Abstract
Magnetic nanoparticles and magnetic nano-species of complex topology (e.g., nanorods, nanowires, nanotubes, etc.) are overviewed briefly in the paper, mostly giving attention to the synthetic details and particle composition (e.g., core-shell structures made of different materials). Some aspects related to applications of magnetic nano-species are briefly discussed. While not being a comprehensive review, the paper offers a large collection of references, particularly useful for newcomers in the research area.
1. Magnetic Nanoparticles—Motivations and Applications
Magnetic particles of different size (nano- and micro-) and various composition resulting in different magnetization (superparamagnetic and ferromagnetic) have found numerous applications in biotechnology [1] and medicine [2,3,4,5]. Particularly, they are used for magneto-controlled targeting (delivering drugs [6,7], genes [8], radiopharmaceuticals [9]), in magnetic resonance imaging [10], in various diagnostic applications [11], for biosensing [12] (e.g., immunoassays [13]), RNA and DNA purification [14], gene cloning, cell separation and purification [15]. Magnetic nano-species with complex topology (e.g., nanorods, nanowires and nanotubes) [16] have been used in numerous nano-technological devices, including tunable micro-fluidic channels with magnetic control [17], data storage units in nano-circuits [18], and magnetized nano-tips for magnetic force microscopes [19]. Magnetic nano- and micro-particles have been modified with various organic and bioorganic molecules (proteins [20], enzymes [21], antigens, antibodies [22], DNA [23], RNA [24], etc.) as well as with biological cells and cellular components. These species demonstrating magnetic properties and biocatalytic or biorecognition features are usually organized in “core-shell” structures, with the core part made of inorganic magnetic material and the shell composed of biomolecular/biological species chemically bound to the core with organic linkers [25,26]. The chemical (usually covalent) binding of organic linkers to the magnetic core units has been studied and characterized using different analytical methods (e.g., capillary electrophoresis with laser-induced fluorescence detection) [25]. Biomolecular-functionalized magnetic particles have found many applications in various biosensing procedures [27], mostly for immunosensing and DNA analysis, as well as in environmental and homeland security monitoring [28].
2. Core-Shell Structures
The present section is concentrated on the magnetic nanoparticles with a solid magnetic core coated with an organic or bioorganic shell (the shell structures composed of solid materials, e.g., metallic or silicon oxide are overviewed in the next sections). The easiest way of particle modification, particularly with organic polymers, can be based on physical adsorption [29]. However, covalent binding of (bio)organic molecules to the core parts is preferable since it provides more stable immobilization. The core parts of functionalized magnetic particles are frequently made of Fe3O4 or γ-Fe2O3 [30] having many hydroxyl groups at their surfaces, thus allowing silanization of particles followed by covalent binding of biomolecules to functional groups in the organosilane film [31]. While biomolecules bound to the particles are important for biocatalytic or biorecognition features, the core parts are responsible for magnetic properties. Magnetic nanoparticles with controlled size, specific shape and magnetization have been synthesized according to various methods [32,33,34,35,36,37] and then successfully used for various biotechnological [35] and biomedical applications [38]. For example, a synthetic procedure was developed for size-controlled preparation of magnetite (Fe3O4) nanoparticles in organic solvents [39]. One of the most important characteristics of biocompatible magnetic nanoparticles was their size dispersion characterized by atomic force microscopy and transmission electron microscopy (TEM) [40]. Particular attention was given to the synthesis of monodisperse and uniform nanoparticles [41]. Superparamagnetic iron oxide nanoparticles of controllable size (<20 nm) were prepared in the presence of reduced polysaccharides [42]. Nanoparticles synthesized by this method have an organic shell composed of polysaccharide, which increased the particle stability and offered functional groups for additional modification with various biomolecules and redox species. Biocompatible superparamagnetic Fe3O4 nanoparticles were extensively studied and their structural and magnetic features were optimized for their use as labeling units in biomedical applications [43]. Polymer-modified magnetic nanoparticles can be used for isolation and purification of various biomolecules. For example, poly(2-hydroxypropylene imine)-functionalized Fe3O4 magnetic nanoparticles were used for high-efficiency DNA isolation, higher than other studied materials at same conditions, and had excellent specificity in presence of some proteins and metal ions [44]. Magnetic nanoparticles modified with a hydrophobic organic shell (e.g., composed of oleic acid) have been tested for magneto-stimulated solvent extraction and demonstrated fast phase disengagement [45].
Highly crystalline iron oxide (Fe3O4) nanoparticles with a continuous size-spectrum of 6–13 nm were prepared from monodispersed Fe nanoparticles used as precursors by their oxidation under carefully controlled conditions [46]. Chemical stability of magnetic nanoparticles is an important issue. In order to increase it, the organic shell components can be cross-linked, for example, in iron oxide/polystyrene (core/shell) particles [47]. Cross-linking of polymeric chains in the organic shell resulted in additionally stabilization of the shell structure, also protecting the magnetic core from physical and chemical decomposition. Magnetic properties of nanoparticles can be tuned by varying chemical composition and thickness of the coating materials, as it was reported for the composite FePt-MFe2O4 (M = Fe, Co) core-shell nanoparticles [48]. While iron oxide-based magnetic nanoparticles are the most frequently used, some alternative magnetized materials have been suggested for various biomedical and bioanalytical applications [49]. For example, ferromagnetic FeCo nanoparticles demonstrated superior properties that make them promising candidates for magnetically assisted bioseparation methods and analysis, as well as for various electrochemical and bioelectrochemical applications. Magnetic and dielectric properties of magnetic nanoparticles functionalized with organic polymers (a core-shell structure) have been modelled and then the parameters obtained theoretically were compared with the experimental data showing good predictability of the nanoparticle properties using the theoretical model [50].
3. Magnetic Nanoparticles Coated with Noble Metal Shells
Formation of a thin shell-film of noble metals (e.g., Au or Ag) around magnetic cores (e.g., Fe3O4 or CoFe2O4) results in the enhanced chemical stability of the magnetic core [51,52,53,54,55,56] (Figure 1) also providing high electrical conductivity in particle assemblies, which is an important feature for electrochemical and electronic applications. The enhanced stability of magnetic nanoparticles coated with a Au shell allowed their operation under conditions when non-protected particles degrade rapidly.
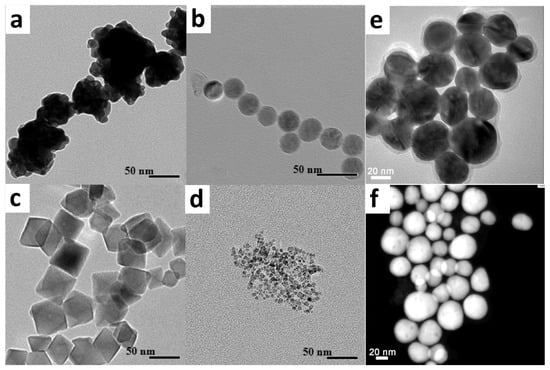
Figure 1.
Various magnetic nanoparticles coated with gold shells: (a–d) TEM images of Fe3O4-core/Au-shell magnetic nanoparticles synthesized according to different experimental procedures: (a) [57], (b) [58], (c) [57,58], (d) [59]; see more details in [54]. (e,f) TEM and STEM (scanning transmission electron microscopy) images, respectively, of the γ-Fe2O3-core/Au-shell magnetic nanoparticles [60] (parts of this figure were adapted from [54] with permission).
For example, Au-coated iron nanoparticles with a specific magnetic moment of 145 emu g−1 and a coercivity of 1664 Oe were synthesized for biomedical applications [61]. Also, Au-coated nanoparticles with magnetic Co cores were synthesized for biomedical applications with the controlled size (5–25 nm; ±1 nm) and morphologies (spheres, discs with specific aspect ratio of 5 × 20 nm) tailored for specific applications [62]. Formation of a Au-shell around a magnetic core results in additional options for modification of nanoparticles with (bio)organic molecules. Indeed, Au surfaces are well known for self-assembling of thiolated molecules resulting in a monolayer formation. Au-coated magnetic nanoparticles of different sizes (50 nm, 70 nm and 100 nm) were prepared by the reduction of AuCl4− ions with hydroxylamine in the presence of Fe3O4 nanoparticles used as seeds [63]. Then, the gold-shell surface was modified with antibodies (rabbit anti-HIVp24 IgG or goat anti-human IgG) through a simple self-assembling of thiolated molecules. The synthesized antibody-functionalized Au-coated magnetic nanoparticles were used in an enzyme-linked immunosorbent assay (ELISA) providing easy separation and purification steps. Importantly for electrochemical and electronic applications, Au-shell-magnetic nanoparticles can be cross-linked with dithiol molecular linkers to yield thin-films with conducting properties [64].
4. Magnetic Nanoparticles Associated with Silicon Oxide Nanoparticles and Nanotubes
Magnetic nanoparticles can be encapsulated in porous silica particles, which were functionalized at their external surfaces with proteins and used for biocatalysis [65,66]. The opposite way of modification resulted in the particles with a magnetic core and a mesoporous silica shell where the pores were filled with biomolecules or drugs [67]. These species allowed magneto-controlled transportation of the molecules included in the porous material of the shells. This approach was successfully used for modifying iron oxide magnetic nanoparticles (γ-Fe2O3 20 nm or Fe3O4 6–7 nm) with a SiO2 shell (thickness of 2–5 nm) using wet chemical synthesis [68,69] (Figure 2).
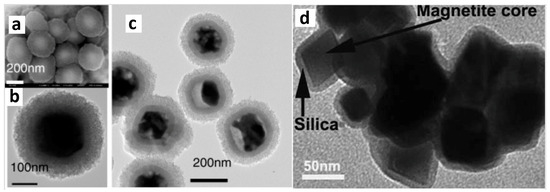
Figure 2.
Various magnetic nanoparticles coated with silica shells: Backscattered electrons image (a) and TEM image (b) of Fe3O4-core/SiO2-mesoporous-shell magnetic nanoparticles [67]. TEM image (c) of Fe3O4-core/SiO2-mesoporous-shell magnetic nanoparticles [67]. TEM image (d) of Fe3O4-core/SiO2-shell magnetic nanoparticles [71] (parts of this figure were adapted from [67,71] with permission).
Different approach was used to load magnetic nanoparticles on one-dimensional nano-objects (nanotubes), thus allowing deposition of many particles with a large total magnetization on one nanotube. SiO2 nanotubes were prepared in an alumina template and then their inner surfaces were modified with Fe3O4 magnetic nanoparticles [70]. The resulting magnetic nanotubes were applied for the magnetic-field-assisted bioseparation, biointeraction, and drug delivery, benefiting from a large magnetization originating from the presence of many magnetic nanoparticles and a large external SiO2 nanotube surface area.
5. Magnetic Nanoparticles with Fluorescent Features
Fe3O4 magnetic nanoparticles (5–15 nm) with unique optical properties were prepared with an inorganic fluorescent shell composed of ytterbium and erbium co-doped sodium yttrium fluoride (NaYF4/Y/Er), which provided infrared-to-visible up-conversion with the high efficiency [72]. The two-component hybrid core-shell magnetic nanoparticles with fluorescent properties were furthercoated with a second shell made of SiO2 allowing covalent immobilization of biomolecules (e.g., streptavidin). The produced multi-functional nanoparticles demonstrated efficient magnetization, fluorescence and bioaffinity features, thus allowing magneto-controlled separation of biomolecules, their fluorescent analysis and formation of affinity complexes with complementary biotinylated molecules. Many different approaches have been studied for combining magnetic properties and fluorescent features in one hybrid bi-functional nano-object. For example, magnetic Fe3O4 nanoparticles (8.5 nm) were modified with polyelectrolyte films using layer-by-layer deposition of differently charged polyelectrolytes, positively charged polyallylamine and the negatively charged polystyrene sulfonate [73]. The thickness of the polymer-shell around the magnetic core and the charge of the external layer were controlled by the number of deposited layers. The electrical charge of the external layer allowed electrostatic binding of secondary fluorescent nanoparticles. Negatively charged thioglycolic acid-capped CdTe nanoparticles were electrostatically bound to the positively charged polyallylamine exterior layer in the polyelectrolyte shell of the magnetic nanoparticles. The distance between the secondary satellite fluorescent CdTe nanoparticles and the magnetic Fe3O4 core was controlled by the number of the deposited polyelectrolyte layers. The developed method allowed further system sophistication by depositing additional layers of polyelectrolytes above the CdTe nanoparticles followed by deposition of another layer of the satellite CdTe nanoparticles. The distance between the primary and secondary fluorescent CdTe nanoparticles was controlled by the number of the polyelectrolyte layers between them, thus allowing tuning of the fluorescent properties of the multi-functional nano-system. Many other magnetic-fluorescent assemblies with different compositions have been reported for different applications. One more example is Co-CdSe core-shell magnetic-fluorescent assembly prepared by deposition of fluorescent CdSe layer on the pre-formed magnetic Co core. The deposition process was performed in a non-aqueous solution using dimethyl cadmium as an organic precursor [74]. Many different magnetic nano-species functionalized with fluorescent labels have been used as versatile labels for biomolecules, demonstrating advantages of both fluorescent reporting part and magnetic separating/transporting part of the assembly. It should be noted that careful optimization of the distance separating the magnetic core and fluorescent species (organic dyes or inorganic quantum dots) should be done to minimize quenching of the photo-excited species by the core part.
6. Magnetic Nanoparticles Combined with Metallic Nano-Species or Quantum Dots
Combining two different nanoparticles (e.g., magnetic and metal or semiconductor) in one nano-assembly where the particles are bound to each other results in unique multi-functional species. In these species two nanoparticles composed of different materials with different properties can be organized as Siamese twins (dumbbell-like bifunctional particles) [75,76,77]. There are different procedures for binding two nanoparticles in one composite assembly, some of the procedures are based on the controlled growth of the second particle next to the primary particle. For example, magnetic nanoparticles, Fe3O4 or FePt, (8 nm) with a protecting/stabilizing organic shell composed of a surfactant were dispersed in an organic solvent (e.g., dichlorobenzene) and added to an aqueous solution of Ag+ salt [75]. The bi-phase aqueous/organic system was ultrasonicated to yield micelles with the magnetic nanoparticles self-assembled on the liquid/liquid interface. Then, Ag+ ions penetrated through defects in the surfactant shell being then catalytically reduced by Fe2+ sites to yield the seeding of a Ag nanoparticle. Further reduction of Ag+ ions on the Ag seed resulted in the grows of the seed and formation of a Ag nanoparticle at a side of the magnetic nanoparticle yielding a twin-particles shown in the transmission electron microscopy (TEM) image (Figure 3a). Another Ag nanoparticle was produced at a side of an FePt magnetic nanoparticle in a similar process (Figure 3b). The size of the produced Ag nanoparticle was controlled by the time allowed for the growing process.
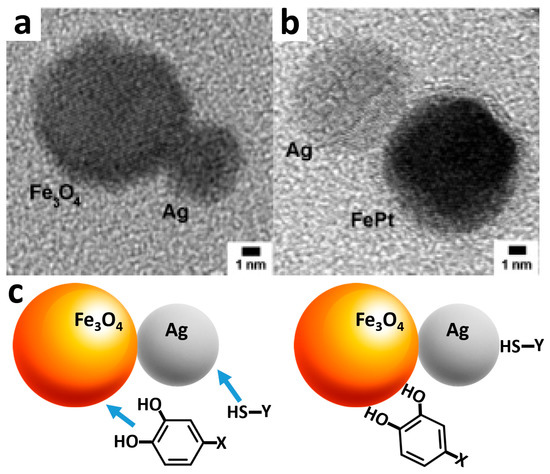
Figure 3.
(a,b) TEM images of Fe3O4-Ag and FePt-Ag hetero-dimers composed of the magnetic nanoparticle and connected Ag nanoparticle [75]. (c) Directed functionalization of the Fe3O4 nanoparticle and Ag nanoparticle with different functional units, such as dopamine-derivatized and thiol-derivatized species, respectively. X and Y might be represented by different molecular and biomolecular species (part of this figure was adapted with permission from [75], American Chemical Society, 2005).
The two parts of the synthesized hetero-dimeric nanoparticles can be conveniently modified with different molecules using the difference of the surface properties of the two parts of the dimer. For example, the Ag nanoparticle in the dimeric hybrid was functionalized with self-assembled thiolated molecules, while the Fe3O4 magnetic nanoparticle was modified using dopamine units as anchor groups bound to Fe2+/3+ sites of the iron oxide surface (Figure 3c). In a different synthetic approach hetero-dimeric species were produced from FePt two-metal alloy nanoparticles coated by an amorphous CdS shell. The metastable amorphous CdS layer had tendency of changing to a crystalized form upon temperature increase. When the multi-component core-shell nanoparticles were heated, FePt and CdS components were transformed into hetero-dimers due to incompatibility of the FePt and CdS lattices, thus resulting in their separation and formation of individual FePt and CdS nanoparticles (less than 10 nm size) connected to each other [76]. Importantly, the hetero-dimeric species demonstrated superparamagnetism characteristic of the FePt part and fluorescence produced by the CdS quantum dot, providing excellent means for labeling of biomaterials. In a different approach, separately synthesized superparamagnetic γ-Fe2O3 nanoparticles (ca. 11.8 nm) and fluorescent CdSe quantum dots (ca. 3.5 nm) were mixed and encapsulated together in a silicon oxide shell yielding a complex multifunctional assembly that demonstrated a unique combination of the magnetic property of γ-Fe2O3 and fluorescent features of CdSe [78]. The silicon oxide shell served as a matrix keeping together the functional nano-components, preserving their individual properties, and providing accessibility of the two-component hybrid system for additional chemical modification of both components with different molecules.
7. Modification of Magnetic Nanoparticles with Various Biomolecules
Various organic shells exhibiting different chemical functional groups (e.g., aminosiloxane, dextran or dimercaptosuccinic acid) were prepared around magnetic nanoparticles [71,79,80]. Organic functional groups available at the outer-layer of the organic shell have been used for numerous chemical coupling reactions resulting in covalent immobilization of different (bio)molecules [81,82] to allow various biochemical, bioanalytical and biomedical applications [83]. For example, covalent immobilization of a polyclonal IgG anti-horseradish peroxidase antibody bound to dextran-coated magnetic particles allowed the use of the functionalized particles for the capturing and separation of horseradish peroxidase enzyme from a crude protein extract from Escherichia coli [83]. In another example, magnetic core of Fe3O4 nanoparticles was silanized and then covalently modified with polyamidoamine (PAMAM) dendrimer [84]. The amino groups added to the nanoparticles upon their modification with PAMAM were used for covalent binding of streptavidin with the load 3.4-fold greater comparing to the direct binding of streptavidin to the silanized magnetite nanoparticles. The increased streptavidin load originated from the increase of the organic shell diameter and the increased number of the amino groups available for the covalent binding of streptavidin. While silanization of metal-oxide magnetic nanoparticles is the most frequently used technique for their primary modification [22], dopamine was also suggested as a robust anchor group to bind biomolecules to magnetic Fe3O4 particles [85]. Dopamine ligands bind to iron oxide magnetic nanoparticles through coordination of the dihydroxyphenyl units with Fe+2 surface sites of the particles providing amino groups for further covalent attachment of various biomolecules, usually through carbodiimide coupling reactions.
Immobilization of proteins (e.g., bovine serum albumin) [31,86,87] or enzymes (e.g., horseradish peroxidase (HRP) or lipase) [88,89,90,91] upon their binding to organic shells of magnetic nanoparticles has been extensively studied and reported for many applications. Immobilization of various enzymes on magnetic nanoparticles preserves the enzyme catalytic activity and, sometimes, results in the enzyme stabilization comparing with the soluble state. For example, alcohol dehydrogenase covalently immobilized on Fe3O4 magnetic particles demonstrated excellent biocatalytic activity [92,93]. In many experimentally studied systems magnetic nanoparticles functionalized with redox enzymes demonstrated bioelectrocatalytic activities upon direct contacting with electrode surfaces [91].
While covalent binding or any other permanent immobilization of enzymes on magnetic nanoparticles is beneficial for many applications (e.g., in magneto-controlled biosensors), reversible binding of enzymes might be important for other special applications. Reversible binding of positively charged proteins/enzymes to negatively charged polyacrylic-shell/Fe3O4-core magnetic nanoparticles has been reported as an example of electrostatically controlled reversible immobilization [94]. The protein molecules, positively charged at low pH values (pH < pI, isoelectric point), were electrostatically attracted and bound to the negatively charged organic shells, while at higher pH values (pH > pI) the negatively charged protein molecules were electrostatically repulsed and removed from the core-shell magnetic nanoparticles. The demonstrated reversible attraction/repulsion of the proteins controlled by pH values was applied for collecting, purification, and transportation of the proteins with the help of magnetic nanoparticles in the presence of an external magnetic field. Many other applications are feasible, for example, magnetic particles functionalized with carbohydrate oligomers yielding multivalent binding of the magnetic labels to proteins or cells via specific carbohydrate-protein interactions have been used in imaging procedures [95].
DNA molecules have been used as templates for formation of magnetic nanoparticles. A mixture of Fe2+/Fe3+ ions was deposited electrostatically on the negatively charged single-stranded DNA molecules [96]. Then, the iron ions associated with DNA were used as seeds to produce Fe3O4 magnetic particles associated with the DNA molecules. The magneto-labeled single-stranded DNA was hybridized with complementary oligonucleotides yielding the double-stranded DNA complex with the bound magnetic nanoparticles. This allowed magneto-induced separation of the oligonucleotide, which can be later dissociated from the magneto-labeled DNA by the temperature increase.
8. Controlled Aggregation of Magnetic Nanoparticles and Formation of Magnetic Nanowires
The controlled assembling of magnetic nanoparticles using different kinds of cross-linking species or organic matrices has been studied for preparing novel materials with unique properties. Different mechanisms and interactions can be responsible for the nanoparticle assembling. For example, assembling of magnetic nanoparticles in the presence of amino acid-based polymers resulted in the controlled organization of these components due to electrostatic interactions between the block co-polypeptides and nanoparticles [97]. Depending on the kind of the added polypeptide the results of their interaction with magnetic nanoparticles can be different. The addition of polyaspartic acid initiated the aggregation of maghemite (γ-Fe2O3) nanoparticles into clusters, without their precipitation. On the other hand, the addition of the block co-polypeptide poly(EG2-Lys)100-b-poly(Asp)30 resulted in the assembling of the magnetic nanoparticles in more sophisticated structures composed of micelles with cores consisting of the nanoparticles electrostatically bound to the polyaspartic acid end of the block co-polypeptide. The micelle shell stabilizing the core clusters and controlling their size was composed of the poly(EG2-Lys) ends of the copolymers. The size and stability of the nanoparticle assembly can be tuned by changing the composition of the block co-polypeptide, thus adjusting the composite structures for their use in different applications.
Magnetic nanowires of different types, sizes and materials have been created for various applications, mostly using alumina membrane template method [98,99]. This method is based on the formation of nanowires inside the pores of the ordered aluminum oxide membrane, usually with electrochemical deposition of the material selected for the nanowires formation, Figure 4. Variation and optimization of the electrochemical deposition parameters allows the control of the nanowires length and structure, while the nanowires diameter depends on the membrane pores. The magnetic properties as well as some other features of the one-dimensional nanowires are unique and allow their use in the fabrication of magnetic nanodevices with high performance and controllability. For example, an ordered hexagonal array of highly aligned strontium ferrite nanowires was produced by dip coating in alumina templates, with magnetic properties dependent on the nanowire diameter and length [100]. The diameter of nanowires, synthesized with high aspect ratios, was changed from 30 to 60 nm while maintaining the same center-to-center distance between the wires. Nickel nanowires (98 nm diameter and 17 µm length) were fabricated by electrodeposition in anodic aluminum oxide membranes [101].
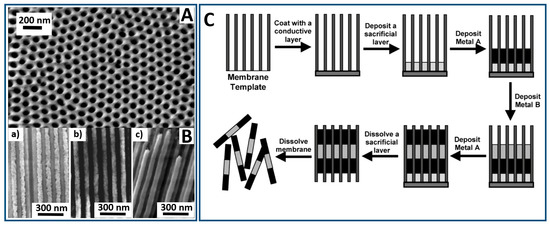
Figure 4.
(A) Scanning electron micrograph, SEM, (top view) of a typical hexagonally ordered nanoporous alumina template with a pore diameter of 70 nm and an interpore distance of 100 nm. (B) SEM cross-sectional view of alumina membranes filled with Fe nanowires deposited from electrolytes containing: (a) 0.1 M FeSO4, (b) 1 M FeSO4 and (c) 0.5 M FeSO4 + 0.4 M H3BO3. (C) Schematic description of the membrane-template electrochemical preparation of multifunctional nanowires. (Parts A and B were adapted from [102] with permission; part C was adopted from [103] with permission).
The synthetic method based on the alumina template can be applied to formation of multi-segment nanowires [104], which include magneto-responsive domains (usually represented by metallic Ni or Fe) and domains made of other materials (e.g., Au for deposition of thiolated redox species and biomolecules). The multi-segment nanowires can demonstrate multi-functional behavior with the magnetic properties combined with biocatalytic or biorecognition features depending on the biomolecule species bound to the non-magnetic segments. It is particularly easy to fabricate nanowires made of different metals, each with different properties. For example, Ni-Cu-Co composite magnetic nanowires have been successfully synthesized by electrochemical deposition inside the alumina template [105]. A few examples of magnetic nanowires are shown in Figure 5 and Figure 6.
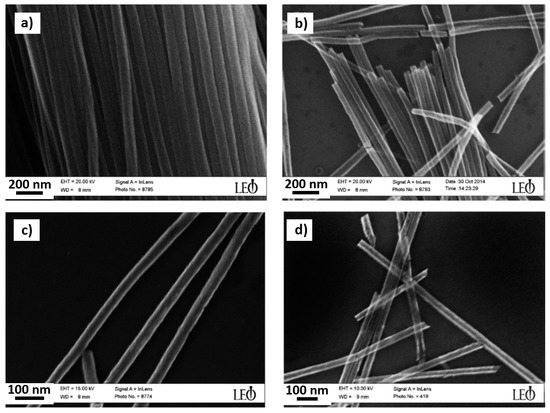
Figure 5.
Field emission scanning electron microscope (FESEM) images of released strontium ferrite magnetic nanowires with diameter of (a) 60, (b) 50, (c) 40 and (d) 30 nm, after removal of alumina templates (figure adapted from [100] with permission).
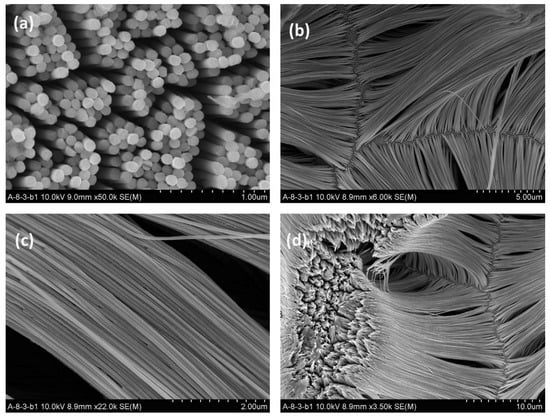
Figure 6.
Scanning electron micrographs (SEM) of Ni nanowires with average diameter of 98 nm and length of 17 µm after removal of alumina templates. (The figure was adapted from ref. [101] with permission.). Images (a-d) show various examples of Ni nanowires prepared in alumina templates.
9. Conclusions and Perspectives
The state-of-the-art in the synthesis, functionalization, characterization, and application of (bio)molecule-functionalized magnetic particles and other related micro-/nano-objects, such as nanowires or nanotubes, allows efficient performance of various in vitro and in vivo biosensors and bioelectronic devices. Many of these devices are aimed for biomedical and biotechnological applications. For example, CoFe2O4-core/Au-shell nanoparticles have been successfully used to design a biosensor for foot-and-mouth viral disease biomarkers [53]. In this example a system with biomimetic oligo peptide-nucleic acid (PNA) was assembled on a gold shell of the magnetic nanoparticles and then hybridized with the complementary DNA sequence which is the disease biomarker. The biosensing was performed upon intercalation of the double-stranded PNA/DNA with a fluorescence probe, Rhodamine 6G. The magnetic features of the nano-species allowed easy separation of the analyzed species from a multi-component biofluid. The present example demonstrates powerful applicability of the biomolecule-functionalized magnetic nanoparticles in biomedical biosensors. Many other applications are feasible using various types of magneto-active nanospecies. Discussion on biological issues related to the biocompatibility, toxicity, etc. are outside the scope of this short review and can be found elsewhere [106,107,108,109,110,111]. While the present review offers a brief introduction to the topic, interested readers can find comprehensive reviews published recently [112,113,114,115,116,117].
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Shen, W.Z.; Cetinel, S.; Montemagno, C. Application of biomolecular recognition via magnetic nanoparticle in nanobiotechnology. J. Nanoparticle Res. 2018, 20, 130. [Google Scholar] [CrossRef]
- Wu, K.; Su, D.Q.; Liu, J.M.; Saha, R.; Wang, J.P. Magnetic nanoparticles in nanomedicine: a review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Abd Elrahman, A.A.; Mansour, F.R. Targeted magnetic iron oxide nanoparticles: Preparation, functionalization and biomedical application. J. Drug Deliv. Sci. Technol. 2019, 52, 702–712. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Zheng, J.J.; Wu, A.G. Applications of iron oxide-based magnetic nanoparticles in the diagnosis and treatment of bacterial infections. Front. Bioeng. Biotechnol. 2019, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Darton, N.J.; Ionescu, A.; Justin Llandro, J. (Eds.) Magnetic Nanoparticles in Biosensing and Medicine; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Ziarani, G.M.; Malmir, M.; Lashgari, N.; Badiei, A. The role of hollow magnetic nanoparticles in drug delivery. RSC Adv. 2019, 9, 25094–25106. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Stanicki, D.; Laurent, S. Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Exp. Opin. Drug Deliv. 2019, 16, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Kami, D.; Takeda, S.; Itakura, Y.; Gojo, S.; Watanabe, M.; Toyoda, M. Application of magnetic nanoparticles to gene delivery. Int. J. Mol. Sci. 2011, 12, 3705–3722. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, D.; Radovic, M.; Mirkovic, M.; Vukadinovic, A.; Peric, M.; Petrovic, D.; Antic, B.; Vranjes-Djuric, S. Y-90-labeled of phosphates-coated magnetic nanoparticles as a potential tumor treatment radiopharmaceuticals. Eur. J. Nuclear Med. Mol. Imaging 2018, 45, S649. [Google Scholar]
- Yang, F.; Lei, P.G.; Jiao, J. Recent advances in the use of magnetic nanoparticles in bio-imaging applications. Nanosci. Nanotechnol. Lett. 2019, 11, 901–922. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S.; Karakoti, A.S. Magnetic nanoparticles: Current trends and future aspects in diagnostics and nanomedicine. Curr. Drug Metab. 2019, 20, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Gloag, L.; Mehdipour, M.; Chen, D.F.; Tilley, R.D.; Gooding, J.J. Advances in the application of magnetic nanoparticles for sensing. Adv. Mater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pastucha, M.; Farka, Z.; Lacina, K.; Mikusova, Z.; Skladal, P. Magnetic nanoparticles for smart electrochemical immunoassays: a review on recent developments. Microchim. Acta 2019, 186, 312. [Google Scholar] [CrossRef] [PubMed]
- Percin, I.; Karakoc, V.; Akgol, S.; Aksoz, E.; Denizli, A. Poly(hydroxyethyl methacrylate) based magnetic nanoparticles for plasmid DNA purification from Escherichia coli lysate. Mater. Sci. Eng. C 2012, 32, 1133–1140. [Google Scholar] [CrossRef]
- Häfeli, U.; Schütt, W.; Teller, J.; Zborowski, M. (Eds.) Scientific and Clinical Applications of Magnetic Carriers; Plenum Press: New York, NY, USA, 2010. [Google Scholar]
- Liu, Z.; Zhang, D.; Han, S.; Li, C.; Lei, B.; Lu, W.; Fang, J.; Zhou, C. Single crystalline magnetite nanotubes. J. Am. Chem. Soc. 2005, 127, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Wicke, W.; Ahmadzadeh, A.; Jamali, V.; Unterweger, H.; Alexiou, C.; Schober, R. Magnetic nanoparticle-based molecular communication in microfluidic environments. IEEE Trans. Nanobiosci. 2019, 18, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.C.; Huang, Y.H.; Nikles, D.E. FePt and CoPt magnetic nanoparticles film for future high density data storage media. Int. J. Nanotechnol. 2004, 1, 328–346. [Google Scholar] [CrossRef]
- Hendrych, A.; Kubínek, R.; Zhukov, A.V. The magnetic force microscopy and its capability for nanomagnetic studies—The short compendium. In Modern Research and Educational Topics in Microscopy; Méndez-Vilas, A., Díaz, J., Eds.; Formatex: Badajoz, Spain, 2007. [Google Scholar]
- Talelli, M.; Aires, A.; Marciello, M. Protein-modified magnetic nanoparticles for biomedical applications. Curr. Org. Chem. 2016, 20, 1252–1261. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.P.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. B 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, A.H.; Faghih, Z.; Khorasani, M.T.; Farjadian, F. Antibody conjugated onto surface modified magnetic nanoparticles for separation of HER2+breast cancer cells. J. Magn. Magn. Mater. 2019, 490, 165479. [Google Scholar] [CrossRef]
- Lee, M.H.; Leu, C.C.; Lin, C.C.; Tseng, Y.F.; Lin, H.Y.; Yang, C.N. Gold-decorated magnetic nanoparticles modified with hairpin-shaped DNA for fluorometric discrimination of single-base mismatch DNA. Microchim. Acta 2019, 186, 80. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Acuna, M.; Halman, J.R.; Afonin, K.A.; Dobson, J.; Rinaldi, C. Magnetic nanoparticles loaded with functional RNA nanoparticles. Nanoscale 2018, 10, 17761–17770. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-H.; Yoshitake, T.; Kim, D.-K.; Muhammed, M.; Bjelke, B.; Kehr, J. Determination of conjugation efficiency of antibodies and proteins to the superparamagnetic iron oxide nanoparticles by capillary electrophoresis with laser-induced fluorescence detection. J. Nanoparticle Res. 2003, 5, 137–146. [Google Scholar] [CrossRef]
- Bucak, S.; Jones, D.A.; Laibinis, P.E.; Hatton, T.A. Protein separations using colloidal magnetic nanoparticles. Biotechnol. Prog. 2003, 19, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, L.; Won, Y.H.; Ganesana, M.; Andreescu, S. Magnetic particle-based hybrid platforms for bioanalytical sensors. Sensors 2009, 9, 2976–2999. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, S.; Njagi, J.; Ispas, C.; Ravalli, M.T. JEM Spotlight: Applications of advanced nanomaterials for environmental monitoring. J. Environ. Monit. 2009, 11, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chow, G.M. Carboxyl group (–CO2H) functionalized ferrimagnetic iron oxide nanoparticles for potential bio-applications. J. Mater. Chem. 2004, 14, 2781–2786. [Google Scholar] [CrossRef]
- Ling, W.H.; Wang, M.Y.; Xiong, C.X.; Xie, D.F.; Chen, Q.Y.; Chu, X.Y.; Qiu, X.Y.; Li, Y.M.; Xiao, X. Synthesis, surface modification, and applications of magnetic iron oxide nanoparticles. J. Mater. Res. 2019, 34, 1828–1844. [Google Scholar] [CrossRef]
- Liu, X.; Xing, J.; Guan, Y.; Shan, G.; Liu, H. Synthesis of amino-silane modified superparamagnetic silica supports and their use for protein immobilization. Colloids Surf. A Physicochem. Eng. Asp. 2004, 238, 127–131. [Google Scholar] [CrossRef]
- Jana, N.R.; Chen, Y.; Peng, X. Size- and shape-controlled magnetic (Cr, Mn, Fe, Co, Ni) oxide nanocrystals via a simple and general approach. Chem. Mater. 2004, 16, 3931–3935. [Google Scholar] [CrossRef]
- Lai, J.; Shafi, K.V.P.M.; Ulman, A.; Loos, K.; Lee, Y.; Vogt, T.; Lee, W.-L.; Ong, N.P. Controlling the size of magnetic nanoparticles using pluronic block copolymer surfactants. J. Phys. Chem. B 2005, 109, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Tartaj, P.; Morales, M.P.; González-Carreño, T.; Veintemillas-Verdaguer, S.; Serna, C.J. Advances in magnetic nanoparticles for biotechnology applications. J. Magn. Magn. Mater. 2005, 290–291, 28–34. [Google Scholar] [CrossRef]
- Shen, L.; Laibinis, P.E.; Hatton, T.A. Bilayer surfactant stabilized magnetic fluids: Synthesis and interactions at interfaces. Langmuir 1999, 15, 447–453. [Google Scholar] [CrossRef]
- Hyeon, T. Chemical synthesis of magnetic nanoparticles. Chem. Commun. 2003, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.; Lacava, Z.G.M.; Buske, N.; Morais, P.C.; Azevedo, R.B. Atomic force microscopy and transmission electron microscopy of biocompatible magnetic fluids: A comparative analysis. J. Nanoparticle Res. 2004, 6, 209–213. [Google Scholar] [CrossRef]
- Matijević, E. Uniform inorganic colloid dispersions. Achievements and challenges. Langmuir 1994, 10, 8–16. [Google Scholar] [CrossRef]
- Paul, K.G.; Frigo, T.B.; Groman, J.Y.; Groman, E.V. Synthesis of ultrasmall superparamagnetic iron oxides using reduced polysaccharides. Bioconjugate Chem. 2004, 15, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, L.F.; Brito, G.E.S.; Pontuschka, W.M.; Amaro, E.; Parma, A.H.C.; Goya, G.F. Biocompatible superparamagnetic iron oxide nanoparticles used for contrast agents: a structural and magnetic study. J. Magn. Magn. Mater. 2005, 289, 439–441. [Google Scholar] [CrossRef]
- Pan, X.H.; Cheng, S.Y.; Su, T.; Zuo, G.C.; Zhang, C.; Wu, L.P.; Jiao, Y.Z.; Dong, W. Poly (2-hydroxypropylene imines) functionalized magnetic polydopamine nanoparticles for high-efficiency DNA isolation. Appl. Surf. Sci. 2019, 498, 143888. [Google Scholar] [CrossRef]
- Lobato, N.C.C.; Ferreira, A.D.; Weidler, P.G.; Franzreb, M.; Mansur, M.B. Improvement of magnetic solvent extraction using functionalized silica coated Fe3O4 nanoparticles. Sep. Purif. Technol. 2019, 229, 115839. [Google Scholar] [CrossRef]
- Park, J.; Lee, E.; Hwang, N.-M.; Kang, M.; Kim, S.C.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; et al. One-nanometer-scale size-controlled synthesis of monodisperse magnetic iron oxide nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 2872–2877. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Fan, J.; Jiang, R.; Gao, Y. Cross-linking the linear polymeric chains in the ATRP synthesis of iron oxide/polystyrene core/shell nanoparticles. Chem. Mater. 2004, 16, 1835–1837. [Google Scholar] [CrossRef]
- Zeng, H.; Sun, S.; Li, J.; Wang, Z.L.; Liu, J.P. Tailoring magnetic properties of core∕shell nanoparticles. Appl. Phys. Lett. 2004, 85, 792–794. [Google Scholar] [CrossRef]
- Hütten, A.; Sudfeld, D.; Ennen, I.; Reiss, G.; Wojczykowski, K.; Jutzi, P. Ferromagnetic FeCo nanoparticles for biotechnology. J. Magn. Magn. Mater. 2005, 293, 93–101. [Google Scholar] [CrossRef]
- Hu, Z.; Kanagaraj, J.; Hong, H.P.; Yang, K.; Ji, X.H.; Fan, Q.H.; Kharel, P. Characterization of ferrite magnetic nanoparticle modified polymeric composites by modeling. J. Magn. Magn. Mater. 2020, 493, 165735. [Google Scholar] [CrossRef]
- Pita, M.; Tam, T.K.; Minko, S.; Katz, E. Dual magneto-biochemical logic control of electrochemical processes based on local interfacial pH changes. ACS Appl. Mater. Interfaces 2009, 1, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.; Sheparovych, R.; Pita, M.; Narvaez Garcia, A.; Dominguez, E.; Minko, S.; Katz, E. Magneto-induced self-assembling of conductive nanowires for biosensor applications. J. Phys. Chem. C 2008, 112, 7337–7344. [Google Scholar] [CrossRef]
- Pita, M.; Abad, J.M.; Vaz-Dominguez, C.; Briones, C.; Mateo-Martí, E.; Martín-Gago, J.A.; del Puerto Morales, M.; Fernández, V.M. Synthesis of cobalt ferrite core/metallic shell nanoparticles for the development of a specific PNA/DNA biosensor. J. Colloid Interface Sci. 2008, 321, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.M.; Tavallaie, R.; Alam, M.T.; Chuah, K.; Gooding, J.J. A comparison of differently synthesized gold-coated magnetic nanoparticles as ‘Dispersible Electrodes’. Electroanalysis 2016, 28, 431–438. [Google Scholar] [CrossRef]
- Mandal, M.; Kundu, S.; Ghosh, S.K.; Panigrahi, S.; Sau, T.K.; Yusuf, S.M.; Pal, T. Magnetite nanoparticles with tunable gold or silver shell. J. Colloid Interface Sci. 2005, 286, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Majetich, S.A. Composite magnetic–plasmonic nanoparticles for biomedicine: Manipulation and imaging. Nano Today 2013, 8, 98–113. [Google Scholar] [CrossRef]
- Goon, I.Y.; Lai, L.M.H.; Lim, M.; Munroe, P.; Gooding, J.J.; Amal, R. Fabrication and dispersion of gold-shell-protected magnetite nanoparticles: Systematic control using polyethyleneimine. Chem. Mater. 2009, 21, 673–681. [Google Scholar] [CrossRef]
- Jin, Y.D.; Jia, C.X.; Huang, S.W.; O’Donnell, M.; Gao, X.H. Multifunctional nanoparticles as coupled contrast agents. Nature Commun. 2010, 1, 41. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.; Viswanathan, S.; Nouws, H.P.A.; Oliveira, M.B.P.P.; Delerue-Matos, C. Iron oxide/gold core/shell nanomagnetic probes and CdS biolabels for amplified electrochemical immunosensing of Salmonella typhimurium. Biosens. Bioelectron. 2014, 51, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Riskin, M.; Basnar, B.; Huang, Y.; Willner, I. Magnetoswitchable charge transport and bioelectrocatalysis using maghemite-Au core-shell nanoparticle/polyaniline composites. Adv. Mater. 2007, 19, 2691–2695. [Google Scholar] [CrossRef]
- Chen, M.; Yamamuro, S.; Farrell, D.; Majetich, S.A. Gold-coated iron nanoparticles for biomedical applications. J. Appl. Phys. 2003, 93, 7551–7553. [Google Scholar] [CrossRef]
- Bao, Y.; Krishnan, K.M. Preparation of functionalized and gold-coated cobalt nanocrystals for biomedical applications. J. Magn. Magn. Mater. 2005, 293, 15–19. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Hui, W.; Zhang, Z.; Xin, X.; Chen, C. The synthesis of GoldMag nano-particles and their application for antibody immobilization. Biomed. Microdevices 2005, 7, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, J.; Maye, M.M.; Fan, Q.; Rendeng, Q.; Engelhard, M.H.; Wang, C.; Lin, Y.; Zhong, C.-J. Iron oxide–gold core–shell nanoparticles and thin film assembly. J. Mater. Chem. 2005, 15, 1821–1832. [Google Scholar] [CrossRef]
- Gao, X.; Yu, K.M.K.; Tam, K.Y.; Tsang, S.C. Colloidal stable silica encapsulated nano-magnetic composite as a novel bio-catalyst carrier. Chem. Commun. 2003, 2998–2999. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-H.; Zhang, S.-Q.; Chen, X.-L.; Zhuang, Z.-X.; Xu, J.-G.; Wang, X.-R. Magnetite-containing spherical silica nanoparticles for biocatalysis and bioseparations. Anal. Chem. 2004, 76, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Gu, J.; Zhang, L.; Chen, H.; Shi, J. Fabrication of uniform magnetic nanocomposite spheres with a magnetic core/mesoporous silica shell structure. J. Am. Chem. Soc. 2005, 127, 8916–8917. [Google Scholar] [CrossRef] [PubMed]
- SYun, S.H.; Lee, C.W.; Lee, J.S.; Seo, C.W.; Lee, E.K. Fabrication of SiO2-coated magnetic nanoparticles for applications to protein separation and purification. Mater. Sci. Forum 2004, 449, 1033–1036. [Google Scholar]
- He, Y.P.; Wang, S.Q.; Li, C.R.; Miao, Y.M.; Wu, Z.Y.; Zou, B.S. Synthesis and characterization of functionalized silica-coated Fe3O4 superparamagnetic nanocrystals for biological applications. J. Phys. D Appl. Phys. 2005, 38, 1342–1350. [Google Scholar] [CrossRef]
- Son, S.J.; Reichel, J.; He, B.; Schuchman, M.; Lee, S.B. Magnetic nanotubes for magnetic-field-assisted bioseparation, biointeraction, and drug delivery. J. Am. Chem. Soc. 2005, 127, 7316–7317. [Google Scholar] [CrossRef] [PubMed]
- del Campo, A.; Sen, T.; Lellouche, J.-P.; Bruce, I.J. Multifunctional magnetite and silica–magnetite nanoparticles: Synthesis, surface activation and applications in life sciences. J. Magn. Magn. Mater. 2005, 293, 33–40. [Google Scholar] [CrossRef]
- Lu, H.; Yi, G.; Zhao, S.; Chen, D.; Guo, L.-H.; Cheng, J. Synthesis and characterization of multi-functional nanoparticles possessing magnetic, up-conversion fluorescence and bio-affinity properties. J. Mater. Chem. 2004, 14, 1336–1341. [Google Scholar] [CrossRef]
- Hong, X.; Li, J.; Wang, M.; Xu, J.; Guo, W.; Li, J.; Bai, Y.; Li, T. Fabrication of magnetic luminescent nanocomposites by a layer-by-layer self-assembly approach. Chem. Mater. 2004, 16, 4022–4027. [Google Scholar] [CrossRef]
- Kim, H.; Achermann, M.; Balet, L.P.; Hollingsworth, J.A.; Klimov, V.I. Synthesis and characterization of Co/CdSe core/shell nanocomposites: Bifunctional magnetic-optical nanocrystals. J. Am. Chem. Soc. 2005, 127, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Yang, Z.; Gao, J.; Chang, C.K.; Xu, B. Heterodimers of nanoparticles: Formation at a liquid−liquid interface and particle-specific surface modification by functional molecules. J. Am. Chem. Soc. 2005, 127, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Zheng, R.; Zhang, X.-X.; Xu, B. Facile one-pot synthesis of bifunctional heterodimers of nanoparticles: A conjugate of quantum dot and magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 5664–5665. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-like bifunctional Au-Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.K.; Selvan, S.T.; Lee, S.S.; Papaefthymiou, G.C.; Kundaliya, D.; Ying, J.Y. Silica-coated nanocomposites of magnetic nanoparticles and quantum dots. J. Am. Chem. Soc. 2005, 127, 4990–4991. [Google Scholar] [CrossRef] [PubMed]
- Morais, P.C.; Santos, J.G.; Silveira, L.B.; Gansau, C.; Buske, N.; Nunes, W.C.; Sinnecker, J.P. Susceptibility investigation of the nanoparticle coating-layer effect on the particle interaction in biocompatible magnetic fluids. J. Magn. Magn. Mater. 2004, 272-276, 2328–2329. [Google Scholar] [CrossRef]
- Bruce, I.J.; Sen, T. Surface modification of magnetic nanoparticles with alkoxysilanes and their application in magnetic bioseparations. Langmuir 2005, 21, 7029–7035. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Matsunaga, T. Fully automated chemiluminescence immunoassay of insulin using antibody−protein A−bacterial magnetic particle complexes. Anal. Chem. 2000, 72, 3518–3522. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Mitchell, D.T. Peer reviewed: Nanomaterials in analytical chemistry. Anal. Chem. 1998, 70, 322A–327A. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Preparation of inert magnetic nano-particles for the directed immobilization of antibodies. Biosens. Bioelectron. 2005, 20, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Pan, B.-F.; Zheng, W.-M.; Ao, L.-M.; Gu, H.-C. Study of streptavidin coated onto PAMAM dendrimer modified magnetite nanoparticles. J. Magn. Magn. Mater. 2005, 293, 48–54. [Google Scholar] [CrossRef]
- Xu, C.; Xu, K.; Gu, H.; Zheng, R.; Liu, H.; Zhang, X.; Guo, Z.; Xu, B. Dopamine as a robust anchor to immobilize functional molecules on the iron oxide shell of magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 9938–9939. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.G.; Hidajat, K.; Uddin, M.S. Adsorption of bovine serum albumin on nanosized magnetic particles. J. Colloid Interface Sci. 2004, 271, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylova, M.; Kim, D.K.; Berry, C.C.; Zagorodni, A.; Toprak, M.; Curtis, A.S.G.; Muhammed, M. BSA immobilization on amine-functionalized superparamagnetic iron oxide nanoparticles. Chem. Mater. 2004, 16, 2344–2354. [Google Scholar] [CrossRef]
- Dyal, A.; Loos, K.; Noto, M.; Chang, S.W.; Spagnoli, C.; Shafi, K.V.P.M.; Ulman, A.; Cowman, M.; Gross, R.A. Activity of Candida rugosa lipase immobilized on gamma-Fe2O3 magnetic nanoparticles. J. Am. Chem. Soc. 2003, 125, 1684–1685. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, Y.; Yu, W.; Shen, H.; Zhang, H.; Gu, N. Preparation and characterization of magnetite nanoparticles coated by amino silane. Colloids Surf. A Physicochem. Eng. Asp. 2003, 212, 219–226. [Google Scholar] [CrossRef]
- Huang, S.-H.; Liao, M.-H.; Chen, D.-H. Direct binding and characterization of lipase onto magnetic nanoparticles. Biotechnol. Prog. 2003, 19, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; He, P.; Hu, N. Electrochemical biosensors utilising electron transfer in heme proteins immobilised on Fe3O4 nanoparticles. Analyst 2003, 128, 1268–1274. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, M.; Honda, H.; Kobayashi, T. Preparation of fine magnetic particles and application for enzyme immobilization. Biocatalysis 1991, 5, 61–69. [Google Scholar] [CrossRef]
- Liao, M.-H.; Chen, D.-H. Immobilization of yeast alcohol dehydrogenase on magnetic nanoparticles for improving its stability. Biotechnol. Lett. 2001, 23, 1723–1727. [Google Scholar] [CrossRef]
- Liao, M.-H.; Chen, D.-H. Fast and efficient adsorption/desorption of protein by a novel magnetic nano-adsorbent. Biotechnol. Lett. 2002, 24, 1913–1917. [Google Scholar] [CrossRef]
- Sun, X.-L.; Cui, W.; Haller, C.; Chaikof, E.L. Site-specific multivalent carbohydrate labeling of quantum dots and magnetic beads. ChemBioChem 2004, 5, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Mornet, S.; Vekris, A.; Bonnet, J.; Duguet, E.; Grasset, F.; Choy, J.-H.; Portier, J. DNA–magnetite nanocomposite materials. Mater. Lett. 2000, 42, 183–188. [Google Scholar] [CrossRef]
- Euliss, L.E.; Grancharov, S.G.; O’Brien, S.; Deming, T.J.; Stucky, G.D.; Murray, C.B.; Held, G.A. Cooperative assembly of magnetic nanoparticles and block copolypeptides in aqueous media. Nano Lett. 2003, 3, 1489–1493. [Google Scholar] [CrossRef]
- Wang, X.W.; He, Z.C.; Li, J.S.; Yuan, Z.H. Controllable synthesis and magnetic properties of ferromagnetic nanowires and nanotubes. Curr. Nanoscience 2012, 8, 801–809. [Google Scholar] [CrossRef]
- Sousa, C.T.; Leitao, D.C.; Proenca, M.P.; Ventura, J.; Pereira, A.M.; Araujo, J.P. Nanoporous alumina as templates for multifunctional applications. Appl. Phys. Rev. 2014, 1, 031102. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Ashrafizadeh, F.; Bakhshi, S.R. Tuning the magnetic properties of high aligned strontium ferrite nanowires formed in alumina template. J. Alloys Compd. 2016, 656, 237–244. [Google Scholar] [CrossRef]
- Adeela, N.; Maaz, K.; Khan, U.; Karim, S.; Ahmad, M.; Iqbal, M.; Riaz, S.; Han, X.F.; Maqbool, M. Fabrication and temperature dependent magnetic properties of nickel nanowires embedded in alumina templates. Ceram. Int. 2015, 41, 12081–12086. [Google Scholar] [CrossRef]
- Schlörb, H.; Haehnel, V.; Khatri, M.S.; Srivastav, A.; Kumar, A.; Schultz, L.; Fähler, S. Magnetic nanowires by electrodeposition within templates. Phys. Status Solidi B 2010, 247, 2364–2379. [Google Scholar] [CrossRef]
- Wang, J. Adaptive nanowires for on-demand control of electrochemical microsystems. Electroanalysis 2008, 20, 611–615. [Google Scholar] [CrossRef]
- Monzon, L.M.A.; O’Neill, K.; Sheth, Y.; Venkatesan, M.; Coey, J.M.D. Fabrication of multisegmented magnetic wires with micron-length copper spacers. Electrochem. Commun. 2013, 36, 96–98. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, M.; Sun, H.Y.; Nairan, A.; Karim, S.; Nisar, A.; Maqbool, M.; Ahmad, M. Fabrication and temperature dependent magnetic properties of Ni–Cu–Co composite. Nanowires. Physical B 2015, 475, 99–104. [Google Scholar] [CrossRef]
- Jiang, Z.; Shan, K.; Song, J.; Liu, J.; Rajendran, S.; Pugazhendhi, A.; Jacob, J.A.; Chen, B. Toxic effects of magnetic nanoparticles on normal cells and organs. Life Sci. 2019, 220, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Jarockyte, G.; Daugelaite, E.; Stasys, M.; Statkute, U.; Poderys, V.; Tseng, T.-C.; Hsu, S.-H.; Karabanovas, V.; Rotomskis, R. Accumulation and toxicity of superparamagnetic iron oxide nanoparticles in cells and experimental animals. Int. J. Mol. Sci. 2016, 17, 1193. [Google Scholar] [CrossRef] [PubMed]
- Erofeev, A.; Gorelkin, P.; Garanina, A.; Alova, A.; Efremova, M.; Vorobyeva, N.; Edwards, C.; Korchev, Y.; Majouga, A. Novel method for rapid toxicity screening of magnetic nanoparticles. Sci. Rep. 2018, 8, 7462. [Google Scholar] [CrossRef] [PubMed]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.M.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Laurent, S.; Shokrgozar, M.A.; Hosseinkhani, M. Toxicity evaluations of superparamagnetic iron oxide nanoparticles: Cell “vision” versus physicochemical properties of nanoparticles. ACS Nano 2011, 5, 7263–7276. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.; Niu, X. Surface modification of magnetic iron oxide nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Rehman, S.; Rahman, H.U.; Khan, Q. Synthesis and application of magnetic nanoparticles. In Nanomagnetism; Gonzalez Estevez, J.M., Ed.; One Central Press (OCP): Cheshire, UK, 2014; Chapter 6; pp. 135–159. [Google Scholar]
- Boal, A.K. Synthesis and applications of magnetic nanoparticles. In Nanoparticles: Building Blocks for Nanotechnology; Rotello, V., Ed.; Springer: Boston, MA, USA, 2004; Chapter 1; pp. 1–27. [Google Scholar]
- Fermon, C. Introduction to Magnetic Nanoparticles. In Nanomagnetism: Applications and Perspectives; Van de Voorde, M., Fermon, C., Eds.; Wiley: Hoboken, NJ, USA, 2017; Chapter 7; pp. 127–136. [Google Scholar]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic nanoparticles: From design and synthesis to real world applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.M.K.; Ficiarà, E.; Ruffinatti, F.A.; Stura, I.; Argenziano, M.; Abollino, O.; Cavalli, R.; Guiot, C.; D’Agata, F. Magnetic iron oxide nanoparticles: Synthesis, characterization and functionalization for biomedical applications in the central nervous system. Materials 2019, 12, 465. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).