Hexakis (propargyl-1H-tetrazole) Iron(II) X2 [X = BF4, ClO4]—Spin Switchable Complexes with Functionalization Potential and the Myth of the Explosive SCO Compound
Abstract
:1. Introduction
2. Results and Discussion
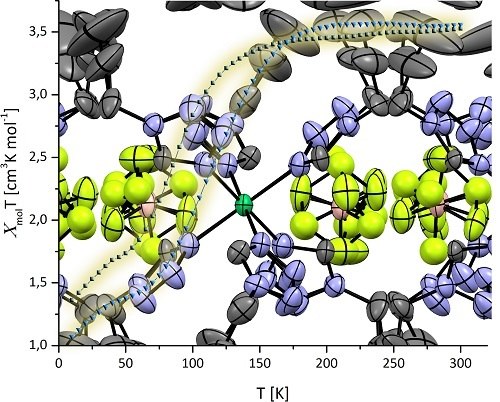
2.1. Magnetic Properties
2.2. Molecular Structure
2.3. Variable Temperature Spectroscopy
2.3.1. MIR-Spectroscopy
2.3.2. FIR-Spectroscopy
2.3.3. Visible/Near-Infrared (VIS/NIR)-Spectroscopy
2.4. Post-Functionalization of the prgTz Complexes
2.5. Thermal and Mechanic Sensitivity
2.6. Theoretical Modeling
3. Experimental Section
3.1. General Methodology
3.2. Sample Analysis
3.3. Ligand Synthesis
Propargyl-1H-tetrazole
3.4. Complexation Reactions
3.4.1. [Fe(prgTz)6](BF4)2 (1)
3.4.2. [Fe(prgTz)6](ClO4)2 (2)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. Spin crossover in transition metal compounds I. In Topics in Current Chemistry; Springer: Berlin, Heidelberg, Germany, 2004. [Google Scholar]
- Real, J.A.; Gaspar, A.B.; Niel, V.; Munoz, M.C. Communication between iron(II) building blocks in cooperative spin transition phenomena. Coord. Chem. Rev. 2003, 236, 121–141. [Google Scholar] [CrossRef]
- Goodwin, H.A. Spin transitions in 6-coordinate iron(II) complexes. Coord. Chem. Rev. 1976, 18, 293–325. [Google Scholar] [CrossRef]
- Gütlich, P.; Garcia, Y.; Goodwin, H.A. Spin crossover phenomena in Fe(II) complexes. Chem. Soc. Rev. 2000, 29, 419–427. [Google Scholar] [CrossRef]
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and optical switching of iron(II) complexes. Angew. Chem. Int. Ed. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Boillot, M.L.; Pillet, S.; Tissot, A.; Riviere, E.; Claiser, N.; Lecomte, C. Ligand-driven light-induced spin change activity and bidirectional photomagnetism of styrylpyridine iron(II) complexes in polymeric media. Inorg. Chem. 2009, 48, 4729–4736. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O.; Martinez, C.J. Spin-transition polymers: From molecular materials toward memory devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Loutete-Dangui, E.D.; Varret, F.; Codjovi, E.; Dahoo, P.R.; Tokoro, H.; Ohkoshi, S.; Eypert, C.; Letard, J.F.; Coanga, J.M.; Boukheddaden, K. Thermal spin transition in [Fe(NH2-trz)3]Br2 investigated by spectroscopic ellipsometry. Phys. Rev. B 2007, 75. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnar, G.; Demont, P.; Menegotto, J. Observation of a thermal hysteresis loop in the dielectric constant of spin crossover complexes: Towards molecular memory devices. J. Mater. Chem. 2003, 13, 2069–2071. [Google Scholar] [CrossRef]
- Mounaix, P.; Freysz, E.; Degert, J.; Daro, N.; Letard, J.F.; Kuzel, P.; Vigneras, V.; Oyenhart, L. One-dimensional tunable photonic crystals with spin crossover material for the terahertz range. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Van Koningsbruggen, P.J.; Garcia, Y.; Kahn, O.; Fournes, L.; Kooijman, H.; Spek, A.L.; Haasnoot, J.G.; Moscovici, J.; Provost, K.; Michalowicz, A.; et al. Synthesis, crystal structure, EXAFS, and magnetic properties of catena [µ-Tris(1,2-bis(tetrazol-1-yl)propane-N1,N1′)iron(II)] Bis(perchlorate). First crystal structure of an iron(II) spin-crossover chain compound. Inorg. Chem. 2000, 39, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Bousseksou, A.; Molnar, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Cambi, L.; Szego, L. The magnetic susceptibility of complex compounds. Ber. Dtsch. Chem. Ges. 1931, 64, 2591–2598. [Google Scholar] [CrossRef]
- Cambi, L.; Szego, L. The magnetic susceptibility of complex compounds (II report). Ber. Dtsch. Chem. Ges. 1933, 66, 656–661. [Google Scholar] [CrossRef]
- Decurtins, S.; Gütlich, P.; Kohler, C.P.; Spiering, H.; Hauser, A. Light-induced excited spin state trapping in a transition-metal complex—The hexa-1-propyltetrazole-iron (II) tetrafluoroborate spin-crossover system. Chem. Phys. Lett. 1984, 105, 1–4. [Google Scholar] [CrossRef]
- Decurtins, S.; Gütlich, P.; Hasselbach, K.M.; Hauser, A.; Spiering, H. Light-induced excited-spin-state trapping in iron(II) spin-crossover systems—Optical spectroscopic and magnetic-susceptibility study. Inorg. Chem. 1985, 24, 2174–2178. [Google Scholar] [CrossRef]
- Decurtins, S.; Gütlich, P.; Kohler, C.P.; Spiering, H. New examples of light-induced excited spin state trapping (LIESST) in iron(II) spin-crossover systems. J. Chem. Soc. Chem. Commun. 1985, 430–432. [Google Scholar] [CrossRef]
- Bonhommeau, S.; Molnar, G.; Galet, A.; Zwick, A.; Real, J.A.; McGarvey, J.J.; Bousseksou, A. One shot laser pulse induced reversible spin transition in the spin-crossover complex [Fe(C4H4N2){Pt(CN)4}] at room temperature. Angew. Chem. 2005, 44, 4069–4073. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Isozaki, H.; Tajima, H. Reproducible on-off switching of the light emission from the electroluminescent device containing a spin crossover complex. Thin Solid Films 2008, 517, 1465–1467. [Google Scholar] [CrossRef]
- Titos-Padilla, S.; Herrera, J.M.; Chen, X.W.; Delgado, J.J.; Colacio, E. Bifunctional hybrid SiO2 nanoparticles showing synergy between core spin crossover and shell luminescence properties. Angew. Chem. Int. Ed. 2011, 50, 3290–3293. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, P.G.; Malfant, I.; Real, J.A.; Rodriguez, V. From magnetic to nonlinear optical switches in spin-crossover complexes. Eur. J. Inorg. Chem. 2013, 2013, 615–627. [Google Scholar] [CrossRef]
- Neville, S.M.; Halder, G.J.; Chapman, K.W.; Duriska, M.B.; Moubaraki, B.; Murray, K.S.; Kepert, C.J. Guest tunable structure and spin crossover properties in a nanoporous coordination framework material. J. Am. Chem. Soc. 2009, 131, 12106–12108. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Gimenez-Marques, M.; Espallargas, G.M.; Rey, F.; Vitorica-Yrezabal, I.J. Spin-crossover modification through selective CO2 sorption. J. Am. Chem. Soc. 2013, 135, 15986–15989. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Lara, F.J.; Gaspar, A.B.; Munoz, M.C.; Lysenko, A.B.; Domasevitch, K.V.; Real, J.A. Fast detection of water and organic molecules by a change of color in an iron(II) microporous spin-crossover coordination polymer. Inorg. Chem. 2012, 51, 13078–13080. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Weinberger, P.; Mereiter, K.; Werner, F.; Molnar, G.; Bousseksou, A.; Valtiner, M.; Linert, W. Comparative investigations on a series of [hexakis(1-(tetrazol-1-yl)alkane-N4)iron(II)] bis(tetrafluoroborate) spin crossover complexes: Methyl- to butyl-substituted species. Inorg. Chim. Acta 2008, 361, 1291–1297. [Google Scholar] [CrossRef]
- Gütlich, P. Spin crossover in iron(II)-complexes. Struct. Bond. 1981, 44, 83–195. [Google Scholar]
- Franke, P.L.; Haasnoot, J.G.; Zuur, A.P. Tetrazoles as ligands Iron(II) complexes of monofunctional tetrazole ligands, showing high-spin reversible low-spin transitions. Inorg. Chim. Acta 1982, 59, 5–9. [Google Scholar] [CrossRef]
- Dirtu, M.M.; Rotaru, A.; Gillard, D.; Linares, J.; Codjovi, E.; Tinant, B.; Garcia, Y. Prediction of the spin transition temperature in FeII one-dimensional coordination polymers: An anion based database. Inorg. Chem. 2009, 48, 7838–7852. [Google Scholar] [CrossRef] [PubMed]
- Absmeier, A.; Bartel, M.; Carbonera, C.; Jameson, G.N.L.; Werner, F.; Reissner, M.; Caneschi, A.; Letard, J.F.; Linert, W. Mutual influence of spacer length and noncoordinating anions on thermal and light-induced spin-crossover properties of iron(II)-α,ω-Bis(tetrazol-1-yl)alkane coordination polymers. Eur. J. Inorg. Chem. 2007, 3047–3054. [Google Scholar] [CrossRef]
- Valtiner, M.; Paulsen, H.; Weinberger, P.; Linert, W. Theoretical investigations of a series of [hexakis(1-(tetrazol-1-yl)alkane-N4)iron(II)]bis(tetrafluoroborate) spin crossover complexes: Methyl-to-pentyl substituted species in the approximation of free cations. Match-Commun. Math. Comput. Chem. 2007, 57, 749–761. [Google Scholar]
- Lucon, J.; Abedin, M.J.; Uchida, M.; Liepold, L.; Jolley, C.C.; Young, M.; Douglas, T. A click chemistry based coordination polymer inside small heat shock protein. Chem. Commun. 2010, 46, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Schweinfurth, D.; Demeshko, S.; Khusniyarov, M.M.; Dechert, S.; Gurram, V.; Buchmeiser, M.R.; Meyer, F.; Sarkar, B. Capped-tetrahedrally coordinated Fe(II) and Co(II) complexes using a “click”-derived tripodal ligand: Geometric and electronic structures. Inorg. Chem. 2012, 51, 7592–7597. [Google Scholar] [CrossRef] [PubMed]
- Schweinfurth, D.; Demeshko, S.; Hohloch, S.; Steinmetz, M.; Brandenburg, J.G.; Dechert, S.; Meyer, F.; Grimme, S.; Sarkar, B. Spin crossover in Fe(II) and Co(II) complexes with the same click-derived tripodal ligand. Inorg. Chem. 2014, 53, 8203–8212. [Google Scholar] [CrossRef] [PubMed]
- Fair, H.D.; Walker, R.F. Energetic Materials; Plenum Press: New York, NY, USA, 1977. [Google Scholar]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the colle-salvetti correlation-energy formula into a functional of the electron-density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab-initio calculation of vibrational absorption and circular-dichroism spectra using density-functional force-fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Papai, M.; Vanko, G.; de Graaf, C.; Rozgonyi, T. Theoretical investigation of the electronic structure of Fe(II) complexes at spin-state transitions. J. Chem. Theory Comput. 2013, 9, 509–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cirera, J.; Paesani, F. Theoretical prediction of spin-crossover temperatures in ligand-driven light-induced spin change systems. Inorg. Chem. 2012, 51, 8194–8201. [Google Scholar] [CrossRef] [PubMed]
- Droghetti, A.; Alfe, D.; Sanvito, S. Assessment of density functional theory for iron(II) molecules across the spin-crossover transition. J. Chem. Phys. 2012, 137. [Google Scholar] [CrossRef] [PubMed]
- Reiher, M.; Salomon, O.; Hess, B.A. Reparameterization of hybrid functionals based on energy differences of states of different multiplicity. Theor. Chem. Acc. 2001, 107, 48–55. [Google Scholar] [CrossRef]
- Ditchfield, R.; Miller, D.P.; Pople, J.A. Self-consistent molecular orbital methods. Molecular orbital theory of nmr chemical shifts. J. Chem. Phys. 1971, 54, 4186–4193. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann Kidlington: Oxford, UK; Waltham, MA, USA, 2013. [Google Scholar]
- Bruker Analytical X-ray Instruments, I. Available online: https://www.bruker.com/products (accessed on 19 February 2016).
- Sheldrick, G.M. Shelxs 97, Program. for the Solution of Crystal Structure; University of Göttingen: Göttingen, Germany, 1997; Volume 125. [Google Scholar]
- Sheldrick, G.M. Shelxl 97, Program. for X-Ray Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Muttenthaler, M.; Bartel, M.; Weinberger, P.; Hilscher, G.; Linert, W. Synthesis and characterisation of new ditetrazole-ligands as more rigid building blocks of envisaged iron(II) spin-crossover coordination polymers. J. Mol. Struct. 2005, 741, 159–169. [Google Scholar] [CrossRef]








| Empirical Formula | C24H21B2F8N24Ni | FW | 877.89 |
|---|---|---|---|
| Z= | 12 | Space group | R |
| λ= | 0.71073 Å | T, K | 200 |
| a, Å | 20.221(2) | - | - |
| c, Å | 35.398(4) | - | - |
| V, Å3 | 12534.7 | calc, g·cm−3 | 1.396 |
| Final R1 | 0.102 | Final wR2 | 0.37 |
| Atom Number | Bond length [Å] | Atom Number | Angles [deg] |
|---|---|---|---|
| Ni(1)–N4 | 2.110 | N4–Ni(1)–N4 | 180.00 |
| Ni(1)–N4A | 2.089 | N4C–Ni(2)–N4C | 180.00 |
| Ni(1)–N4B | 2.095 | N4–Ni(1)–N4A | 89.78 |
| Ni(2)–N4C | 2.083 | N4–Ni(1)–N4B | 90.59 |
| N2–N3 | 1.302 | N4C–Ni(1)–N4C | 90.69 |
| N2A–N3A | 1.287 | - | - |
| N2B–N3B | 1.271 | - | - |
| N2C–N3C | 1.296 | - | - |
| Vibration | LS exp. (cm−1) | HS exp. (cm−1) | (Δν) exp. (cm−1) | LS calc. (cm−1) | HS calc. (cm−1) | (Δν) calc. (cm−1) |
|---|---|---|---|---|---|---|
| ν(CNTz) | 1507 | 1500 | −7 | 1441 | 1436 | −5 |
| ν(C≡C) | 2140 | 2140 | 0 | 2222 | 2222 | 0 |
| ν(CHTz) | 3146 | 3149 | +3 | 3308 | 3304 | −4 |
| ν(≡CH) | 3294 | 3297 | +3 | 3454 | 3454 | 0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifried, M.; Knoll, C.; Giester, G.; Reissner, M.; Müller, D.; Weinberger, P. Hexakis (propargyl-1H-tetrazole) Iron(II) X2 [X = BF4, ClO4]—Spin Switchable Complexes with Functionalization Potential and the Myth of the Explosive SCO Compound. Magnetochemistry 2016, 2, 12. https://doi.org/10.3390/magnetochemistry2010012
Seifried M, Knoll C, Giester G, Reissner M, Müller D, Weinberger P. Hexakis (propargyl-1H-tetrazole) Iron(II) X2 [X = BF4, ClO4]—Spin Switchable Complexes with Functionalization Potential and the Myth of the Explosive SCO Compound. Magnetochemistry. 2016; 2(1):12. https://doi.org/10.3390/magnetochemistry2010012
Chicago/Turabian StyleSeifried, Marco, Christian Knoll, Gerald Giester, Michael Reissner, Danny Müller, and Peter Weinberger. 2016. "Hexakis (propargyl-1H-tetrazole) Iron(II) X2 [X = BF4, ClO4]—Spin Switchable Complexes with Functionalization Potential and the Myth of the Explosive SCO Compound" Magnetochemistry 2, no. 1: 12. https://doi.org/10.3390/magnetochemistry2010012
APA StyleSeifried, M., Knoll, C., Giester, G., Reissner, M., Müller, D., & Weinberger, P. (2016). Hexakis (propargyl-1H-tetrazole) Iron(II) X2 [X = BF4, ClO4]—Spin Switchable Complexes with Functionalization Potential and the Myth of the Explosive SCO Compound. Magnetochemistry, 2(1), 12. https://doi.org/10.3390/magnetochemistry2010012