Synthesis, Structure and Magnetic and Electrochmical Properties of Tetrakis(benzamidato)diruthenium(II,III) Tetrafluoroborate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterizations
2.2. Cyclic Voltammogram (CV) of [Ru2(HNOCPh)4(BF4)(H2O)]
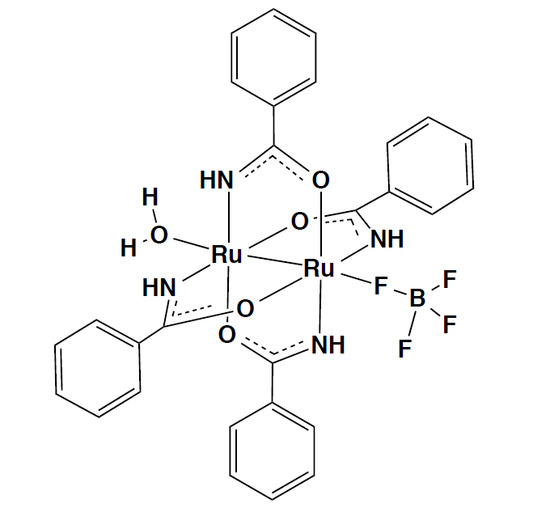
2.3. Crystal Structure of [Ru2(HNOCPh)4(BF4)(H2O)]·2(acetone)
2.4. Magnetic Properties
2.5. DFT Calculations
3. Materials and Methods
3.1. General Aspects
3.2. Synthesis of Complexes
3.2.1. Synthesis of [Ru2(HNOCPh)4Cl]n
3.2.2. Synthesis of [Ru2(HNOCPh)4(BF4)(H2O)]
3.3. Crystal Structure Determination
3.4. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cotton, F.A.; Murillo, C.A.; Walton, R.A. Multiple Bonds between Metal Atoms, 3rd ed.; Springer: New York, NY, USA, 2005. [Google Scholar]
- Liddle, S.T. Molecular Metal-Metal Bonds, Compounds, Synthesis, Properties; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Aquino, M.A.S. Diruthenium and diosmium tetracarboxylates: Synthesis, physical properties and applications. Coord. Chem. Rev. 1998, 170, 141–202. [Google Scholar] [CrossRef]
- Aquino, M.A.S. Recent developments in the synthesis and properties of diruthnium tetracarboxylates. Coord. Chem. Rev. 2004, 248, 1025–1045. [Google Scholar] [CrossRef]
- Mikuriya, M.; Yoshioka, D.; Handa, M. Magnetic interactions in one-, two-, and three-dimensional assemblies of dinuclear ruthenium carboxylates. Coord. Chem. Rev. 2006, 250, 2194–2211. [Google Scholar] [CrossRef]
- Cotton, F.A.; Ren, T. Preparation and Properties of Ru2(DtolF)4Cl: A Surprosing Electronic Structure Change Compared to Ru2(DtolF)4 (DtolF = [(p-tol)NCHN(p-tol)]−). Inorg. Chem. 1995, 34, 3190–3193. [Google Scholar] [CrossRef]
- Chen, W.-Z.; Cotton, F.A.; Dalal, N.S.; Murillo, C.A.; Ramsey, C.M.; Ren, T.; Wang, X. Proof of Large Positive Zero-Field Splitting in a Ru25+ Paddlewheel. J. Am. Chem. Soc. 2005, 127, 12691–12696. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.C.; Gallo, T.; Herrero, S.; Jiménez-Aparicio, R.; Torres, M.R.; Urbanos, F.A. The First Open-Paddlewheel Structures in Diruthenium Chemistry: Examples of Intermediate Magnetic Behaviour between Low and High Spin in Ru25+ species. Chem. Eur. J. 2007, 13, 10088. [Google Scholar] [CrossRef] [PubMed]
- Angaridis, P.; Cotton, F.A.; Murillo, C.A.; Villagrán, D.; Wang, X. Structural and Magnetic Evidnece Concerning Spin Crossover in Formamidinate Compounds with Ru25+ Cores. J. Am. Chem. Soc. 2005, 127, 5008–5009. [Google Scholar] [CrossRef] [PubMed]
- Chiarella, G.M.; Cotton, F.A.; Murillo, C.A.; Ventura, K.; Villagrán, D.; Wang, X. Manipulating Magnetism: Ru25+ Paddlewheels Devoid of Axial Interactions. J. Am. Chem. Soc. 2014, 136, 9580–9589. [Google Scholar] [CrossRef] [PubMed]
- Malinski, T.; Chang, D.; Feldmann, F.N.; Bear, J.L.; Kadish, K.M. Electrochmical Studies of a Novel Ruthenium(II,III) Dimer, Ru2(HNOCCF3)4Cl. Inorg. Chem. 1983, 22, 3225–3233. [Google Scholar] [CrossRef]
- Kadish, K.M.; Lancon, D.; Cocolios, P.; Guilard, R. Electrochemical Generation of New Dinuclear Ruthenium Acetamidate Complexes. Inorg. Chem. 1984, 23, 2373–2375. [Google Scholar] [CrossRef]
- Chakravarty, A.R.; Cotton, F.A.; Tocher, D.A. Displative Transfer of a Phenyl Group from Triphenylphosphine to a Metal Atom: Synthesis and Molecular Structure of Ru2Ph2(PhCONH)2[Ph2POC(Ph)N]2. J. Am. Chem. Soc. 1984, 106, 6409–6413. [Google Scholar] [CrossRef]
- Chakravarty, A.R.; Cotton, F.A.; Tocher, D.A. Synthesis and Structure of a Binuclear Ruthenium 4-Chlorobenzoamidato Complex. Polyhedron 1985, 4, 1097–1102. [Google Scholar] [CrossRef]
- Chakravarty, A.R.; Cotton, F.A. Structure of a Diruthenium(II,III) Complex with Benzoato Bridging Ligands. Polyhedron 1985, 4, 1957–1958. [Google Scholar] [CrossRef]
- Barral, M.C.; Jiménez-Aparicio, R.; Priego, J.L.; Royer, E.C.; Urbanos, F.A.; Monge, A.; Ruíz-Valero, C. Tert-butylbenzamidate Diruthenium(II,III) Compounds. Crystal Structure of [Ru2(μ-HNOC6H4-p-CMe3)4(OPPh3)2]BF4. Polyhedron 1993, 12, 2947–2953. [Google Scholar] [CrossRef]
- Barral, M.C.; de la Fuente, I.; Jiménez-Aparicio, R.; Priego, J.L.; Torres, M.R. Synthesis of diruthenium(II,III) amidate compounds. Crystal Structure of [Ru2(μ-HNOCC4H3S)4(thf)2]SbF6·0.5 cyclohexane. Polyhedron 2001, 20, 2537–2544. [Google Scholar] [CrossRef]
- Villalobos, L.; Cao, Z.; Fanwick, P.E.; Ren, T. Diruthenium(II,III) tetraamidates as new class of oxygenation catalysts. Dalton Trans. 2012, 41, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.; González-Prieto, R.; Jiménez-Aparicio, R.; Perles, J.; Priego, J.L.; Torres, R.M. Comparative study of different methods for the preparation of tetraamidato- and tetracarboxylatodiruthenium compounds. Structural and magnetic characterization. Dalton Trans. 2012, 41, 11866–11874. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Martinez, P.; GonzEalez-Prieto, R.; Gómez-García, C.J.; Jiménez-Aparicio, R.; Priego, J.L.; Torres, M.R. Structural, magnetic and electrical properties of one-dimensional tetraamidatodiruthnium compounds. Dalton Trans. 2014, 43, 3227–3237. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Martínez, P.; Freire, C.; Conzález-Prieto, R.; Jiménez-Aparicio, R.; Priego, J.L.; Torres, M.R. Synthesis, Crystal Structuyre, and Magnetic Properties of Amidate and Carboxylate Dimers of Ruthenium. Crystals 2017, 7, 192. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra, 4th ed.; John Wile & Sons: New York, NY, USA, 1986. [Google Scholar]
- Kataoka, Y.; Mikami, S.; Sakiyama, H.; Mitsumi, M.; Kawamoto, T.; Handa, M. A neutral paddlewheel-type diruthenium(III) complex with benzamidinato ligands: Synthesis, crystal structure, magnetism, and electrochemical and absorption properties. Polyhedron 2017, 136, 87–92. [Google Scholar] [CrossRef]
- Xu, G.-L.; Jablonski, C.G.; Ren, T. Ru2(DMBA)4(BF4)2 and Ru2(DMBA)4(NO3)3: The first exapmles of diruthenium compounds containg BF4− and NO3− as Ligands. Inorg. Chim. Acta 2003, 343, 387–390. [Google Scholar] [CrossRef]
- Corcos, A.R.; Berry, J.F. Anilinpyridinate-supported Ru2X+ (x = 5 or 6) paddlewheel complexes with labile axial ligands. Dalton Trans. 2017, 46, 5532–5539. [Google Scholar] [CrossRef] [PubMed]
- Fuma, Y.; Ebihara, M. Tetra-μ-acetamidati-κ4N:O; κ4O:N-bis[aquaruthenium(II,III)](Ru-Ru) perchlorate. Acta Crstallogr. Sect. E 2006, 62, m2802–m2804. [Google Scholar] [CrossRef]
- Fuma, Y.; Ebihara, M. Tetra-μ-acetamidati-κ4N:O;κ4O:N-bis[aquaruthenium(II,III)](Ru-Ru)nitrate. Acta Crstallogr. Sect. E 2006, 62, m2805–m2807. [Google Scholar] [CrossRef]
- Fuma, Y.; Ebihara, M. Tetra-μ-acetamidati-κ4N:O;κ4O:N-bis[aquaruthenium(II,III)](Ru-Ru) tetraphenylborate monohydrate. Acta Crstallogr. Sect. E 2006, 62, m2808–m2810. [Google Scholar] [CrossRef]
- Telser, J.; Drago, R.S. Reinvesigation of the Electronic and Magnetic Properties of Ruthenium Butyrate Chloride. Inorg. Chem. 1984, 23, 3114. [Google Scholar] [CrossRef]
- Telser, J.; Drago, R.S. Correction: Reinvesigation of the Electronic and Magnetic Properties of Ruthenium Butyrate Chloride. Inorg. Chem. 1985, 24, 4765. [Google Scholar] [CrossRef]
- O’Connor, C.J. Magnetochemistry–Advances in Theory and Experimentaion. Prog. Inorg. Chem. 1982, 29, 203. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Ikeue, T.; Sakiyama, H.; Gúgan, F.; Luneau, D.; Gillon, B.; Hiromitsu, I.; Yoshioka, D.; Mikuriya, M.; Kataoka, Y.; et al. An unprecedented up-field shift in the 13C NMR spectrum of the carboxyl carbons of the lantern-type dinuclear complex TBA[Ru2(O2CCH3)4Cl2] (TBA+ = tetra(n-butyl)ammonium cation. Dalton Trans. 2015, 44, 13439–13443. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.A.; Wilkinson, G. New ruthenium carboxylate complexes. J. Inorg. Nucl. Chem. 1966, 28, 2285. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: Cambridge, UK, 1993; Chapter 1. [Google Scholar]










| Parameter Values a | |
|---|---|
| Empirical formula | C34H38BF4N4O7Ru2 |
| Formula mass | 903.63 |
| Temperature | 123(2) K |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a | 14.412(2) Å |
| b | 15.669(3) Å |
| c | 16.388(3) Å |
| α | 90° |
| β | 93.743(2)° |
| γ | 90° |
| Unit-cell volume, V | 3692.7(10) Å3 |
| Formula per unit cell, Z | 4 |
| Density, Dcalcd | 1.625 g cm−3 |
| Crystal size | 0.200 × 0.170 × 0.050 mm |
| Absorption coefficient, μ | 0.890 mm−1 |
| θ range for data collection | 2.833–27.499˚ |
| Reflections collected/unique | 8333/7421 |
| R indices [I > 2σ(I)] b | R1 = 0.0361, wR2 = 0.0866 |
| Goodness-of-fit on F2 | 1.045 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handa, M.; Yano, N.; Okuno, A.; Nakai, H.; Mitsumi, M.; Mikuriya, M.; Kataoka, Y. Synthesis, Structure and Magnetic and Electrochmical Properties of Tetrakis(benzamidato)diruthenium(II,III) Tetrafluoroborate. Magnetochemistry 2018, 4, 21. https://doi.org/10.3390/magnetochemistry4020021
Handa M, Yano N, Okuno A, Nakai H, Mitsumi M, Mikuriya M, Kataoka Y. Synthesis, Structure and Magnetic and Electrochmical Properties of Tetrakis(benzamidato)diruthenium(II,III) Tetrafluoroborate. Magnetochemistry. 2018; 4(2):21. https://doi.org/10.3390/magnetochemistry4020021
Chicago/Turabian StyleHanda, Makoto, Natsumi Yano, Airi Okuno, Hiroki Nakai, Minoru Mitsumi, Masahiro Mikuriya, and Yusuke Kataoka. 2018. "Synthesis, Structure and Magnetic and Electrochmical Properties of Tetrakis(benzamidato)diruthenium(II,III) Tetrafluoroborate" Magnetochemistry 4, no. 2: 21. https://doi.org/10.3390/magnetochemistry4020021
APA StyleHanda, M., Yano, N., Okuno, A., Nakai, H., Mitsumi, M., Mikuriya, M., & Kataoka, Y. (2018). Synthesis, Structure and Magnetic and Electrochmical Properties of Tetrakis(benzamidato)diruthenium(II,III) Tetrafluoroborate. Magnetochemistry, 4(2), 21. https://doi.org/10.3390/magnetochemistry4020021