Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles
Abstract
:1. Introduction
1.1. Effects and Categories of Hyperthermia
1.2. Magnetic Nanoparticles for Local Hyperthermia
2. Heat Generation
2.1. Magnetic Relaxation Processes
2.2. Power Loss in MNPs in AMF
3. Intrinsic Parameters
3.1. Overview of Intrinsic Parameters
3.2. The Effective Magnetic Anisotropy
4. Extrinsic Parameters
4.1. Parameters of the AMF
4.2. Role of Interparticle Interactions on the Heating Efficiency
4.3. Beyond the LRT
5. Experimental and Theoretical Limitations in the Determination of SAR
6. Thermometry in Magnetic Hyperthermia
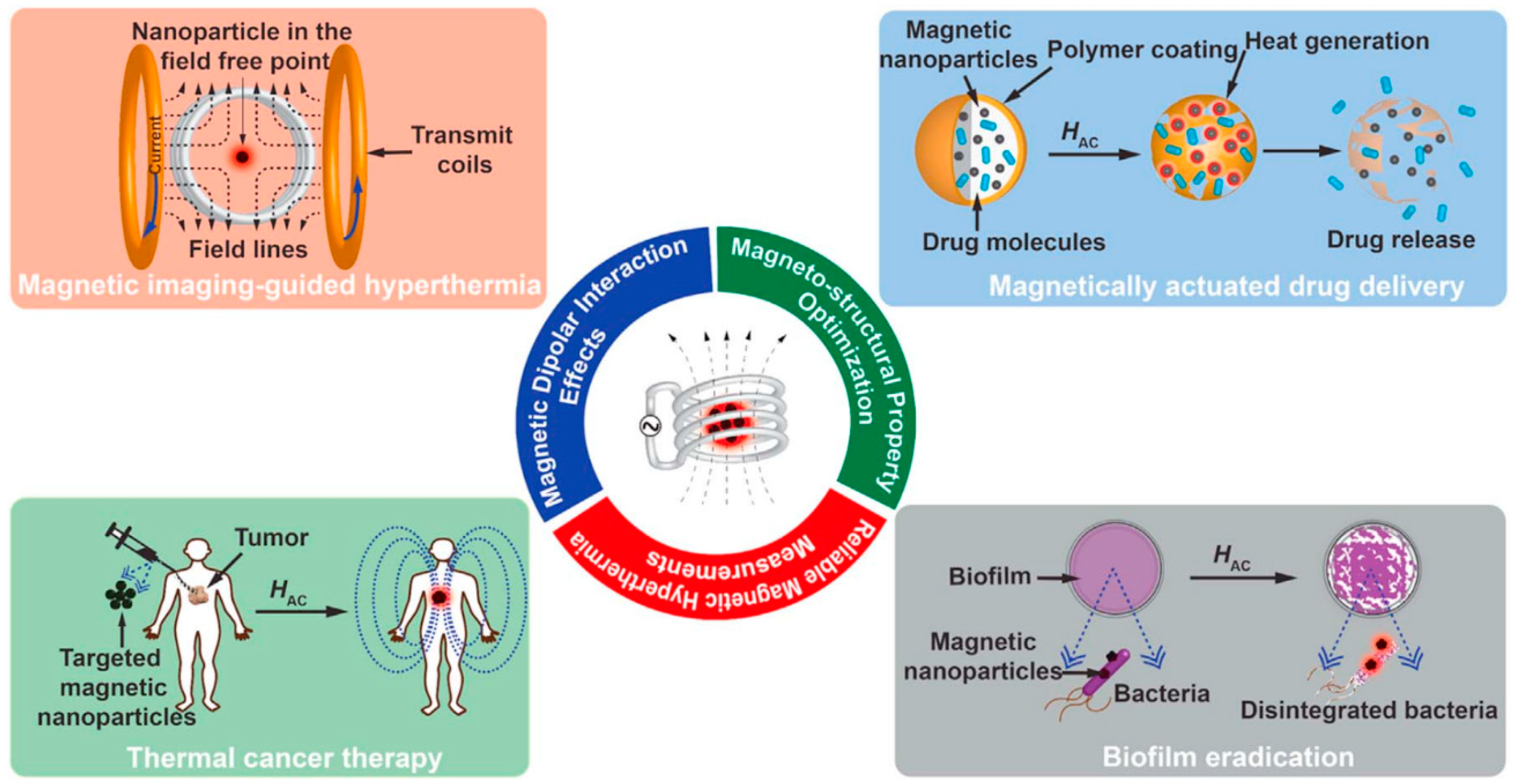
7. Multifunctional Hybrid Magnetic Nanoparticles for hyperthermia Based Biomedical Applications
7.1. Graphene oxide-Fe3O4 Nanocomposites for Hyperthermia
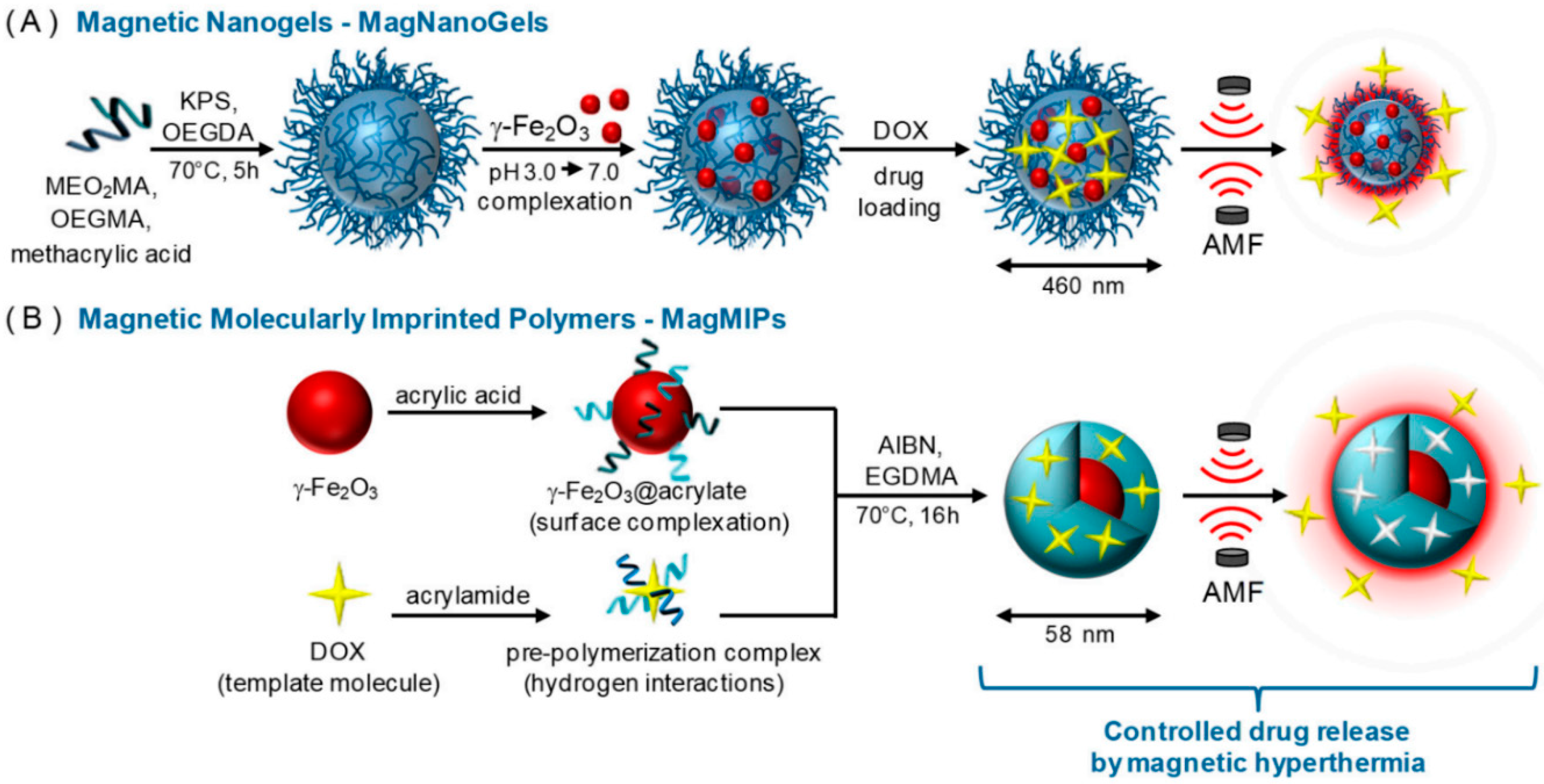
7.2. Magnetic Nanogels for Thermosensitive Drug Delivery
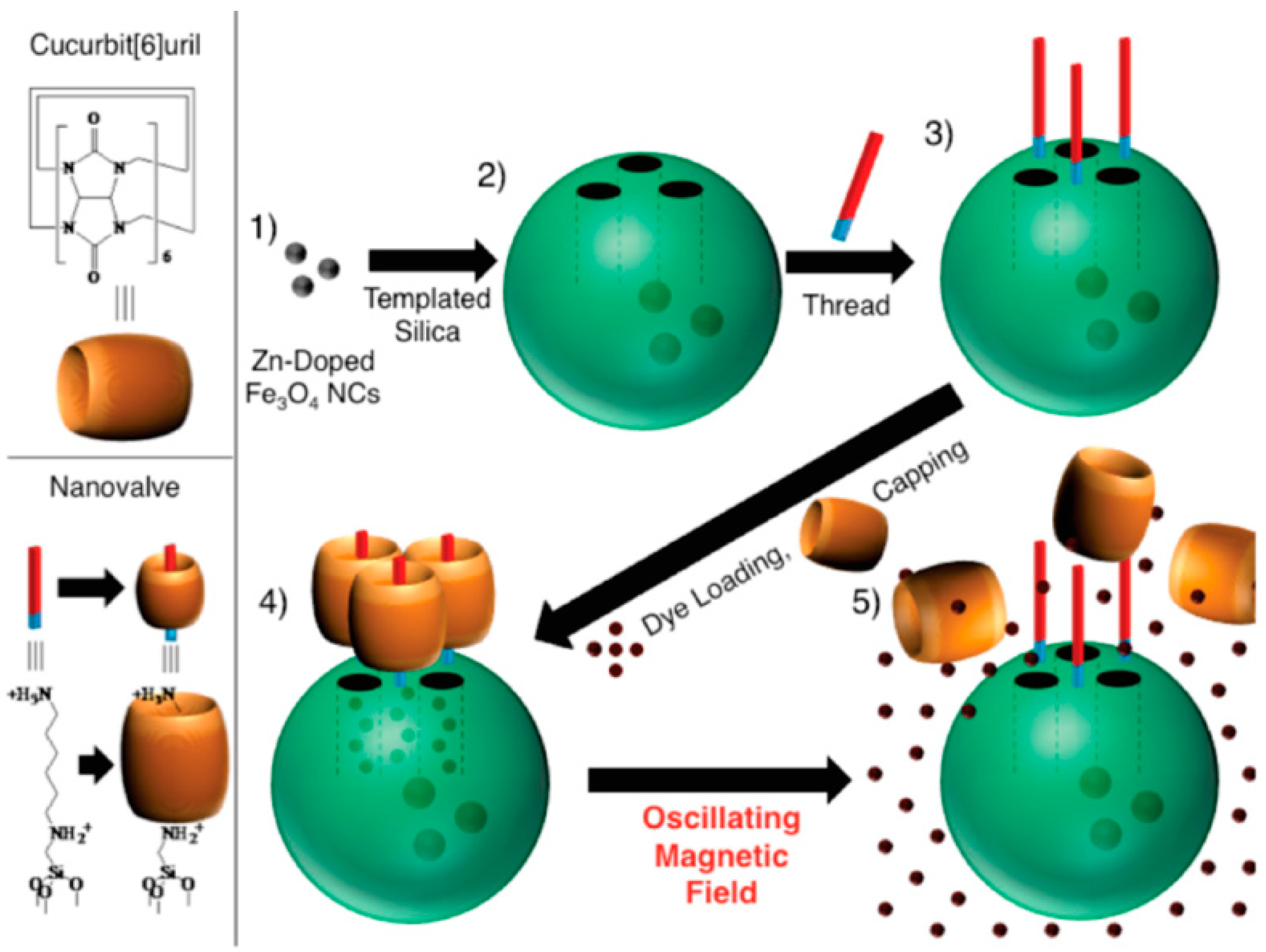
7.3. Magnetic Mesoporus Silica Nanoparticles for High Dose Delivery of Anticancer Drugs
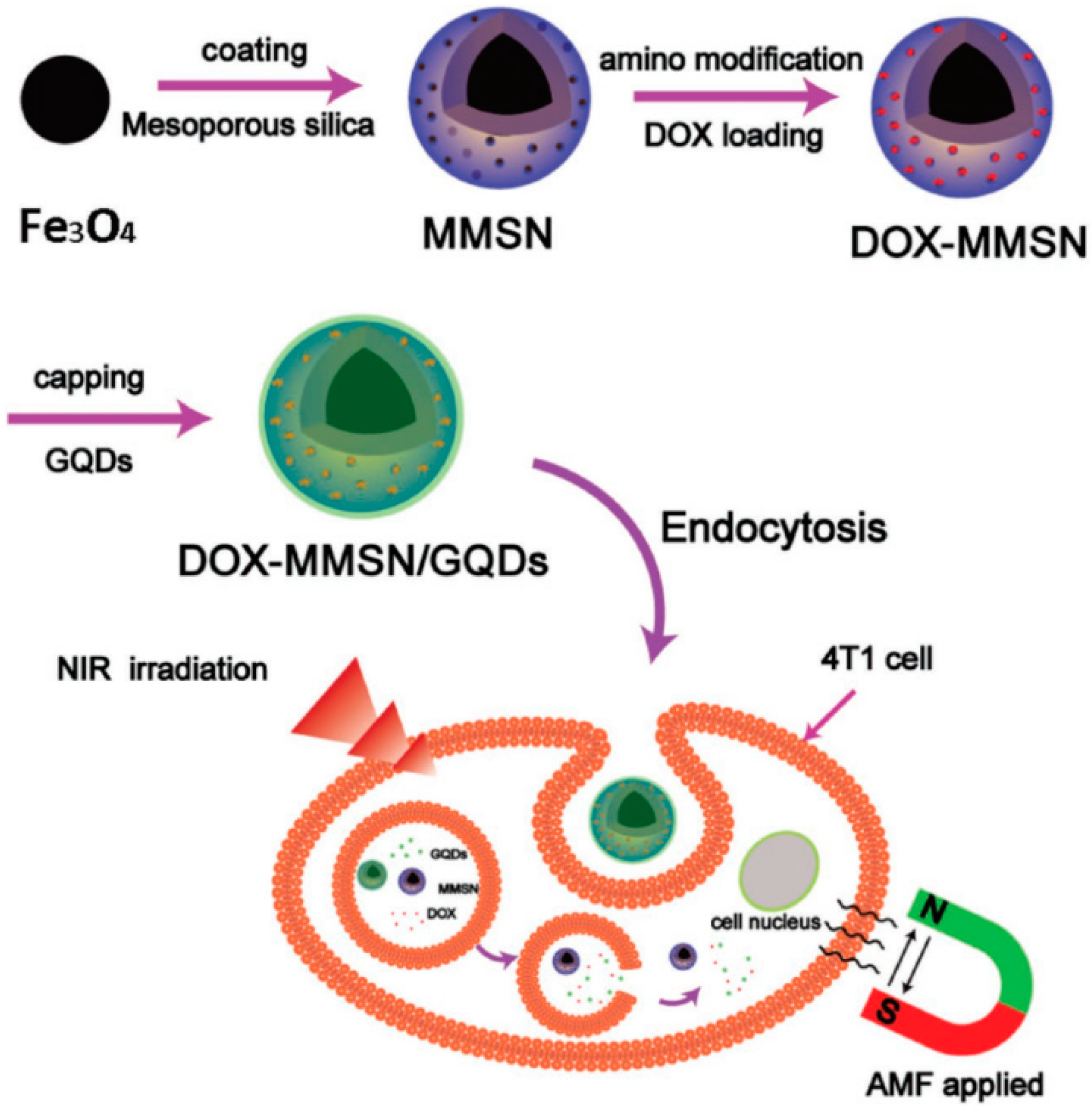
7.4. Multifunctional Drug Delivery Agents Through Magnetic and Photothermal Therapy
7.5. Magnetic Particle Imaging and Hyperthermia in Vivo Applications
8. Synthesis of IONPs
8.1. Thermal Decomposition
8.2. Hydrothermal Synthesis
8.3. Microwave-Assisted Synthesis
8.4. Template Assisted Fabrication
- (i)
- Template use in the fabrication process determines the final size and morphology of the nanostructures.
- (ii)
- Complex nanostructures such as nanobarcodes (segmented nanorods) nanoprism, nanocubes hexagons, and octahedrons MNPs can be fabricated in an easy manner, with full control on size and morphology.
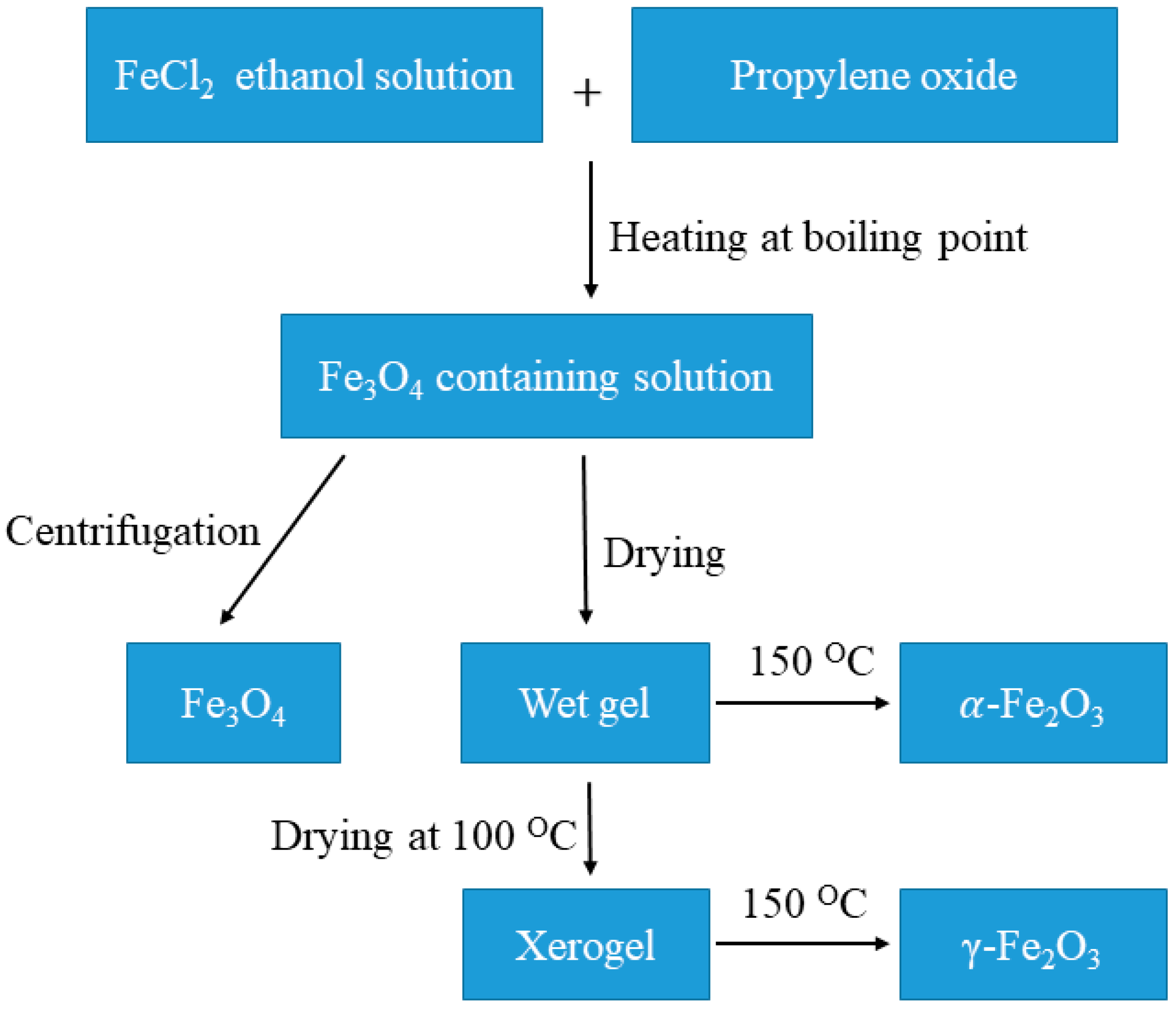
8.5. Sol–Gel Method
8.6. Synthesis of GO-Fe3O4 Nanocomposite
8.6.1. Coprecipitation Method
8.6.2. Organometallic Decomposition and Ligand Exchange Method
8.7. Cytotoxicity of Ferrite Nanoparticles
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dou, Y.; Hynynen, K.; Allen, C. To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. J. Control Release 2017, 249, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Mallory, M.; Gogineni, E.; Jones, G.C.; Greer, L.; Simone, C.B. Therapeutic hyperthermia: The old, the new, and the upcoming. Crit. Rev. Oncol. Hematol. 2016, 97, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Fratila, R.; Fuente, J. Nanomaterials for Magnetic and Optical Hyperthermia Applications, 1st ed.; Elsevier: Amsterdam, The Nederland, 2019; pp. 1–10. ISBN 978-0-12-813928-8. [Google Scholar]
- Pérez-Hernández, M. Chapter 8—Mechanisms of Cell Death Induced by Optical Hyperthermia. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Micro and Nano Technologies; Fratila, R.M., De La Fuente, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 201–228. ISBN 978-0-12-813928-8. [Google Scholar]
- Dhavalikar, R.; Bohórquez, A.C.; Rinaldi, C. Chapter 10—Image-Guided Thermal Therapy Using Magnetic Particle Imaging and Magnetic Fluid Hyperthermia. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Micro and Nano Technologies; Fratila, R.M., De La Fuente, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–286. ISBN 978-0-12-813928-8. [Google Scholar]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control Release 2016, 235, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. NOD/SCID/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef]
- Di Corato, R.; Espinosa, A.; Lartigue, L.; Tharaud, M.; Chat, S.; Pellegrino, T.; Ménager, C.; Gazeau, F.; Wilhelm, C. Magnetic hyperthermia efficiency in the cellular environment for different nanoparticle designs. Biomaterials 2014, 35, 6400–6411. [Google Scholar] [CrossRef]
- Fratila, R.M.; Fuente, J.M.D.L. Nanomaterials for Magnetic and Optical Hyperthermia Applications; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-813929-5. [Google Scholar]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Kumar, C.S.S.R.; Mohammad, F. Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Adv. Drug Deliv. Rev. 2011, 63, 789–808. [Google Scholar] [CrossRef] [Green Version]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919–S2934. [Google Scholar] [CrossRef]
- Vallejo-Fernandez, G.; Whear, O.; Roca, A.G.; Hussain, S.; Timmis, J.; Patel, V.; Grady, K. Mechanisms of hyperthermia in magnetic nanoparticles. J. Phys. D Appl. Phys. 2013, 46, 312001. [Google Scholar] [CrossRef]
- Piñeiro, Y.; Vargas, Z.; Rivas, J.; López-Quintela, M.A. Iron Oxide Based Nanoparticles for Magnetic Hyperthermia Strategies in Biological Applications. Eur. J. Inorganic Chem. 2015, 2015, 4495–4509. [Google Scholar]
- Dennis, C.L.; Ivkov, R. Physics of heat generation using magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2013, 29, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, I.M.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Périgo, E.A.; Hemery, G.; Sandre, O.; Ortega, D.; Garaio, E.; Plazaola, F.; Teran, F.J. Fundamentals and advances in magnetic hyperthermia. Appl. Phys. Rev. 2015, 2, 041302. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [Green Version]
- Angelakeris, M. Magnetic nanoparticles: A multifunctional vehicle for modern theranostics. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1642–1651. [Google Scholar] [CrossRef]
- Spirou, S.V.; Costa Lima, S.A.; Bouziotis, P.; Vranješ-Djurić, S.; Efthimiadou, E.Κ.; Laurenzana, A.; Barbosa, A.I.; Garcia-Alonso, I.; Jones, C.; Jankovic, D.; et al. Recommendations for in Vitro and In Vivo Testing of Magnetic Nanoparticle Hyperthermia Combined with Radiation Therapy. Nanomaterials 2018, 8, 306. [Google Scholar] [CrossRef] [Green Version]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic Hyperthermia and Radiation Therapy: Radiobiological Principles and Current Practice. Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef] [Green Version]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [Green Version]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Mehdaoui, B.; Meffre, A.; Carrey, J.; Lachaize, S.; Lacroix, L.-M.; Gougeon, M.; Chaudret, B.; Respaud, M. Optimal Size of Nanoparticles for Magnetic Hyperthermia: A Combined Theoretical and Experimental Study. Adv. Funct. Mater. 2011, 21, 4573–4581. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Boehm, I.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, I.M.; Nayek, C.; Manna, K.; Bhattacharjee, G.; Al-Omari, I.A.; Gismelseed, A. Investigating Exchange Bias and Coercivity in Fe3O4–γ-Fe2O3 Core–Shell Nanoparticles of Fixed Core Diameter and Variable Shell Thicknesses. Nanomaterials 2017, 7, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obaidat, I.M.; Nayek, C.; Manna, K. Investigating the Role of Shell Thickness and Field Cooling on Saturation Magnetization and Its Temperature Dependence in Fe3O4/γ-Fe2O3 Core/Shell Nanoparticles. Appl. Sci. 2017, 7, 1269. [Google Scholar] [CrossRef] [Green Version]
- Rachakatla, R.S.; Balivada, S.; Seo, G.-M.; Myers, C.B.; Wang, H.; Samarakoon, T.N.; Dani, R.; Pyle, M.; Kroh, F.O.; Walker, B.; et al. Attenuation of mouse melanoma by A/C magnetic field after delivery of bi-magnetic nanoparticles by neural progenitor cells. ACS Nano 2010, 4, 7093–7104. [Google Scholar] [CrossRef]
- Ruiz, A.; Gutiérrez, L.; Cáceres-Vélez, P.R.; Santos, D.; Chaves, S.B.; Fascineli, M.L.; Garcia, M.P.; Azevedo, R.B.; Morales, M.P. Biotransformation of magnetic nanoparticles as a function of coating in a rat model. Nanoscale 2015, 7, 16321–16329. [Google Scholar] [CrossRef]
- Guimarães, A.P. The Basis of Nanomagnetism. In Principles of Nanomagnetism; NanoScience and Technology; Guimarães, A.P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–23. ISBN 978-3-319-59409-5. [Google Scholar]
- Deatsch, A.E.; Evans, B.A. Heating efficiency in magnetic nanoparticle hyperthermia. J. Magn. Magn. Mater. 2014, 354, 163–172. [Google Scholar] [CrossRef]
- Brown, W.F. Thermal Fluctuations of a Single-Domain Particle. Phys. Rev. 1963, 130, 1677–1686. [Google Scholar] [CrossRef]
- Néel, L. Théorie du traînage magnétique des substances massives dans le domaine de Rayleigh. J. Phys. Radium 1950, 11, 49–61. [Google Scholar] [CrossRef]
- Leslie-Pelecky, D.L.; Rieke, R.D. Magnetic Properties of Nanostructured Materials. Chem. Mater. 1996, 8, 1770–1783. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Delaunay, L.; Neveu, S.; Noyel, G.; Monin, J. A new spectrometric method, using a magneto-optical effect, to study magnetic liquids. J. Magn. Magn. Mater. 1995, 149, L239–L245. [Google Scholar] [CrossRef]
- Glöckl, G.; Hergt, R.; Zeisberger, M.; Dutz, S.; Nagel, S.; Weitschies, W. The effect of field parameters, nanoparticle properties and immobilization on the specific heating power in magnetic particle hyperthermia. J. Phys. Condens. Matter 2006, 18, S2935–S2949. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—Biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Moroz, P.; Jones, S.K.; Gray, B.N. Magnetically mediated hyperthermia: Current status and future directions. Int. J. Hyperth. 2002, 18, 267–284. [Google Scholar] [CrossRef]
- Fantechi, E.; Innocenti, C.; Albino, M.; Lottini, E.; Sangregorio, C. Influence of cobalt doping on the hyperthermic efficiency of magnetite nanoparticles. J. Magn. Magn. Mater. 2015, 380, 365–371. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jang, J.-T.; Choi, J.-S.; Moon, S.H.; Noh, S.-H.; Kim, J.-W.; Kim, J.-G.; Kim, I.-S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Mohite, V.; Issa, B.; Tit, N.; Haik, Y. Predicting a major role of surface spins in the magnetic properties of ferrite nanoparticles. Cryst. Res. Technol. 2009, 44, 489–494. [Google Scholar] [CrossRef]
- Cotin, G.; Perton, F.; Blanco-Andujar, C.; Pichon, B.; Mertz, D.; Bégin-Colin, S. Chapter 2—Design of Anisotropic Iron-Oxide-Based Nanoparticles for Magnetic Hyperthermia. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Micro and Nano Technologies; Fratila, R.M., De La Fuente, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 41–60. ISBN 978-0-12-813928-8. [Google Scholar]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials; Wiley: Hoboken IEEE Press: Chichester, UK, 2009; ISBN 978-0-471-47741-9. [Google Scholar]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Martinez-Boubeta, C.; Simeonidis, K.; Makridis, A.; Angelakeris, M.; Iglesias, O.; Guardia, P.; Cabot, A.; Yedra, L.; Estrade, S.; Peiro, F.; et al. Learning from Nature to Improve the Heat Generation of Iron-Oxide Nanoparticles for Magnetic Hyperthermia Applications. Sci. Rep. 2013. [Google Scholar] [CrossRef] [Green Version]
- Usov, N.A.; Nesmeyanov, M.S.; Gubanova, E.M.; Epshtein, N.B. Heating ability of magnetic nanoparticles with cubic and combined anisotropy. Beilstein J. Nanotechnol. 2019, 10, 305–314. [Google Scholar] [CrossRef]
- Nayek, C.; Manna, K.; Bhattacharjee, G.; Murugavel, P.; Obaidat, I. Investigating Size- and Temperature-Dependent Coercivity and Saturation Magnetization in PEG Coated Fe3O4 Nanoparticles. Magnetochemistry 2017, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhong, W.; Du, Y.W. Magnetic nanoparticles with core/shell structures. J. NanoSci. Nanotechnol. 2008, 8, 2781–2792. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, N.; Qurishi, Y.; Atreya, H.S.; Srivastava, C. Effect of core–shell nanoparticle geometry on the enhancement of the proton relaxivity value in a nuclear magnetic resonance experiment. RSC Adv. 2016, 6, 64605–64610. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Haik, Y.; Mohite, V.; Issa, B.; Tit, N. Peculiar Magnetic Properties of MnZnGdFeO Nanoparticles. Adv. Sci. Lett. 2009, 2, 60–64. [Google Scholar] [CrossRef]
- Venkatesha, N.; Pudakalakatti, S.M.; Qurishi, Y.; Atreya, H.S.; Srivastava, C. MnFe2O4–Fe3O4 core–shell nanoparticles as a potential contrast agent for magnetic resonance imaging. RSC Adv. 2015, 5, 97807–97815. [Google Scholar] [CrossRef]
- Mandal, S.; Chaudhuri, K. Engineered magnetic core shell nanoprobes: Synthesis and applications to cancer imaging and therapeutics. World J. Biol. Chem. 2016, 7, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Huls, N.F.; Sigdel, A.; Sun, S. Tuning exchange bias in core/shell FeO/Fe3O4 nanoparticles. Nano Lett. 2012, 12, 246–251. [Google Scholar] [CrossRef]
- Nayek, C.; Al-Akhras, M.-A.; Narayanaswamy, V.; Khaleel, A.; Al-Omari, I.A.; Rusydi, A.; Obaidat, I.M. Role of Shell Thickness and Applied Field on The Magnetic Anisotropy and Temperature Dependence of Coercivity in Fe3O4 -Fe2O3 Core/shell Nanoparticles. Mater. Express 2019, 9, 2158–5849. [Google Scholar] [CrossRef]
- Thomas, S.; Reethu, K.; Thanveer, T.; Myint, M.T.Z.; Al-Harthi, S.H. Effect of shell thickness on the exchange bias blocking temperature and coercivity in Co-CoO core-shell nanoparticles. J. Appl. Phys. 2017, 122, 063902. [Google Scholar] [CrossRef]
- Dimitriadis, V.; Kechrakos, D.; Chubykalo-Fesenko, O.; Tsiantos, V. Shape-dependent exchange bias effect in magnetic nanoparticles with core-shell morphology. Phys. Rev. B 2015, 92, 064420. [Google Scholar] [CrossRef] [Green Version]
- Nogués, J.; Sort, J.; Langlais, V.; Skumryev, V.; Suriñach, S.; Muñoz, J.S.; Baró, M.D. Exchange bias in nanostructures. Phys. Rep. 2005, 422, 65–117. [Google Scholar] [CrossRef]
- López-Ortega, A.; Estrader, M.; Salazar-Alvarez, G.; Roca, A.G.; Nogués, J. Applications of exchange coupled bi-magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Phys. Rep. 2015, 553, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.-H.; Alonso, J.; Khurshid, H.; Lampen-Kelley, P.; Chandra, S.; Stojak Repa, K.; Nemati, Z.; Das, R.; Iglesias, Ó.; Srikanth, H. Exchange Bias Effects in Iron Oxide-Based Nanoparticle Systems. Nanomaterials 2016, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Desautels, R.D.; Skoropata, E.; Chen, Y.-Y.; Ouyang, H.; Freeland, J.W.; van Lierop, J. Tuning the surface magnetism of γ-Fe2O3 nanoparticles with a Cu shell. Appl. Phys. Lett. 2011, 99, 262501. [Google Scholar] [CrossRef]
- Rostamnejadi, A.; Venkatesan, M.; Alaria, J.; Boese, M.; Kameli, P.; Salamati, H.; Coey, J.M.D. Conventional and inverse magnetocaloric effects in La0.45Sr0.55MnO3 nanoparticles. J. Appl. Phys. 2011, 110, 043905. [Google Scholar] [CrossRef] [Green Version]
- Batlle, X.; Labarta, A. Finite-size effects in fine particles: Magnetic and transport properties. J. Phys. D Appl. Phys. 2002. [Google Scholar] [CrossRef]
- Ho, C.-H.; Lai, C.-H. Size-Dependent Magnetic Properties of PtMn Nanoparticles. IEEE Trans. Magn. 2006, 42, 3069–3071. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.D.; Howerth, E.W.; Chen, Z.G.; Platt, S. Magnetic Nanoparticle-Based Hyperthermia for Head & Neck Cancer in Mouse Models. Theranostics 2012, 2, 113–121. [Google Scholar]
- Nieskoski, M.D.; Trembly, B.S. Comparison of a single optimized coil and a Helmholtz pair for magnetic nanoparticle hyperthermia. IEEE Trans. Biomed. Eng. 2014, 61, 1642–1650. [Google Scholar] [CrossRef]
- Cabrera, D.; Rubia-Rodríguez, I.; Garaio, E.; Plazaola, F.; Dupré, L.; Farrow, N.; Terán, F.J.; Ortega, D. Chapter 5—Instrumentation for Magnetic Hyperthermia. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Micro and Nano Technologies; Fratila, R.M., De La Fuente, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 111–138. ISBN 978-0-12-813928-8. [Google Scholar]
- Mamiya, H.; Jeyadevan, B. Chapter 1—Design Criteria of Thermal Seeds for Magnetic Fluid Hyperthermia—From Magnetic Physics Point of View. In Nanomaterials for Magnetic and Optical Hyperthermia Applications; Micro and Nano Technologies; Fratila, R.M., De La Fuente, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 13–39. ISBN 978-0-12-813928-8. [Google Scholar]
- Hergt, R.; Hiergeist, R.; Hilger, I.; Kaiser, W.A.; Boguslavsky, Y.; Margel, S.; Richter, U. Maghemite nanoparticles with very high AC-losses for application in RF-magnetic hyperthermia. J. Magn. Magn. Mater. 2004, 270, 345–357. [Google Scholar] [CrossRef]
- Dössel, O.; Bohnert, J. Safety considerations for magnetic fields of 10 mT to 100 mT amplitude in the frequency range of 10 kHz to 100 kHz for magnetic particle imaging. Biomed. Tech. (Berl) 2013, 58, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Kita, E.; Oda, T.; Kayano, T.; Sato, S.; Minagawa, M.; Yanagihara, H.; Kishimoto, M.; Mitsumata, C.; Hashimoto, S.; Yamada, K.; et al. Ferromagnetic nanoparticles for magnetic hyperthermia and thermoablation therapy. J. Phys. D Appl. Phys. 2010, 43, 474011. [Google Scholar] [CrossRef]
- Lemal, P.; Balog, S.; Geers, C.; Taladriz-Blanco, P.; Palumbo, A.; Hirt, A.M.; Rothen-Rutishauser, B.; Petri-Fink, A. Heating behavior of magnetic iron oxide nanoparticles at clinically relevant concentration. J. Magn. Magn. Mater. 2019, 474, 637–642. [Google Scholar] [CrossRef] [Green Version]
- Ovejero, J.G.; Cabrera, D.; Carrey, J.; Valdivielso, T.; Salas, G.; Teran, F.J. Effects of inter- and intra-aggregate magnetic dipolar interactions on the magnetic heating efficiency of iron oxide nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 10954–10963. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Obaidat, I.M.; Latiyan, S.; Jain, S.; Nayek, C.; Goankar, S.; AL-Akhras, M.-A.; Al-Omari, I.A. Role of interface quality in iron oxide core/shell nanoparticles on heating efficiency and transverse relaxivity. Mater. Express 2019, 2158–5849. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Obaidat, I.M.; Kamzin, A.S.; Latiyan, S.; Jain, S.; Kumar, H.; Srivastava, C.; Alaabed, S.; Issa, B. Synthesis of Graphene Oxide-Fe3O4 Based Nanocomposites Using the Mechanochemical Method and in Vitro Magnetic Hyperthermia. Int. J. Mol. Sci. 2019, 20, 3368. [Google Scholar] [CrossRef] [Green Version]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Kandasamy, G.; Sudame, A.; Bhati, P.; Chakrabarty, A.; Maity, D. Systematic investigations on heating effects of carboxyl-amine functionalized superparamagnetic iron oxide nanoparticles (SPIONs) based ferrofluids for in vitro cancer hyperthermia therapy. J. Mol. Liq. 2018, 256, 224–237. [Google Scholar] [CrossRef]
- de la Presa, P.; Luengo, Y.; Velasco, V.; Morales, M.P.; Iglesias, M.; Veintemillas-Verdaguer, S.; Crespo, P.; Hernando, A. Particle Interactions in Liquid Magnetic Colloids by Zero Field Cooled Measurements: Effects on Heating Efficiency. J. Phys. Chem. C 2015, 119, 11022–11030. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Andujar, C.; Ortega, D.; Southern, P.; Nesbitt, S.A.; Thanh, N.T.K.; Pankhurst, Q.A. Real-time tracking of delayed-onset cellular apoptosis induced by intracellular magnetic hyperthermia. Nanomedicine (Lond) 2016, 11, 121–136. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Yuan, R.; Zhang, C.; Wu, N.; Yan, F.; Yu, S.; Chen, K. Stable and Biocompatible Colloidal Dispersions of Superparamagnetic Iron Oxide Nanoparticles with Minimum Aggregation for Biomedical Applications. J. Phys. Chem. C 2016, 120, 23799–23806. [Google Scholar] [CrossRef]
- Shetake, N.G.; Kumar, A.; Gaikwad, S.; Ray, P.; Desai, S.; Ningthoujam, R.S.; Vatsa, R.K.; Pandey, B.N. Magnetic nanoparticle-mediated hyperthermia therapy induces tumour growth inhibition by apoptosis and Hsp90/AKT modulation. Int. J. Hyperth. 2015, 31, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Sakellari, D.; Brintakis, K.; Kostopoulou, A.; Myrovali, E.; Simeonidis, K.; Lappas, A.; Angelakeris, M. Ferrimagnetic nanocrystal assemblies as versatile magnetic particle hyperthermia mediators. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Mendo, S.G.; Alves, A.F.; Ferreira, L.P.; Cruz, M.M.; Mendonça, M.H.; Godinho, M.; Carvalho, M.D. Hyperthermia studies of ferrite nanoparticles synthesized in the presence of cotton. New J. Chem. 2015, 39, 7182–7193. [Google Scholar] [CrossRef]
- Burrows, F.; Parker, C.; Evans, R.F.L.; Hancock, Y.; Hovorka, O.; Chantrell, R.W. Energy losses in interacting fine-particle magnetic composites. J. Phys. D Appl. Phys. 2010, 43, 474010. [Google Scholar] [CrossRef]
- Tan, R.P.; Carrey, J.; Respaud, M. Magnetic hyperthermia properties of nanoparticles inside lysosomes using kinetic Monte Carlo simulations: Influence of key parameters and dipolar interactions, and evidence for strong spatial variation of heating power. Phys. Rev. B 2014, 90, 214421. [Google Scholar] [CrossRef] [Green Version]
- Coral, D.F.; Mendoza Zélis, P.; Marciello, M.; Morales, M.d.P.; Craievich, A.; Sánchez, F.H.; Fernández van Raap, M.B. Effect of Nanoclustering and Dipolar Interactions in Heat Generation for Magnetic Hyperthermia. Langmuir 2016, 32, 1201–1213. [Google Scholar] [CrossRef]
- Mamiya, H. Recent Advances in Understanding Magnetic Nanoparticles in AC Magnetic Fields and Optimal Design for Targeted Hyperthermia. Available online: https://www.hindawi.com/journals/jnm/2013/752973/ (accessed on 10 October 2019).
- Mamiya, H.; Jeyadevan, B. Hyperthermic effects of dissipative structures of magnetic nanoparticles in large alternating magnetic fields. Sci. Rep. 2011, 1, 157. [Google Scholar] [CrossRef] [Green Version]
- Usov, N.A. Low frequency hysteresis loops of superparamagnetic nanoparticles with uniaxial anisotropy. J. Appl. Phys. 2010, 107, 123909. [Google Scholar] [CrossRef]
- Raikher, Y.L.; Stepanov, V.I. Physical aspects of magnetic hyperthermia: Low-frequency ac field absorption in a magnetic colloid. J. Magne. Magn. Mater. 2014, 368, 421–427. [Google Scholar] [CrossRef]
- Raikher, Y.; Stepanov, V. Absorption of AC field energy in a suspension of magnetic dipoles. J. Magn. Magn. Mater. 2008, 320, 2692–2695. [Google Scholar] [CrossRef]
- Andreu, I.; Natividad, E. Accuracy of available methods for quantifying the heat power generation of nanoparticles for magnetic hyperthermia. Int. J. Hyperth. 2013, 29, 739–751. [Google Scholar] [CrossRef] [Green Version]
- Drayton, A.; Zehner, J.; Timmis, J.; Patel, V.; Vallejo-Fernandez, G.; O’Grady, K. A comparative measurement technique of nanoparticle heating for magnetic hyperthermia applications. J. Phys. D Appl. Phys. 2017, 50, 495003. [Google Scholar] [CrossRef]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Lemal, P.; Geers, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Measuring the heating power of magnetic nanoparticles: An overview of currently used methods. Mater. Today Proc. 2017, 4, S107–S117. [Google Scholar] [CrossRef]
- Bordelon, D.E.; Cornejo, C.; Grüttner, C.; Westphal, F.; DeWeese, T.L.; Ivkov, R. Magnetic nanoparticle heating efficiency reveals magneto-structural differences when characterized with wide ranging and high amplitude alternating magnetic fields. J. Appl. Phys. 2011, 109, 124904. [Google Scholar] [CrossRef]
- Verde, E.L.; Landi, G.T.; Carrião, M.S.; Drummond, A.L.; Gomes, J.A.; Vieira, E.D.; Sousa, M.H.; Bakuzis, A.F. Field dependent transition to the non-linear regime in magnetic hyperthermia experiments: Comparison between maghemite, copper, zinc, nickel and cobalt ferrite nanoparticles of similar sizes. Aip Adv. 2012, 2, 032120. [Google Scholar] [CrossRef]
- Soetaert, F.; Kandala, S.K.; Bakuzis, A.; Ivkov, R. Experimental estimation and analysis of variance of the measured loss power of magnetic nanoparticles. Sci. Rep. 2017, 7, 6661. [Google Scholar] [CrossRef] [Green Version]
- Natividad, E.; Castro, M.; Mediano, A. Adiabatic vs. non-adiabatic determination of specific absorption rate of ferrofluids. J. Magn. Magn. Mater. 2009, 321, 1497–1500. [Google Scholar] [CrossRef]
- Landi, G.T. Simple models for the heating curve in magnetic hyperthermia experiments. J. Magn. Magn. Mater. 2013, 326, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-Y.; Huang, S.; Borca-Tasciuc, D.-A. Potential Sources of Errors in Measuring and Evaluating the Specific Loss Power of Magnetic Nanoparticles in an Alternating Magnetic Field. IEEE Trans. Magn. 2013, 49, 255–262. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, Y.; Fang, J.; Zhang, H.; Peng, E.; Liu, X.; Xiao, W.; Ding, J. Size dependent magnetic hyperthermia of octahedral Fe3O4 nanoparticles. RSC Adv. 2015, 5, 76764–76771. [Google Scholar] [CrossRef]
- Liu, X.L.; Yang, Y.; Ng, C.T.; Zhao, L.Y.; Zhang, Y.; Bay, B.H.; Fan, H.M.; Ding, J. Magnetic vortex nanorings: A new class of hyperthermia agent for highly efficient in vivo regression of tumors. Adv. Mater. Weinh. 2015, 27, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Lv, Y.; Herng, T.S.; Xu, X.; Xia, W.; Zhang, T.; Fang, J.; Xiao, W.; Ding, J. Orientation Mediated Enhancement on Magnetic Hyperthermia of Fe3O4 Nanodisc. Adv. Funct. Mater. 2015, 25, 812–820. [Google Scholar] [CrossRef]
- Peng, E.; Choo, E.S.G.; Chandrasekharan, P.; Yang, C.-T.; Ding, J.; Chuang, K.-H.; Xue, J.M. Synthesis of Manganese Ferrite/Graphene Oxide Nanocomposites for Biomedical Applications. Small 2012, 8, 3620–3630. [Google Scholar] [CrossRef]
- Niculaes, D.; Lak, A.; Anyfantis, G.C.; Marras, S.; Laslett, O.; Avugadda, S.K.; Cassani, M.; Serantes, D.; Hovorka, O.; Chantrell, R.; et al. Asymmetric Assembling of Iron Oxide Nanocubes for Improving Magnetic Hyperthermia Performance. ACS Nano 2017, 11, 12121–12133. [Google Scholar] [CrossRef]
- Sugumaran, P.J.; Liu, X.-L.; Herng, T.S.; Peng, E.; Ding, J. GO-Functionalized Large Magnetic Iron Oxide Nanoparticles with Enhanced Colloidal Stability and Hyperthermia Performance. ACS Appl. Mater. Interfaces 2019, 11, 22703–22713. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, L.; Rao, F.; Song, Z.; Ren, K.; Ji, X.; Song, S.; Yao, D.; Feng, S. Uniform Ti-doped Sb2Te3 materials for high-speed phase change memory applications. Appl. Phys. Lett. 2014, 104, 053119. [Google Scholar] [CrossRef]
- Ortgies, D.H.; de la Cueva, L.; del Rosal, B.; Sanz-Rodríguez, F.; Fernández, N.; Iglesias-de la Cruz, M.C.; Salas, G.; Cabrera, D.; Teran, F.J.; Jaque, D.; et al. In Vivo Deep Tissue Fluorescence and Magnetic Imaging Employing Hybrid Nanostructures. ACS Appl. Mater. Interfaces 2016, 8, 1406–1414. [Google Scholar] [CrossRef]
- Rieke, V.; Pauly, K.B. MR thermometry. J. Magn. Reson. Imaging 2008, 27, 376–390. [Google Scholar] [CrossRef]
- Dhavalikar, R.; Rinaldi, C. Theoretical Predictions for Spatially-Focused Heating of Magnetic Nanoparticles Guided by Magnetic Particle Imaging Field Gradients. J. Magn. Magn. Mater. 2016, 419, 267–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murase, K.; Takata, H.; Takeuchi, Y.; Saito, S. Control of the temperature rise in magnetic hyperthermia with use of an external static magnetic field. Phys. Med. 2013, 29, 624–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barati, M.R.; Selomulya, C.; Sandeman, K.G.; Suzuki, K. Extraordinary induction heating effect near the first order Curie transition. Appl. Phys. Lett. 2014, 105, 162412. [Google Scholar] [CrossRef]
- Rauwerdink, A.M.; Hansen, E.W.; Weaver, J.B. Nanoparticle temperature estimation in combined ac and dc magnetic fields. Phys. Med. Biol. 2009, 54, L51–L55. [Google Scholar] [CrossRef] [Green Version]
- Weaver, J.B.; Rauwerdink, A.M.; Hansen, E.W. Magnetic nanoparticle temperature estimation. Med. Phys. 2009, 36, 1822–1829. [Google Scholar] [CrossRef] [Green Version]
- Zhong, J.; Liu, W.; Du, Z.; César de Morais, P.; Xiang, Q.; Xie, Q. A noninvasive, remote and precise method for temperature and concentration estimation using magnetic nanoparticles. Nanotechnology 2012, 23, 075703. [Google Scholar] [CrossRef]
- Akin, Y.; Obaidat, I.M.; Issa, B.; Haik, Y. Ni1-x Crx alloy for self controlled magnetic hyperthermia. Cryst. Res. Technol. 2009, 44, 386–390. [Google Scholar] [CrossRef]
- Zhong, J.; Dieckhoff, J.; Schilling, M.; Ludwig, F. Influence of static magnetic field strength on the temperature resolution of a magnetic nanoparticle thermometer. J. Appl. Phys. 2016, 120, 143902. [Google Scholar] [CrossRef]
- Garaio, E.; Collantes, J.-M.; Garcia, J.A.; Plazaola, F.; Sandre, O. Harmonic phases of the nanoparticle magnetization: An intrinsic temperature probe. Appl. Phys. Lett. 2015, 107, 123103. [Google Scholar] [CrossRef] [Green Version]
- Garaio, E.; Collantes, J.M.; Plazaola, F.; Garcia, J.A.; Castellanos-Rubio, I. A multifrequency eletromagnetic applicator with an integrated AC magnetometer for magnetic hyperthermia experiments. Meas. Sci. Technol. 2014, 25, 115702. [Google Scholar] [CrossRef]
- Connord, V.; Mehdaoui, B.; Tan, R.P.; Carrey, J.; Respaud, M. An air-cooled Litz wire coil for measuring the high frequency hysteresis loops of magnetic samples--a useful setup for magnetic hyperthermia applications. Rev. Sci. Instrum. 2014, 85, 093904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garaio, E.; Sandre, O.; Collantes, J.-M.; Garcia, J.A.; Mornet, S.; Plazaola, F. Specific absorption rate dependence on temperature in magnetic field hyperthermia measured by dynamic hysteresis losses (ac magnetometry). Nanotechnology 2015, 26, 015704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharm. 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, L.; Liu, X.L.; Di Fan, D.; Miao, Y.Q.; Zhang, H.; Ma, H.P.; Liu, Q.Y.; Ma, P.; Xue, W.M.; Luo, Y.E.; et al. The efficiency of magnetic hyperthermia and in vivo histocompatibility for human-like collagen protein-coated magnetic nanoparticles. Int. J. Nanomed. 2016, 11, 1175–1185. [Google Scholar]
- Dutz, S.; Hergt, R.; Mürbe, J.; Töpfer, J.; Müller, R.; Zeisberger, M.; Andrä, W.; Bellemann, M.E. Magnetic Nanoparticles for Biomedical Heating Applications. Z. Phys. Chem. 2006, 220, 145–151. [Google Scholar] [CrossRef]
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.; Cao, Y.; Nogami, M. Biomedical Applications of Advanced Multifunctional Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 10091–10107. [Google Scholar] [CrossRef]
- Kim, D.; Shin, K.; Kwon, S.; Hyeon, T. Synthesis and Biomedical Applications of Multifunctional Nanoparticles. Adv. Mater. 2018, 30, 1802309. [Google Scholar] [CrossRef]
- Abenojar, E.C.; Wickramasinghe, S.; Bas-Concepcion, J.; Samia, A.C.S. Structural effects on the magnetic hyperthermia properties of iron oxide nanoparticles. Prog. Nat. Sci. Mater. Int. 2016, 26, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.; Kim, Y.-K.; Shin, D.; Ryoo, S.-R.; Hong, B.H.; Min, D.-H. Biomedical Applications of Graphene and Graphene Oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef]
- Dembereldorj, U.; Kim, M.; Kim, S.; Ganbold, E.-O.; Lee, S.Y.; Joo, S.-W. A spatiotemporal anticancer drug release platform of PEGylated graphene oxide triggered by glutathione in vitro and in vivo. J. Mater. Chem. 2012, 22, 23845–23851. [Google Scholar] [CrossRef]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Fan, H.M.; Yi, J.B.; Yang, Y.; Choo, E.S.G.; Xue, J.M.; Fan, D.D.; Ding, J. Optimization of surface coating on Fe3O4 nanoparticles for high performance magnetic hyperthermia agents. J. Mater. Chem. 2012, 22, 8235–8244. [Google Scholar] [CrossRef]
- Venkatesha, N.; Qurishi, Y.; Atreya, H.S.; Srivastava, C. ZnO coated CoFe2O4 nanoparticles for multimodal bio-imaging. RSC Adv. 2016, 6, 18843–18851. [Google Scholar] [CrossRef]
- Sundar, S.; Chen, Y.; Tong, Y.W. Delivery of therapeutics and molecules using self-assembled peptides. Curr. Med. Chem. 2014, 21, 2469–2479. [Google Scholar] [CrossRef]
- Mazzotta, E.; Tavano, L.; Muzzalupo, R. Thermo-Sensitive Vesicles in Controlled Drug Delivery for Chemotherapy. Pharmaceutics 2018, 10, 150. [Google Scholar] [CrossRef] [Green Version]
- Bi, H.; Xue, J.; Jiang, H.; Gao, S.; Yang, D.; Fang, Y.; Shi, K. Current developments in drug delivery with thermosensitive liposomes. Asian J. Pharm. Sci. 2019, 14, 365–379. [Google Scholar] [CrossRef]
- Yadav, H.K.; Halabi, N.A.A.; Alsalloum, G.A. Nanogels as Novel Drug Delivery Systems—A Review. J. Pharm. Pharm. Res. 2017, 1, 1–8. [Google Scholar]
- Blackburn, W.H.; Dickerson, E.B.; Smith, M.H.; McDonald, J.F.; Lyon, L.A. Peptide-Functionalized Nanogels for Targeted siRNA Delivery. Bioconjug. Chem. 2009, 20, 960–968. [Google Scholar] [CrossRef] [Green Version]
- Cazares-Cortes, E.; Espinosa, A.; Guigner, J.-M.; Michel, A.; Griffete, N.; Wilhelm, C.; Ménager, C. Doxorubicin Intracellular Remote Release from Biocompatible Oligo(ethylene glycol) Methyl Ether Methacrylate-Based Magnetic Nanogels Triggered by Magnetic Hyperthermia. ACS Appl. Mater. Interfaces 2017, 9, 25775–25788. [Google Scholar] [CrossRef] [Green Version]
- Cazares-Cortes, E.; Nerantzaki, M.; Fresnais, J.; Wilhelm, C.; Griffete, N.; Ménager, C. Magnetic Nanoparticles Create Hot Spots in Polymer Matrix for Controlled Drug Release. Nanomaterials 2018, 8, 850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eslami, P.; Rossi, F.; Fedeli, S. Hybrid Nanogels: Stealth and Biocompatible Structures for Drug Delivery Applications. Pharmaceutics 2019, 11, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Bharti, C.; Nagaich, U.; Pal, A.K.; Gulati, N. Mesoporous silica nanoparticles in target drug delivery system: A review. Int. J. Pharm. Investig. 2015, 5, 124–133. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomedicine 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Thomas, C.R.; Ferris, D.P.; Lee, J.-H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.-S.; Cheon, J.; Zink, J.I. Noninvasive Remote-Controlled Release of Drug Molecules in Vitro Using Magnetic Actuation of Mechanized Nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef]
- Coll, C.; Mondragón, L.; Martínez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Soto, J.; Amorós, P.; Pérez-Payá, E. Enzyme-mediated controlled release systems by anchoring peptide sequences on mesoporous silica supports. Angew. Chem. Int. Ed. Engl. 2011, 50, 2138–2140. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Kim, S.; Lee, H.; Kim, J.; Kim, N.; Park, H.J.; Choi, E.K.; Lee, J.S.; Kim, C. A multifunctional mesoporous nanocontainer with an iron oxide core and a cyclodextrin gatekeeper for an efficient theranostic platform. J. Mater. Chem. 2012, 22, 14061–14067. [Google Scholar] [CrossRef]
- Guisasola, E.; Asín, L.; Beola, L.; de la Fuente, J.M.; Baeza, A.; Vallet-Regí, M. Beyond Traditional Hyperthermia: In Vivo Cancer Treatment with Magnetic-Responsive Mesoporous Silica Nanocarriers. ACS Appl. Mater. Interfaces 2018, 10, 12518–12525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Yang, K.; Li, Y.; Zeng, X.; Shao, M.; Lee, S.-T.; Liu, Z. Multifunctional nanoparticles for upconversion luminescence/MR multimodal imaging and magnetically targeted photothermal therapy. Biomaterials 2012, 33, 2215–2222. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Yang, J.; Seo, S.; Kim, K.; Suh, J.; Kim, D.; Haam, S.; Yoo, K.-H. Multifunctional nanoparticles for photothermally controlled drug delivery and magnetic resonance imaging enhancement. Small 2008, 4, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tao, H.; Yang, K.; Feng, L.; Cheng, L.; Shi, X.; Li, Y.; Guo, L.; Liu, Z. A functionalized graphene oxide-iron oxide nanocomposite for magnetically targeted drug delivery, photothermal therapy, and magnetic resonance imaging. Nano Res. 2012, 5, 199–212. [Google Scholar] [CrossRef]
- Yao, X.; Niu, X.; Ma, K.; Huang, P.; Grothe, J.; Kaskel, S.; Zhu, Y. Graphene Quantum Dots-Capped Magnetic Mesoporous Silica Nanoparticles as a Multifunctional Platform for Controlled Drug Delivery, Magnetic Hyperthermia, and Photothermal Therapy. Small 2017, 13, 1602225. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, X.; Tong, S.; Bao, G.; Ren, Q.; Dai, Z. Gold Nanoshell Nanomicelles for Potential Magnetic Resonance Imaging, Light-Triggered Drug Release, and Photothermal Therapy. Adv. Funct. Mater. 2013, 23, 815–822. [Google Scholar] [CrossRef]
- Fu, S.; Ding, Y.; Cong, T.; Yang, X.; Hong, X.; Yu, B.; Li, Y.; Liu, Y. Multifunctional NaYF4:Yb,Er@PE3@Fe3O4 nanocomposites for magnetic-field-assisted upconversion imaging guided photothermal therapy of cancer cells. Dalton Trans. 2019, 48, 12850–12857. [Google Scholar] [CrossRef]
- Buzug, T.M.; Bringout, G.; Erbe, M.; Gräfe, K.; Graeser, M.; Grüttner, M.; Halkola, A.; Sattel, T.F.; Tenner, W.; Wojtczyk, H.; et al. Magnetic particle imaging: Introduction to imaging and hardware realization. Z Med. Phys. 2012, 22, 323–334. [Google Scholar] [CrossRef]
- Lim, J.-W.; Son, S.U.; Lim, E.-K. Recent Advances in Bioimaging for Cancer Research. State of the Art Nano-Bioimaging; Ghamsari, M.S., Ed.; IntechOpen: London, UK, 2018; pp. 11–33. [Google Scholar]
- Wu, L.C.; Zhang, Y.; Steinberg, G.; Qu, H.; Huang, S.; Cheng, M.; Bliss, T.; Du, F.; Rao, J.; Song, G.; et al. A Review of Magnetic Particle Imaging and Perspectives on Neuroimaging. Am. J. Neuroradiol. 2019, 40, 206–212. [Google Scholar] [CrossRef]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef] [PubMed]
- Tay, Z.W.; Chandrasekharan, P.; Chiu-Lam, A.; Hensley, D.W.; Dhavalikar, R.; Zhou, X.Y.; Yu, E.Y.; Goodwill, P.W.; Zheng, B.; Rinaldi, C.; et al. Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699–3713. [Google Scholar] [CrossRef] [PubMed]
- Basly, B.; Popa, G.; Fleutot, S.; Pichon, B.P.; Garofalo, A.; Ghobril, C.; Billotey, C.; Berniard, A.; Bonazza, P.; Martinez, H.; et al. Effect of the nanoparticle synthesis method on dendronized iron oxides as MRI contrast agents. Dalton Trans. 2013, 42, 2146–2157. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and characterization of biocompatible Fe3O4 nanoparticles. J. Biomed. Mater. Res. A 2007, 80, 333–341. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; Han, S.; Li, C.; Lei, B.; Lu, W.; Fang, J.; Zhou, C. Single Crystalline Magnetite Nanotubes. J. Am. Chem. Soc. 2005, 127, 6–7. [Google Scholar] [CrossRef]
- Zhong, Z.; Lin, M.; Ng, V.; Ng, G.X.B.; Foo, Y.; Gedanken, A. A Versatile Wet-Chemical Method for Synthesis of One-Dimensional Ferric and Other Transition Metal Oxides. Chem. Mater. 2006, 18, 6031–6036. [Google Scholar] [CrossRef]
- Jiao, F.; Jumas, J.-C.; Womes, M.; Chadwick, A.V.; Harrison, A.; Bruce, P.G. Synthesis of Ordered Mesoporous Fe3O4 and γ-Fe2O3 with Crystalline Walls Using Post-Template Reduction/Oxidation. J. Am. Chem. Soc. 2006, 128, 12905–12909. [Google Scholar] [CrossRef] [Green Version]
- Du, N.; Xu, Y.; Zhang, H.; Zhai, C.; Yang, D. Selective Synthesis of Fe2O3 and Fe3O4 Nanowires Via a Single Precursor: A General Method for Metal Oxide Nanowires. Nanoscale Res. Lett. 2010, 5, 1295–1300. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wang, C.; Dou, W.; Ma, Q.; Yuan, P.; Su, X. The synthesis of magnetic and fluorescent bi-functional silica composite nanoparticles via reverse microemulsion method. J. Fluoresc. 2009, 19, 939–946. [Google Scholar] [CrossRef]
- Jin, J.; Ohkoshi, S.; Hashimoto, K. Giant Coercive Field of Nanometer- Sized Iron Oxide. Adv. Mater. 2004, 16, 48–51. [Google Scholar] [CrossRef]
- Han, Y.C.; Cha, H.G.; Kim, C.W.; Kim, Y.H.; Kang, Y.S. Synthesis of Highly Magnetized Iron Nanoparticles by a Solventless Thermal Decomposition Method. J. Phys. Chem. C 2007, 111, 6275–6280. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Kang, M. Synthesis of Fe/TiO2 photocatalyst with nanometer size by solvothermal method and the effect of H2O addition on structural stability and photodecomposition of methanol. J. Mol. Catal. A Chem. 2003, 197, 173–183. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Teja, A.; Koh, P.Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Guo, Q.; Teng, X.; Rahman, S.; Yang, H. Patterned Langmuir−Blodgett Films of Monodisperse Nanoparticles of Iron Oxide Using Soft Lithography. J. Am. Chem. Soc. 2003, 125, 630–631. [Google Scholar] [CrossRef]
- Taniguchi, I. Powder properties of partially substituted LiMxMn2−xO4 (M=Al, Cr, Fe and Co) synthesized by ultrasonic spray pyrolysis. Mater. Chem. Phys. 2005, 92, 172–179. [Google Scholar] [CrossRef]
- Dosev, D.; Nichkova, M.; Dumas, R.K.; Gee, S.J.; Hammock, B.D.; Liu, K.; Kennedy, I.M. Magnetic/luminescent core/shell particles synthesized by spray pyrolysis and their application in immunoassays with internal standard. Nanotechnology 2007, 18, 055102. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-Assembly of Metal Oxides into Three-Dimensional Nanostructures: Synthesis and Application in Catalysis. ACS Nano 2009, 3, 728–736. [Google Scholar] [CrossRef]
- Jia, C.-J.; Sun, L.-D.; Luo, F.; Han, X.-D.; Heyderman, L.J.; Yan, Z.-G.; Yan, C.-H.; Zheng, K.; Zhang, Z.; Takano, M.; et al. Large-Scale Synthesis of Single-Crystalline Iron Oxide Magnetic Nanorings. J. Am. Chem. Soc. 2008, 130, 16968–16977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wei, L.; Gao, M.Y.; Lei, H. One-Pot Reaction to Synthesize Biocompatible Magnetite Nanoparticles. Adv. Mater. 2005, 17, 1001–1005. [Google Scholar] [CrossRef]
- Jana, N.R.; Chen, Y.; Peng, X. Size- and Shape-Controlled Magnetic (Cr, Mn, Fe, Co, Ni) Oxide Nanocrystals via a Simple and General Approach. Chem. Mater. 2004, 16, 3931–3935. [Google Scholar] [CrossRef]
- Rockenberger, J.; Scher, E.C.; Alivisatos, A.P. A New Nonhydrolytic Single-Precursor Approach to Surfactant-Capped Nanocrystals of Transition Metal Oxides. J. Am. Chem. Soc. 1999, 121, 11595–11596. [Google Scholar] [CrossRef]
- Zeng, H.; Li, J.; Liu, J.P.; Wang, Z.L.; Sun, S. Exchange-coupled nanocomposite magnets by nanoparticle self-assembly. Nature 2002, 420, 395–398. [Google Scholar] [CrossRef]
- Salazar-Alvarez, G.; Qin, J.; Šepelák, V.; Bergmann, I.; Vasilakaki, M.; Trohidou, K.N.; Ardisson, J.D.; Macedo, W.A.A.; Mikhaylova, M.; Muhammed, M.; et al. Cubic versus Spherical Magnetic Nanoparticles: The Role of Surface Anisotropy. J. Am. Chem. Soc. 2008, 130, 13234–13239. [Google Scholar] [CrossRef]
- Hayashi, H.; Hakuta, Y. Hydrothermal Synthesis of Metal Oxide Nanoparticles in Supercritical Water. Materials 2010, 3, 3794–3817. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Yu, X. Hydrothermal Synthesis and Photocatalytic Activity of Zinc Oxide Hollow Spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef]
- Yang, T.; Li, Y.; Zhu, M.; Huang, J.; Jin, H.; Hu, Y. Room-temperature ferromagnetic Mn-doped ZnO nanocrystal synthesized by hydrothermal method under high magnetic field. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2010, 170, 129–132. [Google Scholar] [CrossRef]
- Hu, J.; Bando, Y. Growth and Optical Properties of Single-Crystal Tubular ZnO Whiskers. Appl. Phys. Lett. 2003, 82, 1401–1403. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) Nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hao, R.; Liu, F.; Su, X.; Hou, Y. Single-crystalline α-Fe2O3 nanostructures: Controlled synthesis and high-index plane-enhanced photodegradation by visible light. J. Mater. Chem. A 2013, 1, 6888–6894. [Google Scholar] [CrossRef]
- Zeng, Y.; Hao, R.; Xing, B.; Hou, Y.; Xu, Z. One-pot synthesis of Fe3O4 nanoprisms with controlled electrochemical properties. Chem. Commun. 2010, 46, 3920–3922. [Google Scholar] [CrossRef] [PubMed]
- Weiwei, W. Microwave-induced polyol-process synthesis of MIIFe2O4 (M = Mn, Co) nanoparticles and magnetic property. Mater. Chem. Phys. 2008, 108, 227–231. [Google Scholar]
- Wang, W.-W.; Zhu, Y.-J.; Ruan, M.-L. Microwave-assisted synthesis and magnetic property of magnetite and hematite nanoparticles. J. Nanopart. Res. 2007, 9, 419–426. [Google Scholar] [CrossRef]
- Sander, D.; Oka, H.; Corbetta, M.; Stepanyuk, V.; Kirschner, J. New insights into nano-magnetism by spin-polarized scanning tunneling microscopy. J. Electron. Spectrosc. Relat. Phenom. 2013, 189, 206–215. [Google Scholar] [CrossRef]
- Hurst, S.J.; Payne, E.K.; Qin, L.; Mirkin, C.A. Multisegmented one-dimensional nanorods prepared by hard-template synthetic methods. Angew. Chem. Int. Ed. Engl. 2006, 45, 2672–2692. [Google Scholar] [CrossRef]
- Lam, T.; Pouliot, P.; Avti, P.K.; Lesage, F.; Kakkar, A.K. Superparamagnetic iron oxide based nanoprobes for imaging and theranostics. Adv. Colloid Interface Sci. 2013, 199–200, 95–113. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Ren, W. Structure switch between alpha-Fe2O3, gamma-Fe2O3 and Fe3O4 during the large scale and low temperature sol-gel synthesis of nearly monodispersed iron oxide nanoparticles. Adv. Powder Technol. 2013, 24, 93–97. [Google Scholar] [CrossRef]
- Xu, J.; Yang, H.; Fu, W.; Du, K.; Sui, Y.; Chen, J.; Yi, Z.; Li, M.; Zou, G. Preparation and Magnetic Properties of Magnetite Nanoparticles by Sol–Gel Method. J. Magn. Magn. Mater. 2010, 309, 307–311. [Google Scholar] [CrossRef]
- Bilecka, I.; Elser, P.; Niederberger, M. Kinetic and Thermodynamic Aspects in the Microwave-Assisted Synthesis of ZnO Nanoparticles in Benzyl Alcohol. ACS Nano 2009, 3, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Bilecka, I.; Kubli, M.; Amstad, E.; Niederberger, M. Simultaneous formation of ferrite nanocrystals and deposition of thin films via a microwave-assisted nonaqueous sol–gel process. J. Sol. Gel Sci. Technol. 2011, 57, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Venkatesha, N.; Poojar, P.; Qurishi, Y.; Geethanath, S.; Srivastava, C. Graphene oxide-Fe3O4 nanoparticle composite with high transverse proton relaxivity value for magnetic resonance imaging. J. Appl. Phys. 2015, 117, 154702. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Maitra, S.S. In vitro and in vivo toxicity assessment of nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef] [Green Version]
- Khalili Fard, J.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef]
- Demirer, G.S.; Okur, A.C.; Kizilel, S. Synthesis and design of biologically inspired biocompatible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. B 2015, 3, 7831–7849. [Google Scholar] [CrossRef] [Green Version]
- Zahraei, M.; Marciello, M.; Lazaro-Carrillo, A.; Villanueva, A.; Herranz, F.; Talelli, M.; Costo, R.; Monshi, A.; Shahbazi-Gahrouei, D.; Amirnasr, M.; et al. Versatile theranostics agents designed by coating ferrite nanoparticles with biocompatible polymers. Nanotechnology 2016, 27, 255702. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages. In Genotoxicity A Predictable Risk to Our Actual World; Larramendy, M., Ed.; IntechOpen: London, UK, 2018; pp. 1–17. [Google Scholar]
- Pasukonienė, V.; Mlynska, A.; Steponkienė, S.; Poderys, V.; Matulionytė, M.; Karabanovas, V.; Statkutė, U.; Purvinienė, R.; Kraśko, J.A.; Jagminas, A.; et al. Accumulation and biological effects of cobalt ferrite nanoparticles in human pancreatic and ovarian cancer cells. Medicina 2014, 50, 237–244. [Google Scholar] [CrossRef]





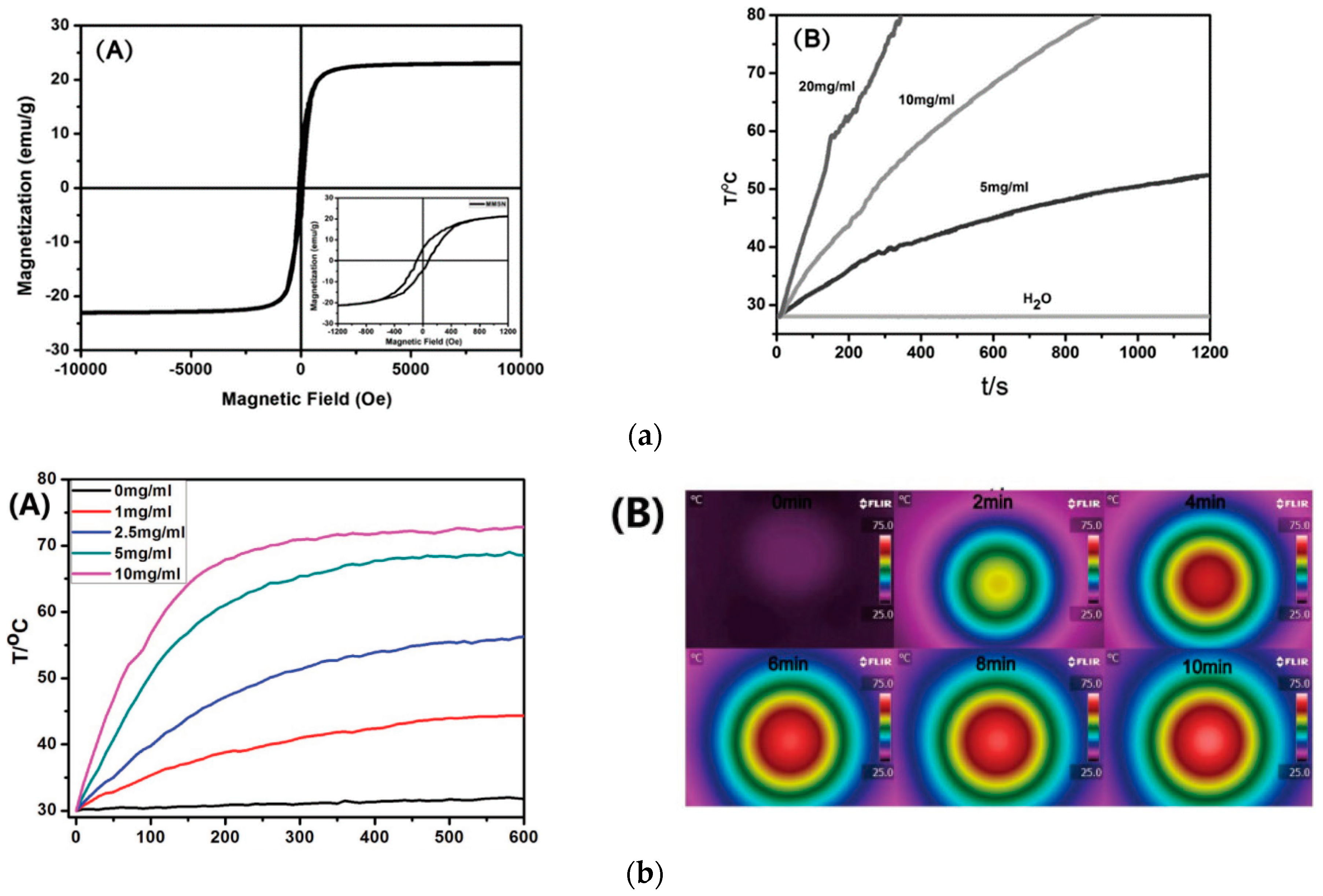











| Shape & Size (nm) | Material | Coating | (kA/m) Field | Frequency (kHz) | (W/g) SAR | Reference |
|---|---|---|---|---|---|---|
| Octahedral-43 | Fe3O4 | CTAB | 63 | 358 | 2483 | [102] |
| Rings-73 | Fe3O4 | mPEG | 35 | 400 | 2213 | [103] |
| Disc-225 | Fe3O4 | CTAB | 47.8 | 488 | 5000 | [104] |
| Cubes-19 | Fe3O4 | PEG | 29 | 520 | 2452 | [48] |
| Sphere-14 | MnFe2O4 | GO | 60 | 240 | 1588 | [105] |
| Core–shell | CoFe2O4 @MnFe2O4 | DMSA | 37.3 | 500 | 2250 | [41] |
| Nanoclusters-33 | Fe3O4 | PMA | 23.8 | 302 | 253 | [106] |
| Sphere-45 | Fe3O4 | GO | 32.5 | 400 | 5160 | [107] |
| Sphere-45 | Fe3O4 | PVP | 32.5 | 400 | 1100 | [107] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obaidat, I.M.; Narayanaswamy, V.; Alaabed, S.; Sambasivam, S.; Muralee Gopi, C.V.V. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry 2019, 5, 67. https://doi.org/10.3390/magnetochemistry5040067
Obaidat IM, Narayanaswamy V, Alaabed S, Sambasivam S, Muralee Gopi CVV. Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry. 2019; 5(4):67. https://doi.org/10.3390/magnetochemistry5040067
Chicago/Turabian StyleObaidat, Ihab M., Venkatesha Narayanaswamy, Sulaiman Alaabed, Sangaraju Sambasivam, and Chandu V. V. Muralee Gopi. 2019. "Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles" Magnetochemistry 5, no. 4: 67. https://doi.org/10.3390/magnetochemistry5040067
APA StyleObaidat, I. M., Narayanaswamy, V., Alaabed, S., Sambasivam, S., & Muralee Gopi, C. V. V. (2019). Principles of Magnetic Hyperthermia: A Focus on Using Multifunctional Hybrid Magnetic Nanoparticles. Magnetochemistry, 5(4), 67. https://doi.org/10.3390/magnetochemistry5040067





