Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties
Abstract
:1. General Introduction
2. Anilato-Based Molecular Magnets
2.1. Introduction
2.2. Molecular Paramagnets
2.3. Molecular Ferrimagnets
3. Anilato-Based Multifunctional Molecular Materials
3.1. Introduction
3.2. Achiral Magnetic Molecular Conductors
3.3. Chiral Magnetic Molecular Conductors
3.4. Spin-Crossover Complexes
3.5. Guests Intercalation of Hydrogen-Bond-Supported Layers
4. Anilato-Based Multifunctional Organic Frameworks (MOFs)
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Khanna, J.M.; Malone, M.H.; Euler, K.L.; Brady, L.R. Atromentin, anticoagulant from hydnellum diabolus. J. Pharm. Sci. 1965, 54, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Salituro, G.; Szalkowski, D.; Li, Z.; Zhang, Y.; Royo, I.; Vilella, D.; Diez, M.T.; Pelaez, F.; Ruby, C.; et al. Discovery of a small molecule insulin mimetic with antidiabetic activity in mice. Science 1999, 284, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Macabalang, A.D.; Abe, T.; Hirota, H.; Ohta, T. Thelephorin a: A new radical scavenger from the mushroom thelephora vialis. Tetrahedron 2002, 58, 1103–1105. [Google Scholar] [CrossRef]
- Puder, C.; Wagner, K.; Vettermann, R.; Hauptmann, R.; Potterat, O. Terphenylquinone inhibitors of the src protein tyrosine kinase from stilbella sp. J. Nat. Prod. 2005, 68, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Xu, L.; Szalkowski, D.; Li, Z.; Ding, V.; Kwei, G.; Huskey, S.; Moller, D.E.; Heck, J.V.; Zhang, B.B.; et al. Discovery of a potent, highly selective, and orally efficacious small-molecule activator of the insulin receptor. J. Med. Chem. 2000, 43, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Wood, H.B., Jr.; Black, R.; Salituro, G.; Szalkowski, D.; Li, Z.; Zhang, Y.; Moller, D.E.; Zhang, B.; Jones, A.B. The basal sar of a novel insulin receptor activator. Bioorgan. Med. Chem. Lett. 2000, 10, 1189–1192. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kawata, S. Coordination compounds of 1,4-dihydroxybenzoquinone and its homologues. Structures and properties. Coord. Chem. Rev. 2002, 224, 11–34. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Burstall, M.L. 435. The synthesis of tetracyclines. Part I. Some model diene reactions. J. Chem. Soc. (Resumed) 1959, 2183–2186. [Google Scholar] [CrossRef]
- Jones, R.G.; Shonle, H.A. The preparation of 2,5-dihydroxyquinone. J. Am. Chem. Soc. 1945, 67, 1034–1035. [Google Scholar] [CrossRef]
- Viault, G.; Gree, D.; Das, S.; Yadav, J.S.; Grée, R. Synthesis of a Focused Chemical Library Based on Derivatives of Embelin, a Natural Product with Proapoptotic and Anticancer Properties. Eur. J. Org. Chem. 2011, 7, 1233–1244. [Google Scholar] [CrossRef]
- Wallenfels, K.; Friedrich, K. Über fluorchinone, ii. Zur hydrolyse und alkoholyse des fluoranils. Chem. Ber. 1960, 93, 3070–3082. [Google Scholar] [CrossRef]
- Stenhouse, J. On chloranil and bromanil. J. Chem. Soc. 1870, 23, 6–14. [Google Scholar] [CrossRef]
- Benmansour, S.; Vallés-García, C.; Gómez-García, C.J. A H-bonded chloranilate chain with an unprecedented topology. Struct. Chem. Crystallogr. Commun. 2015, 1, 1–7. [Google Scholar]
- Torrey, H.A.; Hunter, W.H. The action of iodides on bromanil. Iodanil and some of its derivatives. J. Am. Chem. Soc. 1912, 34, 702–716. [Google Scholar] [CrossRef]
- Meyer, H.O. Eine neue Synthese der Nitranilsäure. Berichte der Deutschen Chemischen Gesellschaft (A and B Series) 1924, 57, 326–328. [Google Scholar] [CrossRef]
- Fatiadi, A.J.; Sager, W.F. Tetrahydroxyquinone. Org. Synth. 1962, 42, 90. [Google Scholar]
- Gelormini, O.; Artz, N.E. The oxidation of inosite with nitric acid. J. Am. Chem. Soc. 1930, 52, 2483–2494. [Google Scholar] [CrossRef]
- Hoglan, F.A.; Bartow, E. Preparation and properties of derivatives of inositol. J. Am. Chem. Soc. 1940, 62, 2397–2400. [Google Scholar] [CrossRef]
- Junek, H.; Unterweger, B.; Peltzmann, R.Z. Notizen: Eine einfache synthese von tetrahydroxybenzochinon-1.4/A simple synthesis of tetrahydroxy-benzoquinone-1,4. Z. Naturforsch. B 1978, 33B, 1201–1203. [Google Scholar] [CrossRef]
- Preisler, P.W.; Berger, L. Preparation of tetrahydroxyquinone and rhodizonic acid salts from the product of the oxidation of inositol with nitric acid. J. Am. Chem. Soc. 1942, 64, 67–69. [Google Scholar] [CrossRef]
- Zaman, B.M.; Morita, Y.; Toyoda, J.; Yamochi, H.; Sekizaki, S.; Nakasuji, K. Convenient preparation and properties of 2,5-dichloro- and 2,5-dibromo-3,6-dicyano-1,4-benzoquinone (cddq and cbdq): Ddq analogs with centrosymmetry. Mol. Cryst. Liq. Cryst. Sci. Technol. Section A Mol. Cryst. Liq. Cryst. 1996, 287, 249–254. [Google Scholar] [CrossRef]
- Wallenfels, K.; Bachmann, G.; Hofmann, D.; Kern, R. Cyansubstituierte chinone—II: 2,3-, 2,5- 2,6-dicyanchinone und tetracyanbenzochinon. Tetrahedron 1965, 21, 2239–2256. [Google Scholar] [CrossRef]
- Rehwoldt, R.E.; Chasen, B.L.; Li, J.B. 2-chloro-5-cyano-3,6-dihydroxybenzoquinone, a new analytical reagent for the spectrophotometric determination of calcium(II). Anal. Chem. 1966, 38, 1018–1019. [Google Scholar] [CrossRef]
- Akutagawa, T.; Nakamura, T. Crystal and electronic structures of hydrogen-bonded 2,5-diamino-3,6-dihydroxy-p-benzoquinone. Cryst. Growth Des. 2006, 6, 70–74. [Google Scholar] [CrossRef]
- Kögl, F.; Lang, A. Über den mechanismus der fichterschen synthese von dialkyl-dioxy-chinonen. Eur. J. Inorg. Chem. 1926, 59, 910–913. [Google Scholar] [CrossRef]
- Fichter, F.; Willmann, A. Ueber synthesen dialkylirter dioxychinone durch ringschluss. Ber. Deutsch. Chem. Ges. 1904, 37, 2384–2390. [Google Scholar] [CrossRef]
- Fichter, F. Ueber synthetische p-dialkylirte dioxychinone. Justus Liebigs Ann. Chem. 1908, 361, 363–402. [Google Scholar] [CrossRef]
- Atzori, M.; Pop, F.; Cauchy, T.; Mercuri, M.L.; Avarvari, N. Thiophene-benzoquinones: Synthesis, crystal structures and preliminary coordination chemistry of derived anilate ligands. Org. Biomol. Chem. 2014, 12, 8752–8763. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; DiPasquale, A.G.; Rheingold, A.L.; White, H.S.; Miller, J.S. Observation of redox-induced electron transfer and spin crossover for dinuclear cobalt and iron complexes with the 2,5-di-tert-butyl-3,6-dihydroxy-1,4-benzoquinonate bridging ligand. J. Am. Chem. Soc. 2009, 131, 6229–6236. [Google Scholar] [CrossRef] [PubMed]
- Semmingsen, D. The crystal and molecular structure of 2,5-dihydroxybenzoquinone at-1620c. Acta Chem. Scand. B 1977, 31, 11–14. [Google Scholar] [CrossRef]
- Munakata, M.; Wu, L.P.; Kuroda-Sowa, T.; Yamamoto, M.; Maekawa, M.; Moriwaki, K. Assembly of a mixed-valence cu(i/ii) system coupled by multiple hydrogen bonding through tetrahydroxybenzoquinone. Inorg. Chim. Acta 1998, 268, 317–321. [Google Scholar] [CrossRef]
- Klug, A. The crystal structure of tetrahydroxy-p-benzoquinone. Acta Crystallogr. 1965, 19, 983–992. [Google Scholar] [CrossRef]
- Robl, C. Crystal structure and hydrogen bonding of 2,5-dihydroxy-3,6-dimethyl-p-benzoquinone. Z. Krist. Cryst. Mater. 1988, 184, 289–293. [Google Scholar] [CrossRef]
- Andersen, E.K.; Andersen, I.G.K. The crystal and molecular structure of hydroxyquinones and salts of hydroxyquinones. Vii. Hydronium cyananilate (cyananilic acid hexahydrate) and hydronium nitranilate (a redetermination). Acta Crystallogra. Sect. B 1975, 31, 379–383. [Google Scholar] [CrossRef]
- Andersen, E.K.; Andersen, I.G.K. The crystal and molecular structure of hydroxyquinones and salts of hydroxyquinones. VIII. Fluoranilic acid. Acta Crystallogr. Sect. B 1975, 31, 384–387. [Google Scholar] [CrossRef]
- Andersen, E. The crystal and molecular structure of hydroxyquinones and salts of hydroxyquinones. I. Chloranilic acid. Acta Crystallogr. 1967, 22, 188–191. [Google Scholar] [CrossRef]
- Andersen, E. The crystal and molecular structure of hydroxyquinones and salts of hydroxyquinones. II. Chloranilic acid dihydrate. Acta Crystallogr. 1967, 22, 191–196. [Google Scholar] [CrossRef]
- Andersen, E. The crystal and molecular structure of hydroxyquinones and salts of hydroxyquinones. V. Hydronium nitranilate, nitranilic acid hexahydrate. Acta Crystallogr. 1967, 22, 204–208. [Google Scholar] [CrossRef]
- Molcanov, K.; Stare, J.; Vener, M.V.; Kojic-Prodic, B.; Mali, G.; Grdadolnik, J.; Mohacek-Grosev, V. Nitranilic acid hexahydrate, a novel benchmark system of the zundel cation in an intrinsically asymmetric environment: Spectroscopic features and hydrogen bond dynamics characterised by experimental and theoretical methods. Phys. Chem. Chem. Phys. 2014, 16, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E. The crystal and molecular structure of hydroxyquinones and salts of hydroxyquinones. III. Ammonium chloranilate monohydrate. Acta Crystallogr. 1967, 22, 196–201. [Google Scholar] [CrossRef]
- Andersen, E. The crystal and molecular structure of hydroxyquinones and salts of hydroxyquinones. IV. Ammonium nitranilate. Acta Crystallogr. 1967, 22, 201–203. [Google Scholar] [CrossRef]
- Biliskov, N.; Kojic-Prodic, B.; Mali, G.; Molcanov, K.; Stare, J. A Partial Proton Transfer in Hydrogen Bond O–H···O in Crystals of Anhydrous Potassium and Rubidium Complex Chloranilates. J. J. Phys. Chem. A 2011, 115, 3154–3166. [Google Scholar] [CrossRef] [PubMed]
- Molcanov, K.; Sabljic, I.; Kojic-Prodic, B. Face-to-face p-stacking in the multicomponent crystals of chloranilic acid, alkali hydrogenchloranilates, and water. CrystEngComm 2011, 13, 4211. [Google Scholar] [CrossRef]
- Molcanov, K.; Juric, M.; Kojic-Prodic, B. Stacking of metal chelating rings with π-systems in mononuclear complexes of copper(II) with 3,6-dichloro-2,5-dihydroxy-1,4-benzoquinone (chloranilic acid) and 2,2′-bipyridine ligands. Dalton Trans. 2013, 42, 15756–15765. [Google Scholar] [CrossRef] [PubMed]
- Molcanov, K.; Kojic-Prodic, B. Face-to-face stacking of quinoid rings of alkali salts of bromanilic acid. Acta Crystallogr. Section B 2012, 68, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Molcanov, K.; Kojic-Prodic, B.; Meden, A. π-stacking of quinoid rings in crystals of alkali diaqua hydrogen chloranilates. CrystEngComm 2009, 11, 1407–1415. [Google Scholar] [CrossRef]
- Robl, C. Complexes with substituted 2,5-dihydroxy-p-benzoquinones: The inclusion compounds [Y(H2O)3]2 (C6Cl2O4)3·6H2O and [Y(H2O)3]2 (C6Br2O4)3·6H2O. Mater. Res. Bull. 1987, 22, 1483–1491. [Google Scholar] [CrossRef]
- Dei, A.; Gatteschi, D.; Pardi, L.; Russo, U. Tetraoxolene radical stabilization by the interaction with transition-metal ions. Inorg. Chem. 1991, 30, 2589–2594. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Gomez-Garcia, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Anil Reddy, M.; Vinayak, B.; Suresh, T.; Niveditha, S.; Bhanuprakash, K.; Prakash Singh, S.; Islam, A.; Han, L.; Chandrasekharam, M. Highly conjugated electron rich thiophene antennas on phenothiazine and phenoxazine-based sensitizers for dye sensitized solar cells. Synth. Metals 2014, 195, 208–216. [Google Scholar] [CrossRef]
- Schweinfurth, D.; Klein, J.; Hohloch, S.; Dechert, S.; Demeshko, S.; Meyer, F.; Sarkar, B. Influencing the coordination mode of tbta (tbta = tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine) in dicobalt complexes through changes in metal oxidation states. Dalton Trans. 2013, 42, 6944–6952. [Google Scholar] [CrossRef] [PubMed]
- Schweinfurth, D.; Khusniyarov, M.M.; Bubrin, D.; Hohloch, S.; Su, C.-Y.; Sarkar, B. Tuning spin–spin coupling in quinonoid-bridged dicopper(II) complexes through rational bridge variation. Inorg. Chem. 2013, 52, 10332–10339. [Google Scholar] [CrossRef] [PubMed]
- Baum, A.E.; Lindeman, S.V.; Fiedler, A.T. Preparation of a semiquinonate-bridged diiron(II) complex and elucidation of its geometric and electronic structures. Chem. Commun. 2013, 49, 6531–6533. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Li, G.-L.; Miao, B.-X.; Ni, Z.-H. Syntheses, Structures and Magnetic Properties of Dinuclear Cobalt(II) Complexes [Co2(TPEA)2(DHBQ)](ClO4)2 and [Co2(TPEA)2(DHBQ)](PF6)2. J. Chem. Crystallogr. 2013, 43, 331. [Google Scholar] [CrossRef]
- Wu, D.-Y.; Huang, W.; Wang, L.; Wu, G. Synthesis, structure, and magnetic properties of a dinuclear antiferromagnetically coupled cobalt complex. Z. Anorg. Allg. Chem. 2012, 638, 401–404. [Google Scholar] [CrossRef]
- Chatterjee, P.B.; Bhattacharya, K.; Kundu, N.; Choi, K.-Y.; Clérac, R.; Chaudhury, M. Vanadium-induced nucleophilic ipso substitutions in a coordinated tetrachlorosemiquinone ring: Formation of the chloranilate anion as a bridging ligand. Inorg. Chem. 2009, 48, 804–806. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx Pieter, C.A.; Viciano-Chumillas, M.; Lutz, M.; Spek, A.L.; Reedijk, J.; van Koten, G.; Klein Gebbink, R.J.M. Oxidative double dehalogenation of tetrachlorocatechol by a bio-inspired cu ii complex: Formation of chloranilic acid. Chemistry 2008, 14, 5567–5576. [Google Scholar] [CrossRef] [PubMed]
- Ghumaan, S.; Sarkar, B.; Maji, S.; Puranik, V.G.; Fiedler, J.; Urbanos, F.A.; Jimenez-Aparicio, R.; Kaim, W.; Lahiri, G.K. Valence-state analysis through spectroelectrochemistry in a series of quinonoid-bridged diruthenium complexes [(acac)2Ru(μ-l)ru(acac)2]n (n = +2, +1, 0, −1, −2). Chemistry 2008, 14, 10816–10828. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; McCusker, J.K. Spin exchange effects on the physicochemical properties of tetraoxolene-bridged bimetallic complexes. Inorg. Chem. 2007, 46, 3257–3274. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Rheingold, A.L.; DiPasquale, A.; Miller, J.S. Characterization of the chloranilate(·3−) π radical as a strong spin-coupling bridging ligand. Inorg. Chem. 2006, 45, 6135–6137. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Xiang, M.; Wu, Q.-G.; He, H.; Cheng, S.-Q.; Cai, X.-Y.; Li, A.-H.; Zhang, Y.-M.; Li, B. Valence tautomerism and photodynamics observed in a dinuclear cobalt-tetraoxolene compound. Inorg. Chim. Acta 2015, 426, 146–149. [Google Scholar] [CrossRef]
- Li, B.; Chen, L.-Q.; Tao, J.; Huang, R.-B.; Zheng, L.-S. Unidirectional charge transfer in di-cobalt valence tautomeric compound finely tuned by ancillary ligand. Inorg. Chem. 2013, 52, 4136–4138. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tao, J.; Sun, H.-L.; Sato, O.; Huang, R.-B.; Zheng, L.-S. Side-effect of ancillary ligand on electron transfer and photodynamics of a dinuclear valence tautomeric complex. Chem. Commun. 2008, 2269–2271. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Maruyama, H.; Sato, O. Valence tautomeric transitions with thermal hysteresis around room temperature and photoinduced effects observed in a cobalt—Tetraoxolene complex. J. Am. Chem. Soc. 2006, 128, 1790–1791. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Horii, Y.; Nakanishi, R.; Ueno, S.; Breedlove, B.K.; Yamashita, M.; Kawata, S. Field-induced single-ion magnetism based on spin-phonon relaxation in a distorted octahedral high-spin cobalt(II) complex. Eur. J. Inorg. Chem. 2016, 3233–3239. [Google Scholar] [CrossRef]
- Horiuchi, S.; Kumai, R.; Tokura, Y. High-temperature and pressure-induced ferroelectricity in Hydrogen-bonded supramolecular crystals of anilic acids and 2,3-di(2-pyridinyl)pyrazine. J. Am. Chem. Soc. 2013, 135, 4492–4500. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, F.; Horiuchi, S.; Minami, N.; Ishibashi, S.; Kobayashi, K.; Kumai, R.; Murakami, Y.; Tokura, Y. Polarization switching ability dependent on multidomain topology in a uniaxial organic ferroelectric. Nano Lett. 2014, 14, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Yakiyama, Y.; Nakasuji, K.; Morita, Y. Proton-transfer salts between an EDT-TTF derivative having imidazole-ring and anilic acids: Multi-dimensional networks by acid-base hydrogen-bonds, pi-stacks and chalcogen atom interactions. Crystengcomm 2011, 13, 3689–3691. [Google Scholar] [CrossRef]
- Horiuchi, S.; Kumai, R.; Tokura, Y. Room-temperature ferroelectricity and gigantic dielectric susceptibility on a supramolecular architecture of phenazine and deuterated chloranilic acid. J. Am. Chem. Soc. 2005, 127, 5010–5011. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.D.; McCleverty, J.A. Non-innocent behaviour in mononuclear and polynuclear complexes: Consequences for redox and electronic spectroscopic properties. J. Chem. Soc. Dalton Trans. 2002, 275–288. [Google Scholar] [CrossRef]
- Tinti, F.; Verdaguer, M.; Kahn, O.; Savariault, J.M. Interaction between copper(II) ions separated by 7.6 A⁰. Crystal structure and magnetic properties of the µ-iodanilato bis[n, n, n′, n′ tetramethylethylenediamine copper(II)] diperchlorate. Inorg. Chem. 1987, 26, 2380–2384. [Google Scholar] [CrossRef]
- Tamaki, H.; Zhong, Z.J.; Matsumoto, N.; Kida, S.; Koikawa, M.; Achiwa, N.; Hashimoto, Y.; Okawa, H. Design of metal-complex magnets. Syntheses and magnetic properties of mixed-metal assemblies {NBu4[MCr(ox)3]}x (NBu4+ = tetra(n-butyl)ammonium ion; ox2− = oxalate ion; M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+). J. Am. Chem. Soc. 1992, 114, 6974–6979. [Google Scholar] [CrossRef]
- Decurtins, S.; Schmalle, H.W.; Oswald, H.R.; Linden, A.; Ensling, J.; Gütlich, P.; Hauser, A. A polymeric two-dimensional mixed-metal network. Crystal structure and magnetic properties of {[P(Ph)4][MnCr(ox)3]}. Inorg. Chim. Acta 1994, 216, 65–73. [Google Scholar] [CrossRef]
- Atovmyan, L.O.; Shilov, G.V.; Lyubovskaya, R.N.; Zhilyaeva, E.I.; Ovanesyan, N.S.; Pirumova, S.I.; Gusakovskaya, I.G.; Morozov, Y.G. Crystal-structure of the molecular ferromagnet NBu4[MnCr(C2O4)3] (Bu = N-C4H9). J. Exp. Theor. Phys. 1993, 58, 766–769. [Google Scholar]
- Mathonière, C.; Nuttall, C.J.; Carling, S.G.; Day, P. Ferrimagnetic mixed-valency and mixed-metal tris(oxalato)iron(III) compounds: Synthesis, structure, and magnetism. Inorg. Chem. 1996, 35, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galan-Mascaros, J.R.; Gómez-García, C.J.; Ensling, J.; Gutlich, P. Hybrid molecular magnets obtained by insertion of decamethylmetallocenium cations in layered bimetallic oxalate complexes. Syntheses, structure and magnetic properties of the series [ZIIICp*2][MIIMIII(ox)3] (ZIII= Co, Fe; MIII = Cr, Fe; MII = Mn, Fe, Co, Ni, Cu; Cp* = pentamethylcyclopentadienyl). Eur. J. Inorg. Chem. 2000, 6, 552–563. [Google Scholar]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Agudo, J.M. Increasing the coercivity in layered molecular-based magnets A[MIIMIII(ox)3] (MII = Mn, Fe, Co, Ni, Cu; MIII = Cr, Fe; ox = oxalate; A = organic or organometallic cation). Adv. Mater. 1999, 11, 558–561. [Google Scholar] [CrossRef]
- Coronado, E.; Clemente-Leon, M.; Galan-Mascaros, J.R.; Gimenez-Saiz, C.; Gomez-Garcia, C.J.; Martinez-Ferrero, E. Design of molecular materials combining magnetic, electrical and optical properties. J. Chem. Soc. Dalton Trans. 2000, 3955–3961. [Google Scholar] [CrossRef]
- Clemente-Leon, M.; Coronado, E.; Galan-Mascaros, J.R.; Gomez-Garcia, C.J. Intercalation of decamethylferrocenium cations in bimetallic oxalate-bridged two-dimensional magnets. Chem. Commun. 1997, 1727–1728. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Agudo, J.M.; Martínez-Ferrero, E.; Waerenborgh, J.C.; Almeida, M. Layered molecule-based magnets formed by decamethylmetallocenium cations and two-dimensional bimetallic complexes [MIIRuIII(ox)3]-(MII=;Mn, Fe, Co, Cu and Zn; ox = oxalate). J. Solid State Chem. 2001, 159, 391–402. [Google Scholar] [CrossRef]
- Bénard, S.; Yu, P.; Audière, J.P.; Rivière, E.; Clément, R.; Guilhem, J.; Tchertanov, L.; Nakatani, K. Structure and NLO properties of layered bimetallic oxalato-bridged ferromagnetic networks containing stilbazolium-shaped chromophores. J. Am. Chem. Soc. 2000, 122, 9444–9454. [Google Scholar] [CrossRef]
- Bénard, S.; Rivière, E.; Yu, P.; Nakatani, K.; Delouis, J.F. A photochromic molecule-based magnet. Chem. Mater. 2001, 13, 159–162. [Google Scholar] [CrossRef]
- Alberola, A.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J. A molecular metal ferromagnet from the organic donor bis(ethylenedithio)tetraselenafulvalene and bimetallic oxalate complexes. J. Am. Chem. Soc. 2003, 125, 10774–10775. [Google Scholar] [CrossRef] [PubMed]
- Aldoshin, S.M.; Nikonova, L.A.; Shilov, G.V.; Bikanina, E.A.; Artemova, N.K.; Smirnov, V.A. The influence of an n-substituent in the indoline fragment of pyrano-pyridine spiropyran salts on their crystalline structure and photochromic properties. J. Mol. Struct. 2006, 794, 103–109. [Google Scholar] [CrossRef]
- Aldoshin, S.M.; Sanina, N.A.; Minkin, V.I.; Voloshin, N.A.; Ikorskii, V.N.; Ovcharenko, V.I.; Smirnov, V.A.; Nagaeva, N.K. Molecular photochromic ferromagnetic based on the layered polymeric tris-oxalate of Cr(III), Mn(II) and 1-[(1′,3′,3′-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2′-indoline]-8-yl)methyl]pyridinium. J. Mol. Struct. 2007, 826, 69–74. [Google Scholar] [CrossRef]
- Kida, N.; Hikita, M.; Kashima, I.; Okubo, M.; Itoi, M.; Enomoto, M.; Kato, K.; Takata, M.; Kojima, N. Control of charge transfer phase transition and ferromagnetism by photoisomerization of spiropyran for an organic—Inorganic hybrid system, (SP)[FeIIFeIII(dto)3] (SP = spiropyran, dto = C2O2S2). J. Am. Chem. Soc. 2009, 131, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Sieber, R.; Decurtins, S.; Stoeckli-Evans, H.; Wilson, C.; Yufit, D.; Howard, J.A.K.; Capelli, S.C.; Hauser, A. A thermal spin transition in [Co(bpy)3][LiCr(ox)3] (ox = C2O42−; bpy = 2,2′-bipyridine). Chemistry 2000, 6, 361–368. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Waerenborgh, J.C. Multifunctional magnetic materials obtained by insertion of spin-crossover FeIII complexes into chiral 3D bimetallic oxalate-based ferromagnets. Inorg. Chem. 2011, 50, 9122–9130. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Mínguez Espallargas, G.; Soriano-Portillo, A.; Waerenborgh, J.C. Multifunctional magnetic materials obtained by insertion of a spin-crossover FeIII complex into bimetallic oxalate-based ferromagnets. Chemistry 2010, 16, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Leon, M.; Coronado, E.; Lopez-Jorda, M.; Desplanches, C.; Asthana, S.; Wang, H.; Letard, J.-F. A hybrid magnet with coexistence of ferromagnetism and photoinduced Fe(III) spin-crossover. Chem. Sci. 2011, 2, 1121–1127. [Google Scholar] [CrossRef]
- Clemente-Leon, M.; Coronado, E.; Lopez-Jorda, M. 2D and 3D bimetallic oxalate-based ferromagnets prepared by insertion of different FeIII spin crossover complexes. Dalton Trans. 2010, 39, 4903–4910. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; Giménez-López, M.C.; Soriano-Portillo, A.; Waerenborgh, J.C.; Delgado, F.S.; Ruiz-Pérez, C. Insertion of a spin crossover FeIII complex into an oxalate-based layered material: Coexistence of spin canting and spin crossover in a hybrid magnet. Inorg. Chem. 2008, 47, 9111–9120. [Google Scholar] [CrossRef] [PubMed]
- Train, C.; Gheorghe, R.; Krstic, V.; Chamoreau, L.-M.; Ovanesyan, N.S.; Rikken, G.L.; Gruselle, M.; Verdaguer, M. Strong magneto-chiral dichroism in enantiopure chiral ferromagnets. Nat. Mater. 2008, 7, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Gruselle, M.; Train, C.; Boubekeur, K.; Gredin, P.; Ovanesyan, N. Enantioselective self-assembly of chiral bimetallic oxalate-based networks. Coord. Chem. Rev. 2006, 250, 2491–2500. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Dias, J.C.; Soriano-Portillo, A.; Willett, R.D. Synthesis, structure, and magnetic properties of [(s)-[PhCH(CH3)n(CH3)3]][Mn(CH3CN)2/3Cr(ox)3]·(CH3CN)_(solvate), a 2D chiral magnet containing a quaternary ammonium chiral cation. Inorg. Chem. 2008, 47, 6458–6463. [Google Scholar] [CrossRef] [PubMed]
- Brissard, M.; Gruselle, M.; Malézieux, B.; Thouvenot, R.; Guyard-Duhayon, C.; Convert, O. An anionic {[MnCo(ox)3]−}n network with appropriate cavities for the enantioselective recognition and resolution of the hexacoordinated monocation [Ru(bpy)2(ppy)]+ (bpy = bipyridine, ppy = phenylpyridine). Eur. J. Inorg. Chem. 2001, 2001, 1745–1751. [Google Scholar] [CrossRef]
- Sadakiyo, M.; O̅kawa, H.; Shigematsu, A.; Ohba, M.; Yamada, T.; Kitagawa, H. Promotion of low-humidity proton conduction by controlling hydrophilicity in layered metal–organic frameworks. J. Am. Chem. Soc. 2012, 134, 5472–5475. [Google Scholar] [CrossRef] [PubMed]
- O̅kawa, H.; Shigematsu, A.; Sadakiyo, M.; Miyagawa, T.; Yoneda, K.; Ohba, M.; Kitagawa, H. Oxalate-bridged bimetallic complexes {NH(prol)3}[MCr(ox)3] (M = MnII, FeII, CoII; NH(prol)3+ = tri(3-hydroxypropyl)ammonium) exhibiting coexistent ferromagnetism and proton conduction. J. Am. Chem. Soc. 2009, 131, 13516–13522. [Google Scholar] [CrossRef] [PubMed]
- Fishman, R.S.; Clemente-León, M.; Coronado, E. Magnetic compensation and ordering in the bimetallic oxalates: Why are the 2D and 3D series so different? Inorg. Chem. 2009, 48, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Clément, R.; Decurtins, S.; Gruselle, M.; Train, C. Polyfunctional two- (2D) and three- (3D) dimensional oxalate bridged bimetallic magnets. Mon. Chem. Chem. Mon. 2003, 134, 117–135. [Google Scholar] [CrossRef]
- Kojima, N.; Aoki, W.; Itoi, M.; Ono, Y.; Seto, M.; Kobayashi, Y.; Maeda, Y. Charge transfer phase transition and Ferromagnetism in a mixed-valence iron complex, (N-C3H7)4n[FeIIFeIII(dto)3] (dto = C2O2S2). Solid State Commun. 2001, 120, 165–170. [Google Scholar] [CrossRef]
- Hisashi, O.; Minoru, M.; Masaaki, O.; Masahito, K.; Naohide, M. Dithiooxalato(dto)-bridged bimetallic assemblies {NPr4[MCr(dto)3]}x (M = Fe, Co, Ni, Zn; NPr4 = tetrapropylammonium ion): New complex-based ferromagnets. Bull. Chem. Soc. Jpn 1994, 67, 2139–2144. [Google Scholar]
- Carling, S.G.; Bradley, J.M.; Visser, D.; Day, P. Magnetic and structural characterisation of the layered materials AMnFe(C2S2O2)3. Polyhedron 2003, 22, 2317–2324. [Google Scholar] [CrossRef]
- Bradley, J.M.; Carling, S.G.; Visser, D.; Day, P.; Hautot, D.; Long, G.J. Structural and physical properties of the ferromagnetic tris-dithiooxalato compounds, A[MIICrIII(C2S2O2)3], with A+ = N(n-CnH2n+1)4+ (n = 3–5) and P(C6H5)4+ and MII = Mn, Fe, Co, and Ni. Inorg. Chem. 2003, 42, 986–996. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Riegler, E.; Robl, C. Polymeric 2,5-dihydroxy-1,4-benzoquinone transition metal complexes Na2(H2O)24[M2(C6H2O4)3] (M = Manganese(2+), Cadmium(2+). Z. Naturforsch. Teil B Anorg. Chem.Org. Chem. 1986, 41, 1501–1505. [Google Scholar]
- Shilov, G.V.; Nikitina, Z.K.; Ovanesyan, N.S.; Aldoshin, S.M.; Makhaev, V.D. Phenazineoxonium chloranilatomanganate and chloranilatoferrate: Synthesis, structure, magnetic properties, and mössbauer spectra. Russ. Chem. Bull. 2011, 60, 1209–1219. [Google Scholar] [CrossRef]
- Luo, T.-T.; Liu, Y.-H.; Tsai, H.-L.; Su, C.-C.; Ueng, C.-H.; Lu, K.-L. A novel hybrid supramolecular network assembled from perfect π-π stacking of an anionic inorganic layer and a cationic hydronium-ion-mediated organic layer. Eur. J. Inorg. Chem. 2004, 2004, 4253–4258. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Coleiro, J.; Hoskins, B.F.; Robson, R. Gas hydrate-like pentagonal dodecahedral M2(H2O)18 cages (M = lanthanide or y) in 2,5-dihydroxybenzoquinone-derived coordination polymers. Chem. Commun. 1996, 603–604. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Coleiro, J.; Ha, K.; Hoskins, B.F.; Orchard, S.D.; Robson, R. Dihydroxybenzoquinone and chloranilic acid derivatives of rare earth metals. J. Chemical. Soc. Dalton Trans. 2002, 1586–1594. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Agudo, J.M. Molecule-based magnets formed by bimetallic three-dimensional oxalate networks and chiral tris(bipyridyl) complex cations. The series [ZII(bpy)3][ClO4][MIICrIII(ox)3] (ZII = Ru, Fe, Co, and Ni; MII = Mn, Fe, Co, Ni, Cu, and Zn; ox = oxalate dianion). Inorg. Chem. 2001, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, B.F.; Hudson, T.A.; McCormick, L.J.; Robson, R. Coordination polymers of 2,5-dihydroxybenzoquinone and chloranilic acid with the (10,3)-atopology. Crys. Growth Des. 2011, 11, 2717–2720. [Google Scholar] [CrossRef]
- Frenzer, W.; Wartchow, R.; Bode, H. Crystal structure of disilver 2,5-dichloro-[1,4]benzoquinone-3,6-diolate, Ag2(C6O4Cl2). Z. Kristallogr. Cryst. Mater. 1997, 212, 237. [Google Scholar] [CrossRef]
- Junggeburth, S.C.; Diehl, L.; Werner, S.; Duppel, V.; Sigle, W.; Lotsch, B.V. Ultrathin 2D coordination polymer nanosheets by surfactant-mediated synthesis. J. Am. Chem. Soc. 2013, 135, 6157–6164. [Google Scholar] [CrossRef] [PubMed]
- Saines, P.J.; Tan, J.-C.; Yeung, H.H.-M.; Barton, P.T.; Cheetham, A.K. Layered inorganic-organic frameworks based on the 2,2-dimethylsuccinate ligand: Structural diversity and its effect on nanosheet exfoliation and magnetic properties. Dalton Trans. 2012, 41, 8585–8593. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Marchiò, L.; Clérac, R.; Serpe, A.; Deplano, P.; Avarvari, N.; Mercuri, M.L. Hydrogen-bonded supramolecular architectures based on tris(hydranilato)metallate(III) (M = Fe, Cr) metallotectons. Cryst. Growth Des. 2014, 14, 5938–5948. [Google Scholar] [CrossRef]
- Min, K.S.; Rhinegold, A.L.; Miller, J.S. Tris(chloranilato)ferrate(iii) anionic building block containing the (dihydroxo)oxodiiron(III) dimer cation: Synthesis and characterization of [(Tpa)(OH)Fe(III)OFe(III)(OH)(Tpa)][Fe(CA)3]0.5(BF4)0.5,1.5MeOH‚H2O [Tpa = tris(2-pyridylmethyl)amine; CA = chloranilate]. J. Am. Chem. Soc. 2006, 128, 40–41. [Google Scholar] [PubMed]
- Hazell, A.; Jensen, K.B.; McKenzie, C.J.; Toftlund, H. Synthesis and reactivity of (.Mu.-oxo)diiron(III) complexes of tris(2-pyridylmethyl)amine. X-ray crystal structures of [Tpa(OH)feofe(H2O)tpa](ClO4)3 and [Tpa(Cl)FeOFe(Cl)Tpa](ClO4)2. Inorg. Chem. 1994, 33, 3127–3134. [Google Scholar] [CrossRef]
- Atzori, M.; Artizzu, F.; Sessini, E.; Marchio, L.; Loche, D.; Serpe, A.; Deplano, P.; Concas, G.; Pop, F.; Avarvari, N.; et al. Halogen-bonding in a new family of tris(haloanilato)metallate(III) magnetic molecular building blocks. Dalton Transactions 2014, 43, 7006–7019. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Artizzu, F.; Marchiò, L.; Loche, D.; Caneschi, A.; Serpe, A.; Deplano, P.; Avarvari, N.; Mercuri, M.L. Switching-on luminescence in anilate-based molecular materials. Dalton Trans. 2015, 44, 15727–16178. [Google Scholar] [CrossRef] [PubMed]
- Benmansour, S.; Valles-Garcia, C.; Gomez-Claramunt, P.; Minguez Espallargas, G.; Gomez-Garcia, C.J. 2d and 3d anilato-based heterometallic M(I)M(III) lattices: The missing link. Inorg. Chem. 2015, 54, 5410–5418. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, M.; Deplano, P.; Serpe, A.; Artizzu, F. Multifunctional materials of interest in molecular electronics. In Multifunctional Molecular Materials; Pan Stanford Publishing: Boca Raton, FL, USA, 2013; pp. 219–280. [Google Scholar]
- Kurmoo, M.; Graham, A.W.; Day, P.; Coles, S.J.; Hursthouse, M.B.; Caulfield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W. Superconducting and semiconducting magnetic charge transfer salts: (BEDT-TTF)4AFe(C2O4)3.C6H5CN (A = H2O, K, NH4). J. Am. Chem. Soc. 1995, 117, 12209–12217. [Google Scholar] [CrossRef]
- Coronado, E.; Day, P. Magnetic molecular conductors. Chem. Rev. 2004, 104, 5419–5448. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Benmansour, S.; Minguez Espallargas, G.; Clemente-Leon, M.; Abherve, A.; Gomez-Claramunt, P.; Coronado, E.; Artizzu, F.; Sessini, E.; Deplano, P.; et al. A Family of layered chiral porous magnets exhibiting tunable ordering temperatures. Inorg. Chem. 2013, 52, 10031–10040. [Google Scholar] [CrossRef] [PubMed]
- Kherfi, H.; Hamadène, M.; Guehria-Laïdoudi, A.; Dahaoui, S.; Lecomte, C. Synthesis, structure and thermal behavior of oxalato-bridged Rb+ and H3O+ extended frameworks with different dimensionalities. Materials 2010, 3, 1281. [Google Scholar] [CrossRef]
- Cañadillas-Delgado, L.; Fabelo, O.; Rodríguez-Velamazán, J.A.; Lemée-Cailleau, M.-H.; Mason, S.A.; Pardo, E.; Lloret, F.; Zhao, J.-P.; Bu, X.-H.; Simonet, V.; et al. The role of order–disorder transitions in the quest for molecular multiferroics: Structural and magnetic neutron studies of a mixed valence Iron(II)–Iron(III) formate framework. J. Am. Chem. Soc. 2012, 134, 19772–19781. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Cui, H.; Kobayashi, A. Organic metals and superconductors based on bets (bets = bis(ethylenedithio)tetraselenafulvalene). Chem. Rev. 2004, 104, 5265–5288. [Google Scholar] [CrossRef] [PubMed]
- Enoki, T.; Miyazaki, A. Magnetic ttf-based charge-transfer complexes. Chem. Rev. 2004, 104, 5449–5478. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Giménez-Saiz, C.; Gómez-García, C.J. Recent advances in polyoxometalate-containing molecular conductors. Coord. Chem. Rev. 2005, 249, 1776–1796. [Google Scholar] [CrossRef]
- Schlueter, J.A.; Geiser, U.; Whited, M.A.; Drichko, N.; Salameh, B.; Petukhov, K.; Dressel, M. Two alternating BEDT-TTF packing motifs in α-κ-(BEDT-TTF)2Hg(SCN)3. Dalton Trans. 2007, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Turner, S.S.; Day, P.; Howard, J.A.K.; Guionneau, P.; McInnes, E.J.L.; Mabbs, F.E.; Clark, R.J.H.; Firth, S.; Biggs, T. New superconducting charge-transfer salts (BEDT-TTF)4[A·M(C2O4)3]·C6H5NO2 (A = H3O or NH4, M = Cr or Fe, BEDT-TTF = bis(ethylenedithio)tetrathiafulvalene). J. Mater. Chem. 2001, 11, 2095–2101. [Google Scholar] [CrossRef]
- Martin, L.; Turner, S.S.; Day, P.; Mabbs, F.E.; McInnes, E.J.L. New molecular superconductor containing paramagnetic Chromium(III) ions. Chem. Commun. 1997, 1367–1368. [Google Scholar] [CrossRef]
- Uji, S.; Shinagawa, H.; Terashima, T.; Yakabe, T.; Terai, Y.; Tokumoto, M.; Kobayashi, A.; Tanaka, H.; Kobayashi, H. Magnetic-field-induced superconductivity in a two-dimensional organic conductor. Nature 2001, 410, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Fujiwara, E.; Nakazawa, Y.; Narymbetov, B.Z.; Kato, K.; Kobayashi, H.; Kobayashi, A.; Tokumoto, M.; Cassoux, P. A novel antiferromagnetic organic superconductor κ-(BETS)2FeBr4 [where BETS = bis(ethylenedithio)tetraselenafulvalene]. J. Am. Chem. Soc. 2001, 123, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Day, P.; Kurmoo, M.; Mallah, T.; Marsden, I.R.; Friend, R.H.; Pratt, F.L.; Hayes, W.; Chasseau, D.; Gaultier, J. Structure and properties of tris[bis(ethylenedithio)tetrathiafulvalenium]tetrachlorocopper(II) hydrate, (BEDT-TTF)3CuCl4·H2O: First evidence for coexistence of localized and conduction electrons in a metallic charge-transfer salt. J. Am. Chem. Soc. 1992, 114, 10722–10729. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Clegg, W.; Harrington, R.W.; Horton, P.N.; Bingham, A.; Hursthouse, M.B.; McMillan, P.; Firth, S. Multi-layered molecular charge-transfer salts containing alkali metal ions. J. Mater. Chem. 2007, 17, 3324–3329. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J.; Alberola, A. Radical salts of bis(ethylenediseleno)tetrathiafulvalene with paramagnetic tris(oxalato)metalate anions. Inorg. Chem. 2006, 45, 10815–10824. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. The series of molecular conductors and superconductors ET4[AFe(C2O4)3]·PhX (ET = bis(ethylenedithio)tetrathiafulvalene; (C2O4)2− = oxalate; A+ = H3O+, K+; X = F, Cl, Br, and I): Influence of the halobenzene guest molecules on the crystal structure and superconducting properties. Inorg. Chem. 2012, 51, 1111–1126. [Google Scholar] [PubMed]
- Coronado, E.; Curreli, S.; Gimenez-Saiz, C.; Gomez-Garcia, C.J. A novel paramagnetic molecular superconductor formed by bis(ethylenedithio)tetrathiafulvalene, tris(oxalato)ferrate(III) anions and bromobenzene as guest molecule: Et4[(H3O)Fe(C2O4)3]·C6H5Br. J. Mater. Chem. 2005, 15, 1429–1436. [Google Scholar] [CrossRef]
- Fourmigué, M.; Batail, P. Activation of hydrogen- and halogen-bonding interactions in tetrathiafulvalene-based crystalline molecular conductors. Chem. Rev. 2004, 104, 5379–5418. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J.; Deplano, P.; Mercuri, M.L.; Serpe, A.; Pilia, L.; Faulmann, C.; Canadell, E. New BEDT-TTF/[Fe(C5O5)3]3- hybrid system: Synthesis, crystal structure, and physical properties of a chirality-induced α phase and a novel magnetic molecular metal. Inorg. Chem. 2007, 46, 4446–4457. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Garcia, C.J.; Coronado, E.; Curreli, S.; Gimenez-Saiz, C.; Deplano, P.; Mercuri, M.L.; Pilia, L.; Serpe, A.; Faulmann, C.; Canadell, E. A chirality-induced alpha phase and a novel molecular magnetic metal in the BEDT-TTF/tris(croconate)Ferrate(III) hybrid molecular system. Chem. Commun. 2006, 4931–4933. [Google Scholar] [CrossRef] [PubMed]
- Avarvari, N.; Wallis, J.D. Strategies towards chiral molecular conductors. J. Mater. Chem. 2009, 19, 4061. [Google Scholar] [CrossRef]
- Pop, F.; Auban-Senzier, P.; Canadell, E.; Rikken, G.L.J.A.; Avarvari, N. Electrical magnetochiral anisotropy in a bulk chiral molecular conductor. Nat. Commun. 2014, 5, 3757. [Google Scholar] [CrossRef] [PubMed]
- Rikken, G.L.J.A.; Fölling, J.; Wyder, P. Electrical magnetochiral anisotropy. Phys. Rev. Lett. 2001, 87, 236602. [Google Scholar] [CrossRef] [PubMed]
- Krstic, V.; Roth, S.; Burghard, M.; Kern, K.; Rikken, G.L.J.A. Magneto-chiral anisotropy in charge transport through single-walled carbon nanotubes. J. Chem. Phys. 2002, 117, 11315. [Google Scholar] [CrossRef]
- De Martino, A.; Egger, R.; Tsvelik, A.M. Nonlinear magnetotransport in interacting chiral nanotubes. Phys. Rev. Lett. 2006, 97, 076402. [Google Scholar] [CrossRef] [PubMed]
- Rethore, C.; Fourmigue, M.; Avarvari, N. Tetrathiafulvalene based phosphino-oxazolines: A new family of redox active chiral ligands. Chem. Commun. 2004, 1384–1385. [Google Scholar] [CrossRef] [PubMed]
- Réthoré, C.; Avarvari, N.; Canadell, E.; Auban-Senzier, P.; Fourmigué, M. Chiral molecular metals: Syntheses, structures, and properties of the AsF6- salts of Racemic (±)-, (R)-, and (S)-tetrathiafulvalene—Oxazoline derivatives. J. Am. Chem. Soc. 2005, 127, 5748–5749. [Google Scholar] [CrossRef] [PubMed]
- Madalan, A.M.; Rethore, C.; Fourmigue, M.; Canadell, E.; Lopes, E.B.; Almeida, M.; Auban-Senzier, P.; Avarvari, N. Order versus disorder in chiral tetrathiafulvalene-oxazoline radical-cation salts: Structural and theoretical investigations and physical properties. Chemistry 2010, 16, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Pop, F.; Auban-Senzier, P.; Frąckowiak, A.; Ptaszyński, K.; Olejniczak, I.; Wallis, J.D.; Canadell, E.; Avarvari, N. Chirality driven metallic versus semiconducting behavior in a complete series of radical cation salts based on dimethyl-ethylenedithio-tetrathiafulvalene (DM-EDT-TTF). J. Am. Chem. Soc. 2013, 135, 17176–17186. [Google Scholar] [CrossRef] [PubMed]
- Karrer, A.; Wallis, J.D.; Dunitz, J.D.; Hilti, B.; Mayer, C.W.; Bürkle, M.; Pfeiffer, J. Structures and electrical properties of some new organic conductors derived from the donor molecule tmet (s,s,s,s,-bis(dimethylethylenedithio) tetrathiafulvalene). Helvetica Chim. Acta 1987, 70, 942–953. [Google Scholar] [CrossRef]
- Wallis, J.D.; Karrer, A.; Dunitz, J.D. Chiral metals? A chiral substrate for organic conductors and superconductors. Helvetica Chim. Acta 1986, 69, 69–70. [Google Scholar] [CrossRef]
- Pop, F.; Laroussi, S.; Cauchy, T.; Gomez-Garcia, C.J.; Wallis, J.D.; Avarvari, N. Tetramethyl-bis(ethylenedithio)-tetrathiafulvalene (TM-BEDT-TTF) revisited: Crystal structures, chiroptical properties, theoretical calculations, and a complete series of conducting radical cation salts. Chirality 2013, 25, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Galán-Mascarós, J.R.; Coronado, E.; Goddard, P.A.; Singleton, J.; Coldea, A.I.; Wallis, J.D.; Coles, S.J.; Alberola, A. A chiral ferromagnetic molecular metal. J. Am. Chem. Soc. 2010, 132, 9271–9273. [Google Scholar] [CrossRef] [PubMed]
- Madalan, A.M.; Canadell, E.; Auban-Senzier, P.; Branzea, D.; Avarvari, N.; Andruh, M. Conducting mixed-valence salt of bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) with the paramagnetic heteroleptic anion [CrIII(oxalate)2(2,2′-bipyridine)]. New J. Chem. 2008, 32, 333–339. [Google Scholar] [CrossRef]
- Martin, L.; Day, P.; Horton, P.; Nakatsuji, S.I.; Yamada, J.I.; Akutsu, H. Chiral conducting salts of BEDT-TTF containing a single enantiomer of tris(oxalato)chromate(III) crystallised from a chiral solvent. J. Mater. Chem. 2010, 20, 2738–2742. [Google Scholar] [CrossRef]
- Pop, F.; Allain, M.; Auban-Senzier, P.; Martínez-Lillo, J.; Lloret, F.; Julve, M.; Canadell, E.; Avarvari, N. Enantiopure conducting salts of dimethylbis(ethylenedithio)tetrathiafulvalene (DM-BEDT-TTF) with the hexachlororhenate(IV) anion. Eur. J. Inorg. Chem. 2014, 2014, 3855–3862. [Google Scholar] [CrossRef]
- Coronado, E.; Minguez Espallargas, G. Dynamic Magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Goodwin, H.A. Spin Crossover in Transition Metal Compounds i; Springer: Berlin, Germany, 2004; Volume 233. [Google Scholar]
- Halcrow, M.A. Spin-Crossover Materials: Properties and Applications; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Min, K.S.; DiPasquale, A.G.; Golen, J.A.; Rheingold, A.L.; Miller, J.S. Synthesis, structure, and magnetic properties of valence ambiguous dinuclear antiferromagnetically coupled cobalt and ferromagnetically coupled iron complexes containing the chloranilate(2−) and the significantly stronger coupling chloranilate(3−) radical trianion. J. Am. Chem. Soc. 2007, 129, 2360–2368. [Google Scholar] [PubMed]
- Atzori, M.; Pop, F.; Auban-Senzier, P.; Gomez-Garcia, C.J.; Canadell, E.; Artizzu, F.; Serpe, A.; Deplano, P.; Avarvari, N.; Mercuri, M.L. Structural diversity and physical properties of paramagnetic molecular conductors based on bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) and the tris(chloranilato)FerrateIII) complex. Inorg. Chem. 2014, 53, 7028–7039. [Google Scholar] [CrossRef] [PubMed]
- Takehiko, M. Structural genealogy of BEDT-TTF-based organic conductors i. Parallel molecules: Β and β″ phases. Bull. Chem. Soc. Jpn. 1998, 71, 2509–2526. [Google Scholar]
- Benmansour, S.; Coronado, E.; Giménez-Saiz, C.; Gómez-García, C.J.; Rößer, C. Metallic charge-transfer salts of bis(ethylenedithio)tetrathiafulvalene with paramagnetic tetrachloro(oxalato)rhenate(IV) and tris(chloranilato)ferrate(III) anions. Eur. J. Inorg. Chem. 2014, 3949–3959. [Google Scholar] [CrossRef]
- Atzori, M.; Pop, F.; Auban-Senzier, P.; Clerac, R.; Canadell, E.; Mercuri, M.L.; Avarvari, N. Complete series of chiral paramagnetic molecular conductors based on tetramethyl-bis(ethylenedithio)-tetrathiafulvalene (TM-BEDT-TTF) and chloranilate-bridged heterobimetallic honeycomb layers. Inorg. Chem. 2015, 54, 3643–3653. [Google Scholar] [CrossRef] [PubMed]
- Abherve, A.; Clemente-Leon, M.; Coronado, E.; Gomez-Garcia, C.J.; Verneret, M. One-dimensional and two-dimensional anilate-based magnets with inserted spin-crossover complexes. Inorg. Chem. 2014, 53, 12014–12026. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Leo, M.; Coronado, E.; Martí-Gastaldoza, C.; Romero, F.M. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Teppei, Y.; Shota, M.; Hiroshi, K. Structures and proton conductivity of one-dimensional M(dhbq)·nH2O (M = Mg, Mn, Co, Ni, and Zn, H2(dhbq) = 2,5-dihydroxy-1,4-benzoquinone) promoted by connected hydrogen-bond networks with absorbed water. Bull. Chem. Soc. Jpn. 2010, 83, 42–48. [Google Scholar]
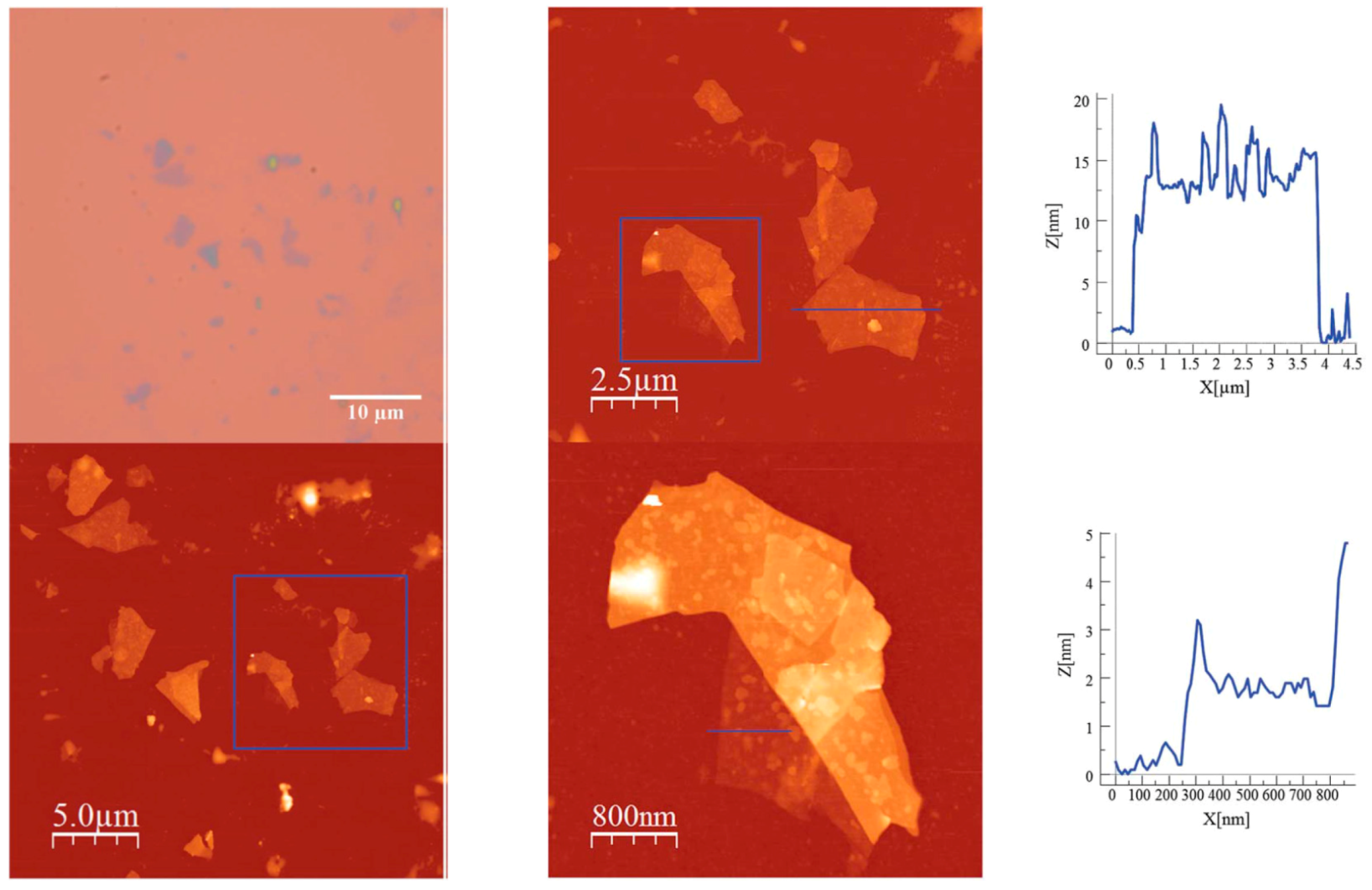
- Abhervé, A.; Mañas-Valero, S.; Clemente-León, M.; Coronado, E. Graphene related magnetic materials: Micromechanical exfoliation of 2D layered magnets based on bimetallic anilate complexes with inserted [FeIII(acac2-trien)]+ and [FeIII(sal2-trien)]+ molecules. Chem. Sci. 2015, 6, 4665–4673. [Google Scholar] [CrossRef]
- Castellanos-Gomez, A.; Buscema, M.; Molenaar, R.; Singh, V.; Janssen, L.; van der Zant, H.S.J.; Steele, G.A. Deterministic transfer of two-dimensional materials by all-dry viscoelastic stamping. 2D Mater. 2014, 1, 011002. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, J.; Guo, W.; Jiang, L. Mechanical exfoliation of track-etched two-dimensional layered materials for the fabrication of ultrathin nanopores. Chem. Commun. 2014, 50, 14149–14152. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, J.; Yin, Z.; Zhang, H. Preparation and applications of mechanically exfoliated single-layer and multilayer MoS2 and WSe2 nanosheets. Acc. Chem. Res. 2014, 47, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Z.; Maeda, Y.; Xu, Q. Top-down fabrication of crystalline metal-organic framework nanosheets. Chem. Commun. 2011, 47, 8436–8438. [Google Scholar] [CrossRef] [PubMed]
- Gallego, A.; Hermosa, C.; Castillo, O.; Berlanga, I.; Gómez-García, C.J.; Mateo-Martí, E.; Martínez, J.I.; Flores, F.; Gómez-Navarro, C.; Gómez-Herrero, J.; et al. Solvent-induced delamination of a multifunctional two dimensional coordination polymer. Adv. Mater. 2013, 25, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Amo-Ochoa, P.; Welte, L.; Gonzalez-Prieto, R.; Sanz Miguel, P.J.; Gomez-Garcia, C.J.; Mateo-Marti, E.; Delgado, S.; Gomez-Herrero, J.; Zamora, F. Single layers of a multifunctional laminar Cu(I,II) coordination polymer. Chem. Commun. 2010, 46, 3262–3264. [Google Scholar] [CrossRef] [PubMed]
- Beldon, P.J.; Tominaka, S.; Singh, P.; Saha Dasgupta, T.; Bithell, E.G.; Cheetham, A.K. Layered structures and nanosheets of pyrimidinethiolate coordination polymers. Chem. Commun. 2014, 50, 3955–3957. [Google Scholar] [CrossRef] [PubMed]
- Saines, P.J.; Steinmann, M.; Tan, J.-C.; Yeung, H.H.M.; Li, W.; Barton, P.T.; Cheetham, A.K. Isomer-directed structural diversity and its effect on the nanosheet exfoliation and magnetic properties of 2,3-dimethylsuccinate hybrid frameworks. Inorg. Chem. 2012, 51, 11198–11209. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.-C.; Saines, P.J.; Bithell, E.G.; Cheetham, A.K. Hybrid nanosheets of an Inorganic–Organic framework material: Facile synthesis, structure, and elastic properties. ACS Nano 2012, 6, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, H.; Kawata, S.; Kitagawa, S. Fabrication of infinite two-dimensional sheets of tetragonal metal(II) lattices X-ray crystal structures and magnetic properties of [M(CA)(pyz)]n (M2+ = Mn2+ and Co2+;H2CA=chloranilic acid; pyz=pyrazine). Inorg. Chim. Acta 2002, 337, 387–392. [Google Scholar] [CrossRef]
- Nielsen, R.B.; Kongshaug, K.O.; Fjellvag, H. Delamination, synthesis, crystal structure and thermal properties of the layered metal-organic compound Zn(C12H14O4). J. Mater. Chem. 2008, 18, 1002–1007. [Google Scholar] [CrossRef]
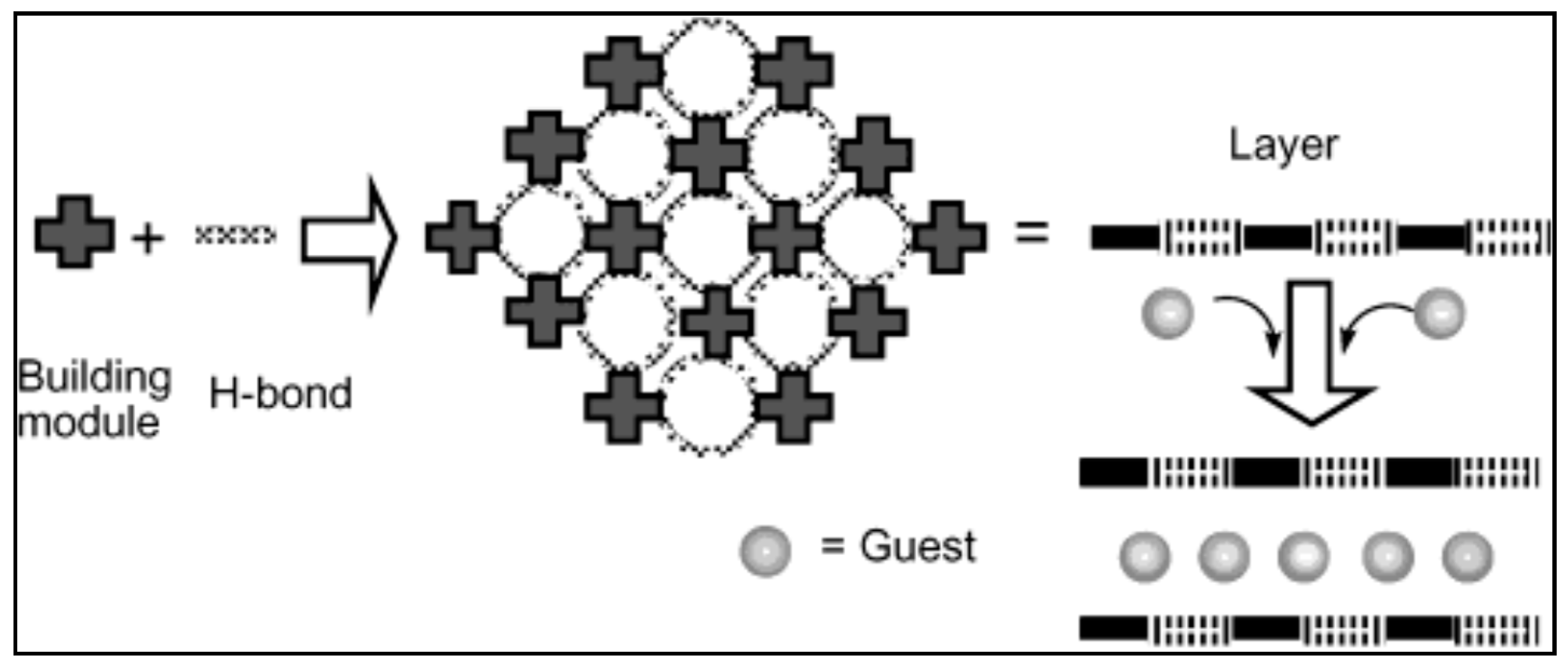
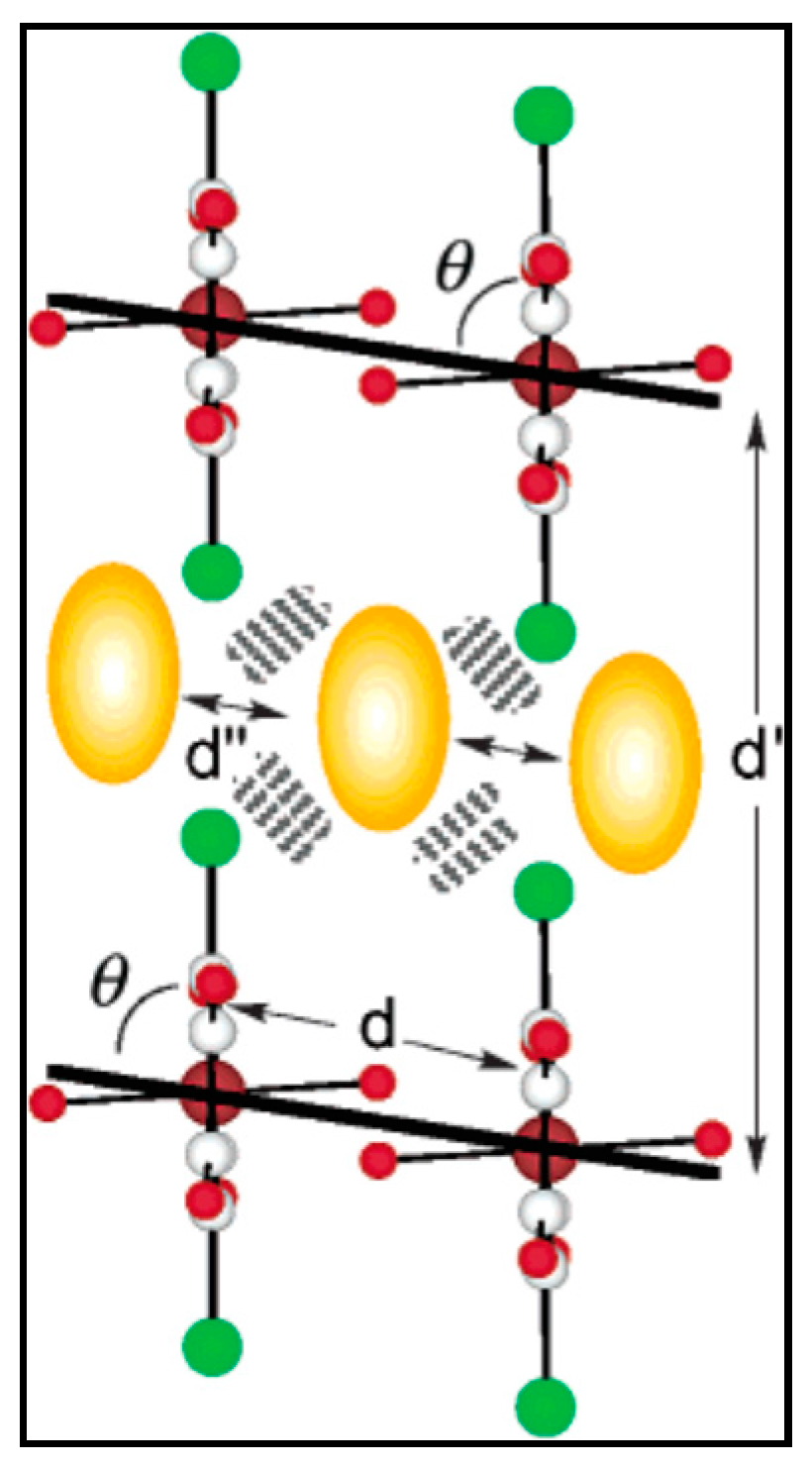
- Nagayoshi, K.; Kabir, M.K.; Tobita, H.; Honda, K.; Kawahara, M.; Katada, M.; Adachi, K.; Nishikawa, H.; Ikemoto, I.; Kumagai, H.; et al. Design of novel inorganic—Organic hybrid materials based on iron-chloranilate mononuclear complexes: Characteristics of hydrogen-bond-supported layers toward the intercalation of guests. J. Am. Chem. Soc. 2003, 125, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Darago, L.E.; Aubrey, M.L.; Yu, C.J.; Gonzalez, M.I.; Long, J.R. Electronic conductivity, ferrimagnetic ordering, and reductive insertion mediated by organic mixed-valence in a ferric semiquinoid metal—Organic framework. J. Am. Chem. Soc. 2015, 137, 15703–15711. [Google Scholar] [CrossRef] [PubMed]
- Kawata, S.; Kitagawa, S.; Kumagai, H.; Ishiyama, T.; Honda, K.; Tobita, H.; Adachi, K.; Katada, M. Novel intercalation host system based on transition metal (Fe2+, Co2+, Mn2+)—Chloranilate coordination polymers. Single crystal structures and properties. Chem. Mater. 1998, 10, 3902–3912. [Google Scholar] [CrossRef]
- Wrobleski, J.T.; Brown, D.B. Synthesis, magnetic susceptibility, and moessbauer spectra of Iron(III) dimers and Iron(II) polymers containing 2,5-dihydroxy-1,4-benzoquinones. Inorg. Chem. 1979, 18, 498–504. [Google Scholar] [CrossRef]
- Wrobleski, J.T.; Brown, D.B. Synthesis, magnetic susceptibility, and spectroscopic properties of single- and mixed-valence iron oxalate, squarate, and dihydroxybenzoquinone coordination polymers. Inorg. Chem. 1979, 18, 2738–2749. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Gómez-García, C.J.; Soriano-Portillo, A. Increasing the ordering temperatures in oxalate-based 3d chiral magnets: The series [Ir(ppy)2(bpy)][MIIMIII(ox)3]·0.5H2O (MIIMIII = MnCr, FeCr, CoCr, NiCr, ZnCr, MnFe, FeFe); bpy = 2,2′-bipyridine; ppy = 2-phenylpyridine; ox = oxalate dianion). Inorg. Chem. 2006, 45, 5653–5660. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Ferrero, E.; Almeida, M.; Waerenborgh, J.C. Oxalate-based 3D chiral magnets: The series [ZII(bpy)3][ClO4][MIIFeIII(ox)3] (ZII = Fe, Ru; MII = Mn, Fe; bpy = 2,2′-bipyridine; ox = oxalate dianion). Eur. J. Inorg. Chem. 2005, 2005, 2064–2070. [Google Scholar] [CrossRef]
- Decurtins, S.; Schmalle, H.W.; Schneuwly, P.; Ensling, J.; Guetlich, P. A concept for the synthesis of 3-dimensional homo- and bimetallic oxalate-bridged networks [M2(ox)3]n. Structural, moessbauer, and magnetic studies in the field of molecular-based magnets. J. Am. Chem. Soc. 1994, 116, 9521–9528. [Google Scholar] [CrossRef]
- Shaikh, N.; Goswami, S.; Panja, A.; Wang, X.-Y.; Gao, S.; Butcher, R.J.; Banerjee, P. New route to the mixed valence semiquinone-catecholate based mononuclear FeIII and catecholate based dinuclear MnIII complexes: First experimental evidence of valence tautomerism in an iron complex. Inorg. Chem. 2004, 43, 5908–5918. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.M.; Keene, F.R. Current trends and future challenges in the experimental, theoretical and computational analysis of intervalence charge transfer (IVCT) transitions. Chem. Soc. Rev. 2006, 35, 424–440. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.M.; Keene, F.R. Intervalence charge transfer (IVCT) in trinuclear and tetranuclear complexes of Iron, Ruthenium, and Osmium. Chem. Rev. 2006, 106, 2270–2298. [Google Scholar] [CrossRef] [PubMed]
- Demadis, K.D.; Hartshorn, C.M.; Meyer, T.J. The localized-to-delocalized transition in mixed-valence chemistry. Chem. Rev. 2001, 101, 2655–2686. [Google Scholar] [CrossRef] [PubMed]
- Hankache, J.; Wenger, O.S. Organic mixed valence. Chem. Rev. 2011, 111, 5138–5178. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S. Magnetically ordered molecule-based materials. Chem. Soc. Rev. 2011, 40, 3266–3296. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.D. A dinuclear Ruthenium(II) complex with the dianion of 2,5-dihydroxy-1,4-benzoquinone as bridging ligand. Redox, spectroscopic, and mixed-valence properties. Inorg. Chem. 1996, 35, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, A.; Yamazaki, H.; Aimatsu, M.; Enoki, T.; Watanabe, R.; Ogura, E.; Kuwatani, Y.; Iyoda, M. Crystal structure and physical properties of conducting molecular antiferromagnets with a halogen-substituted donor: (EDO-TTFBr2)2FeX4 (X = Cl, Br). Inorg. Chem. 2007, 46, 3353–3366. [Google Scholar] [CrossRef] [PubMed]
- Clérac, R.; O’Kane, S.; Cowen, J.; Ouyang, X.; Heintz, R.; Zhao, H.; Bazile, M.J.; Dunbar, K.R. Glassy magnets composed of metals coordinated to 7,7,8,8-tetracyanoquinodimethane: M(tcnq)2 (M = Mn, Fe, Co, Ni). Chem. Mater. 2003, 15, 1840–1850. [Google Scholar] [CrossRef]
- Jeon, I.-R.; Negru, B.; Van Duyne, R.P.; Harris, T.D. A 2D semiquinone radical-containing microporous magnet with solvent-induced switching from Tc = 26 to 80 k. J. Am. Chem. Soc. 2015, 137, 15699–15702. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.-H.; Yin, Z.; Tan, Y.-X.; Zhang, W.-X.; He, Y.-P.; Kurmoo, M. Nanoporous Cobalt(II) mof exhibiting four magnetic ground states and changes in gas sorption upon post-synthetic modification. J. Am. Chem. Soc. 2014, 136, 4680–4688. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hendon, C.H.; Minier, M.A.; Walsh, A.; Dincă, M. Million-fold electrical conductivity enhancement in Fe2(DEBDC) versus Mn2(DEBDC) (E = S, O). J. Am. Chem. Soc. 2015, 137, 6164–6167. [Google Scholar] [CrossRef] [PubMed]
- Murdock, C.R.; Hughes, B.C.; Lu, Z.; Jenkins, D.M. Approaches for synthesizing breathing MOFs by exploiting dimensional rigidity. Coord. Chem. Rev. 2014, 258–259, 119–136. [Google Scholar] [CrossRef]
- Ferey, G.; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules and consequences. Chem. Soc. Rev. 2009, 38, 1380–1399. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, K.; Ohkoshi, S.-I. Monometallic lanthanoid assembly showing ferromagnetism with a curie temperature of 11 k. Inorg. Chem. 2009, 48, 8647–8649. [Google Scholar] [CrossRef] [PubMed]
- Przychodzeń, P.; Pełka, R.; Lewiński, K.; Supel, J.; Rams, M.; Tomala, K.; Sieklucka, B. Tuning of magnetic properties of polynuclear lanthanide(III)—Octacyanotungstate(V) systems: Determination of ligand-field parameters and exchange interaction. Inorg. Chem. 2007, 46, 8924–8938. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, N.; Sugita, M.; Wernsdorfer, W. Nuclear spin driven quantum tunneling of magnetization in a new lanthanide single-molecule magnet: Bis(phthalocyaninato)Holmium anion. J. Am. Chem. Soc. 2005, 127, 3650–3651. [Google Scholar] [CrossRef] [PubMed]

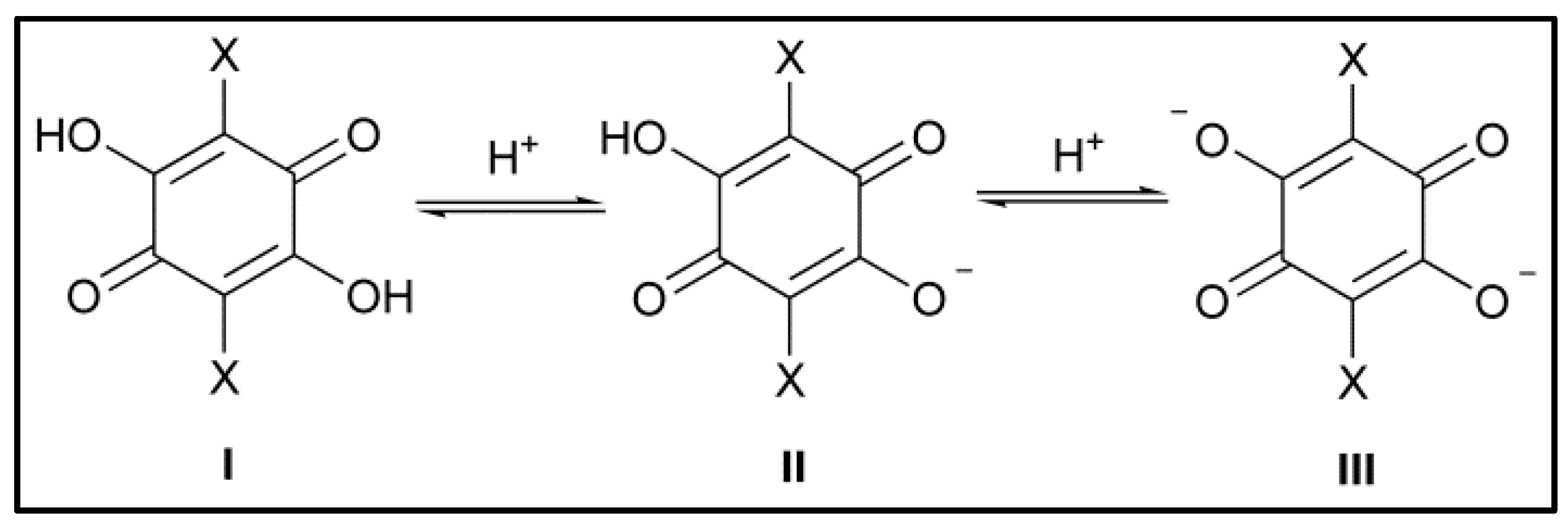

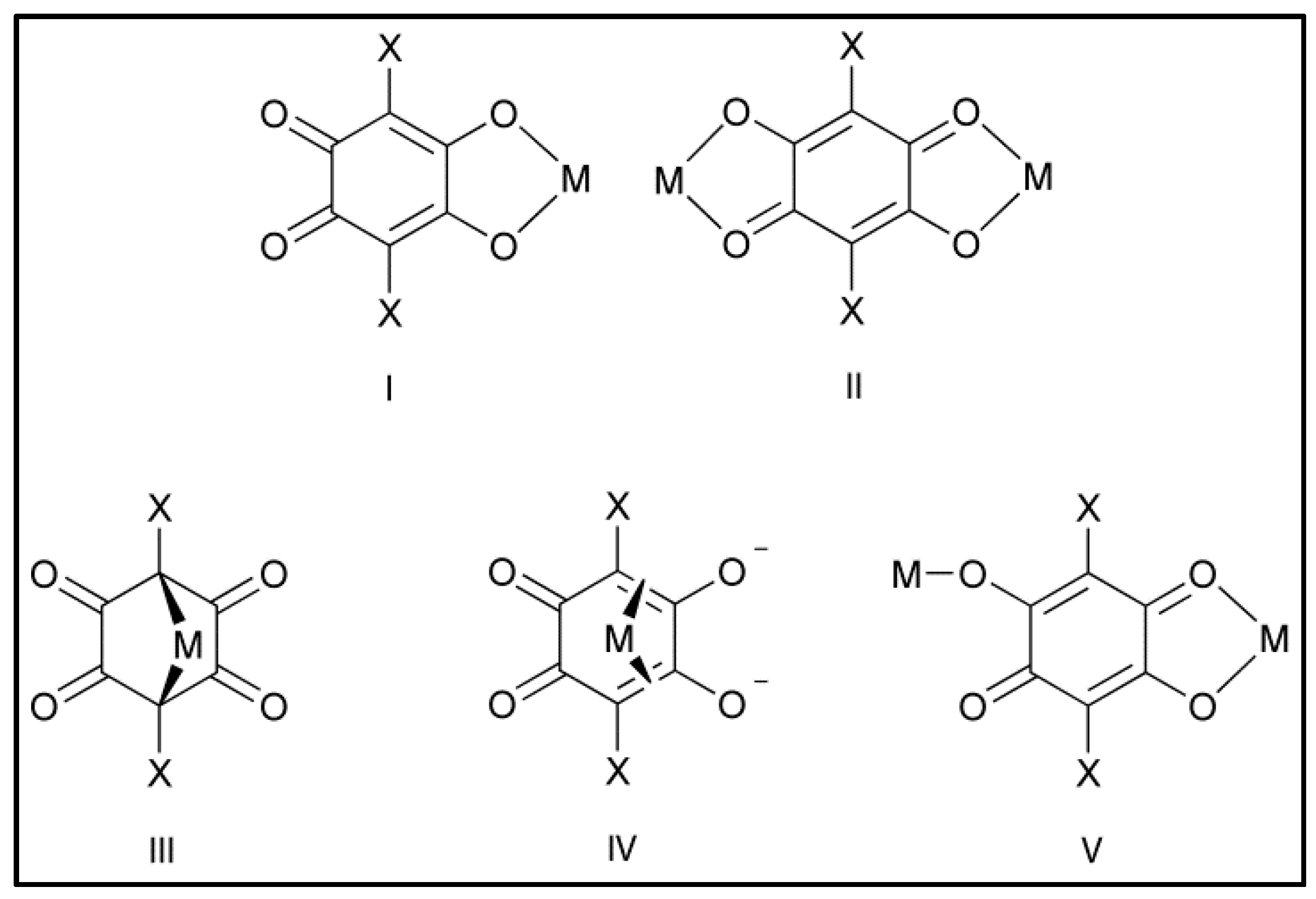
| Cation | Cl2An2− | Br2An2− | I2An2− | |||
|---|---|---|---|---|---|---|
| Cr(III) | Fe(III) | Cr(III) | Fe(III) | Cr(III) | Fe(III) | |
| (n-Bu)4N+ | 4a | 5a | 6a | 7a | 8a | 9a |
| (Ph)4P+ | 4b | 5b | 6b | 7b | 8b | 9b |
| (Et)3NH+ | 4c | 5c | - | - | - | - |

| Cation | ClCNAn2− | ||
|---|---|---|---|
| Cr(III) | Fe(III) | Al(III) | |
| (n-Bu)4N+ | 10a | 11a | 12a |
| (Ph)4P+ | 10b | 11b | 12b |

| Cationic Layer | Anionic Layer |
|---|---|
| [H3O(phz)3]+ | MnIICrIII (X-Cl) 17 |
| [H3O(phz)3]+ | MnIICrIII (X-Br) 18 |
| [H3O(phz)3]+ | MnIIFeIII (X-Br) 19 |
| [(n-Bu4)N]+ | MnIICrIII (X-Cl) 20 |
| [(n-Bu4)N]+ | MnIICrIII (X-Br) 21 |
| [(n-Bu4)N]+ | MnIICrIII (X-I) 22 |
| [(n-Bu4)N]+ | MnIICrIII (X-H) 23 |
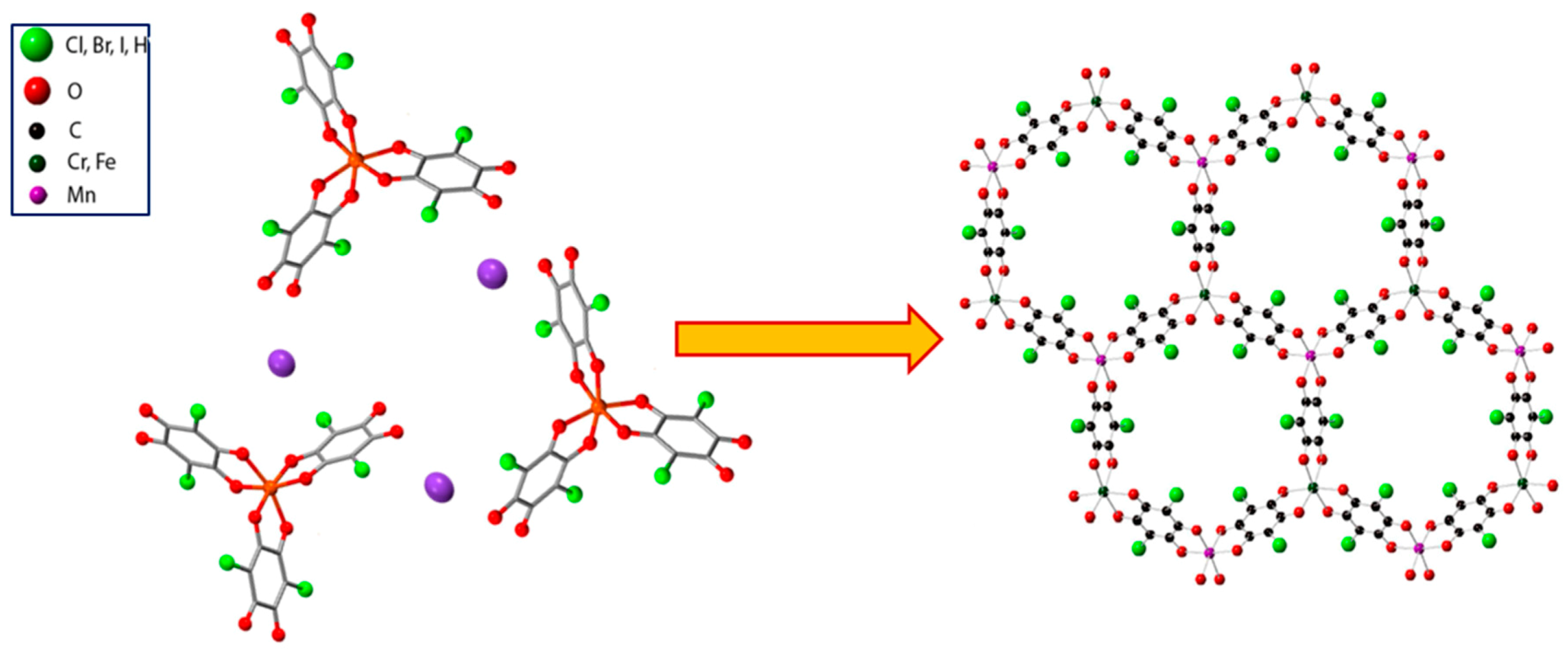
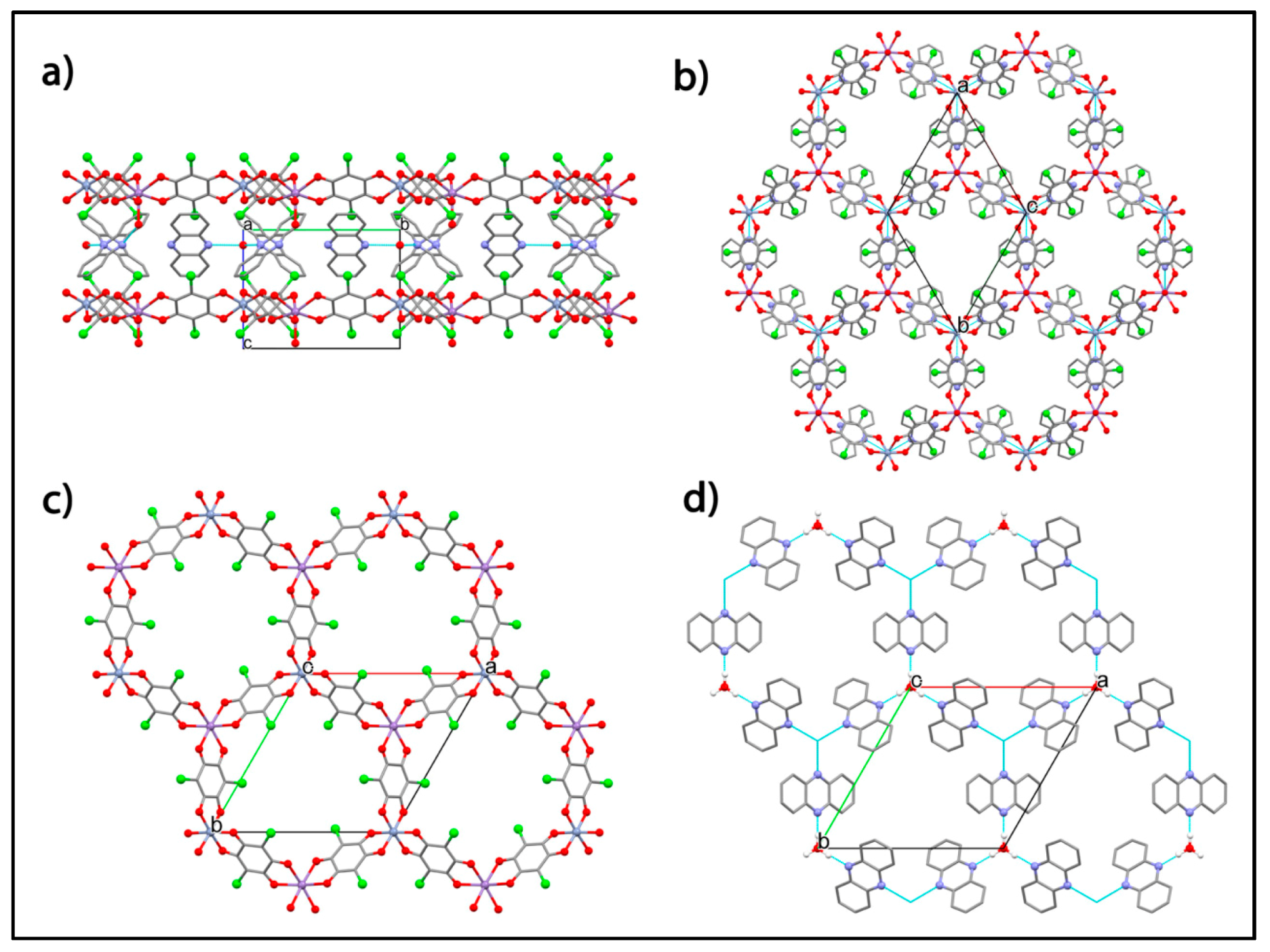
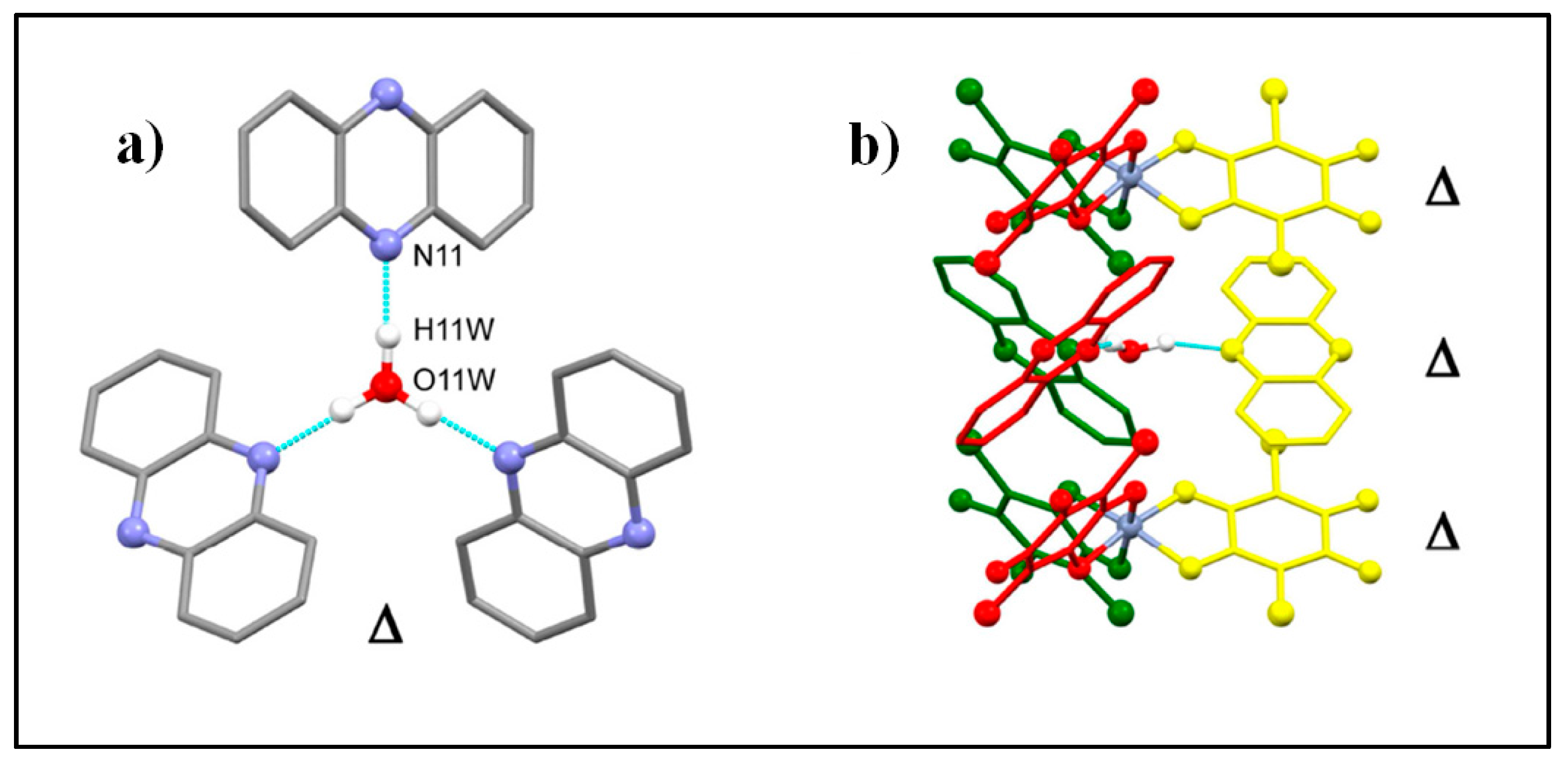
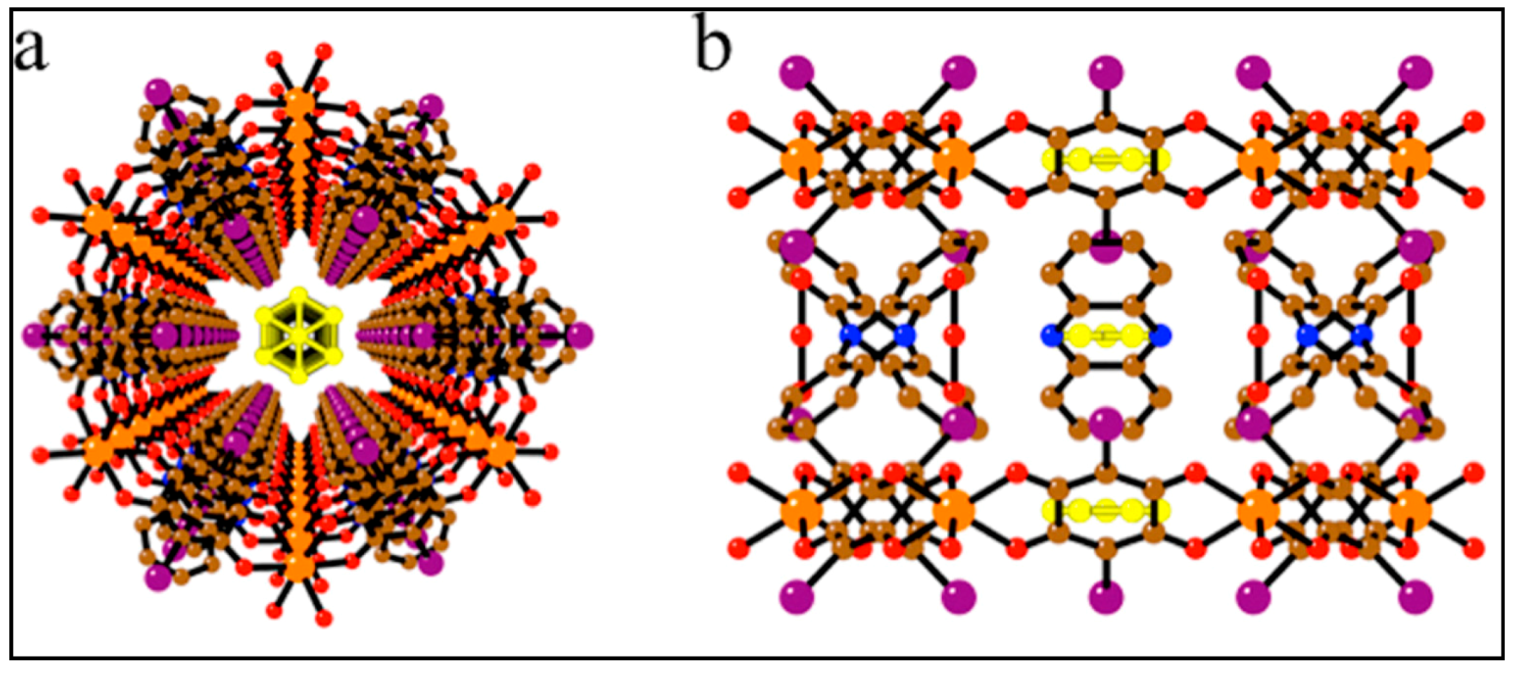


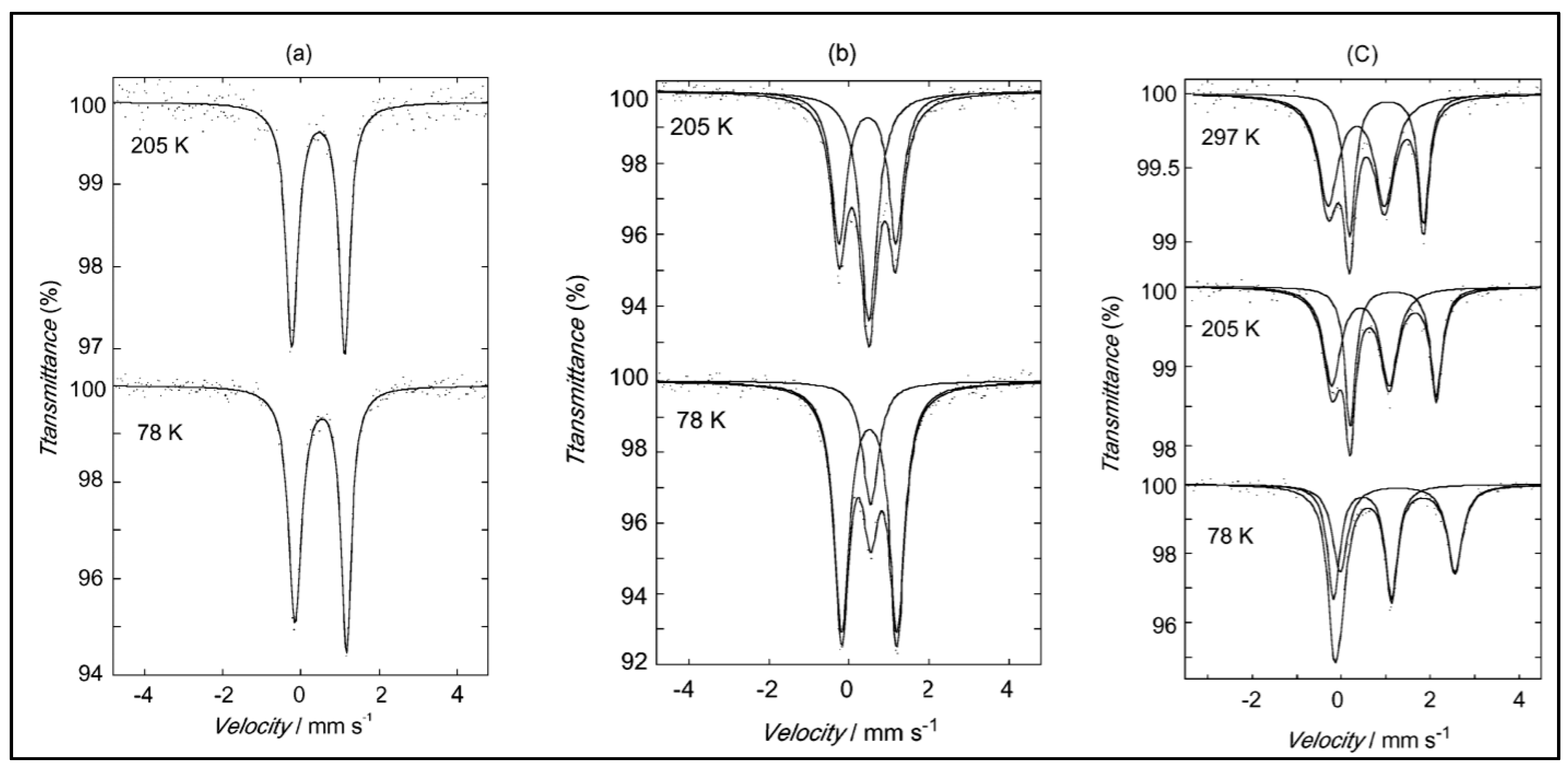
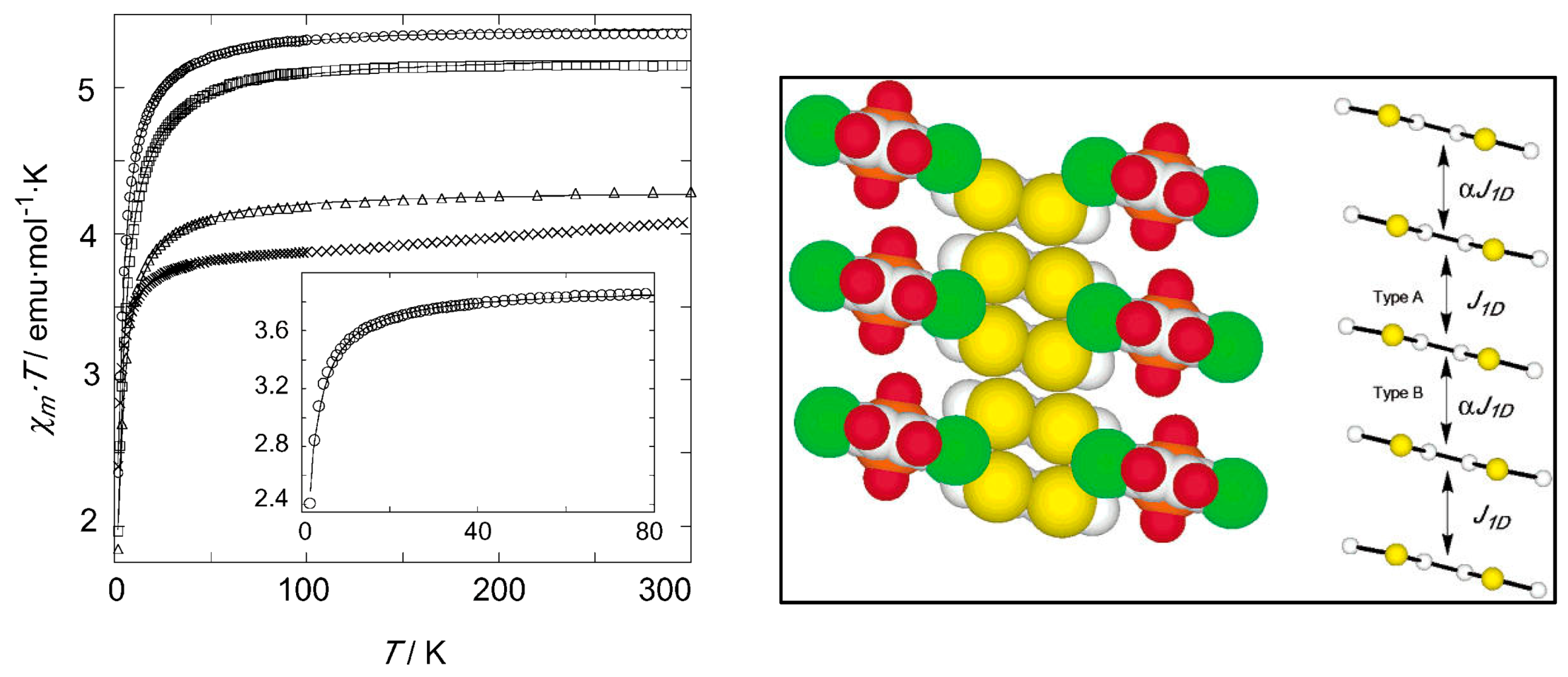
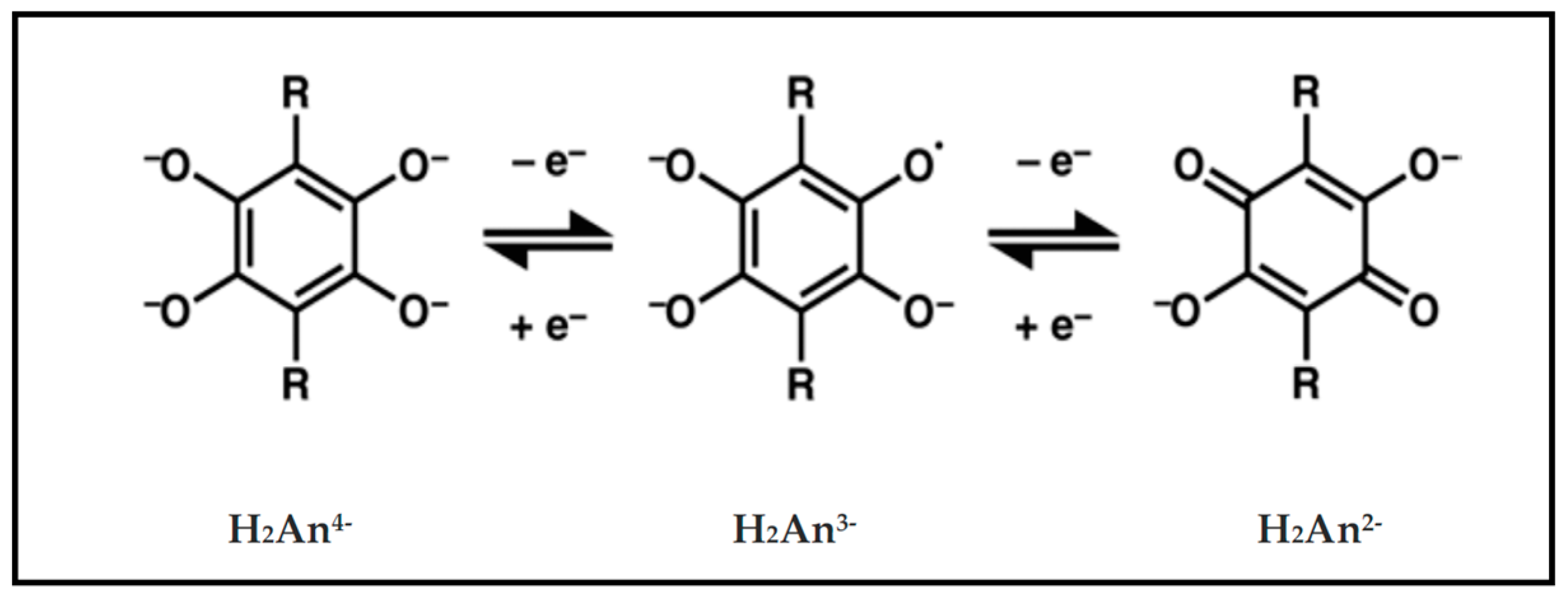
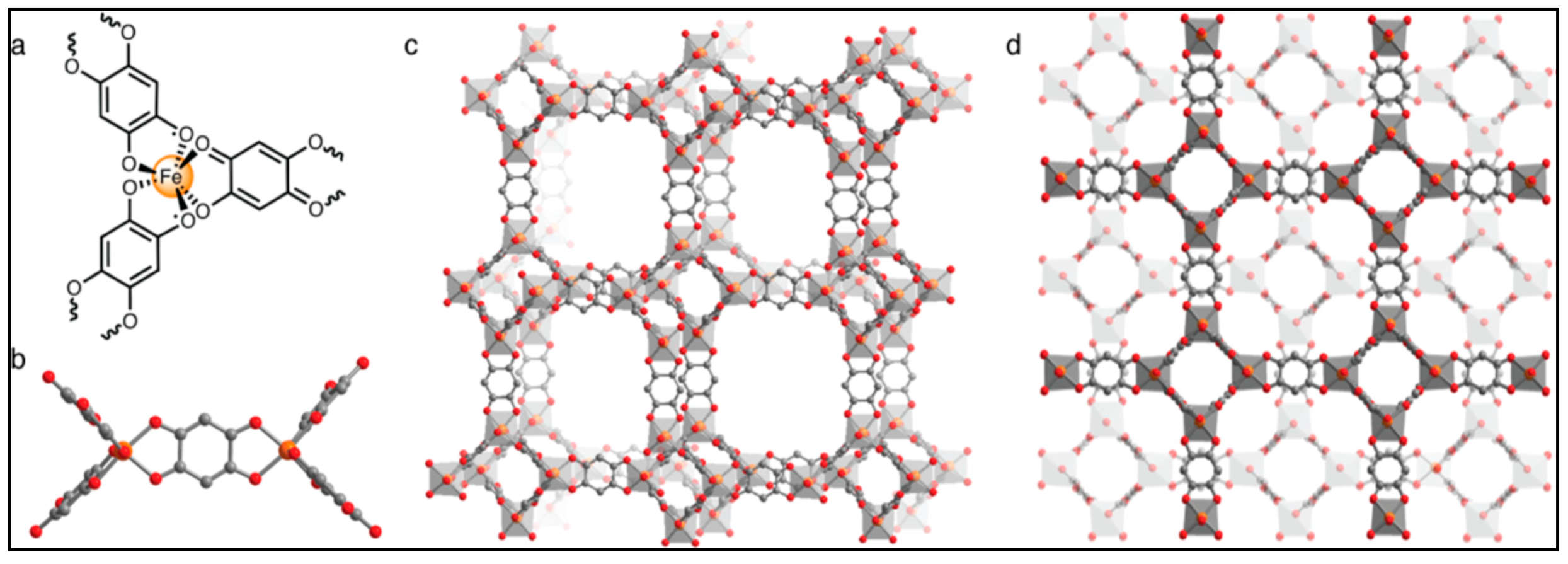
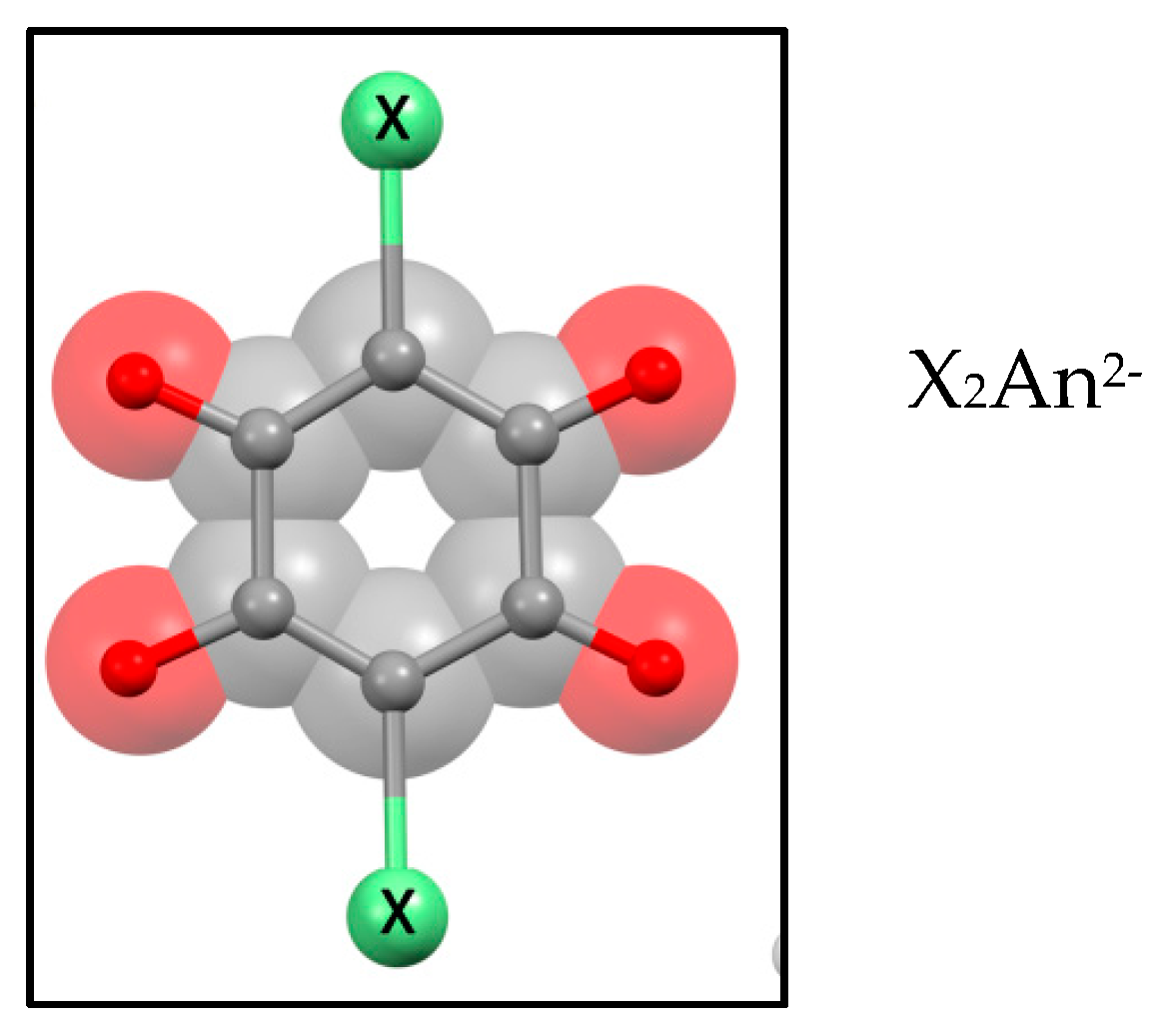
| Substituent, X | Formula | Anilic Acid Name | Acronyms | Anilate Dianion Name | Acronyms | Ref. |
|---|---|---|---|---|---|---|
| H | H4C6O4 | Hydranilic acid | H2H2An | Hydranilate | H2An2− | [8,9,10] |
| F | H2F2C6O4 | Fluoranilic acid | H2F2An | Fluoranilate | F2An2− | [11] |
| Cl | H2Cl2C6O4 | Chloranilic acid | H2Cl2An | Chloranilate | Cl2An2− | [12,13] |
| Br | H2Br2C6O4 | Bromanilic acid | H2Br2An | Bromanilate | Br2An2− | [14] |
| I | H2I2C6O4 | Iodanilic acid | H2F2An | Iodanilate | I2An2− | [14] |
| NO2 | H2N2C6O8 | Nitranilic acid | H2(NO2)2An | Nitranilate | (NO2)2An2− | [15] |
| OH | H4C6O6 | Hydroxyanilic acid | H2(OH)2An | Hydroxyanilate | (OH2)2An2− | [16,17,18,19,20] |
| CN | H2N2C8O4 | Cyananilic acid | H2(CN)2An | Cyananilate | (CN)2An2− | [21,22] |
| Cl/CN | H2ClNC7O4 | Chlorocyananilic acid | H2ClCNAn | Chlorocyananilate | ClCNAn2− | [23] |
| NH2 | H6N2C6O4 | Aminanilic acid | H2(NH2)2An | Aminanilate | (NH2)2An2− | [24] |
| CH3 | H8C8O4 | Methylanilic acid | H2Me2An | Methylanilate | Me2An2− | [25] |
| CH2CH3 | H12C10O4 | Ethylanilic acid | H2Et2An | Ethylanilate | Et2An2− | [25] |
| iso-C3H7 | H16C12O4 | Isopropylanilic acid | H2iso-Pr2An | Isopropylanilate | iso-Pr2An2− | [26] |
| C6H5 | H12C18O4 | Phenylanilic acid | H2Ph2An | Phenylanilate | Ph2An2− | [10,27] |
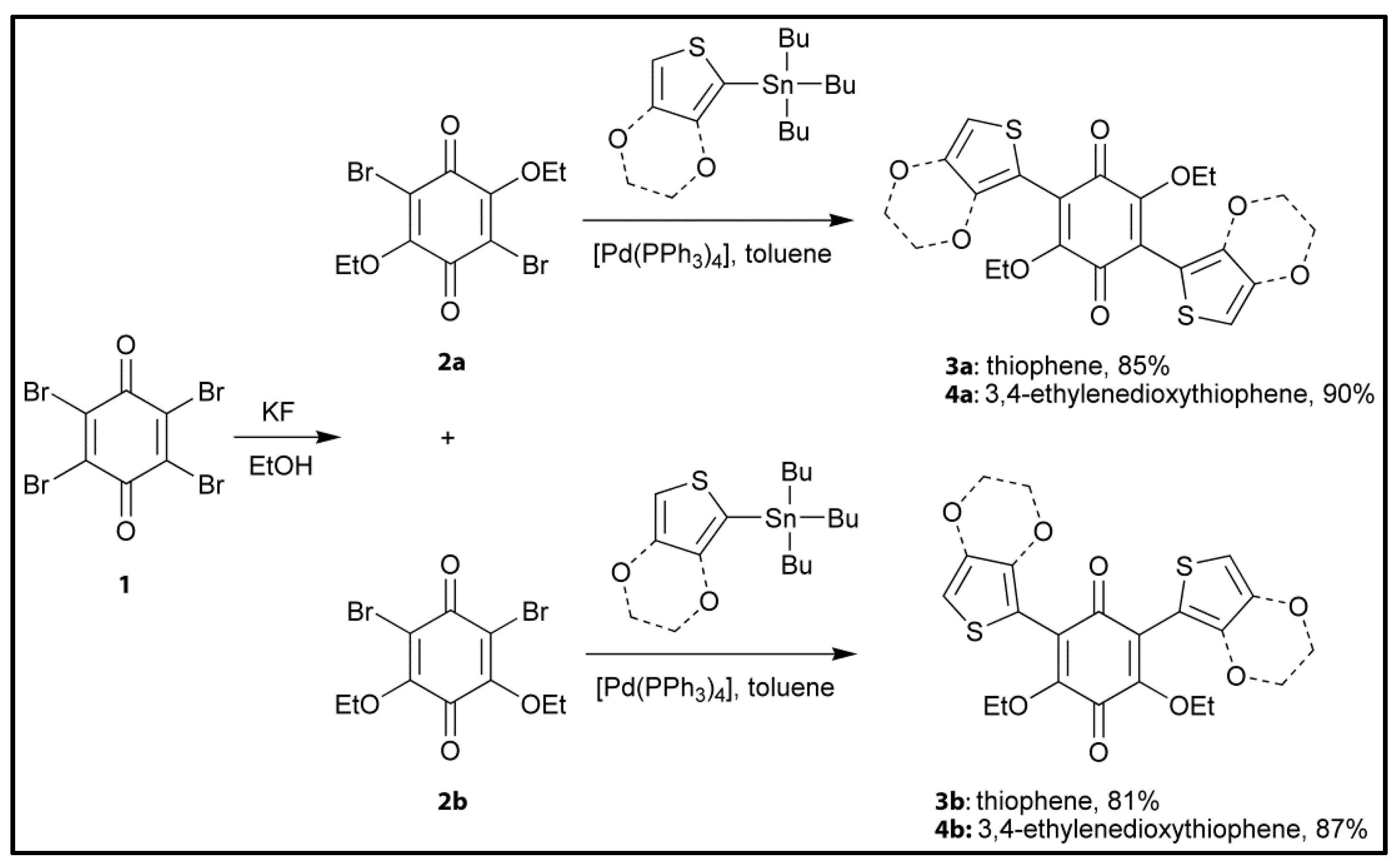
| C4H3S | H8C14O4S2 | Thiophenylanilic acid | H2Th2An | Thiophenylanilate | Th2An2− | [28] |
| C6H5O2S | H12C18O8S2 | 3,4-ethylene dioxythiophenyl anilic acid | H2EDOT2An | 3,4-ethylene dioxythiophenyl anilate | EDOTAn2− | [28] |
| C4H9 | H20C14O4 | 2,3,5,6-tetrahydroxy-1,4-benzo quinone | H2THBQ | 2,5-di-tert-butyl-3,6-dihydroxy-1,4-benzoquinonate | THBQ2− | [29] |
| Compound | Molecular Packing | Physical Properties | Ref. |
|---|---|---|---|
| [(Ph)4P]3[Fe(H2An)3]·6H2O 1 | Homoleptic tris-chelated octahedral complex. strong HBs between oxygen atoms of the ligand and crystallization water molecules | PM J/kB = −0.020 K | [116] |
| [(Ph)4P]3[Cr(H2An)3]·6H2O 2 | Homoleptic tris-chelated octahedral complex. π-π interactions | PM Weak magnetic coupling due to charge transfer between the Cr metal ions and the hydranilate ligands | [116] |
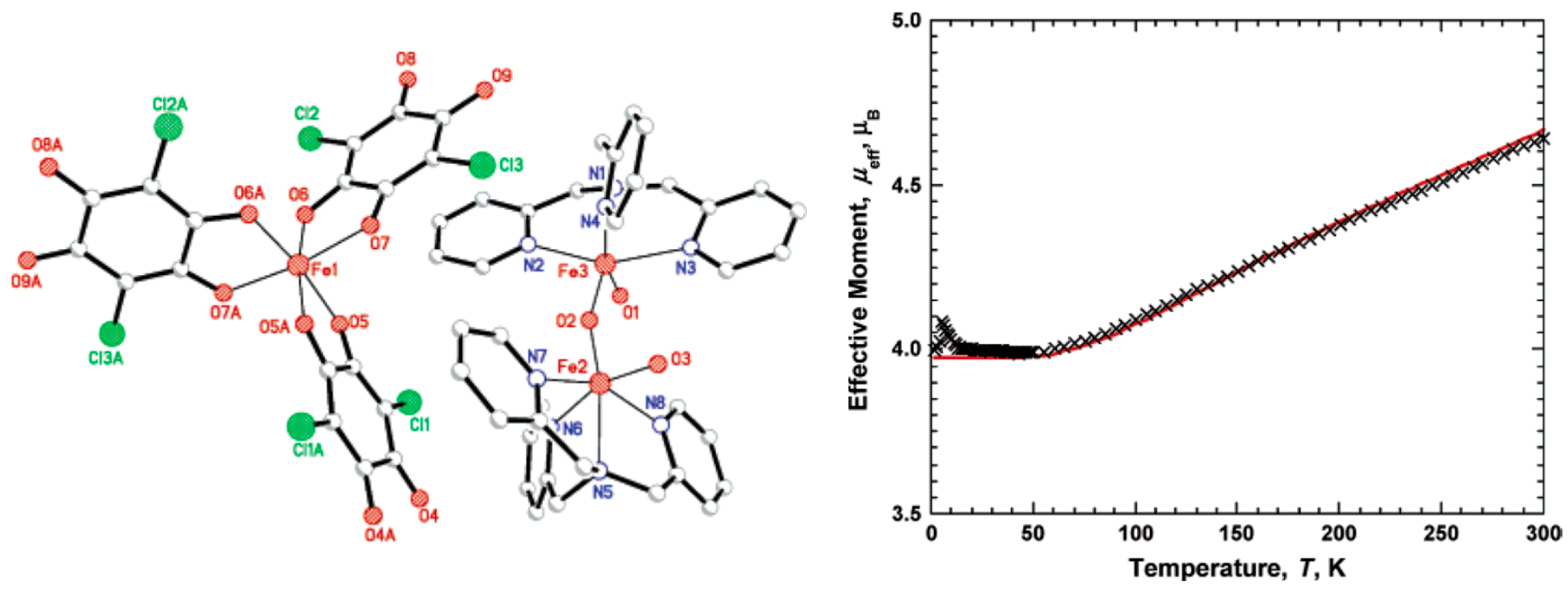
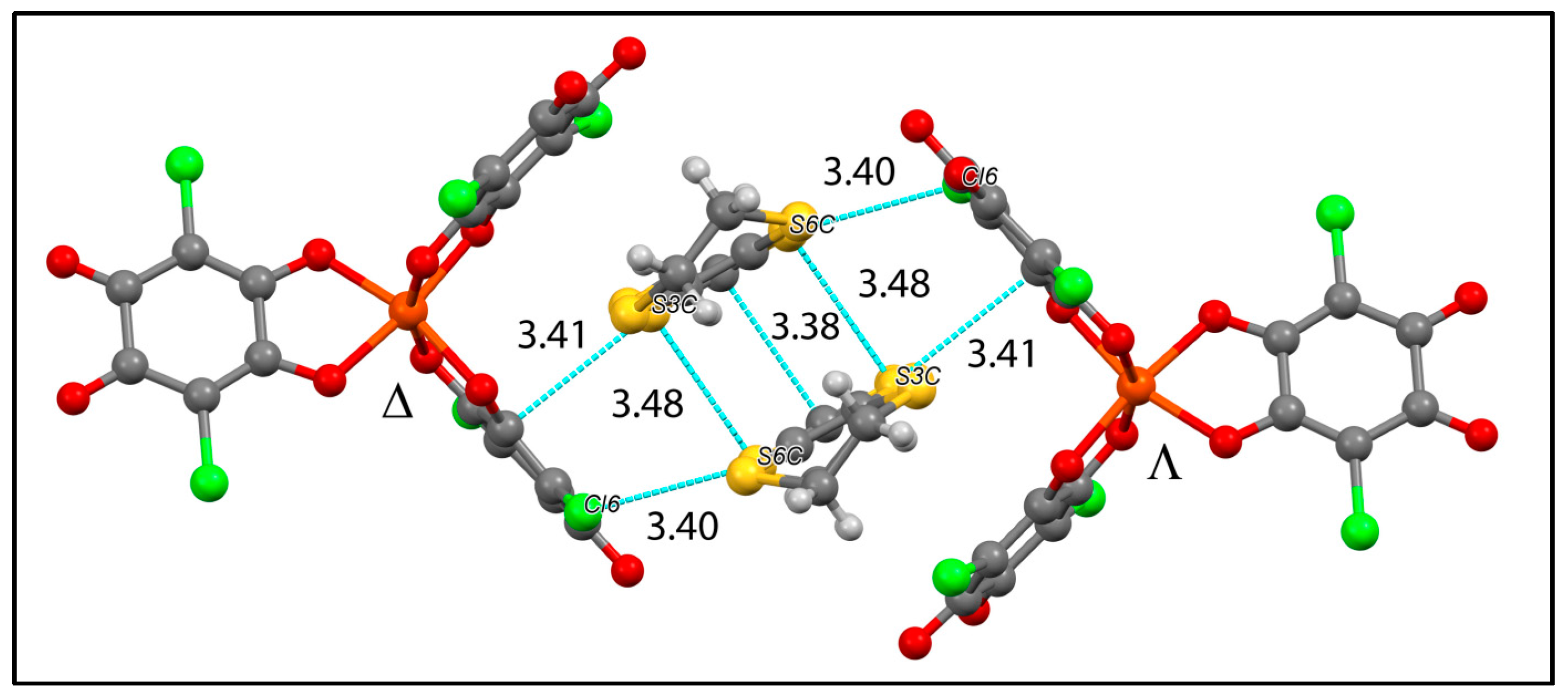
| [(TPA)(OH)FeIIIOFeIII(OH)(TPA)][Fe(Cl2An)3]0.5(BF4)0.5∙1.5MeOH∙H2O 3 | Homoleptic trischelated complex | μeff(RT) = 2.93 μB Strong AFM interaction within FeIIIOFeIII with a plateau at 55 K. Below 55 K, μ(T) is constant at 4.00 μB J/kB = −165 K | [117] |
| [(n-Bu)4N]3[Cr(Cl2An)3] 4a | Homoleptic tris-chelated octahedral complex. | PM ZFS | [119] |
| [(Ph)4P]3 [Cr(Cl2An)3] 4b | Homoleptic tris-chelated octahedral complex. π-π interactions | PM ZFS | [119] |
| [(Et)3NH]3 [Cr(Cl2An)3] 4c | Homoleptic tris-chelated octahedral complex. | PM ZFS | [119] |
| [(n-Bu)4N]3[Fe(Cl2An)3] 5a | Homoleptic tris-chelated octahedral complex. | Curie-Weiss PM J/kB = −2.2 K | [119] |
| [(Ph)4P]3 [Fe(Cl2An)3] 5b | Homoleptic tris-chelated octahedral complex. π-π interactions | PM ZFS | [119] |
| [(Et)3NH]3 [Fe(Cl2An)3] 5c | Homoleptic tris-chelated octahedral complex. | PM ZFS | [119] |
| [(n-Bu)4N]3[Cr(Br2An)3] 6a | Homoleptic tris-chelated octahedral complex. | PM ZFS | [119] |
| [(Ph)4P]3 [Cr(Br2An)3] 6b | Homoleptic tris-chelated octahedral complex. π-π interactions | PM ZFS | [119] |
| [(n-Bu)4N]3[Fe(Br2An)3] 7a | Homoleptic tris-chelated octahedral complex. | Curie-Weiss PM (r.t.−4.1 K), AFM coupling via halogen-bonding between the complexes forming the dimers | [119] |
| [(Ph)4P]3 [Fe(Br2An)3] 7b | Homoleptic tris-chelated octahedral complex. π-π interactions | Curie-Weiss PM | [119] |
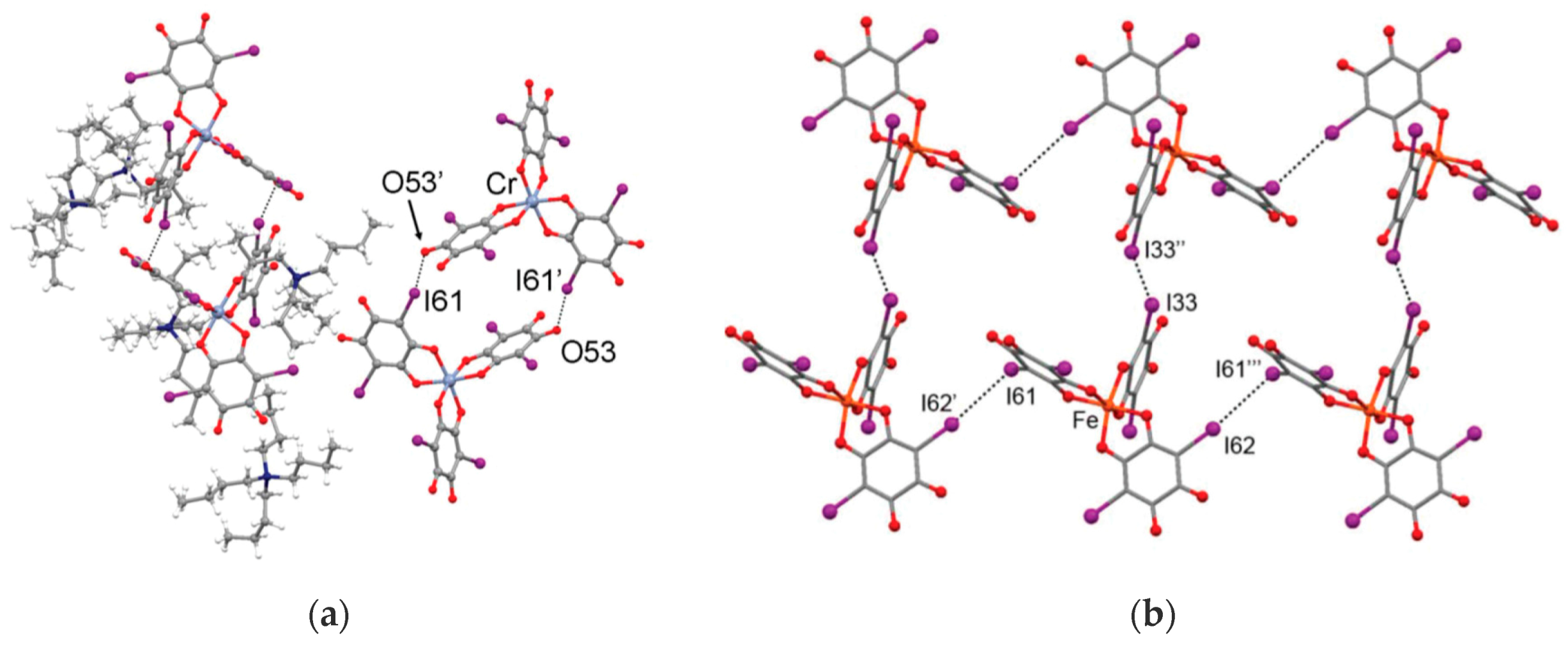
| [(n-Bu)4N]3[Cr(I2An)3] 8a | Supramolecular dimers that are held together by two symmetry-related I···O interactions | Curie-Weiss PM (r.t.−4.1 K), AFM coupling via halogen-bonding between the complexes forming the dimers | [119] |
| [(Ph)4P]3 [Cr(I2An)3] 8b | Homoleptic tris-chelated octahedral complex. π-π interactions | Curie-Weiss PM | [119] |
| [(n-Bu)4N]3Fe(I2An)3] 9a | Homoleptic tris-chelated octahedral complex. | Curie-Weiss PM J/kB = 0.011 K | [119] |
| [(Ph)4P]3[Fe(I2An)3] 9b | Homoleptic tris-chelated octahedral complex. iodine–iodine interactions XB interactions π-π interactions | Curie-Weiss PM J/kB = 0.34 K | [119] |
| [(n-Bu)4N]3[Cr(ClCNAn)3] 10a | Homoleptic tris-chelated octahedral complex. C–N···Cl interactions between complex anions having an opposite stereochemical configuration (Λ, Δ) | Curie-Weiss PM J/kB = 0.0087 K | [120] |
| [(Ph)4P]3 [Cr(ClCNAn)3] 10b | Homoleptic tris-chelated octahedral complex. π-π interactions | Curie-Weiss PM J/kB = −0.24 K | [120] |
| [(n-Bu)4N]3[Fe(ClCNAn)3] 11a | Homoleptic tris-chelated octahedral complex. C–N···Cl interactions between complex anions having an opposite stereochemical configuration (Λ, Δ) | Curie-Weiss PM | [120] |
| [(Ph)4P]3 [Fe(ClCNAn)3] 11b | Homoleptic tris-chelated octahedral complex. π-π interactions | Curie-Weiss PM | [120] |
| [(n-Bu)4N]3[Al(ClCNAn)3] 12a | Homoleptic tris-chelated octahedral complex. C–N···Cl interactions between complex anions having an opposite stereochemical configuration (Λ, Δ) | Red luminophore Ligand centred emission | [120] |
| [(Ph)4P]3 [Al(ClCNAn)3] 12b | Homoleptic tris-chelated octahedral complex. | Red luminophore Ligand centred emission | [120] |
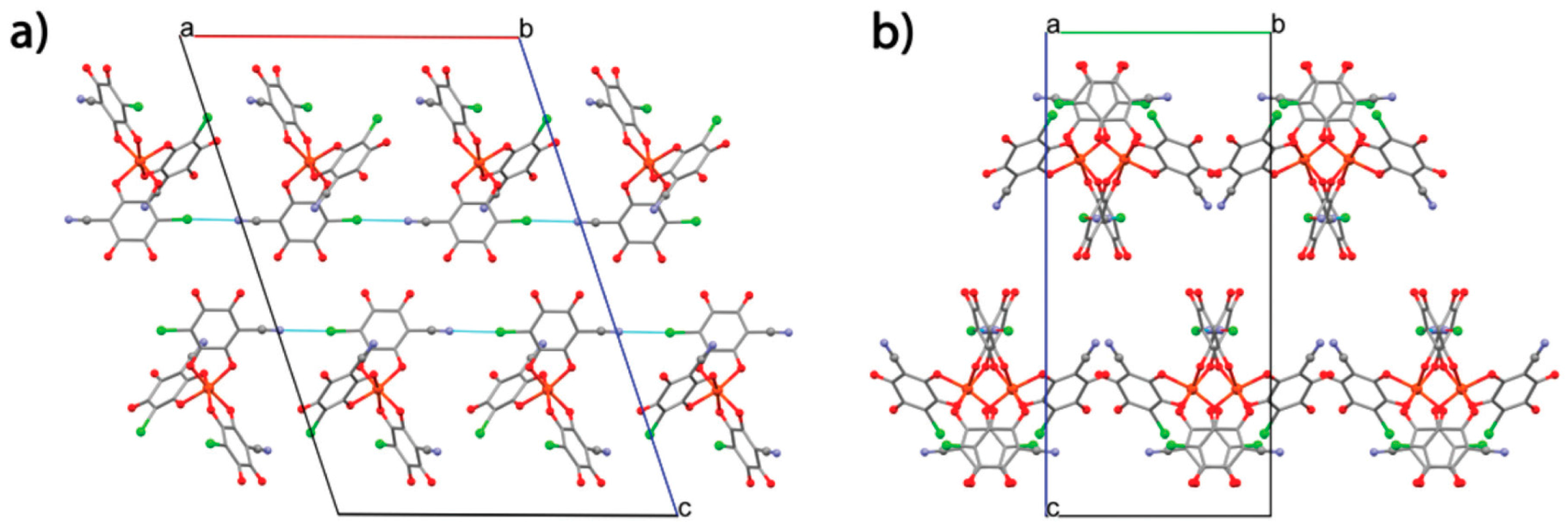
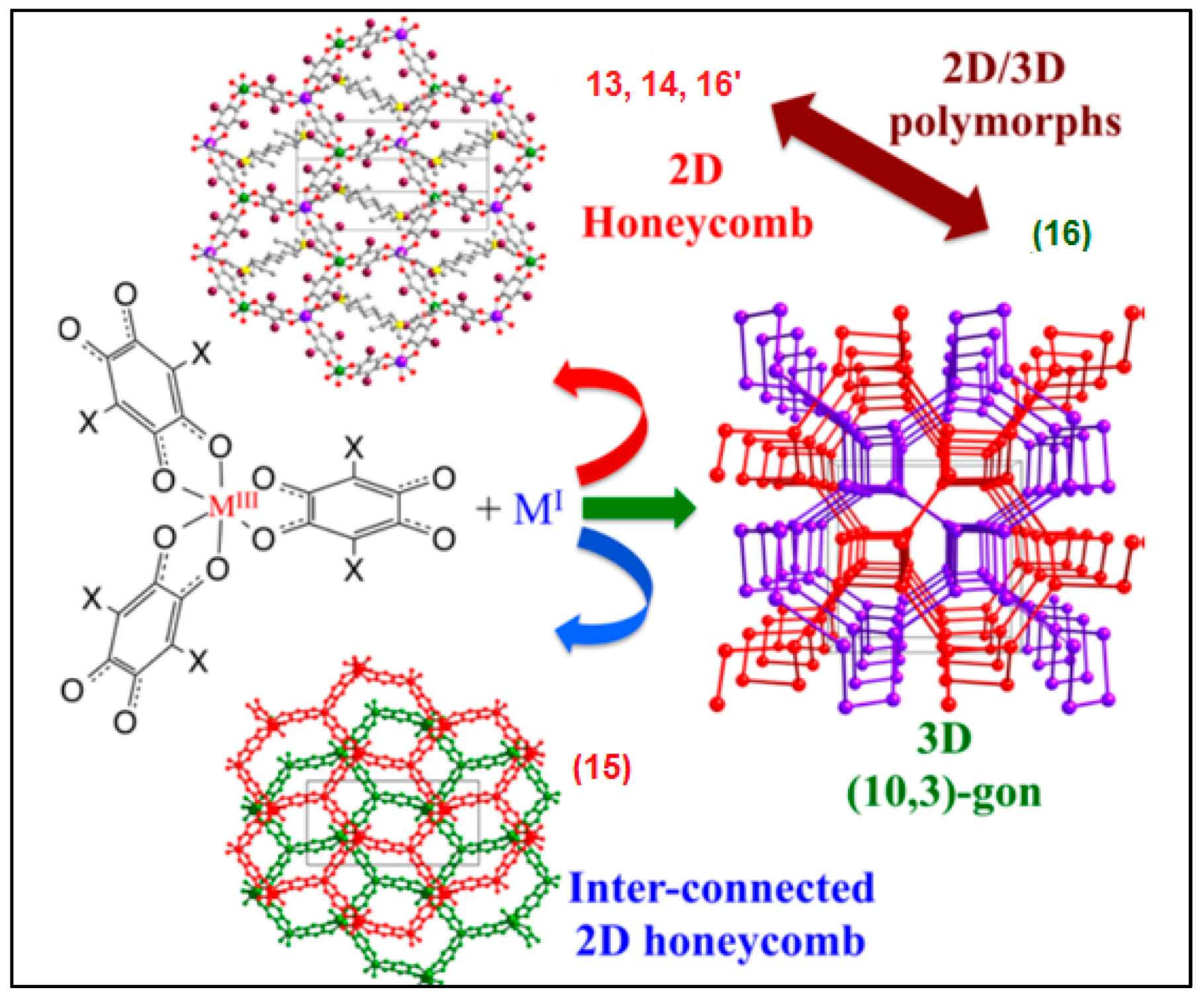
| (PBu3Me)2[NaCr(Br2An)3] 13 | 2D lattice Heterometallic anionic Honeycomb layers alternated with cationic layer in alternated manner | PM ZFS | [121] |
| (PPh3Et)2[KFe(Cl2An)3] (dmf)2 14 | 2D lattice Heterometallic anionic Honeycomb layers alternated with cationic layer in alternated manner | PM ZFS | [121] |
| (NEt3Me)[Na(dmf)]-[NaFe(Cl2An)3] 15 | Inter-connected 2D honeycomb | PM ZFS | [121] |
| (NBu3Me)2[NaCr(Br2An)3] 16 | 3D lattice | PM ZFS | [121] |
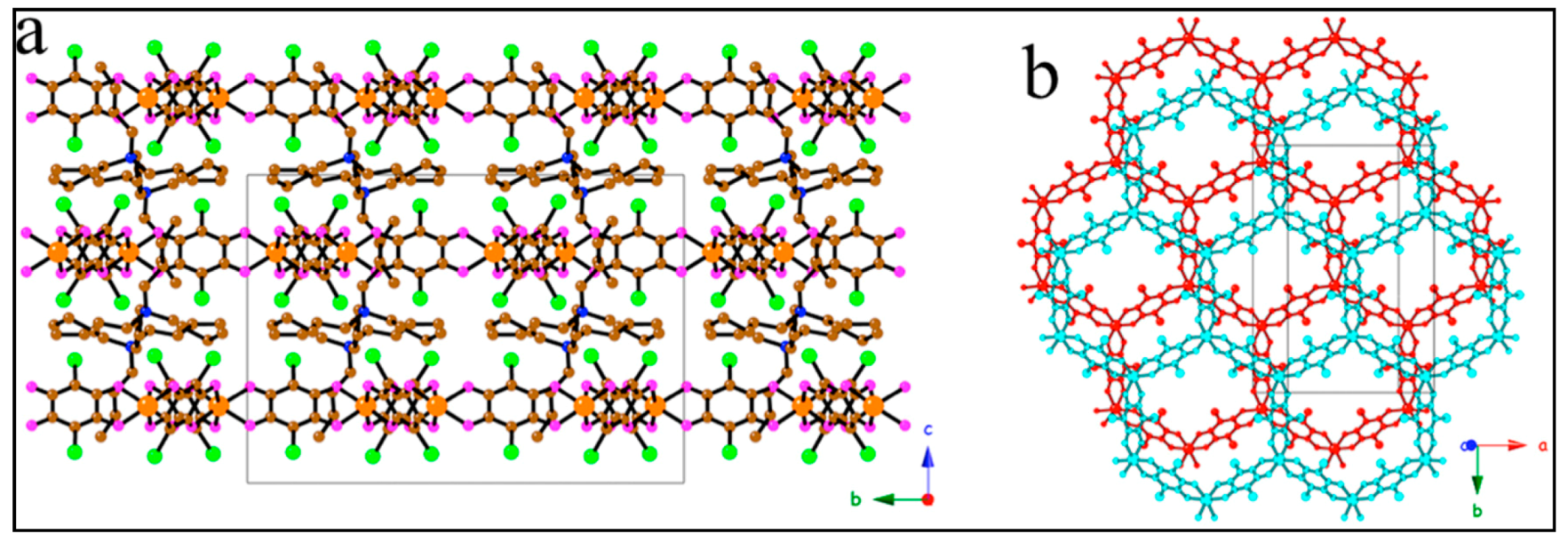
| [(H3O)(phz)3][MnCr(Cl2An)3 (H2O)] 17 | Eclipsed Heterometallic anionic Honeycomb layers alternated with cationic layers | Ferrimagnet Tc = ca. 5.0 K | [125] |
| [(H3O)(phz)3][MnCr(Br2An)3]·H2O 18 | Eclipsed Heterometallic anionic Honeycomb layers alternated with cationic layers | Ferrimagnet Tc = ca. 5.0 K | [125] |
| [(H3O)(phz)3][MnFe(Br2An)3]·H2O 19 | Eclipsed Heterometallic anionic honeycomb layers | Weak FM due to long-range AF ordering with spin canting at ca. 3.5 K | [125] |
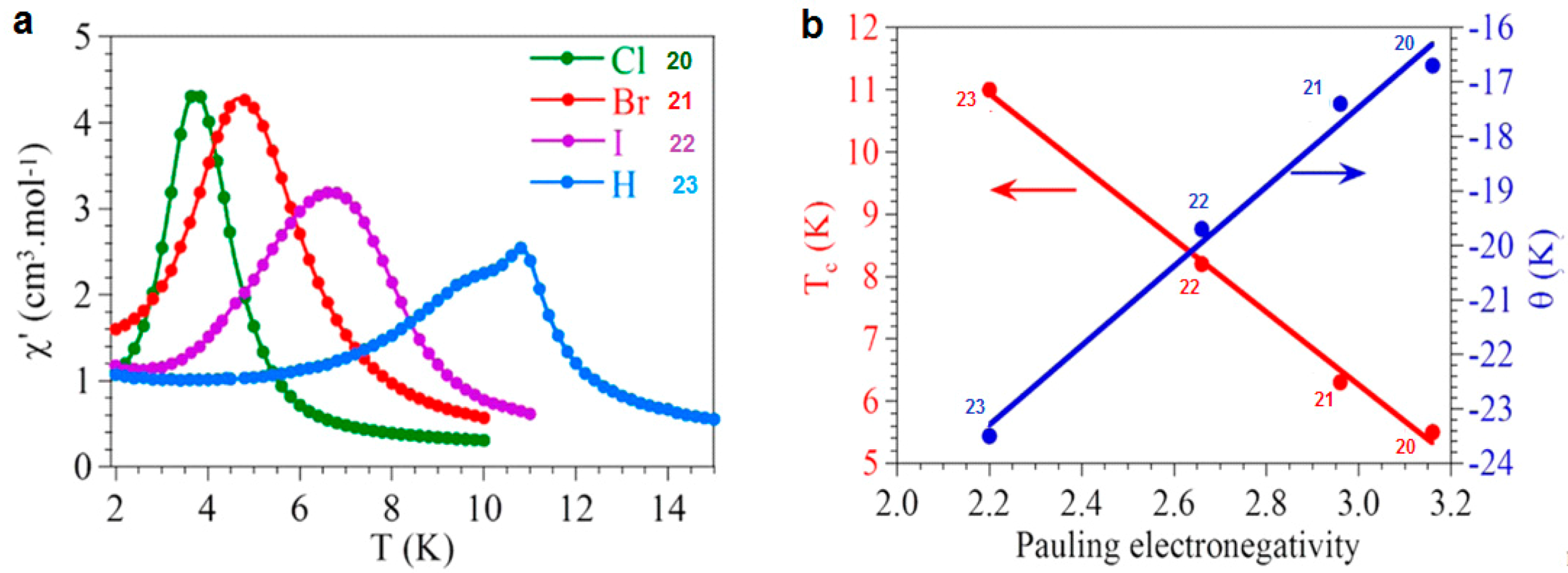
| [(n-Bu)4N]3[MnCr(Cl2An)3 (H2O)] 20 | Alternated Heterometallic anionic honeycomb layers | Ferrimagnet Tc = 5.5 K J/kB = −8.7 K | [125] |
| [(n-Bu)4N]3[MnCr(Br2An)3 (H2O)] 21 | Alternated Heterometallic anionic honeycomb layers | Ferrimagnet Tc = 6.3 K J/kB = −8.7 K | [125] |
| [(n-Bu)4N]3[MnCr(I2An)3(H2O)] 22 | Alternated heterometallic anionic honeycomb layers | Ferrimagnet Tc = 8.2 K J/kB = −10 K | [125] |
| Bu)4N]3[MnCr(H2An)3(H2O)] 23 | Alternated Heterometallic anionic honeycomb layers | Ferrimagnet Tc = 11.0 K J/kB = −12 K | [125] |
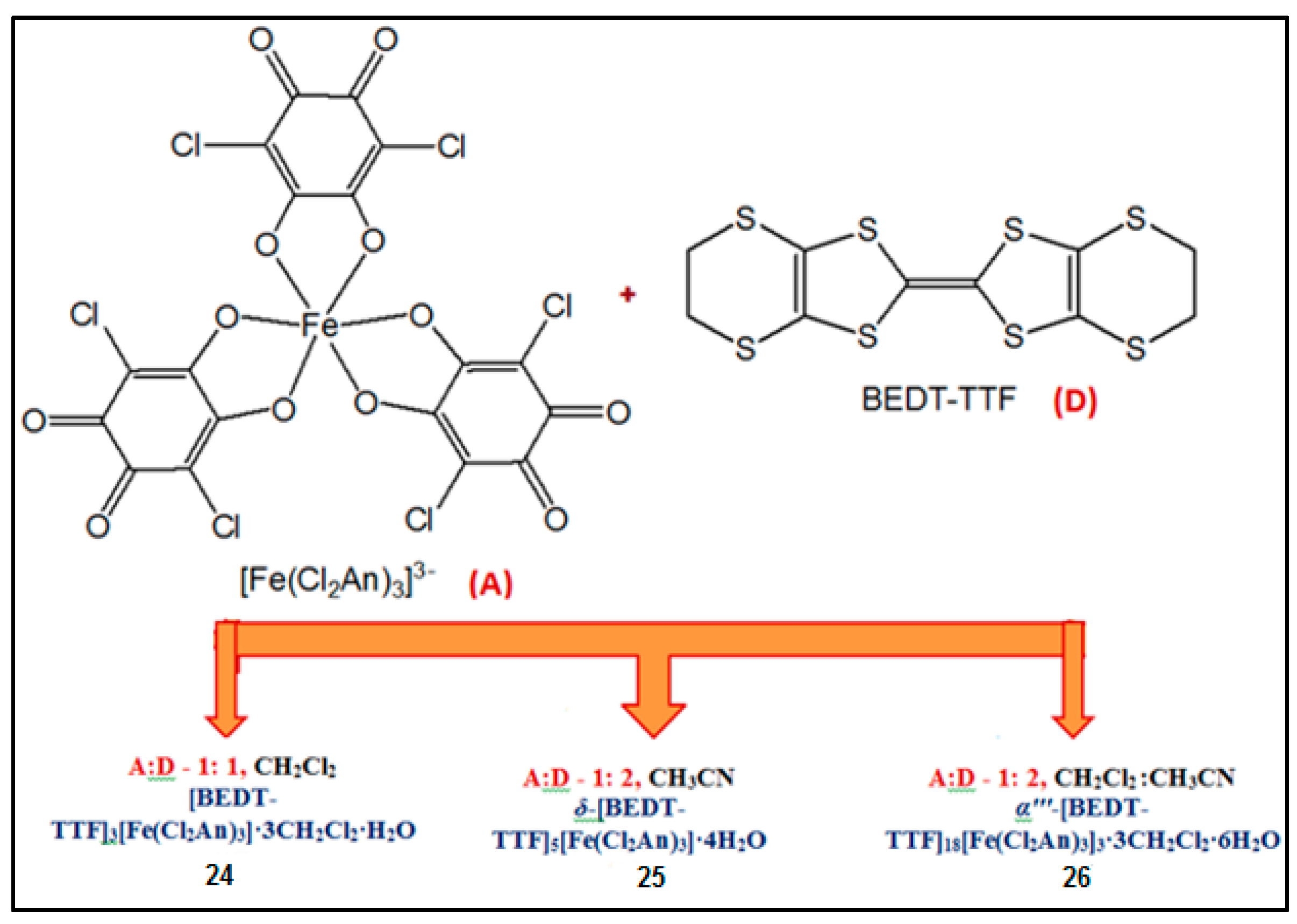
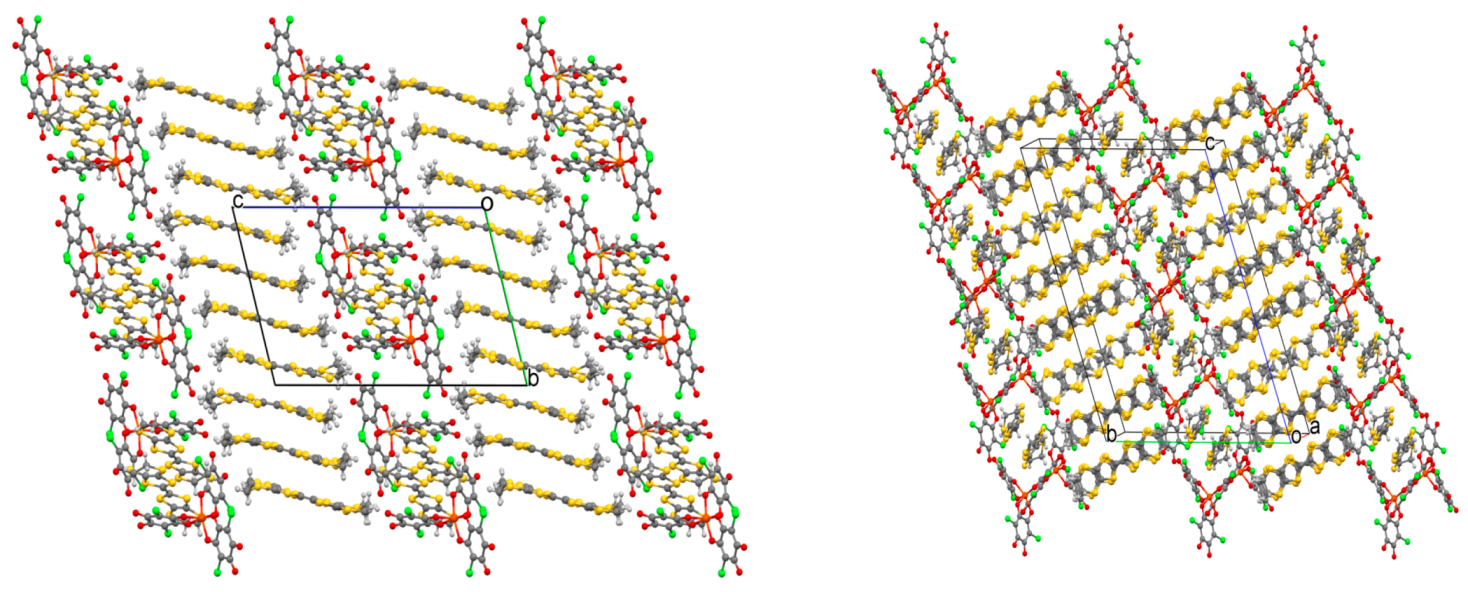
| [BEDT-TTF]3[Fe(Cl2An)3]· 3CH2Cl2·H2O 24 | BEDT-TTF dimers not-layered structure Cl···S interactions | PM with a contribution at high temperatures from BEDT-TTF radical cations semiconductor σRT = 3 × 10−4 S cm−1 Intradimer Coupling Constant JCC = −2.6 × 1033 K | [164] |
| δ-[BEDT-TTF]5[Fe(Cl2An)3]·4H2O 25 | organic-inorganic layers segregation δ packing of BEDT TTF Cl···S interactions | PM with a contribution at high temperatures from BEDT-TTF radical cations Semiconductor σRT = 2 S cm−1 | [164] |
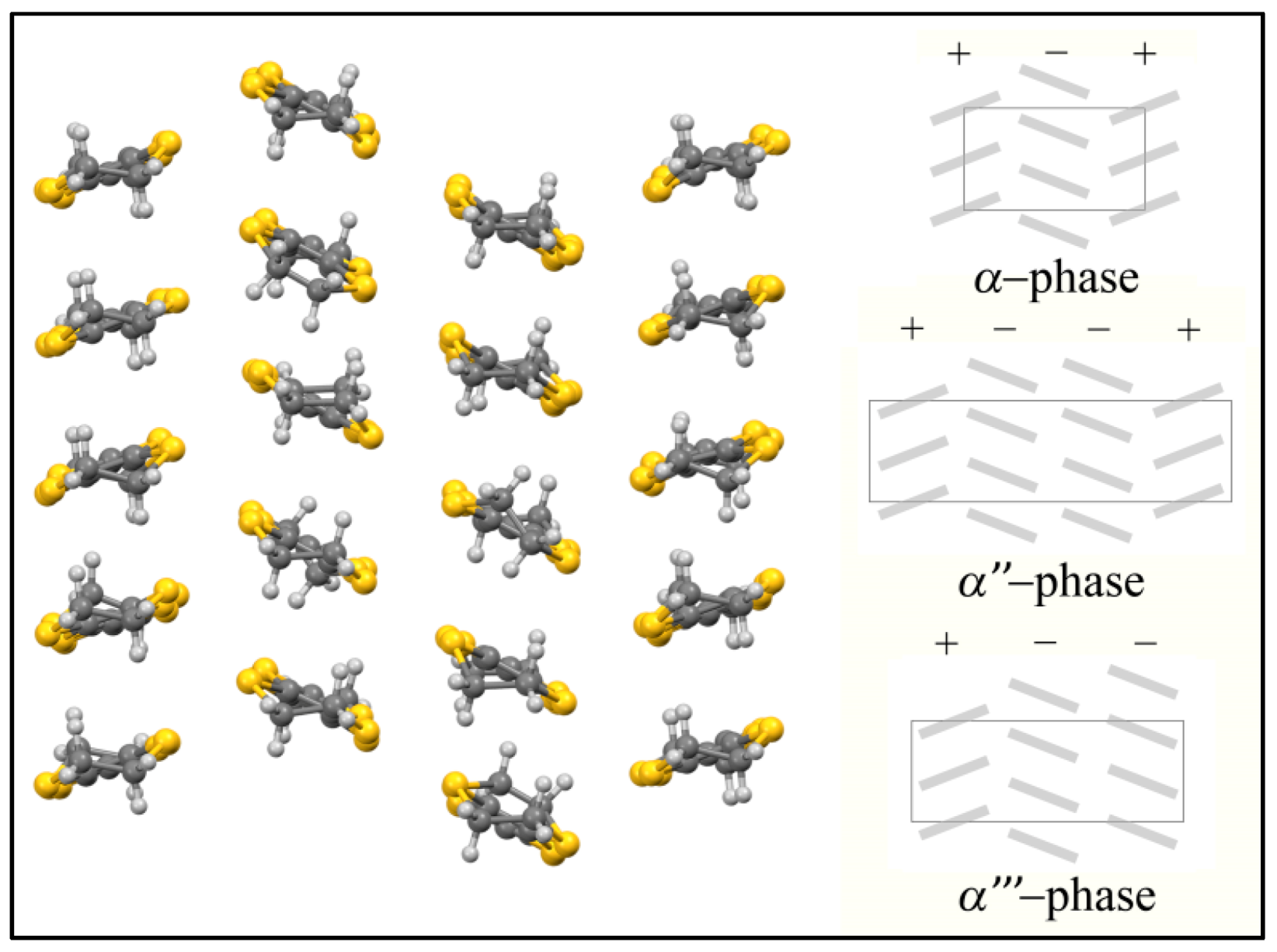
| α'''-[BEDT-TTF]18 [Fe(Cl2An)3]3·3CH2Cl2·6H2O 26 | organic-inorganic layers segregation α''' packing of BEDT TTF Cl···S interaction | PM with a contribution at high temperatures from BEDT-TTF radical cations Semiconductor σRT = 8 S cm−1 | [164] |
| [(BEDT-TTF)6 [Fe(Cl2An)3]·(H2O)1.5·(CH2Cl2)0.5 27 | organic-inorganic layers segregation θ21 phase of BEDT TTF Cl···S interaction | PM with Pauli PM contribution Semiconductor σRT = ca. 10 S cm−1 | [166] |
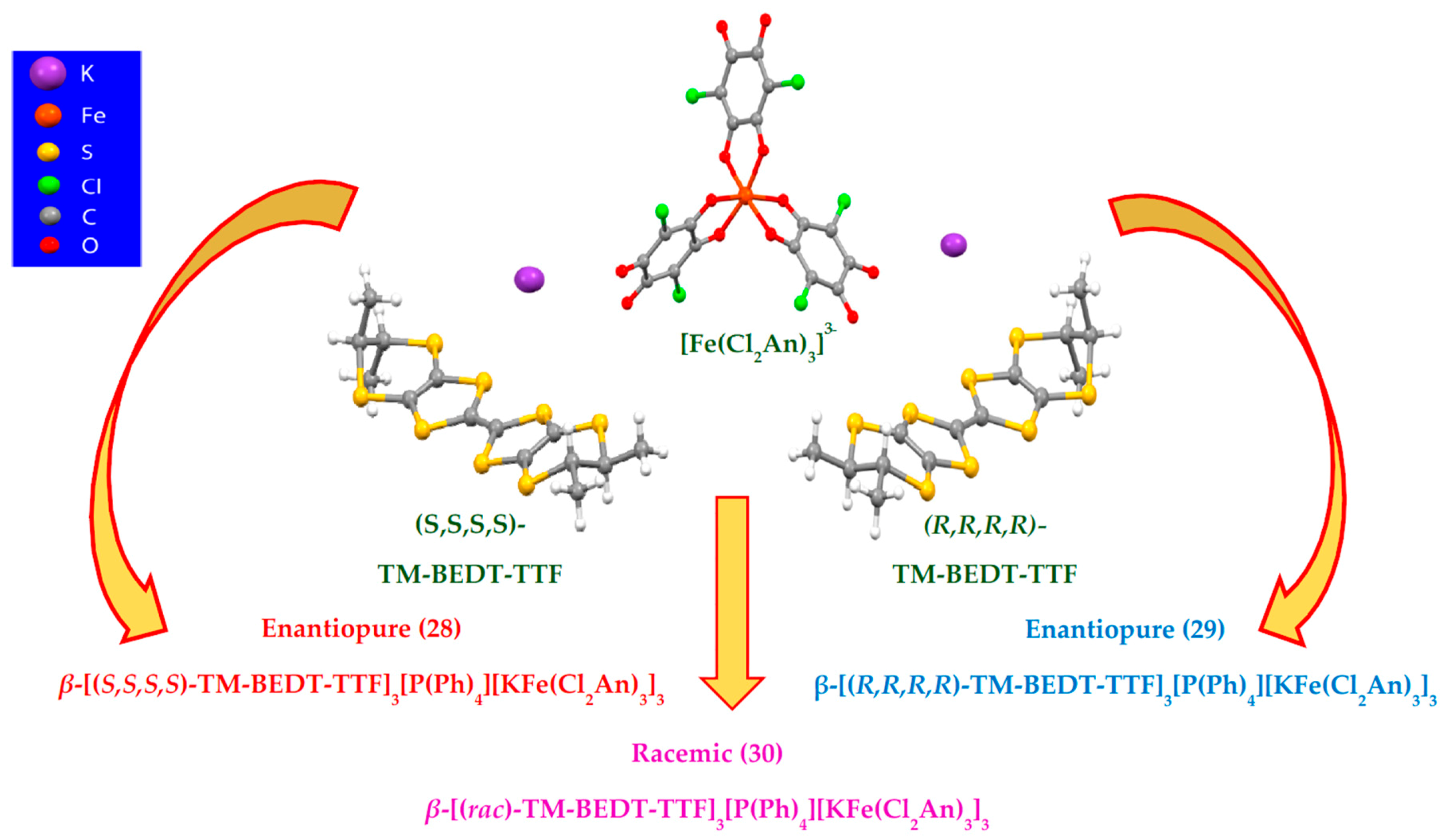
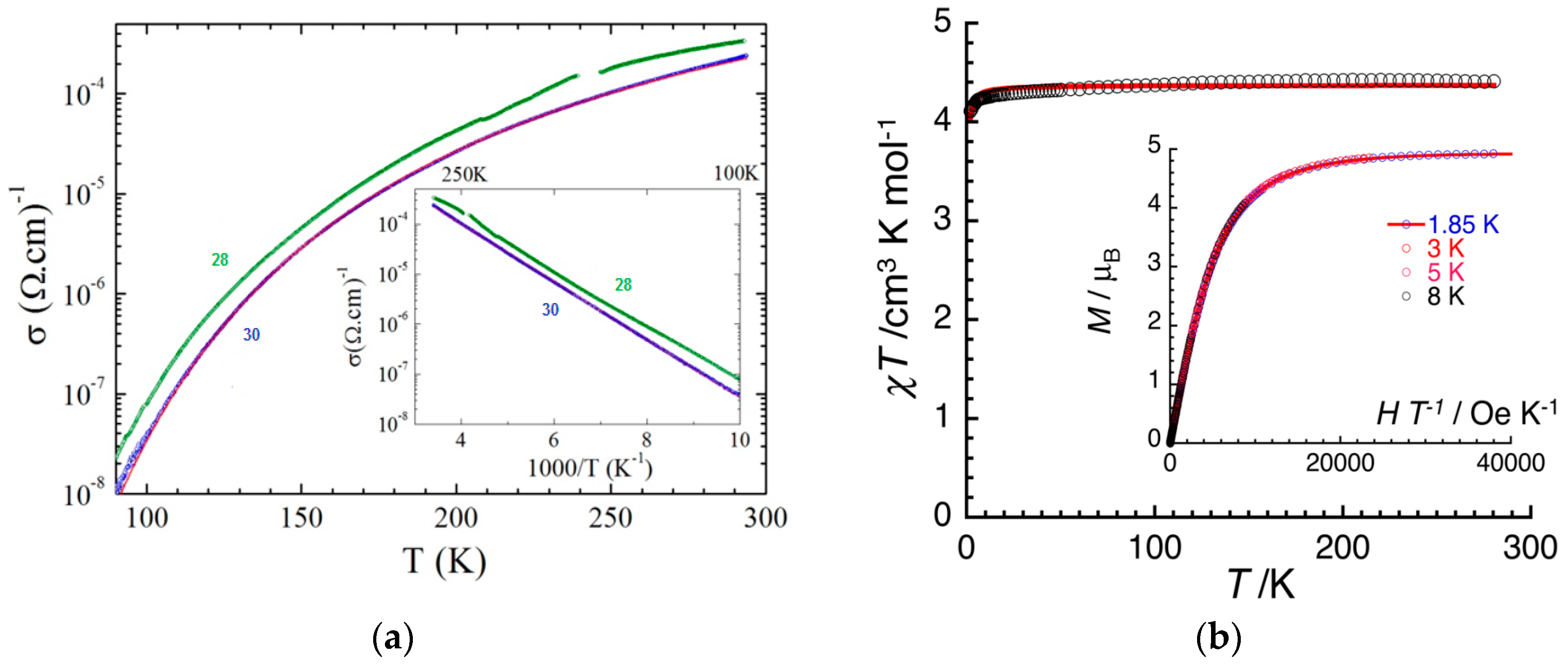
| β-[(S,S,S,S)-TM-BEDT-TTF]3PPh4[KIFeIII(Cl2An)3]·3H2O 28 β-[(R,R,R,R)-TM-BEDT-TTF]3PPh4[KIFeIII(Cl2An)3]·3H2O 29 | heterobimetallic anionic honeycomb layers alternated with cationic chiral donors Cl···Cl contact, π-π stacking terminal CH3···O contacts (segregated columns of cations and anions) β packing of TM-BEDT-TTF | Curie-Weiss PM Semiconductors σRT = 3 × 10−4 S cm−1 | [167] |
| β-[(rac)-TM-BEDT-TTF]3 PPh4[KIFeIII(Cl2An)3]·3H2O 30 | heterobimetallic anionic honeycomb layers alternated with cationic chiral -(rac)-donors Cl···Cl contact, π-π stacking terminal CH3···O contacts (segregated columns of cations and anions) β packing of TM-BEDT-TTF | Curie-Weiss PM Semiconductors σRT = 3 × 10−4 S cm−1 | [167] |
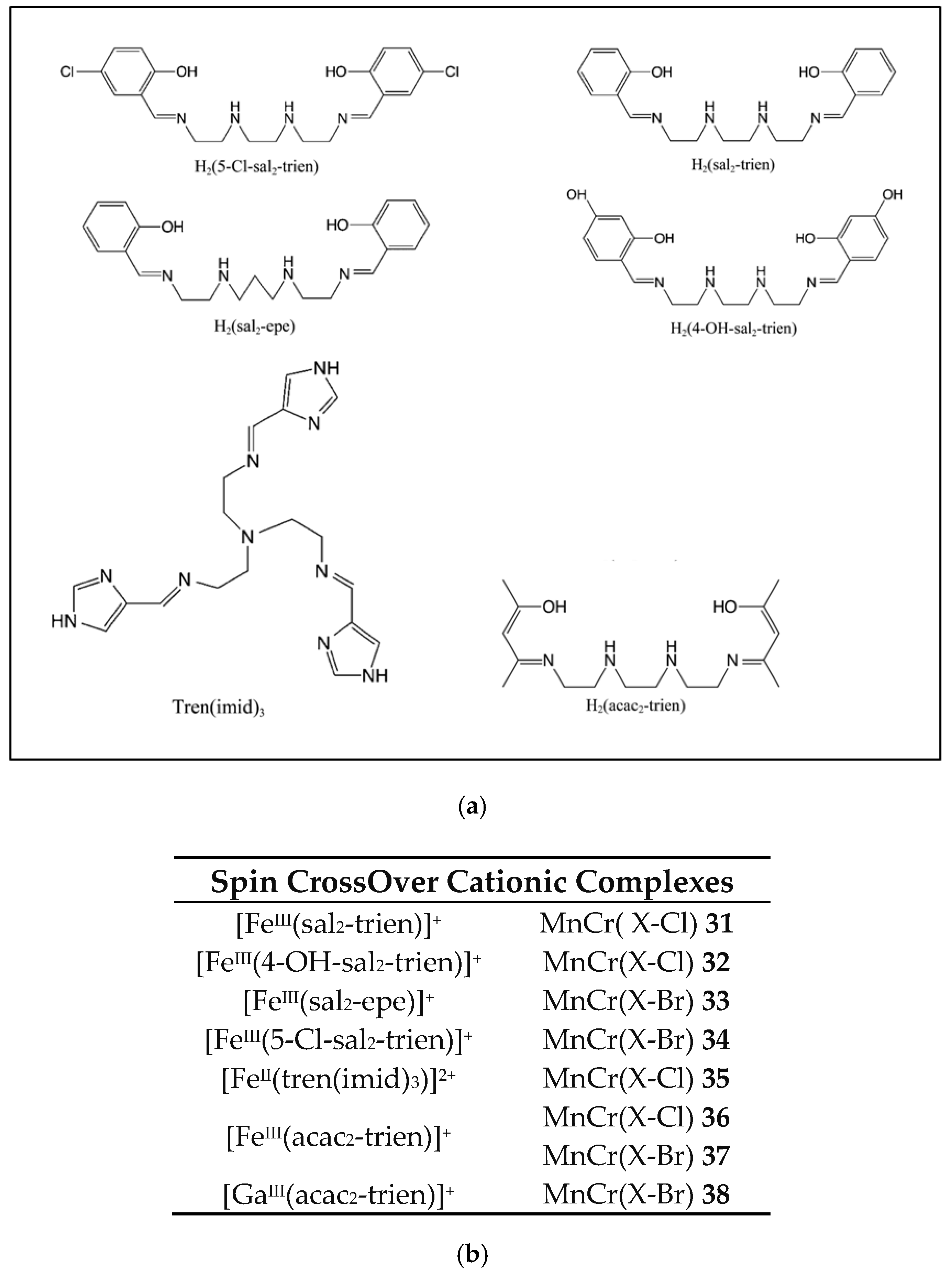
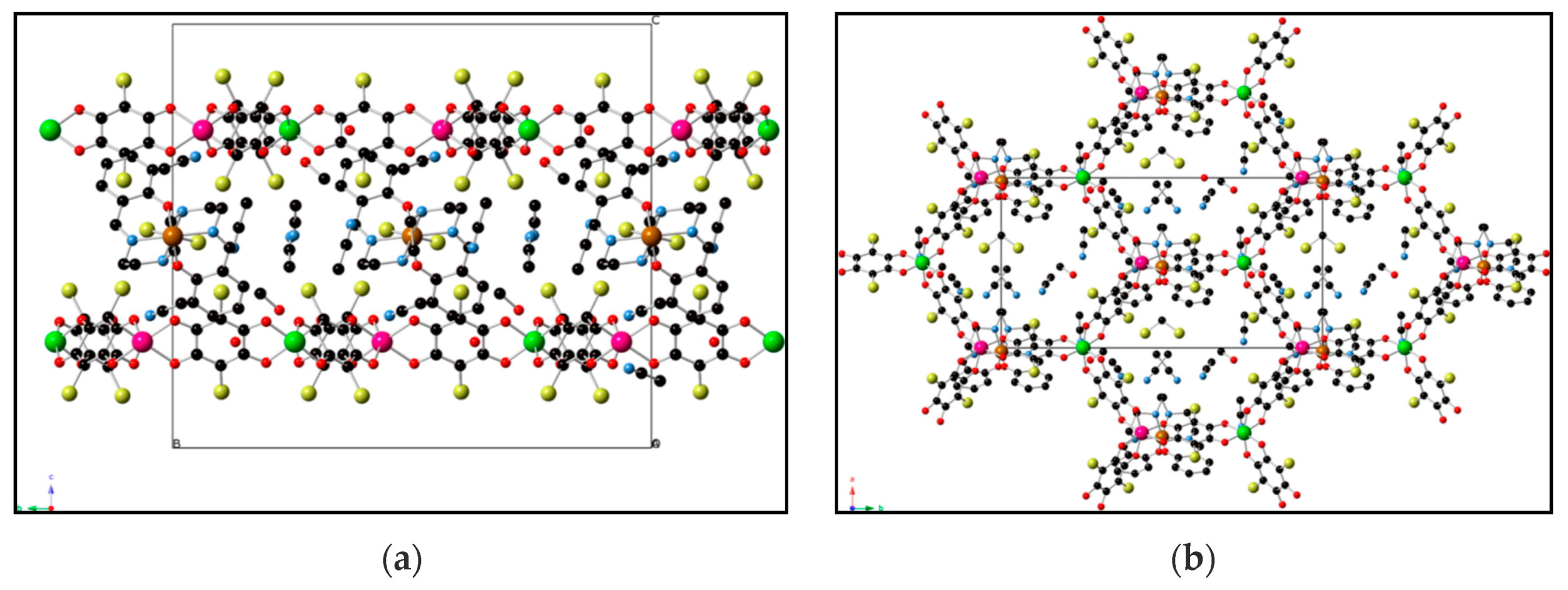
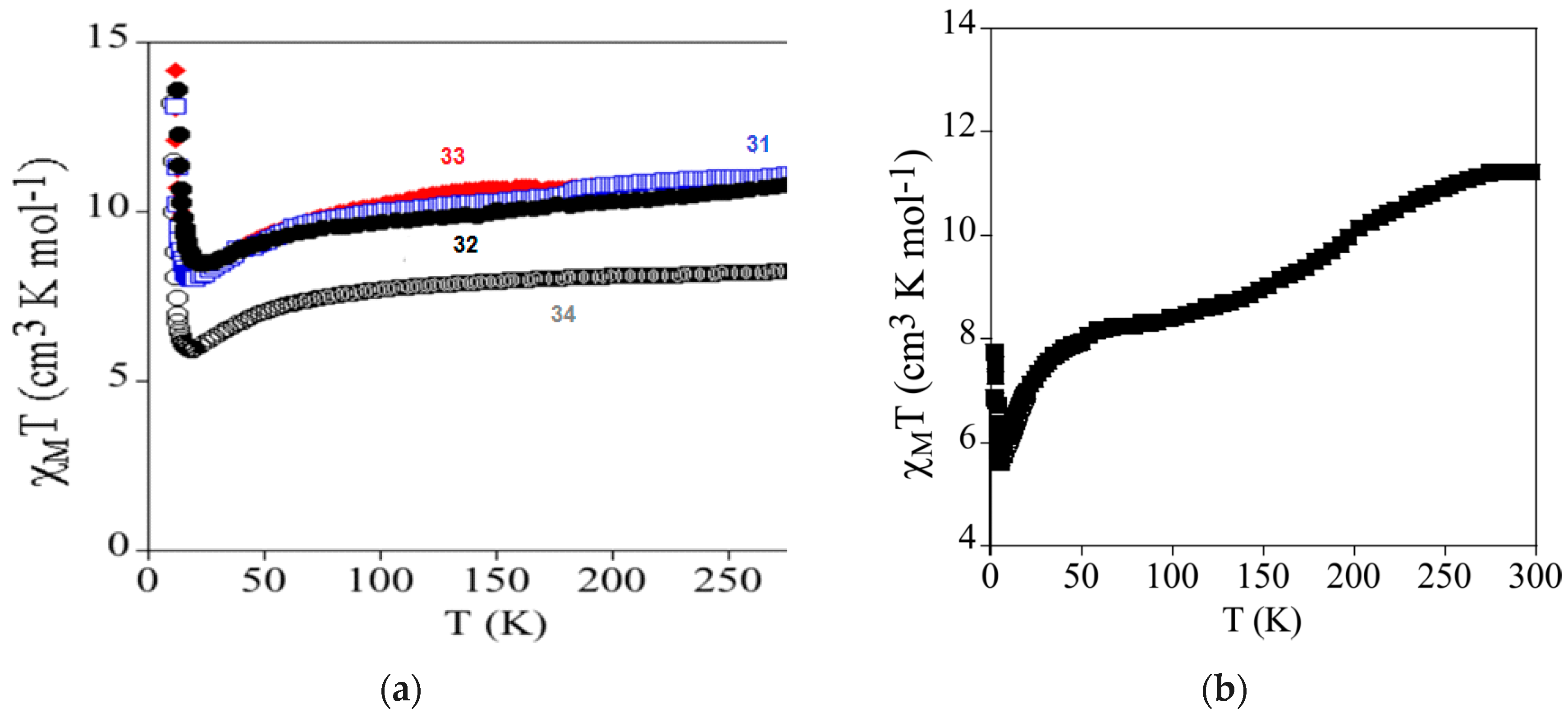
| [FeIII(sal2-trien)]MnCr(Cl2An)3 31 | 2D Honeycomb bimetallic anionic layers with inserted Fe(III) cationic complexes and solvent molecules. | FerriM Inserted HS Fe(III) cations Tc = 10K J/kB = −10 K Exfoliation | [168] |
| [FeIII(4-OH-sal2-trien)] MnCr(Cl2An)3 32 | 2D Honeycomb bimetallic anionic layers with inserted Fe(III) cationic complexes and solvent molecules. | FerriM Inserted HS Fe(III) cations Tc = 10.4 K J/kB = −7.2 K | [168] |
| [FeIII(sal2-epe)] MnCr(Br2An)3 33 | 2D Honeycomb bimetallic anionic layers with inserted Fe(III) cationic complexes and solvent molecules. | FerriM Inserted HS Fe(III) cations Tc = 10.2 K J/kB = −6.5 K | [168] |
| [FeIII(5-Cl-sal2-trien)] MnCr(Br2An)3 34 | 2D honeycomb bimetallic anionic layers with inserted Fe(III) cationic complexes and solvent molecules. | FerriM Inserted LS Fe(III) cations Tc = 9.8 K J/kB = −6.7 K | [168] |
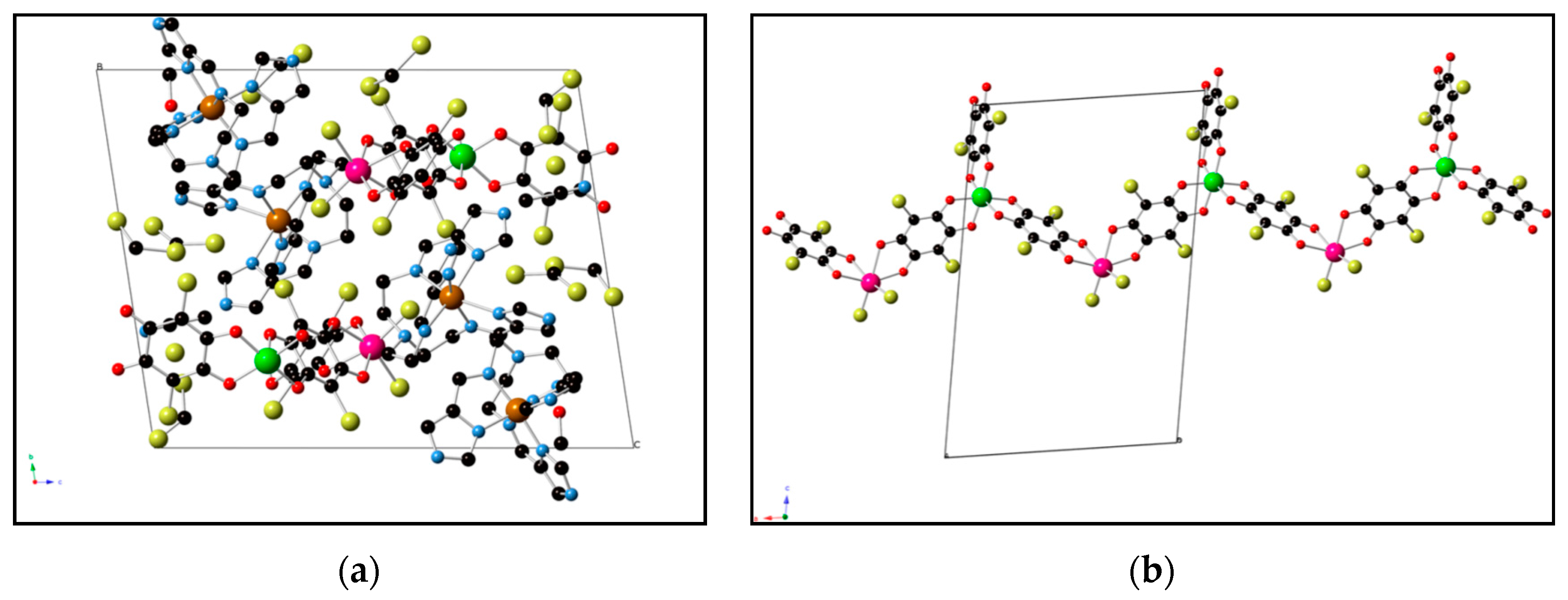
| [FeII(tren- (imid)3)]2 MnIICl2CrIII(Cl2An)3]Cl·solvent 35 | 1D anionic chain formed by CrIIIcomplexes bonded to two Mn(II) ions through two bis-bidentate chloranilate bridges, and terminal third choranilate. | FerriM coupling within the chains that gives rise to a magnetic ordering below 2.6 K | [168] |
| [FeIII(acac2-trien)] [MnIICrIII(Cl2An)3]3(CH3CN)2 36 | Neutral layers formed by 2D honeycomb bimetallic anionic layers with cationic complexes inside the hexagonal channels. van der Waals interactions between the layers. | FerriM at ca. 10.8 K, inserted HS Fe(III) cations Exfoliation | [172] |
| [FeIII(acac2-trien)] [MnIICrIII(Br2An)3]3(CH3CN)2 37 | Neutral layers formed by 2D Honeycomb bimetallic anionic layers with cationic complexes inside the hexagonal channels. Van der Waals interactions between the layers | FerriM at ca. 11.4 K, inserted HS Fe(III) cations Exfoliation | [172] |
| [GaIII(acac2-trien)] [MnIICrIII(Br2An)3]3(CH3CN)2 38 | Neutral layers formed by 2D Honeycomb bimetallic anionic layers with cationic complexes inside the hexagonal channels. Van der Waals interactions between the layers | FerriM at ca. 11.6 K | [172] |
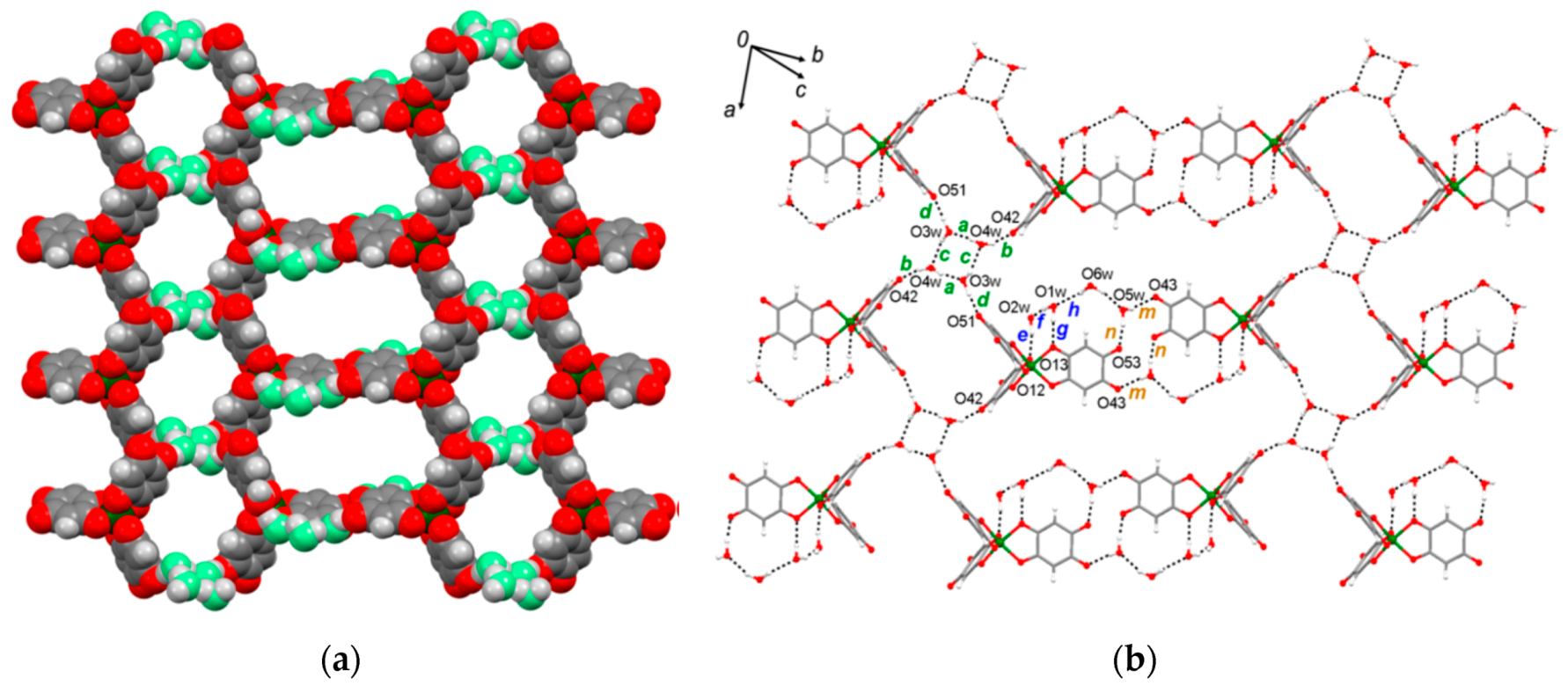
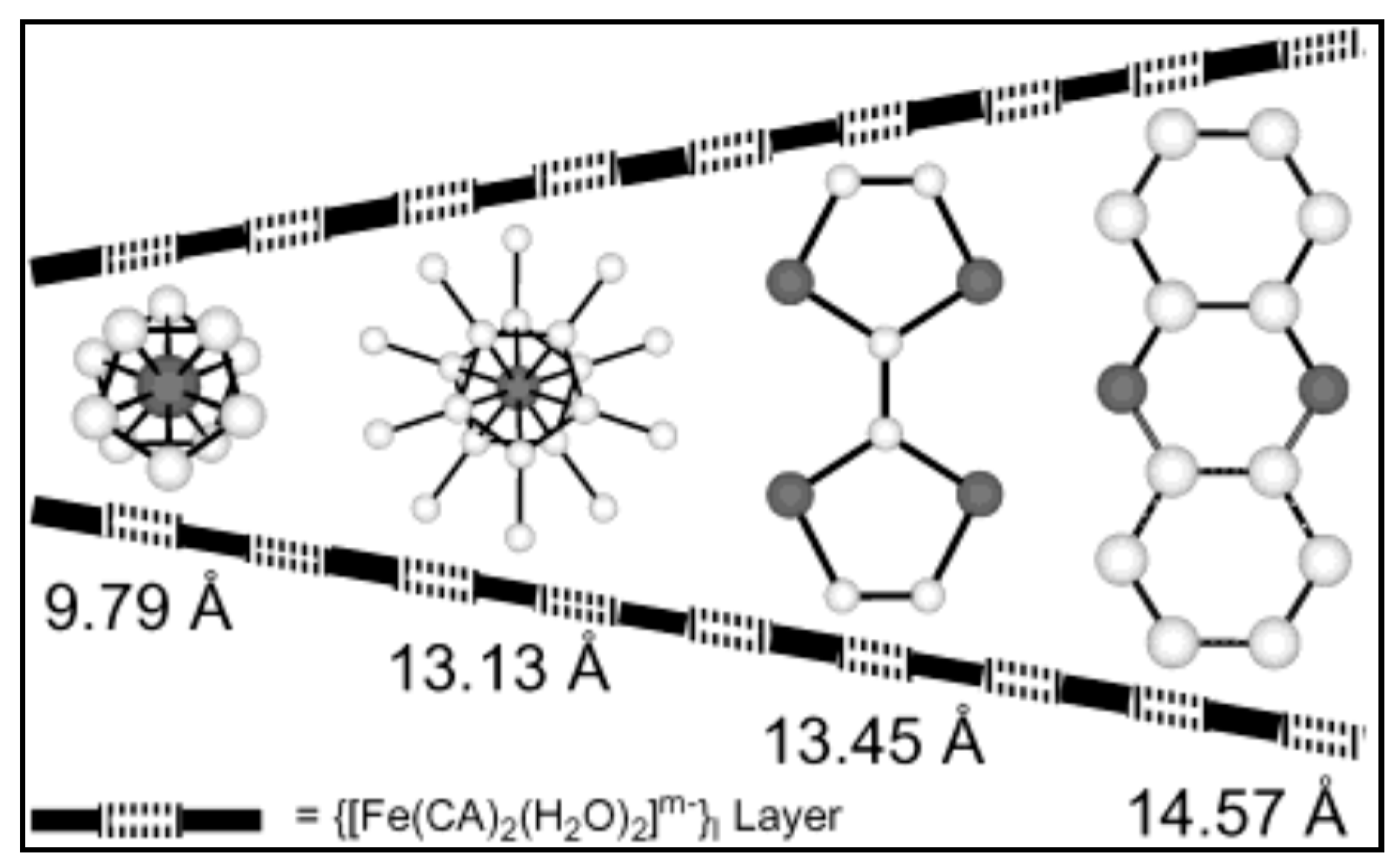
| {(H0.5phz)2[Fe(Cl2An)2(H2O)2]∙2H2O}n 39 | Supramolecular Framework Novel Intercalation Compounds Electrostatic interactions | Interlayer distances (Fe(1)-Fe(1′′))14.57 Å In 77–300 K temperature range, EPR silent. Intralayer AFM exchange via Hydrogen-Bonds and stacking interactions among [Fe(Cl2An)2(H2O)2]− monomers J2D/kB = −0.10 K | [184]. |
| {[Fe(Cp)2][Fe(Cl2An)2(H2O)2]}n 40 | Supramolecular Framework Novel Intercalation Compounds Electrostatic interactions | Interlayer distances (Fe(1)-Fe(1′′)) 9.79 Å In 77–300 K temperature range, EPR silent. Intralayer AFM exchange via Hydrogen-Bonds and stacking interactions among [Fe(Cl2An)2(H2O)2]− monomers and Heisenberg AFM intrachain stacking interactions in 1D arrays of [Fe(Cp)2]+ cations J2D/kB = −0.13 K J1D/kB = −2.4 K | [184] |
| [Fe(Cp*)2][Fe(Cl2An)2(H2O)2]}n 41 | Supramolecular Framework Novel Intercalation Compounds Electrostatic interactions π-π stacking tilted columns of stacked decamethylferrocene cations | Interlayer distances (Fe(1)-Fe(1′′)) 13.13 Å. In 77–300 K temperature range, EPR silent High-spin (S = 5/2)Fe(III) ions in {[Fe(Cl2An)2(H2O)2]}m− anions Low-spin (S = 1/2) Fe(III) ions in [Fe(Cp*)2]+ cations Intralayer AFM exchange via hydrogen-bonds and stacking interactions among [Fe(Cl2An)2(H2O)2]− monomers and Heisenberg AFM intrachains stacking interaction in 1D arrays of [Fe(Cp)2]+ cations J/kB = −9.5 K J1D/kB = −1.9 K | [184] |
| {(TTF)2[Fe(Cl2An)2(H2O)2]}n 42 | Novel intercalation compounds formed by the 2D hydrogen-bond supported layers and functional guests. Electrostatic interactions π-π stacking Face to Face stacking of TTF cations in columnar structure S∙∙∙S distances (type A; 3.579(3) Å, and type B; 3.618(3) Å). Head-to-Tail arrangement for TTF cations in the stacked column | Interlayer distances (Fe(1)-Fe(1′′)) 13.45 Å. EPR active with g = 2.008 (2 signals) indicating TTF is present as radical species High-spin Fe(II) and Fe(III) ions (the iron-chloranilate anionic layer has a valence-trapped mixed-valence state) Isotropic intralayer AFM exchange via hydrogen-bonds and stacking interaction among iron(III)- and iron(II)-chloranilate monomers (1:1) Heisenberg alternating AFM linear chain for isotropic exchange in the 1D array of TTF cations via intrachain stacking interactions J/kB = −6.5 K J1D/kB = −443 K | [184] |
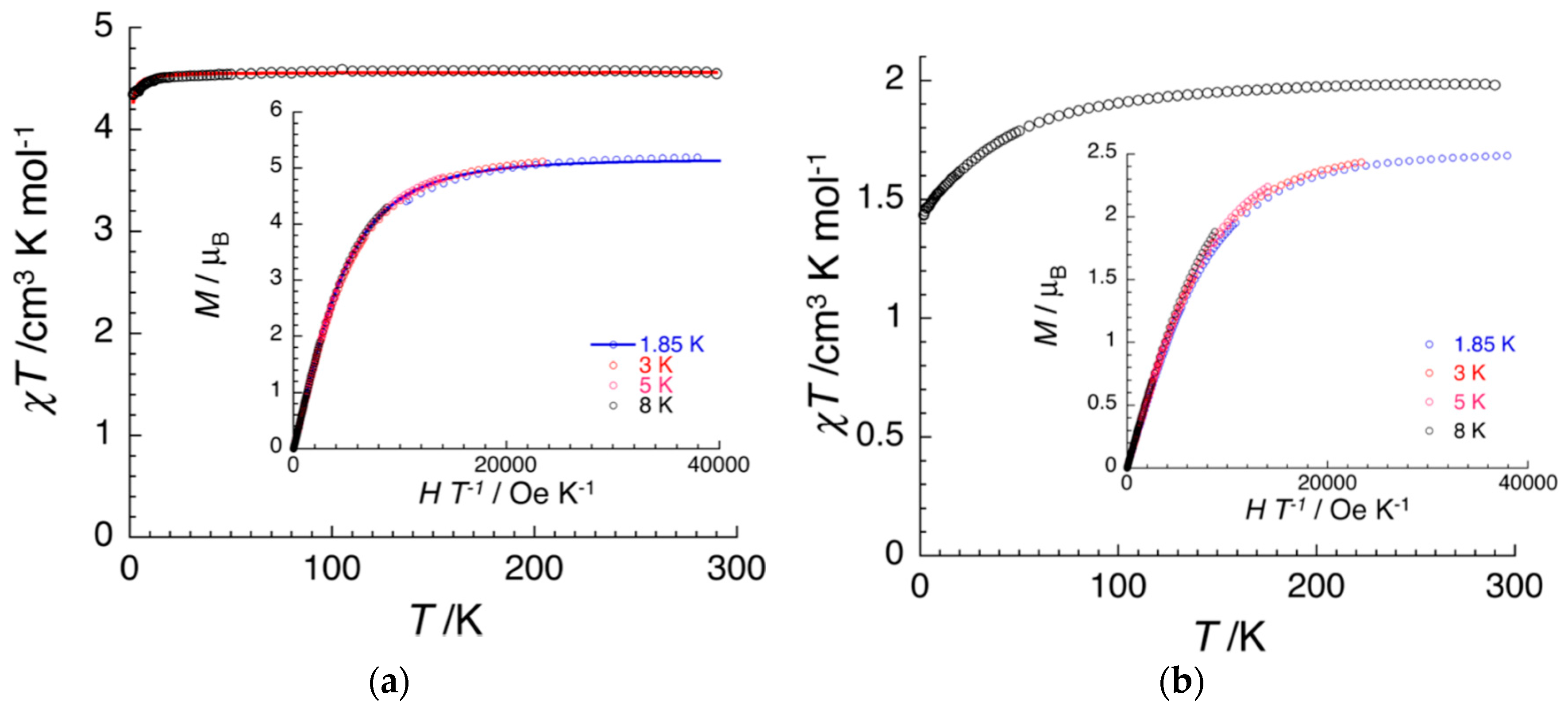
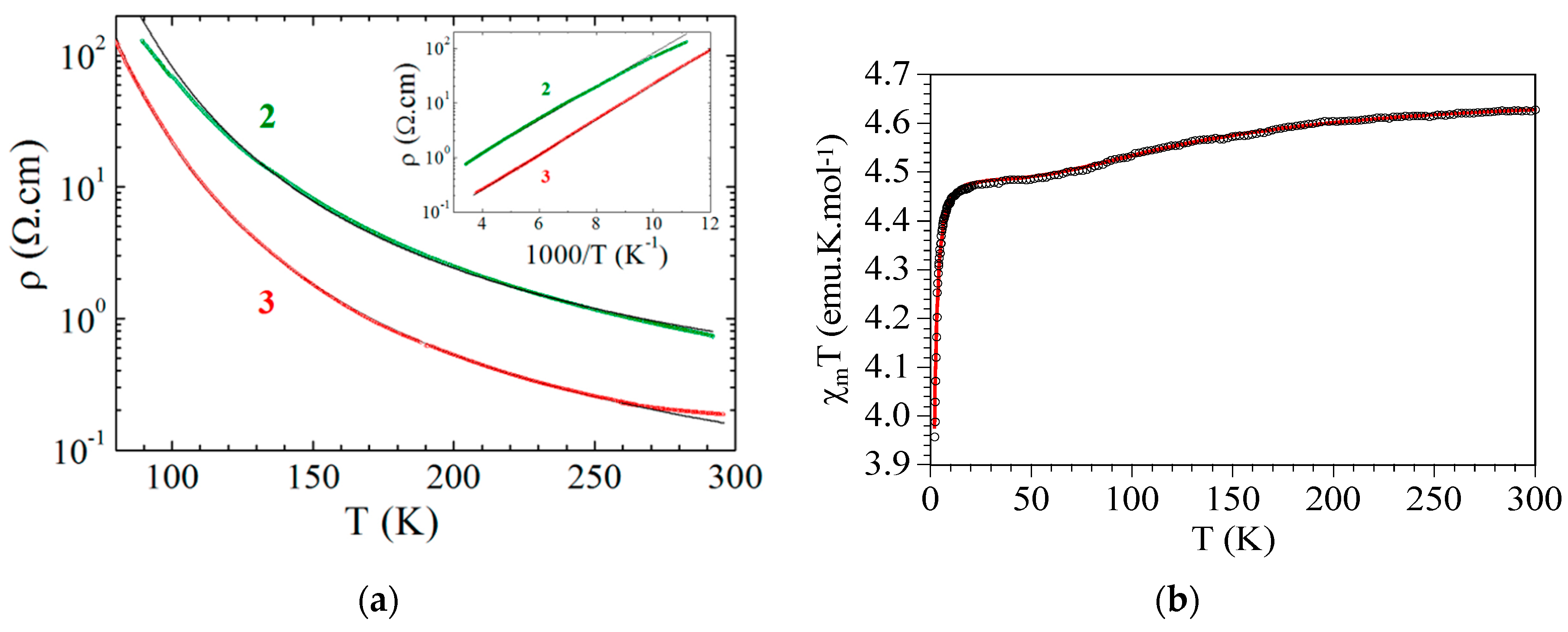
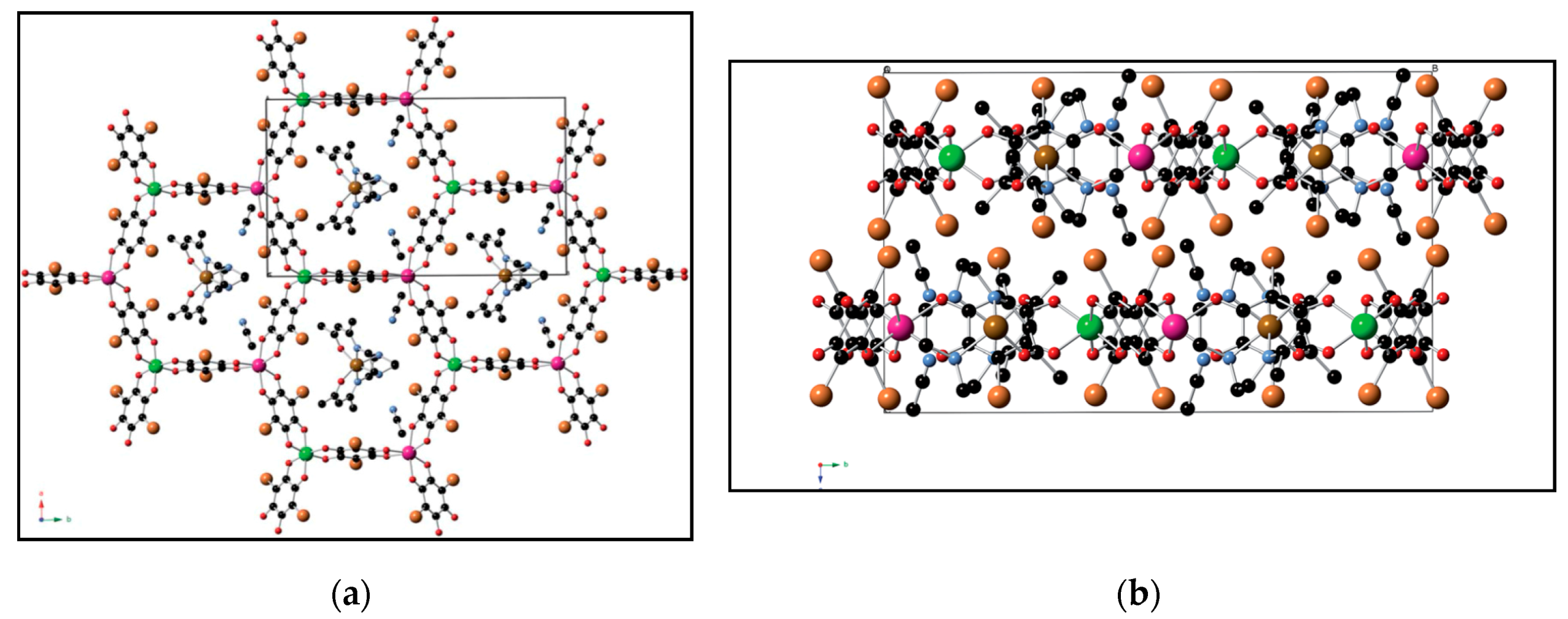
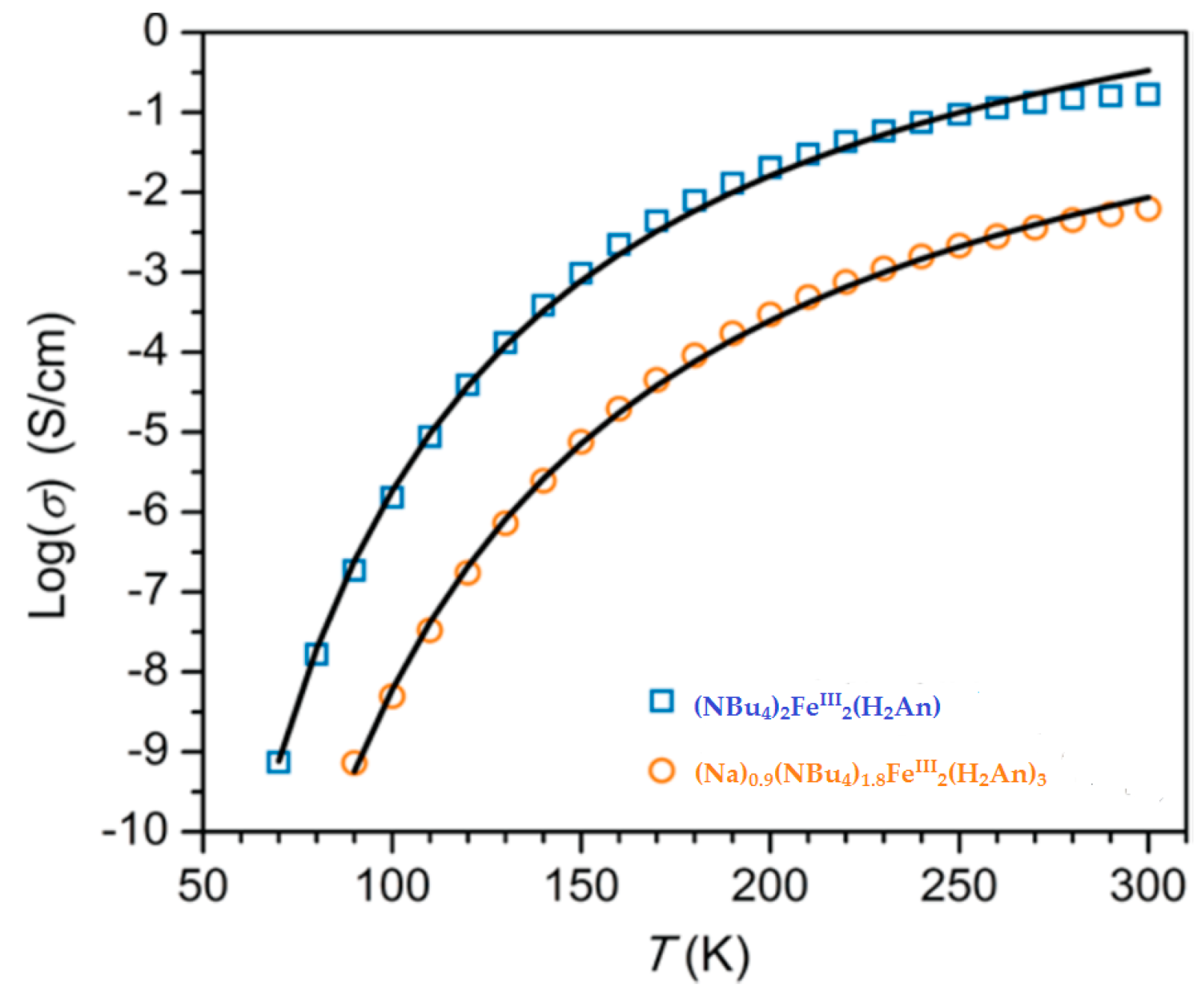
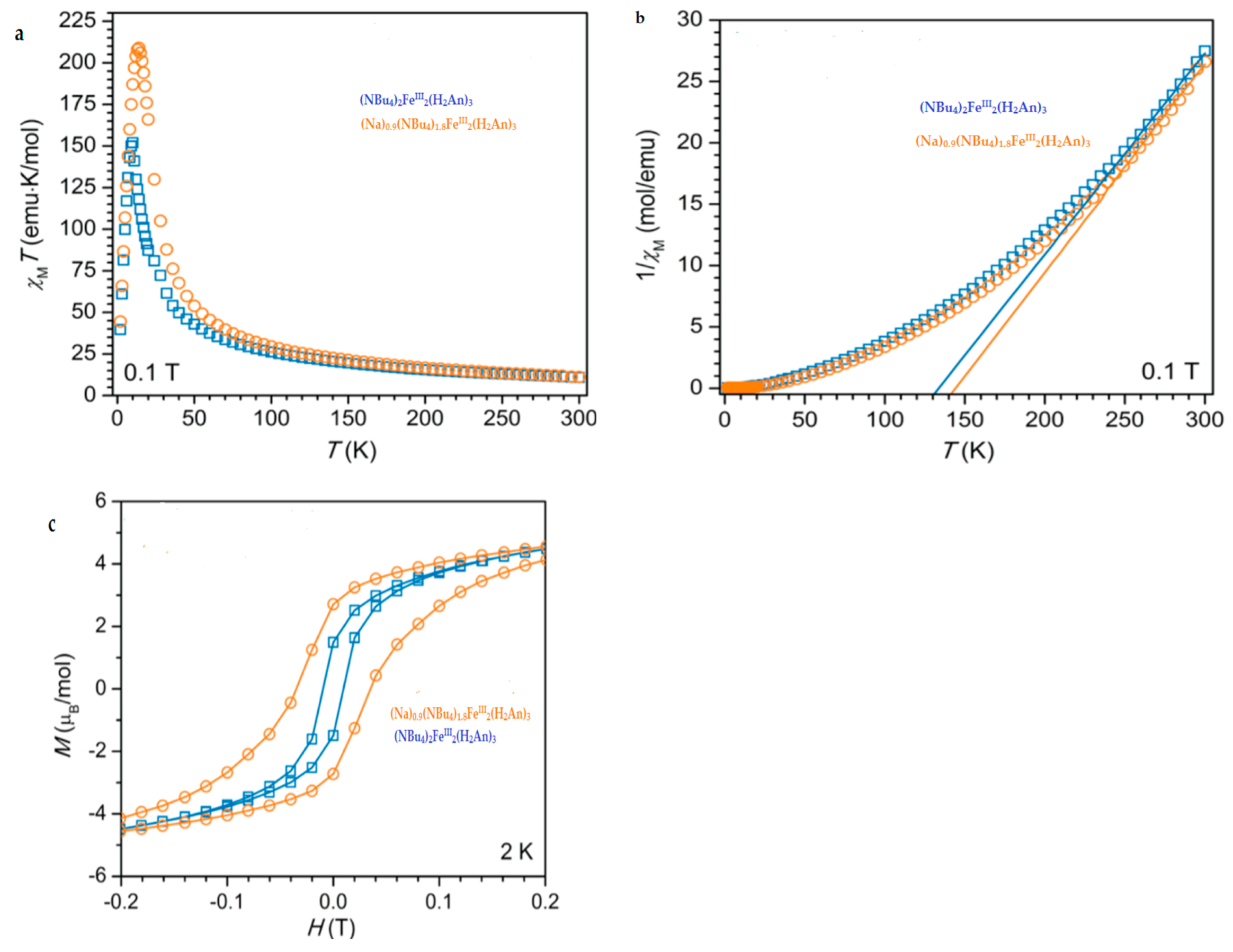
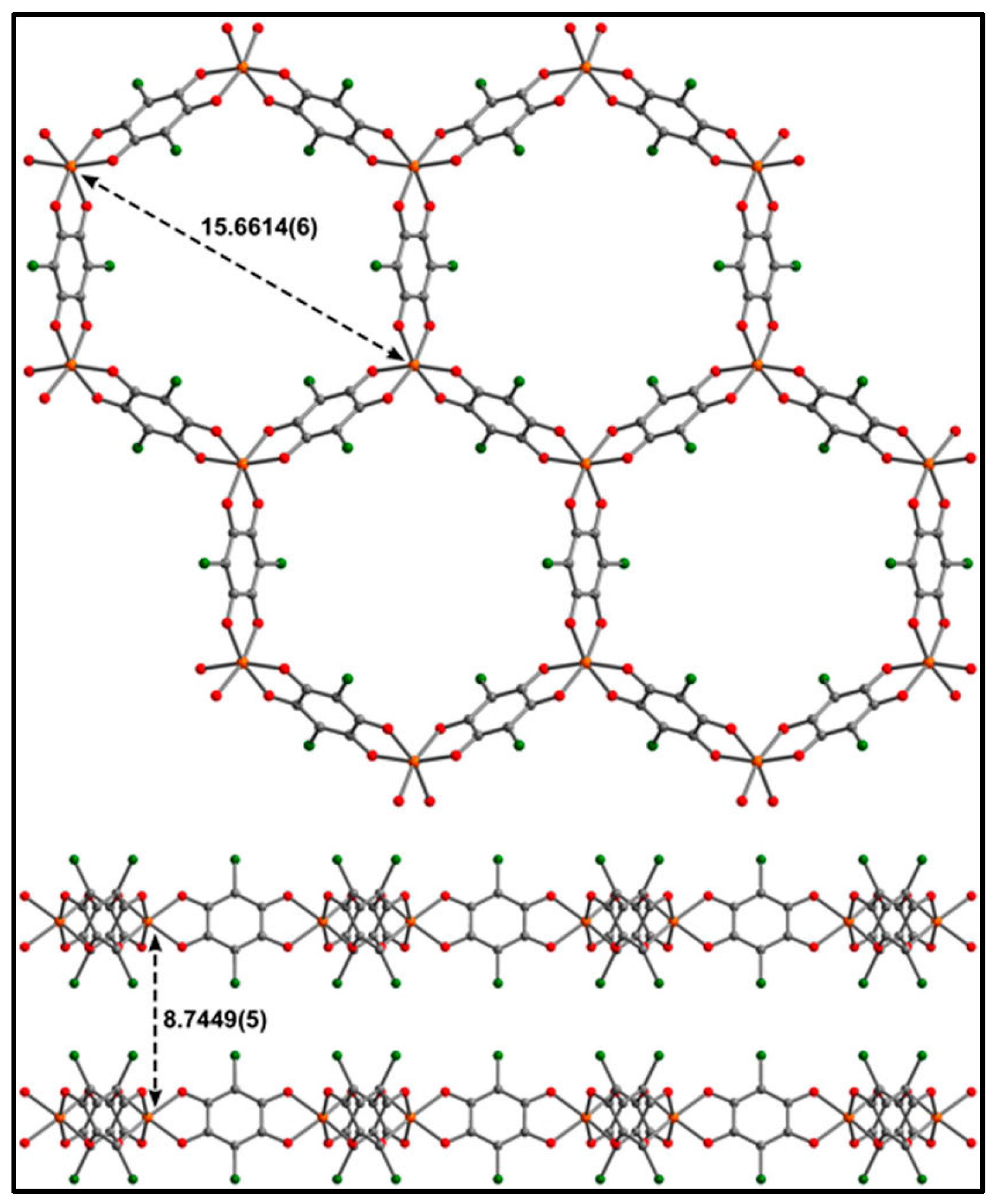
| (NBu4)2FeIII2(H2An)3 43 | 3D structure MOF with Robin-Day Class II/III mixed-valency ligand | Curie-Weiss PM J1D/kB = 0.89 K (250–300 K) High T- FM Low T- FerriM interactions Arrhenius semiconductor σRT = 0.16(1) S cm−1 | [185] |
| (Na)0.9(NBu4)1.8FeIII2(H2An)3 44 | Isostructural to 46 (PXRD) MOF with Robin-Day Class II/III mixed-valency ligand | Curie-Weiss PM J1D/kB = 0.95 K (250-300K) Arrhenius semiconductor σRT = 0.0062(1) S cm−1 | [185] |
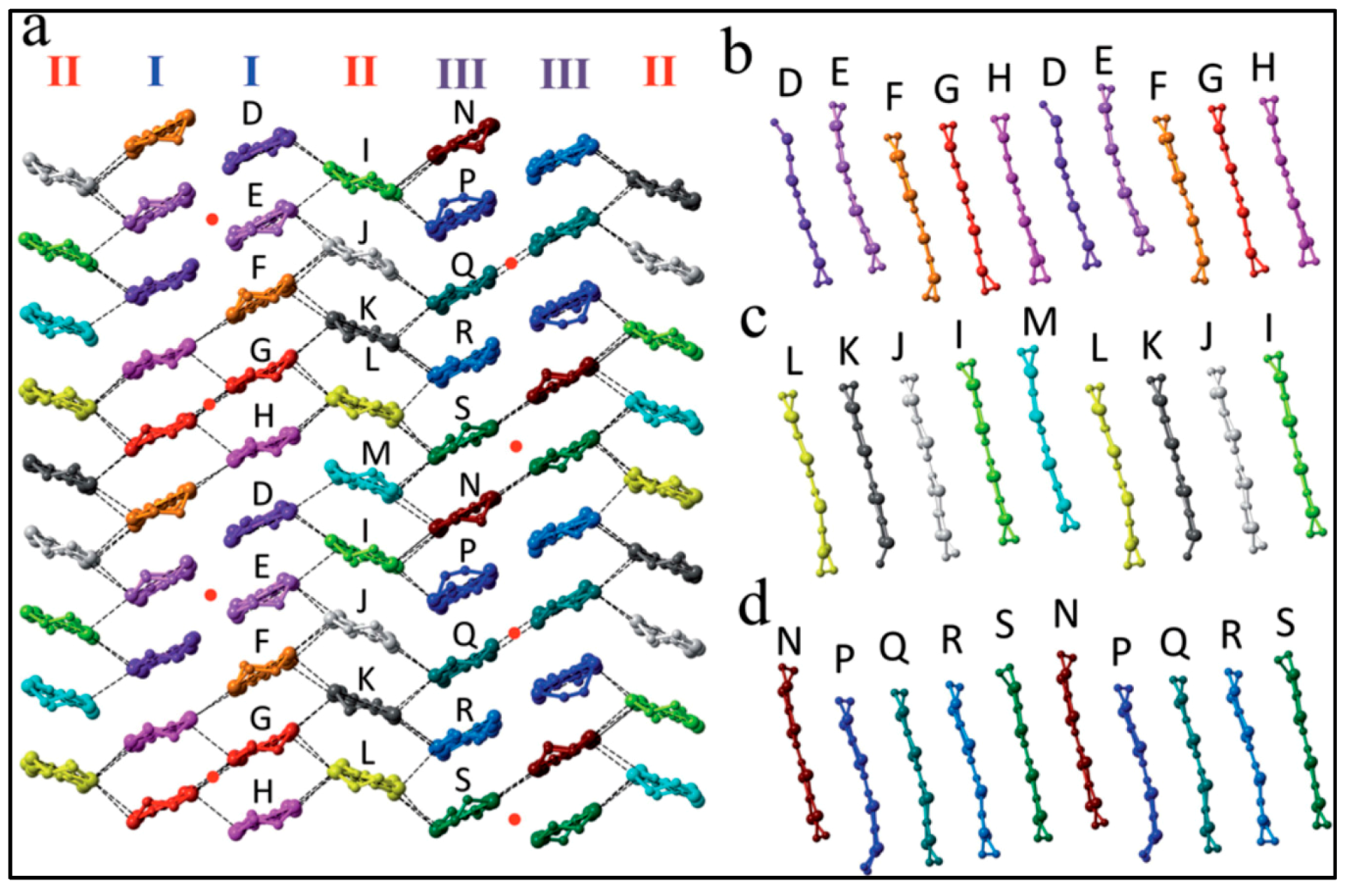
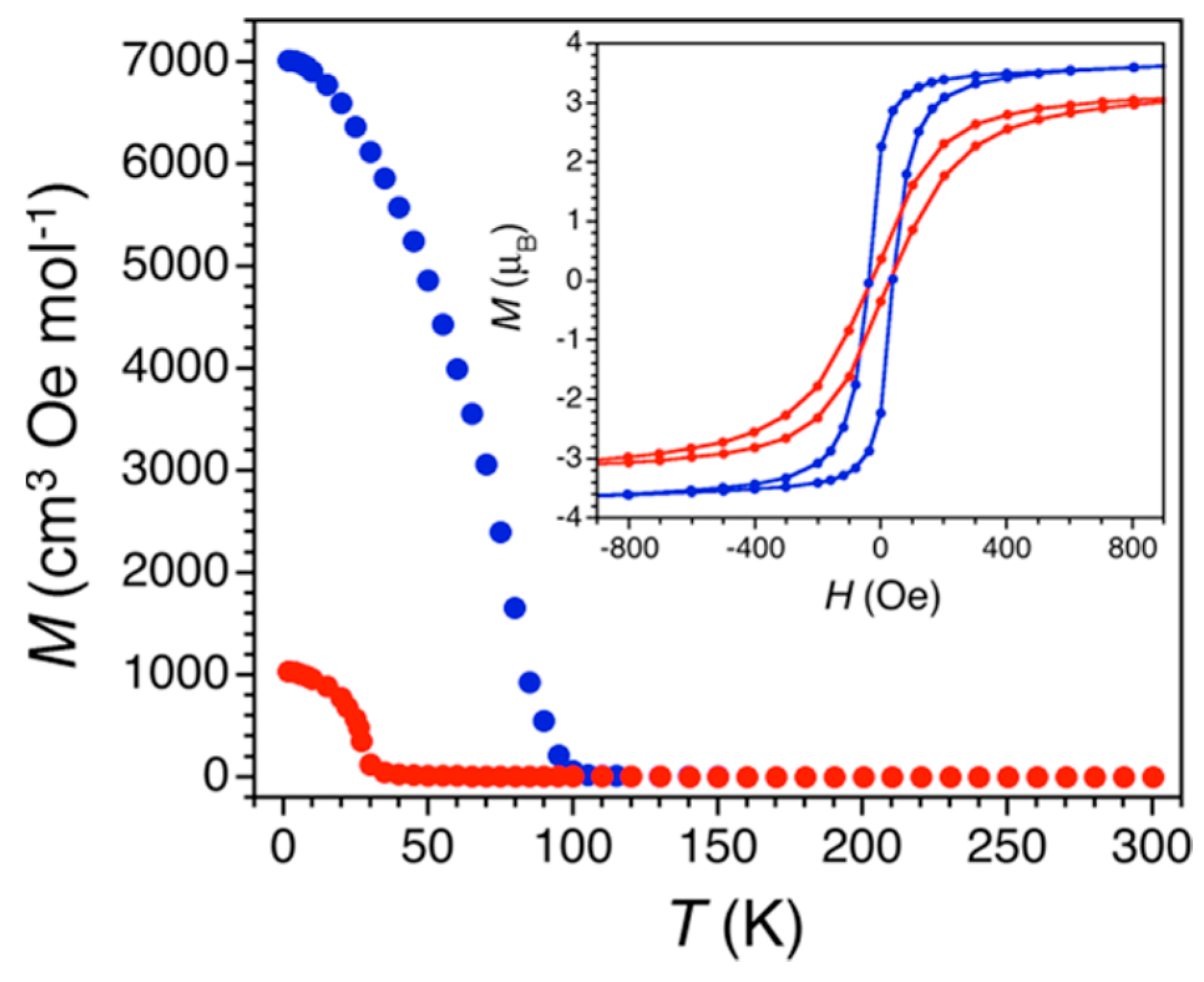
| (Me2NH2)2[Fe2Cl2An3]·2H2O·6DMF 45 | Eclipsed 2D honeycomb layered packing with a H2O between Fe centers, leading to the formation of 1D hexagonal channels | 2D Microporous magnet with strong magnetic coupling. Intralayer AFM interactions Tc = 80 K, glassy Magnet, Mydosh parameter, φ = 0.023 | [201] |
| (Me2NH2)2[Fe2Cl2An3] 45a | Eclipsed 2D honeycomb layered packing Desolvated phase of 48 | Intralayer AFM interactions Tc = 26 K. Permanent porosity with BET surface area of 885(105) m2/g | [201] |
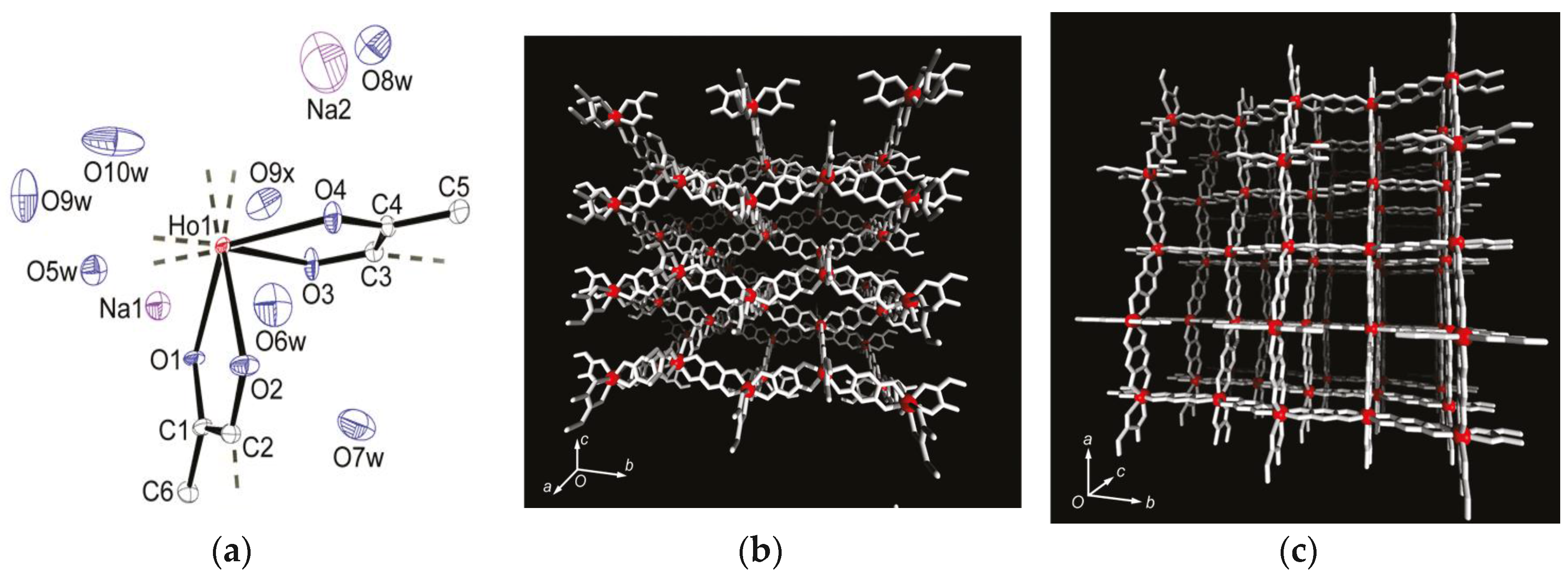
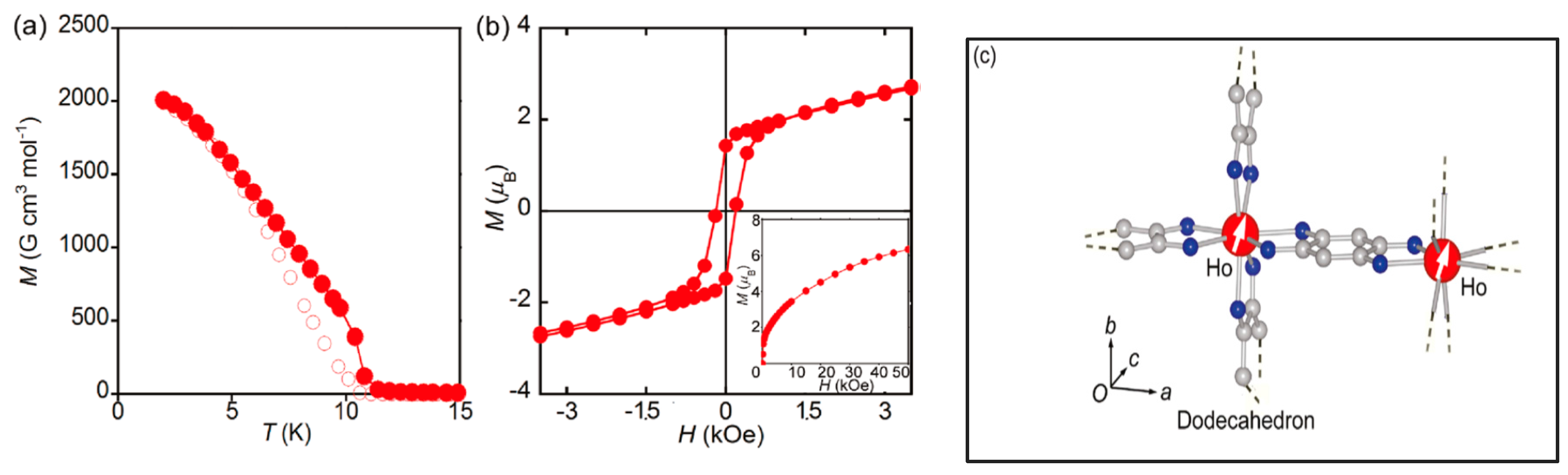
| Na5[Ho(H2An4−)2]3 7H2O 46 | 3D monometallic lanthanoid assembly Ho3+ ion adopts a dodecahedron (D4d) geometry with regular square-grid channels | FM with a Curie Temperature of 11 K | [206] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercuri, M.L.; Congiu, F.; Concas, G.; Sahadevan, S.A. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry 2017, 3, 17. https://doi.org/10.3390/magnetochemistry3020017
Mercuri ML, Congiu F, Concas G, Sahadevan SA. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry. 2017; 3(2):17. https://doi.org/10.3390/magnetochemistry3020017
Chicago/Turabian StyleMercuri, Maria Laura, Francesco Congiu, Giorgio Concas, and Suchithra Ashoka Sahadevan. 2017. "Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties" Magnetochemistry 3, no. 2: 17. https://doi.org/10.3390/magnetochemistry3020017
APA StyleMercuri, M. L., Congiu, F., Concas, G., & Sahadevan, S. A. (2017). Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry, 3(2), 17. https://doi.org/10.3390/magnetochemistry3020017