Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment
Abstract
1. Introduction
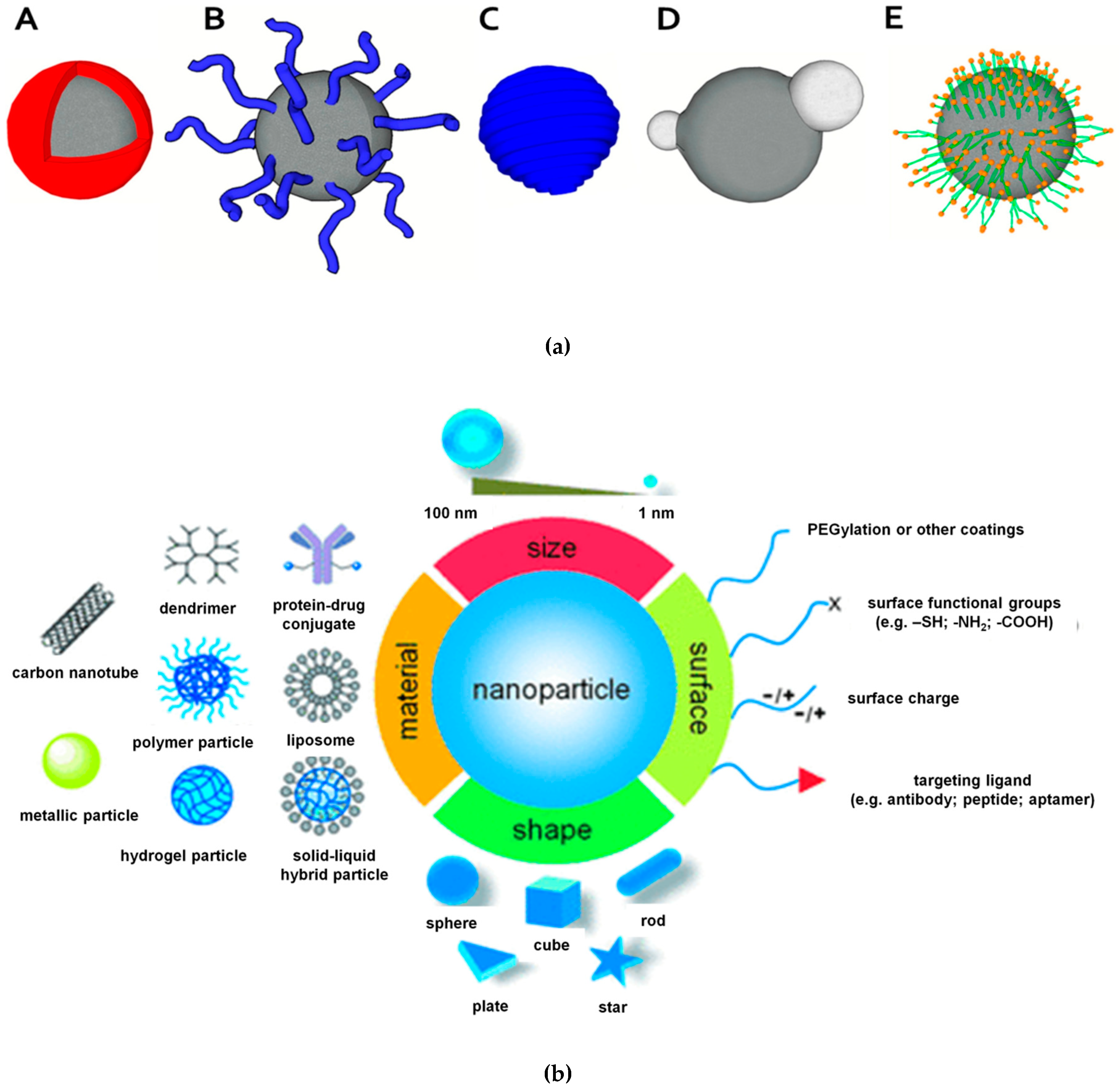
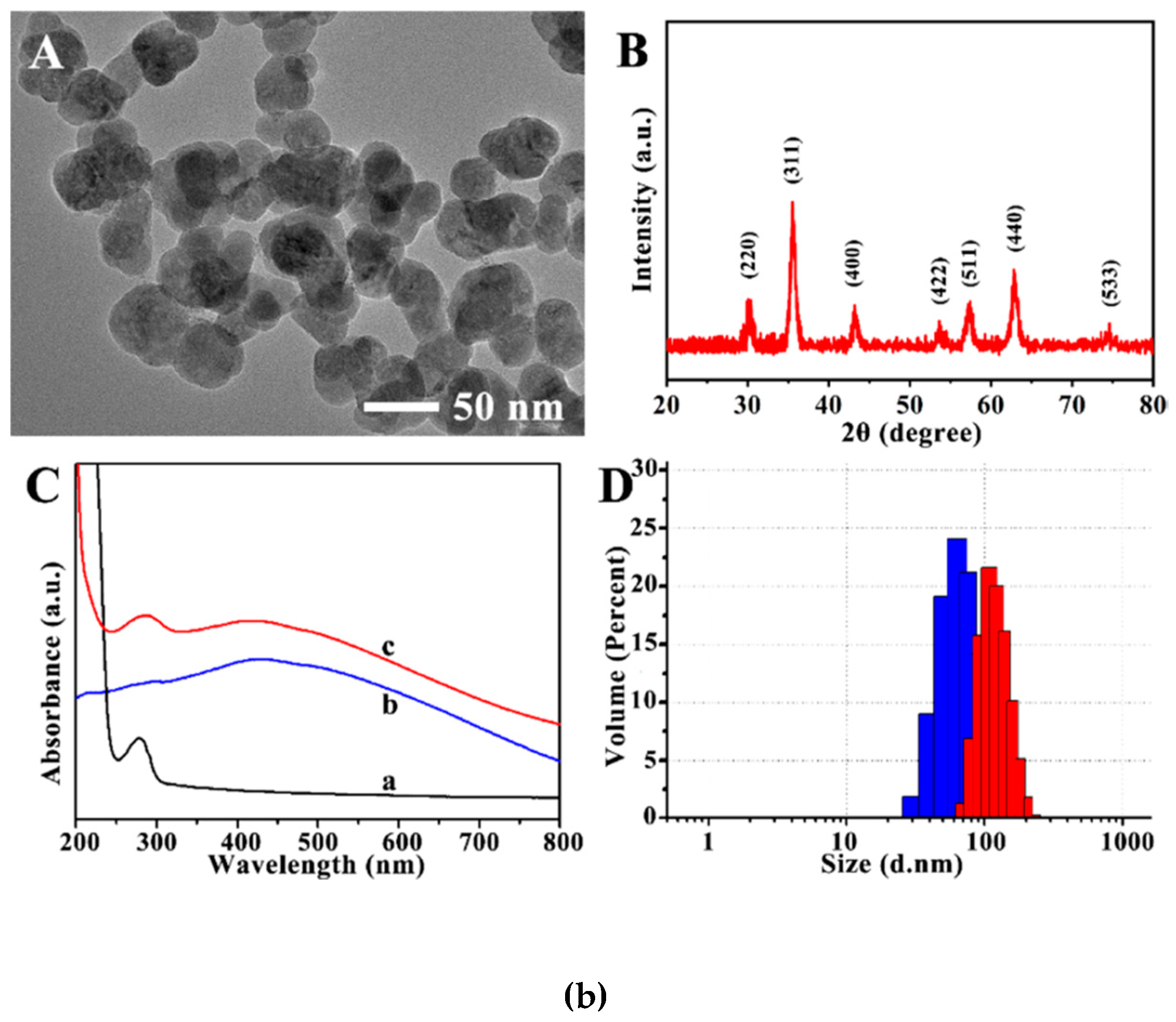
2. Synthesis and Characterization of Magnetic Nanoparticles
- iron oxide nanoparticles or oxides (ferrites): hematite (α-Fe2O3), maghemite (γ-Fe2O3) and magnetite (Fe3O4); their involvement in biomedical application is based on their easy surface modification with various compounds for increased stability in aqueous media (e.g. surfactants, silica) [27];
- metallic nanoparticles with only a metallic core: more suitable for biomedical applications due to their higher magnetic moment compared to oxides; reported drawbacks are pyrophoric property, and presence of high reactivity to oxidizing agents;
- shell-based ferrites: chemically inert MNPs core covered by a silica shell for further functionalization through covalent bonding;
- shell-based metallic nanoparticles: metallic core covered by a shell made of polymers, precious metals or modified surfactants [27].
3. Functionalization and Stabilization of Magnetic Nanoparticles
4. Applications of Magnetic Nanoparticles in Cancer Biomedicine
4.1. Cancer Biomarker Detection Using Magnetic Nanoparticles
4.1.1. Biomolecules Conjugation
4.1.2. Bioseparation
4.1.3. Biosensing
4.2. Cancer Screening Using Magnetic Nanoparticles
Magnetic Resonance Imaging (MRI)
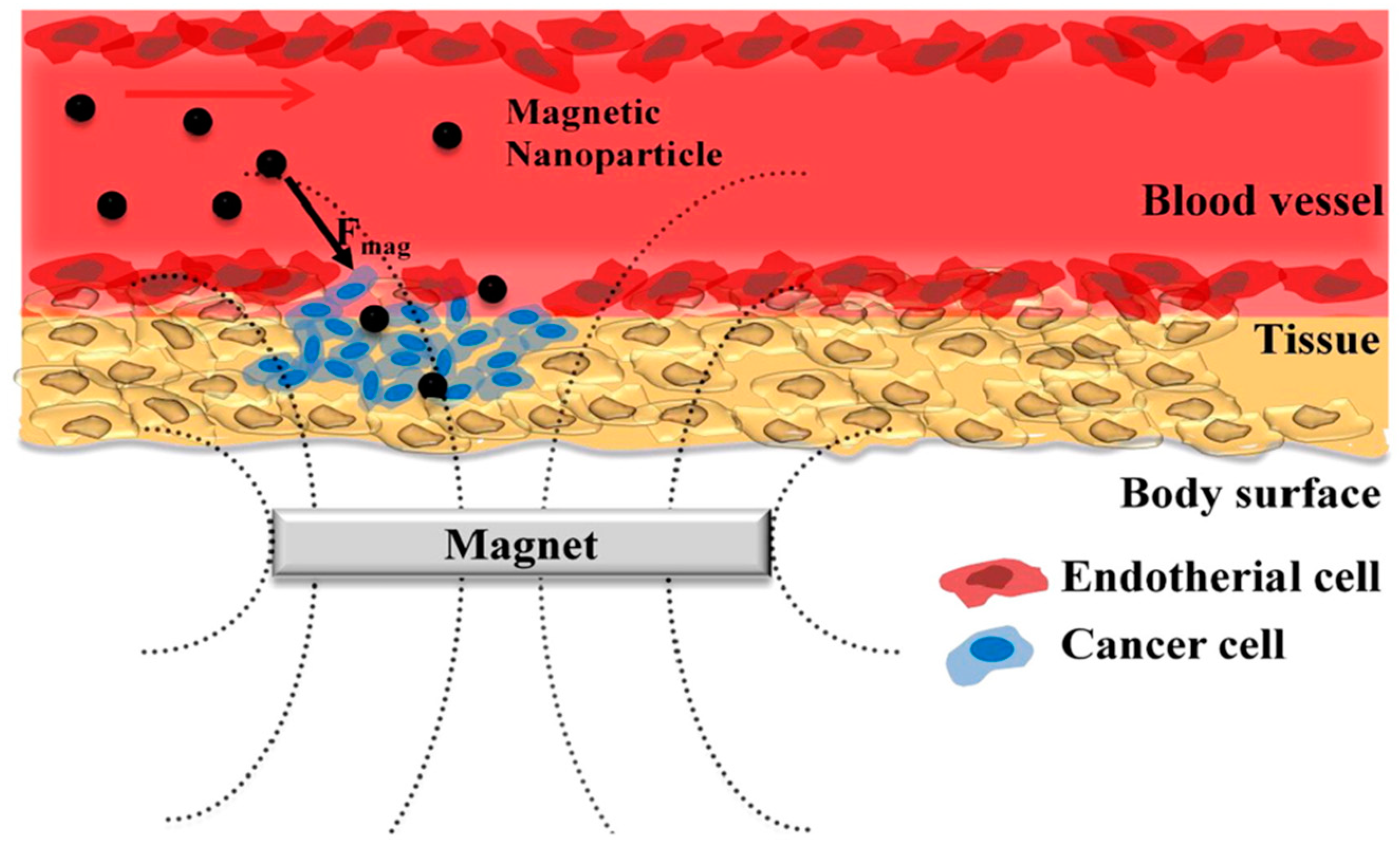
4.3. Cancer Treatment Using Magnetic Nanoparticles
4.3.1. Drug Delivery
4.3.2. Therapeutic Viruses
4.3.3. Hyperthermia
- the MNPs need to be functionalized for increase biocompatibility and low toxicity;
- only MNPs that are located in tumors must be heated;
- MNPs must absorb enough power to achieve cytolytic tumor temperatures without significant heating of the surrounding cells;
- these MNPs must be observable when in vivo using a non-invasive technique (MRI or fluorescence imaging) in order to prove their presence in the tumor;
- the temperature variations must be monitored in real time during the hyperthermia treatment;
- the effectiveness of the hyperthermia treatment needs to be accurately determined for optimization of all the required parameters (e.g., nanoparticle dose, administration technique, temperature, duration of the treatment);
- functionalization of the MNPs for increased selectivity when used for metastases treatment.
4.3.4. Photodynamic Therapy
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, R.; Billone, P.S.; Mullett, W.M. Nanomedicine in action: An overview of cancer nanomedicine on the market and in clinical trials. J. Nanomater. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- WHO. Cancer; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Wu, M.; Huang, S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment (Review). Mol. Clin. Oncol. 2017, 7, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Khmara, I.; Strbak, O.; Zavisova, V.; Koneracka, M.; Kubovcikova, M.; Antal, I.; Kavecansky, V.; Lucanska, D.; Dobrota, D.; Kopcansky, P. Chitosan-stabilized iron oxide nanoparticles for magnetic resonance imaging. J. Magn. Magn. Mater. 2019, 474, 319–325. [Google Scholar] [CrossRef]
- Abu-Dief, A.M. Abdel-Mawgoud AAH. Functionalization of Magnetic Nanoparticles for Drug Delivery. SF J. Nanochem. Nanotechnol. 2018, 1, 1005. [Google Scholar]
- Price, P.M.; Mahmoud, W.E.; Al-Ghamdi, A.A.; Bronstein, L.M. Magnetic drug delivery: Where the field is going. Front. Chem. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; McKeague, M.; DeRosa, M.C. Synthesis, transfer, and characterization of core-shell gold-coated magnetic nanoparticles. MethodsX 2019, 6, 333–354. [Google Scholar] [CrossRef]
- Rikken, R.S.M.; Nolte, R.J.M.; Maan, J.C.; van Hest, J.C.M.; Wilson, D.A.; Christianen, P.C.M. Manipulation of micro- and nanostructure motion with magnetic fields. Soft Matter 2014, 10, 1295–1308. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic Iron Oxide Nanoparticles as MRI contrast agents for Non-invasive Stem Cell Labeling and Tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic Nanoparticle Hyperthermia in Cancer Treatment. Nano Life 2010, 01, 17–32. [Google Scholar] [CrossRef]
- Simeonidis, K.; Mourdikoudis, S.; Kaprara, E.; Mitrakas, M.; Polavarapu, L. Inorganic engineered nanoparticles in drinking water treatment: A critical review. Environ. Sci. Water Res. Technol. 2016, 2, 43–70. [Google Scholar] [CrossRef]
- Baresel, C.; Schaller, V.; Jonasson, C.; Johansson, C.; Bordes, R.; Chauhan, V.; Sugunan, A.; Sommertune, J.; Welling, S. Functionalized magnetic particles for water treatment. Heliyon 2019, 5, e02325. [Google Scholar] [CrossRef] [PubMed]
- Rabias, I.; Tsitrouli, D.; Karakosta, E.; Kehagias, T.; Diamantopoulos, G.; Fardis, M.; Stamopoulos, D.; Maris, T.G.; Falaras, P.; Zouridakis, N.; et al. Rapid magnetic heating treatment by highly charged maghemite nanoparticles on Wistar rats exocranial glioma tumors at microliter volume. Biomicrofluidics 2010, 4, 024111. [Google Scholar] [CrossRef] [PubMed]
- Xianyu, Y.; Wang, Q.; Chen, Y. Magnetic particles-enabled biosensors for point-of-care testing. TrAC Trends Anal. Chem. 2018, 106, 213–224. [Google Scholar] [CrossRef]
- Kralj, S.; Rojnik, M.; Kos, J.; Makovec, D. Targeting EGFR-overexpressed A431 cells with EGF-labeled silica-coated magnetic nanoparticles. J. Nanoparticle Res. 2013, 15, 1666. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C. Magnetic Nanoparticles and Their Bioapplications. In Smart Nanoparticles for Biomedicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 131–142. ISBN 9780128141564. [Google Scholar]
- Liu, G.; Li, R.-W.; Chen, Y. Magnetic Nanoparticle for Biomedicine Applications. Nanotechnol. Nanomed. Nanobiotechnol. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Jamshaid, T.; Neto, E.T.T.; Eissa, M.M.; Zine, N.; Kunita, M.H.; El-Salhi, A.E.; Elaissari, A. Magnetic particles: From preparation to lab-on-a-chip, biosensors, microsystems and microfluidics applications. TrAC Trends Anal. Chem. 2016, 79, 344–362. [Google Scholar] [CrossRef]
- Chen, Y.T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing using magnetic particle detection techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Rhee, I. Medical Applications of Magnetic Nanoparticles. New Phys. Sae Mulli 2015, 65, 411–431. [Google Scholar] [CrossRef]
- Bucak, S.; Altan, C.L. Magnetic nanoparticles and cancer. In Nanotechnology in Cancer; Elsevier: Amsterdam, The Netherlands, 2016; pp. 105–137. ISBN 9780323390811. [Google Scholar]
- Hajba, L.; Guttman, A. The use of magnetic nanoparticles in cancer theranostics: Toward handheld diagnostic devices. Biotechnol. Adv. 2016, 34, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.A.P. Sensors and biosensors based on magnetic nanoparticles. TrAC Trends Anal. Chem. 2014, 62, 28–36. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, C.; Zhang, Z.; Wu, W.; Wang, X.; Yu, Z. Synthesis, functionalization, and nanomedical applications of functional magnetic nanoparticles. Chin. Chem. Lett. 2018, 29, 1601–1608. [Google Scholar] [CrossRef]
- Cristea, C.; Tertis, M.; Galatus, R. Magnetic nanoparticles for antibiotics detection. Nanomaterials 2017, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.K.; Zhang, F.F.; Sun, J.J.; Sheng, J.; Wang, F.; Sun, M. Bio and nanomaterials based on Fe3O4. Molecules 2014, 19, 21506–21528. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Reverse micelle synthesis and characterization of superparamagnetic MnFe2O4 spinel ferrite nanocrystallites. J. Phys. Chem. B 2000, 104, 1143–1145. [Google Scholar] [CrossRef]
- Karade, V.C.; Dongale, T.D.; Sahoo, S.C.; Kollu, P.; Chougale, A.D.; Patil, P.S.; Patil, P.B. Effect of reaction time on structural and magnetic properties of green-synthesized magnetic nanoparticles. J. Phys. Chem. Solids 2018, 120, 161–166. [Google Scholar] [CrossRef]
- Magdziarz, A.; Colmenares, J.C. In situ coupling of ultrasound to electro-and photo-deposition methods for materials synthesis. Molecules 2017, 22, 216. [Google Scholar] [CrossRef]
- Abd Elrahman, A.A.; Mansour, F.R. Targeted magnetic iron oxide nanoparticles: Preparation, functionalization and biomedical application. J. Drug Deliv. Sci. Technol. 2019, 52, 702–712. [Google Scholar] [CrossRef]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A review of small molecules and drug delivery applications using gold and iron nanoparticles. Int. J. Nanomed. 2019, 14, 1633–1657. [Google Scholar] [CrossRef]
- Mehta, R.V. Synthesis of magnetic nanoparticles and their dispersions with special reference to applications in biomedicine and biotechnology. Mater. Sci. Eng. C 2017, 79, 901–916. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, A.; Varghese, S.S.; Meena, S.S.; Prajapat, C.L.; Gupta, N.; Prasad, N.K. Fe 3 C nanoparticles for magnetic hyperthermia application. J. Magn. Magn. Mater. 2019, 481, 251–256. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered nanoparticles for drug delivery in cancer therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef] [PubMed]
- Ayubi, M.; Karimi, M.; Abdpour, S.; Rostamizadeh, K.; Parsa, M.; Zamani, M.; Saedi, A. Magnetic nanoparticles decorated with PEGylated curcumin as dual targeted drug delivery: Synthesis, toxicity and biocompatibility study. Mater. Sci. Eng. C 2019, 104, 109810. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Yuan, X.; Chang, J. Self-aggregates of cholic acid hydrazide-dextran conjugates as drug carriers. J. Appl. Polym. Sci. 2005, 95, 487–493. [Google Scholar] [CrossRef]
- de Mendonça, E.S.D.T.; de Faria, A.C.B.; Dias, S.C.L.; Aragón, F.F.H.; Mantilla, J.C.; Coaquira, J.A.H.; Dias, J.A. Effects of silica coating on the magnetic properties of magnetite nanoparticles. Surfaces Interfaces 2019, 14, 34–43. [Google Scholar] [CrossRef]
- Hosu, O.; Selvolini, G.; Cristea, C.; Marrazza, G. Electrochemical Immunosensors for Disease Detection and Diagnosis. Curr. Med. Chem. 2017, 25, 4119–4137. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef]
- Kouassi, G.K.; Irudayaraj, J. Magnetic and gold-coated magnetic nanoparticles as a DNA sensor. Anal. Chem. 2006, 78, 3234–3241. [Google Scholar] [CrossRef]
- Wu, L.; Qu, X. Cancer biomarker detection: Recent achievements and challenges. Chem. Soc. Rev. 2015, 44, 2963–2997. [Google Scholar] [CrossRef] [PubMed]
- Hosu, O.; Florea, A.; Cristea, C.; Sandulescu, R. Functionalized Advanced Hybrid Materials for Biosensing Applications. In Advanced Biosensors for Health Care Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–207. ISBN 9780128157435. [Google Scholar]
- Soloducho, J.; Cabaj, J. Electrochemical and Optical Biosensors in Medical Applications. In Biosensors—Micro and Nanoscale Applications; Toonika, R., Ed.; IntechOpen: London, UK, 2015; pp. 321–346. ISBN 9789537619343. [Google Scholar]
- Hosu, O.; Selvolini, G.; Marrazza, G. Recent advances of immunosensors for detecting food allergens. Curr. Opin. Electrochem. 2018, 10, 149–156. [Google Scholar] [CrossRef]
- Ge, S.; Sun, M.; Liu, W.; Li, S.; Wang, X.; Chu, C.; Yan, M.; Yu, J. Disposable electrochemical immunosensor based on peroxidase-like magnetic silica-graphene oxide composites for detection of cancer antigen 153. Sens. Actuators B Chem. 2014, 192, 317–326. [Google Scholar] [CrossRef]
- Ge, S.; Liu, W.; Ge, L.; Yan, M.; Yan, J.; Huang, J.; Yu, J. In situ assembly of porous Au-paper electrode and functionalization of magnetic silica nanoparticles with HRP via click chemistry for Microcystin-LR immunoassay. Biosens. Bioelectron. 2013, 49, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, N.; Salimi, A.; Hallaj, R. Magnetoimmunosensor for simultaneous electrochemical detection of carcinoembryonic antigen and α-fetoprotein using multifunctionalized Au nanotags. J. Electroanal. Chem. 2018, 811, 8–15. [Google Scholar] [CrossRef]
- Guerrero, S.; Cadano, D.; Agüí, L.; Barderas, R.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Click chemistry-assisted antibodies immobilization for immunosensing of CXCL7 chemokine in serum. J. Electroanal. Chem. 2019, 837, 246–253. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Tian, L.; Qi, J.; Qian, K.; Oderinde, O.; Cai, Y.; Yao, C.; Song, W.; Wang, Y. An ultrasensitive electrochemical cytosensor based on the magnetic field assisted binanozymes synergistic catalysis of Fe3O4 nanozyme and reduced graphene oxide/molybdenum disulfide nanozyme. Sens. Actuators B Chem. 2018, 260, 676–684. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Xu, H.; Yan, W.; Jin, Q.; Cui, D. Detection platforms for point-of-care testing based on colorimetric, luminescent and magnetic assays: A review. Talanta 2019, 202, 96–110. [Google Scholar] [CrossRef]
- Xu, Q.; Liang, K.; Liu, R.Y.; Deng, L.; Zhang, M.; Shen, L.; Liu, Y.N. Highly sensitive fluorescent detection of p53 protein based on DNA functionalized Fe 3 O 4 nanoparticles. Talanta 2018, 187, 142–147. [Google Scholar] [CrossRef]
- Hu, Y.; Li, L.; Guo, L. The sandwich-type aptasensor based on gold nanoparticles/DNA/magnetic beads for detection of cancer biomarker protein AGR2. Sens. Actuators B Chem. 2015, 209, 846–852. [Google Scholar] [CrossRef]
- Wang, J.X.; Zhuo, Y.; Zhou, Y.; Yuan, R.; Chai, Y.Q. Electrochemiluminescence immunosensor based on multifunctional luminol-capped AuNPs@Fe3O4 nanocomposite for the detection of mucin-1. Biosens. Bioelectron. 2015, 71, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, G.C.; Gao, F.; Cui, Y.; Wang, W.; Luo, X. High-activity Fe3O4 nanozyme as signal amplifier: A simple, low-cost but efficient strategy for ultrasensitive photoelectrochemical immunoassay. Biosens. Bioelectron. 2019, 127, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Teoh, C.L.; Samanta, A.; Kang, N.Y.; Park, S.J.; Chang, Y.T. The development of a highly photostable and chemically stable zwitterionic near-infrared dye for imaging applications. Chem. Commun. 2015, 51, 3989–3992. [Google Scholar] [CrossRef] [PubMed]
- Gui, C.; Wang, K.; Li, C.; Dai, X.; Cui, D. A CCD-based reader combined with CdS quantum dot-labeled lateral flow strips for ultrasensitive quantitative detection of CagA. Nanoscale Res. Lett. 2014, 9, 57. [Google Scholar] [CrossRef]
- Peng, J.; Guan, J.; Yao, H.; Jin, X. Magnetic colorimetric immunoassay for human interleukin-6 based on the oxidase activity of ceria spheres. Anal. Biochem. 2016, 492, 63–68. [Google Scholar] [CrossRef]
- Domingo-Domenech, J.; Oliva, C.; Rovira, A.; Codony-Servat, J.; Bosch, M.; Filella, X.; Montagut, C.; Tapia, M.; Campás, C.; Dang, L.; et al. Interleukin 6, a nuclear factor-κB target, predicts resistance to docetaxel in hormone-independent prostate cancer and nuclear factor-κB inhibition by PS-1145 enhances docetaxel antitumor activity. Clin. Cancer Res. 2006, 12, 5578–5586. [Google Scholar] [CrossRef]
- Lee, Y.W.; Hirani, A.A.; Kyprianou, N.; Toborek, M. Human immunodeficiency virus-1 Tat protein up-regulates interleukin-6 and interleukin-8 expression in human breast cancer cells. Inflamm. Res. 2005, 54, 380–389. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, M.; Hou, L.; Chen, G.; Tang, D. Magnetic bead-based reverse colorimetric immunoassay strategy for sensing biomolecules. Anal. Chem. 2013, 85, 6945–6952. [Google Scholar] [CrossRef]
- Han, S.-J.; Wang, S. Magnetic Nanotechnology for Biodetection. J. Assoc. Lab. Autom. 2010, 15, 93–98. [Google Scholar] [CrossRef]
- Serrate, D.; De Teresa, J.M.; Marquina, C.; Marzo, J.; Saurel, D.; Cardoso, F.A.; Cardoso, S.; Freitas, P.P.; Ibarra, M.R. Quantitative biomolecular sensing station based on magnetoresistive patterned arrays. Biosens. Bioelectron. 2012, 35, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Hira, S.M.; Aledealat, K.; Chen, K.S.; Field, M.; Sullivan, G.J.; Chase, P.B.; Xiong, P.; Von Molnár, S.; Strouse, G.F. Detection of target ssDNA using a microfabricated hall magnetometer with correlated optical readout. J. Biomed. Biotechnol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Osterfeld, S.J.; Yu, H.; Gaster, R.S.; Caramuta, S.; Xu, L.; Han, S.J.; Hall, D.A.; Wilson, R.J.; Sun, S.; White, R.L.; et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl. Acad. Sci. USA 2008, 105, 20637–20640. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chi, B.; Wu, F.; Ma, S.; Zhan, S.; Yi, M.; Xu, H.; Mao, C. A sensitive label-free immunosensor for detection α-Fetoprotein in whole blood based on anticoagulating magnetic nanoparticles. Biosens. Bioelectron. 2017, 95, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Eletxigerra, U.; Martinez-Perdiguero, J.; Merino, S.; Barderas, R.; Ruiz-Valdepeñas Montiel, V.; Villalonga, R.; Pingarrón, J.M.; Campuzano, S. Estrogen receptor α determination in serum, cell lysates and breast cancer cells using an amperometric magnetoimmunosensing platform. Sens. Bio-Sens. Res. 2016, 7, 71–76. [Google Scholar] [CrossRef]
- Lin, R.; Li, Y.; MacDonald, T.; Wu, H.; Provenzale, J.; Peng, X.; Huang, J.; Wang, L.; Wang, A.Y.; Yang, J.; et al. Improving sensitivity and specificity of capturing and detecting targeted cancer cells with anti-biofouling polymer coated magnetic iron oxide nanoparticles. Colloids Surfaces B Biointerfaces 2017, 150, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Dianping, T.; Yuan, R.; Chai, Y. Ultrasensitive Electrochemical Immunosensor for Clinical Immunoassay Using Thionine-Doped Magnetic Gold Nanospheres as Labels and Horseradish Peroxidase as Enhancer. Anal. Chem. 2008, 80, 1582–1588. [Google Scholar]
- Tertiş, M.; Melinte, G.; Ciui, B.; Şimon, I.; Ştiufiuc, R.; Săndulescu, R.; Cristea, C. A Novel Label Free Electrochemical Magnetoimmunosensor for Human Interleukin-6 Quantification in Serum. Electroanalysis 2019, 31, 282–292. [Google Scholar] [CrossRef]
- Taleat, Z.; Cristea, C.; Marrazza, G.; Săndulescu, R. Electrochemical Sandwich Immunoassay for the Ultrasensitive Detection of Human MUC1 Cancer Biomarker. Int. J. Electrochem. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Mani, V.; Chikkaveeraiah, B.V.; Patel, V.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive Immunosensor for Cancer Biomarker Proteins Using Gold Nanoparticle Film Electrodes and Multienzyme-Particle Amplification. ACS Nano 2009, 3, 585–594. [Google Scholar] [CrossRef]
- Marrazza, G.; Florea, A.; Ravalli, A.; Cristea, C.; Sa, R. An Optimized Bioassay for Mucin1 Detection in Serum. Electroanalysis 2015, 27, 1594–1601. [Google Scholar]
- Florea, A.; Taleat, Z.; Cristea, C.; Mazloum-Ardakani, M.; Săndulescu, R. Label free MUC1 aptasensors based on electrodeposition of gold nanoparticles on screen printed electrodes. Electrochem. Commun. 2013, 33, 127–130. [Google Scholar] [CrossRef]
- Hong, W.; Lee, S.; Cho, Y. Dual-responsive immunosensor that combines colorimetric recognition and electrochemical response for ultrasensitive detection of cancer biomarkers. Biosens. Bioelectron. 2016, 86, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.B.; Costa-Rama, E.; Viswanathan, S.; Nouws, H.P.A.; Costa-García, A.; Delerue-Matos, C.; González-García, M.B. Voltammetric immunosensor for the simultaneous analysis of the breast cancer biomarkers CA 15-3 and HER2-ECD. Sens. Actuators B Chem. 2018, 255, 918–925. [Google Scholar] [CrossRef]
- Williams, H.M. The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases. Biosci. Horizons Int. J. Student Res. 2017, 10. [Google Scholar] [CrossRef]
- Wu, S.; Liu, X.; He, J.; Wang, H.; Luo, Y.; Gong, W.; Li, Y.; Huang, Y.; Zhong, L.; Zhao, Y. A Dual Targeting Magnetic Nanoparticle for Human Cancer Detection. Nanoscale Res. Lett. 2019, 14, 228. [Google Scholar] [CrossRef]
- Das, P.; Fatehbasharzad, P.; Colombo, M.; Fiandra, L.; Prosperi, D. Multifunctional Magnetic Gold Nanomaterials for Cancer. Trends Biotechnol. 2019, 37, 995–1010. [Google Scholar] [CrossRef]
- Kubovcikova, M.; Koneracka, M.; Strbak, O.; Molcan, M.; Zavisova, V.; Antal, I.; Khmara, I.; Lucanska, D.; Tomco, L.; Barathova, M.; et al. Poly-L-lysine designed magnetic nanoparticles for combined hyperthermia, magnetic resonance imaging and cancer cell detection. J. Magn. Magn. Mater. 2019, 475, 316–326. [Google Scholar] [CrossRef]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- Belyanina, I.; Kolovskaya, O.; Zamay, S.; Gargaun, A.; Zamay, T.; Kichkailo, A. Targeted magnetic nanotheranostics of cancer. Molecules 2017, 22, 975. [Google Scholar] [CrossRef]
- Pondman, K.M.; Bunt, N.D.; Maijenburg, A.W.; Van Wezel, R.J.A.; Kishore, U.; Abelmann, L.; Ten Elshof, J.E.; Ten Haken, B. Magnetic drug delivery with FePd nanowires. J. Magn. Magn. Mater. 2015, 380, 299–306. [Google Scholar] [CrossRef]
- Wu, C.Y.; Chen, Y.C. Riboflavin immobilized Fe3O4 magnetic nanoparticles carried with n-butylidenephthalide as targeting-based anticancer agents. Artif. Cells Nanomed. Biotechnol. 2019, 47, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, C.; Chen, Y.; Wang, P.; Chen, C.; Geng, D.; Li, L.; Song, T. Magnetically targeted photothemal cancer therapy in vivo with bacterial magnetic nanoparticles. Colloids Surfaces B Biointerfaces 2018, 172, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Bekaroğlu, M.G.; Alemdar, A.; İşçi, S. Comparison of ionic polymers in the targeted drug delivery applications as the coating materials on the Fe3O4 nanoparticles. Mater. Sci. Eng. C 2019, 103, 109838. [Google Scholar] [CrossRef] [PubMed]
- Panda, J.; Satapathy, B.S.; Majumder, S.; Sarkar, R.; Mukherjee, B.; Tudu, B. Engineered polymeric iron oxide nanoparticles as potential drug carrier for targeted delivery of docetaxel to breast cancer cells. J. Magn. Magn. Mater. 2019, 485, 165–173. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.i.; Lee, H.; Kwon, S.-h.; Choi, H.; Park, S. Magnetic nano-particles retrievable biodegradable hydrogel microrobot. Sens. Actuators B Chem. 2019, 289, 65–77. [Google Scholar] [CrossRef]
- Roohi, R.; Emdad, H.; Jafarpur, K.; Mahmoudi, M.R. Determination of Magnetic Nanoparticles Injection Characteristics for Optimal Hyperthermia Treatment of an Arbitrary Cancerous Cells Distribution. J. Test. Eval. 2020, 48, 20170677. [Google Scholar] [CrossRef]
- Alomari, M.; Jermy, B.R.; Ravinayagam, V.; Akhtar, S.; Almofty, S.A.; Rehman, S.; Bahmdan, H.; AbdulAzeez, S.; Borgio, J.F. Cisplatin-functionalized three-dimensional magnetic SBA-16 for treating breast cancer cells (MCF-7). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3079–3086. [Google Scholar] [CrossRef]
- Montazerabadi, A.; Beik, J.; Irajirad, R.; Attaran, N.; Khaledi, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Folate-modified and curcumin-loaded dendritic magnetite nanocarriers for the targeted thermo-chemotherapy of cancer cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 330–340. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.L.; Zhang, Y.F.; Gao, F.; Li, G.L.; He, Y.; Peng, M.L.; Fan, H.M. Magnetic nanoparticles based cancer therapy: Current status and applications. Sci. China Life Sci. 2018, 61, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Namiki, Y.; Namiki, T.; Yoshida, H.; Ishii, Y.; Tsubota, A.; Koido, S.; Nariai, K.; Mitsunaga, M.; Yanagisawa, S.; Kashiwagi, H.; et al. A novel magnetic crystal–lipid nanostructure for magnetically guided in vivo gene delivery. Nat. Nanotechnol. 2009, 4, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Salunkhe, A.B.; Khot, V.M.; Pawar, S.H. Magnetic Hyperthermia with Magnetic Nanoparticles: A Status Review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef] [PubMed]







| Methods | Details | Ref. |
|---|---|---|
| Co-precipitation | —the most facile and efficient method for MNPs synthesis; —iron oxides nanoparticles obtained from Fe2+/Fe3+ salts aqueous solutions; —several parameters need to be well established like pH, Fe2+/Fe3+ ratio, temperature, nature of the solvent, etc; | [27] |
| Thermal and Hydrothermal Decomposition | —synthesis in aqueous media at high pressure and high temperature; —improves the nucleation rate and speed up the growth of the new particles; —hydrolysis and oxidation reaction are the most commonly used; —another route is neutralization of hybrid metal hydroxides; —advantage: generates particles of small diameter size; | [28] |
| Sol-Gel Processes | —hydroxylation and condensation reactions generate a sol of nanoparticles; —condensation reaction of sol generates a three-dimensional network gel of metal oxide; —crystallization form of the gel can be obtained by temperature-controlled treatment; —several parameters need to be well established like pH, concentration of salts precursors and ratio, temperature, nature of the solvent, etc; —surfactants addition influences the synthesis of the 3D gel structure; —major drawback: coagulation of the gels may occur; | [26] |
| Microemulsion and Inverse Micelles | —specific synthesis of MFe2O4-type MNPs, where M could be Mn, Co, Ni, Cu, Zn, Mg, or Cd, etc., important magnetic materials for electronic applications; —the size and shape of the MFe2O4 can be easily tailored depending on the parameters applied; —major drawbacks: harsh experimental conditions (narrow working window, high solvent consumption), low yield of nanoparticles; | [29] |
| Biosynthesis | —environment friendly method which generates biocompatible MNPs; —biosynthesis of MNPs can be performed using reducing agents such as plant phytochemicals, microbial enzymes, bacteria and magnetotactic bacteria; —major drawbacks: the mechanism of biological synthesis has not been yet clearly elucidated; parameters cannot be modulated for shape- and size-controlled synthesis of the nanoparticles; | [30] |
| Sonolysis | —high intensity ultrasound-based method; —oscillating cavities of different size can be achieved by the commutative expansive and compressive acoustic waves; —when the oscillating cavities grow to a certain size, the ultrasonic energy can be accumulated by them; —advantage: mild experimental conditions (pressure, temperature or reaction time); | [31] |
| Spray/laser Pyrolysis | —nucleation of the particles occurs through condensation after spraying an iron salt solution into a hot air or a laser beam; —temperature assisted decomposition of the formed particles is usually followed; —advantage: effective production of small particle size (5–60 nm); —major drawbacks: sophisticated and expensive equipment, oxygen or other gaseous interferences; | [32] |
| Target | Type of assay | Detection method | LOD | Sample | Ref |
|---|---|---|---|---|---|
| AFP | Label-free immunosensor based on graphite electrode modified with Fe3O4-ɛ-PL-Hep nanoparticles with anti-biofouling and anticoagulating MNPs | Electrochemical | 72 pg/mL | Blood | [69] |
| AGR2 | Optical aptasensor based on MNPs | UV-Vis spectroscopy | 6.6 pM | Cell culture | [56] |
| ERα | Sandwich immunoassay based on SPCEs modified HOOC-MNPs and HRP as label | Electrochemical | 19 pg/mL | Serum and cell lysate | [70] |
| D556 CTCs | Polyethyleneglycol-block-ally lglycidy lether copolymer coated iron oxide nanoparticles conjugated with transferrin | Flow cytometry | - | Cell culture and blood | [71] |
| IL-13Rα2 | Disposable detection system based on a hybrid nanomaterial composed of MWCNTs and graphene quantum dots and enzyme label | Electrochemical | 0.8 ng/mL | Cell lysate and extracts from tumor tissues | [51] |
| p53PE | DNA sensors based on DNA functionalized MNPs | Fluorescence | 8 pM | Serum | [55] |
| CagA | CCD-based reader combined with CdS quantum dot-labeled lateral flow strips | Fluorescence | 20 pg/mL | - | [60] |
| LNCaP | Sandwich-based magnetic DNA sensor | Piezoelectric | 0.4 ng/mL | Cell culture | [65] |
| αvβ3 TM | Nanohybrid composite based on MNPs and platinum nanoparticles simultaneously immobilized in the framework of GO | Colorimetric | - | Cell culture | [59] |
| hCG | Lateral-flow magnetoresistive immunoassays based on MNPs | Magnetoresistive sensor | 5.5 ng/mL | Serum | [66] |
| S100β | Magnetosensor based on GMR | Optical | 27 pg/mL | Serum | [67] |
| CEA | Sandwich immunoassay based on carbon fiber microelectrode modified with thionine-doped magnetic gold nanospheres as labels and HRP as enhancer | Electrochemical | 10 pg/mL | Serum | [72] |
| TNF-α | Hall-based magnetic transduction platform 35-base pathogenic DNA target | Fluorescence | 5.7 pM | Serum | [68] |
| IL-6 | Colorimetric immunoassay based on CeNPs | Colorimetric | 40 fg/mL | Serum | [61] |
| Sandwich-based label free magnetoimmunosensor based on ProteinG-functionalized MNPs | Electrochemical | 0.3 pg/mL | Serum | [73] | |
| MUC1 | Sandwich immunosensor based on a multifunctional hybrid materials of luminol-decorated gold-functionalized MNPs | Electrochemiluminescence | 4.5 fg/mL | - | [57] |
| Sandwich immunoassay using graphite SPEs modified with MNPs functionalized with ProteinG and HRP as label | Electrochemical | 1.34 ng/mL | Serum | [74] | |
| PSA | Sandwich-type immunoassay; primary Ab immobilized on MNPs; secondary Ab labelled with HRP | Electrochemical | 0.5 ng/mL | Serum | [75] |
| PEC-based immunoassay based on ZnIn2S4/ZnO-NRs/ITO photoelectrode | UV-Vis spectroscopy | 18 fg/mL | - | [58] | |
| Sandwich-type colorimetric immunoassay based on a reverse strategy based on two nanostructures including MNPs and AuNPs | Colorimetric | 30 pg/mL | Serum | [64] | |
| CA 15-3 | Sandwich immunoassay built on carbon-based SPE modified with graphene oxide and peroxidase-like silica MNPs/GO composites as labels | Electrochemical | 2.8 × 10−4 U/mL | Serum | [48] |
| Sandwich assay; capture aptamer /Ab immobilized on MNPs modified with Protein-G and streptavidin; Detection aptamer / Ab labelled with AP | Electrochemical | 0.07 nM (aptasensor) 0.19 mM (immunosensor) | Serum | [76] | |
| Label-free immunoassay; aptamer immobilized on AuNPs modified graphite and Au SPEs | Electrochemical | 0.95 ng/mL | Serum | [77] | |
| CEA AFP | Sandwich immunoassay based on azide-functionalized sphere-like peroxidase silica MNPs and alkynylated peroxidase as label | Electrochemical | 12 pg/mL 18 pg/mL | Serum | [50] |
| MCF-7 CTCs | Aptamer-functionalized cytosensor based on MNPs nanozyme and rGO/molybdenum disulfide immobilized on magnetic glassy carbon electrode | Electrochemical | 6 cells/mL | Cell culture | [53] |
| PSA CA125 CEA | Nanoroughened, biotin-doped polypyrrole immunosensor based on MNPs with HRP as label | Electrochemical and colorimetric | 0.7 pg/mL 0.005 U/mL 0.8 pg/mL | Plasma | [78] |
| CA15-3 CA 125 CA19-9 | Sandwich immunoassay; primary Ab immobilized on MNPs; secondary Ab labelled with PAMAM dendrimer-metal sulfide QD | Electrochemical | 5 10−3 U/mL | Serum | [79] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosu, O.; Tertis, M.; Cristea, C. Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment. Magnetochemistry 2019, 5, 55. https://doi.org/10.3390/magnetochemistry5040055
Hosu O, Tertis M, Cristea C. Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment. Magnetochemistry. 2019; 5(4):55. https://doi.org/10.3390/magnetochemistry5040055
Chicago/Turabian StyleHosu, Oana, Mihaela Tertis, and Cecilia Cristea. 2019. "Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment" Magnetochemistry 5, no. 4: 55. https://doi.org/10.3390/magnetochemistry5040055
APA StyleHosu, O., Tertis, M., & Cristea, C. (2019). Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment. Magnetochemistry, 5(4), 55. https://doi.org/10.3390/magnetochemistry5040055







