal–Organic Framework. Water 2023, 15, 2531. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef]
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment—A review. J. Water Process Eng. 2022, 45, 102474. [Google Scholar]
- Saitoh, T.; Shibata, K.; Fujimori, K.; Ohtani, Y. Rapid removal of tetracycline antibiotics from water by coagulation-flotation of sodium dodecyl sulfate and poly(allylamine hydrochloride) in the presence of Al(III) ions. Sep. Purif. Technol. 2017, 187, 76–83. [Google Scholar] [CrossRef]
- Adams, C.; Wang, Y.; Loftin, K.; Meyer, M. Removal of Antibiotics from Surface and Distilled Water in Conventional Water Treatment Processes. J. Environ. Eng. 2002, 128, 253–260. [Google Scholar] [CrossRef]
- Saitoh, T.; Shibata, K.; Hiraide, M. Rapid removal and photodegradation of tetracycline in water by surfactant-assisted coagulation-sedimentation method. J. Environ. Chem. Eng. 2014, 2, 1852–1858. [Google Scholar] [CrossRef]
- Nasrollahi, N.; Vatanpour, V.; Khataee, A. Removal of antibiotics from wastewaters by membrane technology: Limitations, successes, and future improvements. Sci. Total Environ. 2022, 838, 156010. [Google Scholar] [CrossRef]
- Oh, J.; Salcedo, D.E.; Medriano, C.A.; Kim, S. Comparison of different disinfection processes in the effective removal of antibiotic-resistant bacteria and genes. J. Environ. Sci. 2014, 26, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Apostolescu, N.; Tataru Farmus, R.E.; Harja, M.; Vizitiu, M.A.; Cernatescu, C.; Cobzaru, C.; Apostolescu, G.A. Photocatalytic Removal of Antibiotics from Wastewater Using the CeO2/ZnO Heterojunction. Materials 2023, 16, 850. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, P.; Álvarez-Torrellas, S.; Larriba, M.; Gil, M.V.; Garrido-Zoido, J.M.; García, J. Efficient removal of antibiotic ciprofloxacin by catalytic wet air oxidation using sewage sludge-based catalysts: Degradation mechanism by DFT studies. J. Environ. Chem. Eng. 2023, 11, 109344. [Google Scholar] [CrossRef]
- Brillas, E. A review on the photoelectro-Fenton process as efficient electrochemical advanced oxidation for wastewater remediation. Treatment with UV light, sunlight, and coupling with conventional and other photo-assisted advanced technologies. Chemosphere 2020, 250, 126198. [Google Scholar] [CrossRef]
- Hu, X.; Hu, X.; Peng, Q.; Zhou, L.; Tan, X.; Jiang, L.; Tang, C.; Wang, H.; Liu, S.; Wang, Y.; et al. Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 2020, 380, 122366. [Google Scholar] [CrossRef]
- Attar, R.M.S.; Alkhamis, K.M.; Alsharief, H.H.; Alaysuy, O.; Alrashdi, K.S.; Mattar, H.; Alkhatib, F.; El-Metwaly, N.M. Remarkable photodegradation breakdown cost, antimicrobial activity, photocatalytic efficiency, and recycling of SnO2 quantum dots throughout industrial hazardous pollutants treatment. Ceram. Int. 2024, 50, 36194–36209. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El-Nemr, M.A.; Elkatory, M.R.; Ragab, S.; Niculescu, V.C.; El Nemr, A. Principles of Photocatalysts and Their Different Applications: A Review; Springer International Publishing: New York, NY, USA, 2023; Volume 381, ISBN 0123456789. [Google Scholar]
- Xu, M.; Wu, C.; Zhou, Y. Advancements in the Fenton Process for Wastewater Treatment; Bustillo-Lecompte, C., Ed.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Yee, L.Y.; Ng, Q.H.; Kartini Enche Ab Rahim, S.; Hoo, P.Y.; Chang, P.T.; Ahmad, A.L.; Low, S.C.; Shuit, S.H. A Novel Tri-Functionality pH-Magnetic-Photocatalytic Hybrid Organic-Inorganic Polyoxometalates Augmented Microspheres for Polluted Water Treatment. Membranes 2023, 13, 174. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Xu, T.; Ji, W.; Zong, X. Recent Advances on Small Band Gap Semiconductor Materials (≤2.1 eV) for Solar Water Splitting. Catalysts 2023, 13, 728. [Google Scholar] [CrossRef]
- Madrakian, T.; Afkhami, A.; Haryani, R.; Ahmadi, M. Synthesis of γ-Fe2O3/TiO2nanocomposite and its application in removal of dyes from water samples by adsorption and degradation processes. RSC Adv. 2014, 4, 44841–44847. [Google Scholar] [CrossRef]
- Ismail, N.; Imran, M.; Ramzan, M.; Anwar, A.; Alsafari, I.A.; Asgher, M.; Iqbal, H.M.N. Functionalized graphene oxide-zinc oxide hybrid material and its deployment for adsorptive removal of levofloxacin from aqueous media. Environ. Res. 2023, 217, 114958. [Google Scholar] [CrossRef]
- Bedin, K.C.; Muche, D.N.F.; Melo, M.A.; Freitas, A.L.M.; Gonçalves, R.V.; Souza, F.L. Role of Cocatalysts on Hematite Photoanodes in Photoelectrocatalytic Water Splitting: Challenges and Future Perspectives. ChemCatChem 2020, 12, 3156–3169. [Google Scholar] [CrossRef]
- Mei, Q.; Zhang, F.; Wang, N.; Yang, Y.; Wu, R.; Wang, W. TiO2/Fe2O3 heterostructures with enhanced photocatalytic reduction of Cr(vi) under visible light irradiation. RSC Adv. 2019, 9, 22764–22771. [Google Scholar] [CrossRef]
- Sakar, M.; Mithun Prakash, R.; Trong-On, D. Insights into the Tio2-based photocatalytic systems and their mechanisms. Catalysts 2019, 9, 680. [Google Scholar] [CrossRef]
- Kiziltaş, H.; Tekin, T.; Tekin, D. Preparation and characterization of recyclable investigation of the photocatalytic activity. Chem. Eng. Commun. 2021, 208, 1041–1053. [Google Scholar] [CrossRef]
- Behzadi, S.; Nonahal, B.; Royaee, S.J.; Asadi, A.A. TiO2/SiO2/Fe3O4 magnetic nanoparticles synthesis and application in methyl orange UV photocatalytic removal. Water Sci. Technol. 2020, 82, 2432–2445. [Google Scholar] [CrossRef]
- Malakootian, M.; Nasiri, A.; Amiri Gharaghani, M. Photocatalytic degradation of ciprofloxacin antibiotic by TiO2 nanoparticles immobilized on a glass plate. Chem. Eng. Commun. 2020, 207, 56–72. [Google Scholar] [CrossRef]
- Hayri-senel, T.; Kahraman, E.; Sezer, S.; Erdol-aydin, N.; Nasun-saygili, G. Photocatalytic degradation of ciprofloxacin from water with waste polystyrene and TiO2 composites. Heliyon 2024, 10, e25433. [Google Scholar] [CrossRef] [PubMed]
- Sakineh, S.; Kakavandi, B.; Noorisepehr, M.; Akbar, A. Photocatalytic oxidation of tetracycline by magnetic carbon-supported TiO2 nanoparticles catalyzed peroxydisulfate: Performance, synergy and reaction mechanism studies. Sep. Purif. Technol. 2021, 258, 117936. [Google Scholar]
- Lv, Y.; Yue, L.; Khan, I.M.; Zhou, Y.; Cao, W.; Niazi, S.; Wang, Z. Fabrication of magnetically recyclable yolk-shell Fe3O4@TiO2 nanosheet/Ag/g-C3N4 microspheres for enhanced photocatalytic degradation of organic pollutants. Nano Res. 2021, 14, 2363–2371. [Google Scholar] [CrossRef]
- Amoli, A.E.; Masoumi, M.; Sharifzadeh, M. Synthesis of TiO2-Fe2O3 nanocomposite for the photocatalytic degradation of Direct Blue 199 and Basic Yellow 28 dyes under visible light irradiation. J. Dispers. Sci. Technol. 2023, 44, 630–638. [Google Scholar] [CrossRef]
- Rani, N.; Dehiya, B.S. Magnetically recyclable copper doped core-shell Fe3O4@TiO2@Cu nanocomposites for wastewater remediation. Environ. Technol. 2022, 43, 4484–4492. [Google Scholar] [CrossRef]
- Brossault, D.F.F.; Mccoy, T.M.; Routh, A.F. Self-assembly of TiO2 Fe3O4/SiO2 microbeads: A green approach to produce magnetic photocatalysts. J. Colloid Interface Sci. 2021, 584, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Dewi, S.H.; Sutanto; Fisli, A.; Wardiyati, S. Synthesis and Characterization of Magnetized Photocatalyst Fe3O4/SiO2/TiO2 by Heteroagglomeration Method. J. Phys. Conf. Ser. 2016, 739, 012113. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Babar, S.; Gavade, N.; Shinde, H.; Gore, A.; Mahajan, P.; Lee, K.H.; Bhuse, V.; Garadkar, K. An innovative transformation of waste toner powder into magnetic g-C3N4-Fe2O3 photocatalyst: Sustainable e-waste management. J. Environ. Chem. Eng. 2019, 7, 103041. [Google Scholar] [CrossRef]
- Synowiec, M.; Dominika, Z.; Trenczek-Zajac, A.; Radecka, M. The impact of nanometric Fe2O3 on the magnetic, electronic, and photocatalytic behavior of TiO2@ Fe2O3 heterostructures. Appl. Surf. Sci. 2023, 608, 155186. [Google Scholar] [CrossRef]
- Qamar, U.; Jazib, S.; Zaidi, A.; Riaz, A.; Atiq, M.; Xin, C.; Abdul, M. Simple two-step development of TiO2/Fe2O3 nanocomposite for oxygen evolution reaction (OER) and photo-bio active applications. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131662. [Google Scholar]
- Makovec, D.; Sajko, M.; Drofenik, M. Magnetically recoverable photocatalytic nanocomposite particles for water treatment. Mater. Chem. Phys. 2011, 129, 83–89. [Google Scholar] [CrossRef]
- Zamani, W.; Rastgar, S.; Hedayati, A. Capability of TiO2 and Fe3O4 nanoparticles loaded onto Algae (Scendesmus sp.) as a novel bio-magnetic photocatalyst to degration of Red195 dye in the sonophotocatalytic treatment process under ultrasonic/UVA irradiation. Sci. Rep. 2023, 13, 18182. [Google Scholar] [CrossRef]
- Zulfiqar, N.; Nadeem, R.; Musaimi, O.A.I. Photocatalytic Degradation of Antibiotics via Exploitation of a Magnetic Nanocomposite: A Green Nanotechnology Approach toward Drug-Contaminated Wastewater Reclamation. ACS Omega 2024, 9, 7986–8004. [Google Scholar] [CrossRef] [PubMed]
- Beketova, D.; Motola, M.; Sopha, H.; Michalicka, J.; Cicmancova, V.; Dvorak, F.; Hromadko, L.; Frumarova, B.; Stoica, M.; Macak, J.M. One-Step Decoration of TiO2 Nanotubes with Fe3O4 Nanoparticles: Synthesis and Photocatalytic and Magnetic Properties. ACS Appl. Nano Mater. 2020, 3, 1553–1563. [Google Scholar] [CrossRef]
- Plohl, O.; Ajdnik, U.; Gyergyek, S.; Ban, I.; Vesel, A.; Glaser, T.K.; Zemljič, L.F. Superior stability and high biosorbent efficiency of carboxymethylchitosan covalently linked to silica-coated core-shell magnetic nanoparticles for application in copper removal. J. Environ. Chem. Eng. 2019, 7, 102913. [Google Scholar] [CrossRef]
- Makovec, D.; Sajko, M.; Selinik, A.; Drofenik, M. Low-temperature synthesis of magnetically recoverable, superparamagnetic, photocatalytic, nanocomposite particles. Mater. Chem. Phys. 2012, 136, 230–240. [Google Scholar] [CrossRef]
- Herrera, W.; Vera, J.; Hermosilla, E.; Diaz, M.; Tortella, G.R.; Albino, R.; Reis, D.; Seabra, A.B.; Rubilar, O. The Catalytic Role of Superparamagnetic Iron Oxide Nanoparticles as a Support Material for TiO2 and ZnO on Chlorpyrifos Photodegradation in an Aqueous Solution. Nanomaterials 2024, 14, 299. [Google Scholar] [CrossRef]
- Suliman, Z.A.; Mecha, A.C.; Mwasiagi, J.I. Effect of TiO2/Fe2O3 nanopowder synthesis method on visible light photocatalytic degradation of reactive blue dye. Heliyon 2024, 10, e29648. [Google Scholar] [CrossRef]
- Esfandiari, N.; Kashefi, M.; Mirjalili, M.; Afsharnezhad, S. On the adsorption kinetics and mechanism of enhanced photocatalytic activity of Fe3O4-SiO2-TiO2 core-multishell nanoparticles against E. coli. J. Biomed. Mater. Res.—Part A 2021, 109, 181–192. [Google Scholar] [CrossRef]
- Wang, J.; Sgarzi, M.; Němečková, Z.; Henych, J.; Licciardello, N.; Cuniberti, G. Reusable and Antibacterial Polymer-Based Nanocomposites for the Adsorption of Dyes and the Visible-Light-Driven Photocatalytic Degradation of Antibiotics. Glob. Chall. 2022, 6, 2200076. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Zi, T.Q.; Zhao, X.R.; Liu, C.; Ren, Q.; Fang, J.B.; Li, W.M.; Li, A.D. Enhanced visible light photocatalytic activity of Fe2O3 modified TiO2 prepared by atomic layer deposition. Sci. Rep. 2020, 10, 13437. [Google Scholar] [CrossRef]
- Xia, Y.; Yin, L. Core-shell structured α-Fe2O3@TiO2 nanocomposites with improved photocatalytic activity in the visible light region. Phys. Chem. Chem. Phys. 2013, 15, 18627–18634. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.; Wu, W.; Tian, Q.; Cui, S.; Dai, Z.; Ren, F.; Xiao, X.; Jiang, C. 3D Flowerlike α-Fe2O3@TiO2 Core-Shell Nanostructures: General Synthesis and Enhanced Photocatalytic Performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Krishnan, S.; Shriwastav, A. Chlorophyll sensitized and salicylic acid functionalized TiO2 nanoparticles as a stable and efficient catalyst for the photocatalytic degradation of ciprofloxacin with visible light. Environ. Res. 2023, 216, 114568. [Google Scholar] [CrossRef] [PubMed]
- Rajiv, P.; Mengelizadeh, N.; McKay, G.; Balarak, D. Photocatalytic degradation of ciprofloxacin with Fe2O3 nanoparticles loaded on graphitic carbon nitride: Mineralisation, degradation mechanism and toxicity assessment. Int. J. Environ. Anal. Chem. 2021, 103, 2193–2207. [Google Scholar] [CrossRef]
- Bazzanella, N.; Bajpai, O.P.; Fendrich, M.; Guella, G.; Miotello, A.; Orlandi, M. Ciprofloxacin degradation with a defective TiO2-x nanomaterial under sunlight. MRS Commun. 2023, 13, 1252–1259. [Google Scholar] [CrossRef]
- Paul, T.; Dodd, M.C.; Strathmann, T.J. Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: Transformation products and residual antibacterial activity. Water Res. 2010, 44, 3121–3132. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Hanafi-Bojd, H.; Shiva, M. Photocatalytic degradation of ciprofloxacin antibiotic in water by biosynthesized silica supported silver nanoparticles. Ceram. Int. 2023, 49, 7717–7726. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y. ZnIn2S4/CoFe2O4 p-n junction-decorated biochar as magnetic recyclable nanocomposite for efficient photocatalytic degradation of ciprofloxacin under simulated sunlight. Sep. Purif. Technol. 2022, 303, 122156. [Google Scholar] [CrossRef]
- Karuppaiah, S.; Annamalai, R.; Muthuraj, A.; Kesavan, S.; Palani, R.; Ponnusamy, S.; Nagarajan, E.R.; Meenakshisundaram, S. Efficient photocatalytic degradation of ciprofloxacin and bisphenol A under visible light using Gd2WO6 loaded ZnO/bentonite nanocomposite. Appl. Surf. Sci. 2019, 481, 1109–1119. [Google Scholar]
- Zhang, N.; Li, X.; Wang, Y.; Zhu, B.; Yang, J. Fabrication of magnetically recoverable Fe3O4/CdS/g-C3N4 photocatalysts for effective degradation of ciprofloxacin under visible light. Ceram. Int. 2020, 46, 20974–20984. [Google Scholar] [CrossRef]
- Tamaddon, F.; Nasiri, A.; Yazdanpanah, G. Photocatalytic degradation of ciprofloxacin using CuFe2O4@methyl cellulose based magnetic nanobiocomposite. MethodsX 2020, 7, 74–81. [Google Scholar] [CrossRef]
- Raikar, L.G.; Patel, A.; Gandhi, J.; Gupta, K.V.K.; Prakash, H. Nano-TiO2 immobilized polyvinylidene fluoride based spongy-spheres for ciprofloxacin photocatalytic degradation: Antibacterial activity removal, mechanisms, UVA LED irradiation and easy recovery. Environ. Sci. Nano, 2024; Advance Article. Available online: https://pubs.rsc.org/en/content/articlehtml/2024/en/d4en00302k (accessed on 29 July 2024).
- Naga Lakshmi, C.; Irfan, M.; Sinha, R.; Singh, N. Magnetically recoverable Ni-doped iron oxide/graphitic carbon nitride nanocomposites for the improved photocatalytic degradation of ciprofloxacin: Investigation of degradation pathways. Environ. Res. 2024, 242, 117812. [Google Scholar] [CrossRef]
- Zeng, Y.; Wu, L.; Luo, J.; Lu, X.; Jia, B.; Liang, L.; Zhang, J. Photocatalytic degradation of ciprofloxacin by Gd-Co/g-C3N4 under low-power light source: Degradation pathways and mechanistic insights. J. Water Process Eng. 2024, 58, 104849. [Google Scholar] [CrossRef]
- Huang, Y.; Nengzi, L.C.; Zhang, X.; Gou, J.; Gao, Y.; Zhu, G.; Cheng, Q.; Cheng, X. Catalytic degradation of ciprofloxacin by magnetic CuS/Fe2O3/Mn2O3 nanocomposite activated peroxymonosulfate: Influence factors, degradation pathways and reaction mechanism. Chem. Eng. J. 2020, 388, 124274. [Google Scholar] [CrossRef]
- Campelj, S.; Makovec, D.; Drofenik, M. Preparation and properties of water-based magnetic fluids. J. Phys. Condens. Matter 2008, 20, 204101. [Google Scholar] [CrossRef]
- Zemljič, L.F.; Čakara, D.; Michaelis, N.; Heinze, T.; Kleinschek, K.S. Protonation behavior of 6-deoxy-6-(2-aminoethyl)amino cellulose: A potentiometric titration study. Cellulose 2011, 18, 33–43. [Google Scholar] [CrossRef]
- Bračič, M.; Mohan, T.; Kargl, R.; Grießer, T.; Heinze, T.; Stana Kleinschek, K. Protein repellent anti-coagulative mixed-charged cellulose derivative coatings. Carbohydr. Polym. 2021, 254, 117437. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y.K. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef] [PubMed]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Reactions, Occurrences and Uses, 2nd ed.; WILEY-VCH: Weinheim, Baden, Germany, 2003. [Google Scholar]
- Plohl, O.; Finšgar, M.; Gyergyek, S.; Ajdnik, U.; Ban, I.; Fras Zemljič, L. Efficient Copper Removal from an Aqueous Anvironment using a Novel and Hybrid Nanoadsorbent Based on Derived-Polyethyleneimine Linked to Silica Magnetic Nanocomposites. Nanomaterials 2019, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Plohl, O.; Simonič, M.; Kolar, K.; Gyergyek, S.; Zemljič, L.F. Magnetic nanostructures functionalized with a derived lysine coating applied to simultaneously remove heavy metal pollutants from environmental systems. Sci. Technol. Adv. Mater. 2021, 22, 55–71. [Google Scholar] [CrossRef]
- Silva, C.G.; Sampaio, M.J.; Marques, R.R.N.; Ferreira, L.A.; Tavares, P.B.; Silva, A.M.T.; Faria, J.L. Photocatalytic production of hydrogen from methanol and saccharides using carbon nanotube-TiO2 catalysts. Appl. Catal. B Environ. 2015, 178, 82–90. [Google Scholar] [CrossRef]
- Novak, U.; Grdadolnik, J. Infrared spectra of hydrogen bond network in lamellar perfluorocarboxylic acid monohydrates. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2021, 253, 119551. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Pan, S.; Liu, X.; Guo, M.; Yu, S.F.; Huang, H.; Fan, H.; Li, G. Engineering the intermediate band states in amorphous Ti3+-doped TiO2 for hybrid dye-sensitized solar cell applications. J. Mater. Chem. A 2015, 3, 11437–11443. [Google Scholar] [CrossRef]
- Sikdar, S.; Pathak, S.; Ghorai, T.K. Aqueous phase photodegradation of rhodamine B and p-nitrophenol desctruction using titania based nanocomposites. Adv. Mater. Lett. 2015, 6, 867–873. [Google Scholar] [CrossRef]
- Plohl, O.; Zemljič, L.F.; Potrč, S.; Luxbacher, T. Applicability of electro-osmotic flow for the analysis of the surface zeta potential. RSC Adv. 2020, 10, 6777–6789. [Google Scholar] [CrossRef]
- Plohl, O.; Kralj, S.; Majaron, B.; Fröhlich, E.; Ponikvar-svet, M.; Makovec, D.; Lisjak, D. Amphiphilic coatings for the protection of upconverting nanoparticles against dissolution in aqueous media. Dalt. Trans. 2017, 46, 6975–6984. [Google Scholar] [CrossRef]
- Gyergyek, S.; Pahovnik, D.; Žagar, E.; Mertelj, A.; Kostanjšek, R.; Beković, M.; Jagodič, M.; Hofmann, H.; Makovec, D. Nanocomposites comprised of homogeneously dispersed magnetic iron-oxide nanoparticles and poly(methyl methacrylate). Beilstein J. Nanotechnol. 2018, 9, 1612–1622. [Google Scholar] [CrossRef]
- Plohl, O.; Fras Zemljič, L.; Vihar, B.; Vesel, A.; Gyergyek, S.; Maver, U.; Ban, I.; Bračič, M. Novel magnetic iron oxide-dextran sulphate nanocomposites as potential anticoagulants: Investigating interactions with blood components and assessing cytotoxicity. Carbohydr. Polym. 2024, 343, 122469. [Google Scholar] [CrossRef] [PubMed]
- Dušak, P.; Benčina, M.; Turk, M.; Bavčar, D.; Košmerl, T.; Berovič, M.; Makovec, D. Application of magneto-responsive Oenococcus oeni for the malolactic fermentation in wine. Biochem. Eng. J. 2016, 110, 134–142. [Google Scholar] [CrossRef]
- Kralj, S.; Makovec, D. The chemically directed assembly of nanoparticle clusters from superparamagnetic iron-oxide nanoparticles. RSC Adv. 2014, 4, 13167–13171. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Meng, X.; Shen, T.; Sun, J.; Han, W.; Wang, X. Effects of particle size on the structure and photocatalytic performance by alkali-treated TiO2. Nanomaterials 2020, 10, 546. [Google Scholar] [CrossRef]
- Amano, F.; Nogami, K.; Tanaka, M.; Ohtani, B. Correlation between surface area and photocatalytic activity for acetaldehyde decomposition over bismuth tungstate particles with a hierarchical structure. Langmuir 2010, 26, 7174–7180. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore structure and fractal characteristics of different shale lithofacies in the dalong formation in the western area of the lower yangtze platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Siemieniewska, T. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Lindgren, T.; Mwabora, J.M.; Avandaño, E.; Jonsson, J.; Hoel, A.; Granqvist, C.G.; Lindquist, S.E. Photoelectrochemical and optical properties of nitrogen doped titanium dioxide films prepared by reactive DC magnetron sputtering. J. Phys. Chem. B 2003, 107, 5709–5716. [Google Scholar] [CrossRef]
- Mallick, P.; Dash, B.N. X-ray Diffraction and UV-Visible Characterizations of α-Fe2O3 Nanoparticles Annealed at Different Temperature. Nanosci. Nanotechnol. 2013, 3, 130–134. [Google Scholar]
- Fang, X.; Lu, G.; Mahmood, A.; Tang, Z.; Liu, Z.; Zhang, L.; Wang, Y.; Sun, J. A novel ternary Mica/TiO2/Fe2O3 composite pearlescent pigment for the photocatalytic degradation of acetaldehyde. J. Photochem. Photobiol. A Chem. 2020, 400, 112617. [Google Scholar] [CrossRef]
- Abazović, N.D.; Čomor, M.I.; Dramićanin, M.D.; Jovanović, D.J.; Ahrenkiel, S.P.; Nedeljković, J.M. Photoluminescence of anatase and rutile TiO2 particles. J. Phys. Chem. B 2006, 110, 25366–25370. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, L.; Hemamalini, R.; Rajendran, S.; Qin, J.; Yola, M.L.; Atar, N.; Gracia, F. Nanosized Fe3O4 incorporated on a TiO2 surface for the enhanced photocatalytic degradation of organic pollutants. J. Mol. Liq. 2019, 287, 2–8. [Google Scholar] [CrossRef]
- Song, S.Y.; Chen, H.D.; Li, C.X.; Shi, D.S.; Ying, Y.; Han, Y.B.; Xu, J.C.; Hong, B.; Jin, H.X.; Jin, D.F.; et al. Magnetic Bi2WO6 nanocomposites: Synthesis, magnetic response and their visible-light-driven photocatalytic performance for ciprofloxacin. Chem. Phys. 2020, 530, 110614. [Google Scholar] [CrossRef]
- Navó, J.A.; Colón, G.; Trillas, M.; Peral, J.; Domènech, X.; Testa, J.J.; Padrón, J.; Rodŕguez, D.; Litter, M.I. Heterogeneous photocatalytic reactions of nitrite oxidation and Cr(VI) reduction on iron-doped titania prepared by the wet impregnation method. Appl. Catal. B Environ. 1998, 16, 187–196. [Google Scholar] [CrossRef]


| Sample/Element (at %) | C | O | Fe | N | Ti |
|---|---|---|---|---|---|
| IO NPs | 14.7 ± 1.0 | 65.8 ± 1.8 | 19.5 ± 1.3 | ||
| IO-bPEI | 18.6 ± 1.1 | 57.3 ± 0.7 | 17.8 ± 1.1 | 6.3 ± 0.7 | |
| Citric acid (CA) | 49.0 ± 1.6 | 51.0 ± 1.4 | |||
| TiO2@CA | 12.4 ± 2.0 | 62.3 ± 1.4 | 25.3 ± 1.1 | ||
| IO-TiO2 | 21.9 ± 2.2 | 56.2 ± 1.0 | 7.5 ± 0.6 | 2.9 ± 1.2 | 11.6 ± 1.1 |
| Sample | SBET | Vpore | dpore |
|---|---|---|---|
| m2/g | cm3/g | nm | |
| IO-bPEI | 55.8 | 0.24 | 14.5 |
| TiO2@CA | 246.8 | 0.47 | 7.6 |
| IO-TiO2 | 165.3 | 0.45 | 10.1 |
| Sample | Band Gap |
|---|---|
| eV | |
| IO-bPEI | 1.7 |
| TiO2@CA | 3.2 |
| IO-TiO2 | 1.8 |
| Studied Catalyst | Photocatalytic Conditions | Ratio of Catalyst/Ciprofloxacin | Illumination Source | Degradation Efficiency of Ciprofloxacin at (Duration) | Reference |
|---|---|---|---|---|---|
| Chlorophyll sensitized and salicylic acid functionalized TiO2 nanoparticles | 0.75 g/L catalyst dosage, initial pH of 6, and 10 ppm of initial ciprofloxacin | 75 | with visible light (λmax = 457 nm) | ~75% (120 min) | [58] |
| Fe2O3 nanoparticles loaded on graphitic carbon nitride | pH of 7, photocatalytic dosage of 0.3 g/L, and 25 mg/L of ciprofloxacin | 12 | under visible light radiation | 100% (60 min) | [59] |
| defective TiO2-x nanomaterial | 10 ppm CIP solution in the presence of 0.5 g/L TiO2 | 50 | the visible range | about 10% (180 min) | [60] |
| magnetite (Fe3O4) nanoparticles | pH 6 for ciprofloxacin, dosage of the photocatalyst (0.12 g), concentration (100 mg/L), | 1.2 | sunlight radiation | 73.51% for magnetic nanocomposites, 54.57% magnetic nanoparticles (240 min) | [47] |
| waste polystyrene and TiO2 composites | a pH value of 3 and an initial ciprofloxacin concentration of 5 mg/L | / | (400–800 nm) used as a light source | 95.01% (180 min) | [34] |
| TiO2 nanoparticles immobilized on a glass plate | pH of 5, ciprofloxacin initial concentration of 3 mg/L. | / | UV-C irradiation | 86.57% (105 min) | [33] |
| Fe3O4/P(NIPAM-co-MAA)/Ag-TiO2 | initial concentration of 5 mg/L; concentration of the nanocomposites: 460 mg/L | 92 | visible-light irradiation | 47% | [54] |
| γ-Fe2O3/Bi2WO6 | 0.1 g photocatalysts and 100 mL ciprofloxacin (15 mg/L) | 67 | visible light irradiation | 65% (120 min) | [98] |
| ZnIn2S4/CoFe2O4 p-n junction-decorated biochar | 50 mL ciprofloxacin solution (20 mg/L, pH = 7) | 25 | simulated sunlight | 96.9% (120 min) | [63] |
| CuFe2O4@methyl cellulose based magnetic nanobiocomposite | pH = 7, ciprofloxacin initial concentration of 3 mg/L, photocatalyst loading of 0.2 g | / | UV-C lamps | 80.74% (90 min) | [66] |
| Ni-doped iron oxide/graphitic carbon nitride nanocomposites | catalyst = 15 mg in 50 mL of ciprofloxacin with 10 mg/L, | 30 | solar light exposure | 82.1% (120 min) | [68] |
| IO-TiO2 heterostructures | optimal pH of 7, and ciprofloxacin initial concentration of 0.008 g/L and 50 mL, 10 mg of nanocomposite | 25 | UV-B irradiation | Approx. 70% (150 min) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radić, J.; Žerjav, G.; Jurko, L.; Bošković, P.; Fras Zemljič, L.; Vesel, A.; Mavrič, A.; Gudelj, M.; Plohl, O. First Utilization of Magnetically-Assisted Photocatalytic Iron Oxide-TiO2 Nanocomposites for the Degradation of the Problematic Antibiotic Ciprofloxacin in an Aqueous Environment. Magnetochemistry 2024, 10, 66. https://doi.org/10.3390/magnetochemistry10090066
Radić J, Žerjav G, Jurko L, Bošković P, Fras Zemljič L, Vesel A, Mavrič A, Gudelj M, Plohl O. First Utilization of Magnetically-Assisted Photocatalytic Iron Oxide-TiO2 Nanocomposites for the Degradation of the Problematic Antibiotic Ciprofloxacin in an Aqueous Environment. Magnetochemistry. 2024; 10(9):66. https://doi.org/10.3390/magnetochemistry10090066
Chicago/Turabian StyleRadić, Josip, Gregor Žerjav, Lucija Jurko, Perica Bošković, Lidija Fras Zemljič, Alenka Vesel, Andraž Mavrič, Martina Gudelj, and Olivija Plohl. 2024. "First Utilization of Magnetically-Assisted Photocatalytic Iron Oxide-TiO2 Nanocomposites for the Degradation of the Problematic Antibiotic Ciprofloxacin in an Aqueous Environment" Magnetochemistry 10, no. 9: 66. https://doi.org/10.3390/magnetochemistry10090066
APA StyleRadić, J., Žerjav, G., Jurko, L., Bošković, P., Fras Zemljič, L., Vesel, A., Mavrič, A., Gudelj, M., & Plohl, O. (2024). First Utilization of Magnetically-Assisted Photocatalytic Iron Oxide-TiO2 Nanocomposites for the Degradation of the Problematic Antibiotic Ciprofloxacin in an Aqueous Environment. Magnetochemistry, 10(9), 66. https://doi.org/10.3390/magnetochemistry10090066










