Abstract
The effects of substitution of Zr and Ga on the structural and magnetic properties of Dy2Fe17 intermetallic compound were investigated in this study. The Rietveld analysis confirmed that the crystalline system was a Th2Ni17 structure. Lattice parameters a (Å) and c (Å), unit cell volume (Å3), and bonding distance (Å) were calculated using Rietveld analysis. The unit cell volume of Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx increased linearly with Zr and Ga substitution. The Curie temperature (Tc) of Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx was found to be Zr content-dependent. The maximum Curie temperatures were observed at 510 K (x = 0.75 Zr content) for Dy2Fe17−xZrx and 505.1 K (x = 0.5 Zr content) for Dy2Fe16Ga1−xZrx, which are 102 K and 97 K higher than the value found for Dy2Fe17, respectively. The room-temperature Mössbauer analysis showed a decrease in the average hyperfine field and increases in the isomer shift with Zr doping. The overall improvement in Curie temperature with the substitution strategy of Zr–Ga substitution in 2:17 intermetallic compounds could find potential use of these magnetic compounds in high-temperature applications.
1. Introduction
Intermetallic compounds (rare-earth elements (R) and 3d-transition elements (T)) possess outstanding magnetic properties because of their high saturation magnetization (Ms). R2Fe17 compounds were studied in late the 1970s, and the values of (BH)max and coercivity (Hc) were found to be about 26 MGOe and 15 kOe, respectively; however, they have low Curie temperature (~473 K for Gd2Fe17 and ~300 K for Dy2Fe17) and low magnetic anisotropies [1]. Many studies were done to improve the Curie temperatures of Dy2Fe17 [2,3,4] either by replacing the Fe atoms with non-magnetic atoms (Al, Si, Ga) [5] or by doping refractory atoms (Ti, V, Mo, Nb, W, Zr) in the Fe site [6,7,8,9,10]. It was also found that substitution of magnetic atoms (Co, Ni, Cr, Mn, Ni) [11] and non-metals (C, N, H) in the R2Fe17 lattice also increased the Curie temperature of R2Fe17 compounds [12,13,14,15]. The substitution of non-magnetic atoms at Fe sites was reported to increase ferromagnetic coupling, which in turn increases the Curie temperature [16,17] and magneto-crystalline anisotropy [18]. The introduction of nitrogen on interstitial sites of R2Fe17Nx also increased the Curie temperature. This happens because the unit cell volume expansion increases the distance between iron atoms with a greater degree of exchange interaction [19,20]. Betancourt et al. (2003) [21] reported that Zr and Nb substitution could also improve magnetic properties. The Curie temperatures of Ce2Fe17 [22], Gd2Fe17 [5] Dy2Fe17 [8], Pr2Fe17 [23], etc. are improved by the addition of elements like Si, Cr, Mn, and Ga. The substitution of a non-magnetic atom such as Ga into the R2Fe17 brings a concomitant decrease in magnetization, which affects the energy product of the permanent magnets [18]. Furthermore, it is possible to increase the magnetic moment via Fe–Zr 3d band hybridization, which can cause band narrowing or increase exchange splitting by moving the 3d↑ states below the Fermi level, or which can allow charge transfer out of the 3d band, provided the spin-down density of states exceeds the spin-up density [24].
The present work focuses on substituting the Fe atom with elements Zr and Ga in Dy2Fe17. The substitution of non-magnetic atoms was limited to up to x = 1 to prevent a significant reduction in saturation magnetization. Doping of Zr and Ga atoms can increase the lattice parameters a and c, as well as the unit cell volume, and improve the Fe–Fe exchange interaction. The dysprosium element was chosen as it has a high Bohr magneton value (10.63 μB), which brings enhancement in the magnetic properties of the intermetallic compound.
2. Experimental
The raw materials of Dy, Fe, Zr, and Ga were used with ~99.9% purity and purchased from Sigma Aldrich. The samples Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx (x = 0.00, 0.25, 0.50, 0.75, 1.00) were prepared via arc melting in a high-purity argon atmosphere. The ingots were melted several times to ensure homogeneity. The prepared alloys were made into fine powders, and the X-ray powder datasets were collected using a Bruker (D8 Advance) diffractometer with a monochromatic incident beam of CuKα (λ ≈ 1.5406 Å) radiation with the 2θ range from 20° to 70° with a step size of 0.042°. The XRD analysis was performed using the well-known refinement Rietveld method [25] with the JANA2006 [26] software package. The purpose of this refinement process is to minimize the difference between the theoretically modeled profile and the observed one as a function, where Whkl is the weight assigned to each observation, and FO and FC are the observed and calculated structure factors.
A vibrating sample magnetometer (VSM) with a maximum field of 1.2 T was used to investigate the magnetic properties of powder samples at room temperature (RT). The samples were compacted at 3000 psi and cut into rectangular parallelepipeds with a ratio of length to width larger than three, and they were embedded in epoxy in order to minimize the effect of the demagnetizing field.
The Mössbauer spectra of the samples were obtained at RT using a 25-mCi 57Co source in Rh foil mounted on a constant acceleration drive system (SEE Co. Minneapolis, USA) in transmission geometry. The velocity scale of the Mössbauer spectrometer was calibrated by measuring the hyperfine field of α-Fe foil, at room temperature. The Mössbauer spectra were analyzed using the WMoss software from SEE Co. The spectra were least-square fitted with the hyperfine field (HF), isomer shift (IS), and quadrupole shift (QS) parameters as variables.
3. Results and Discussion
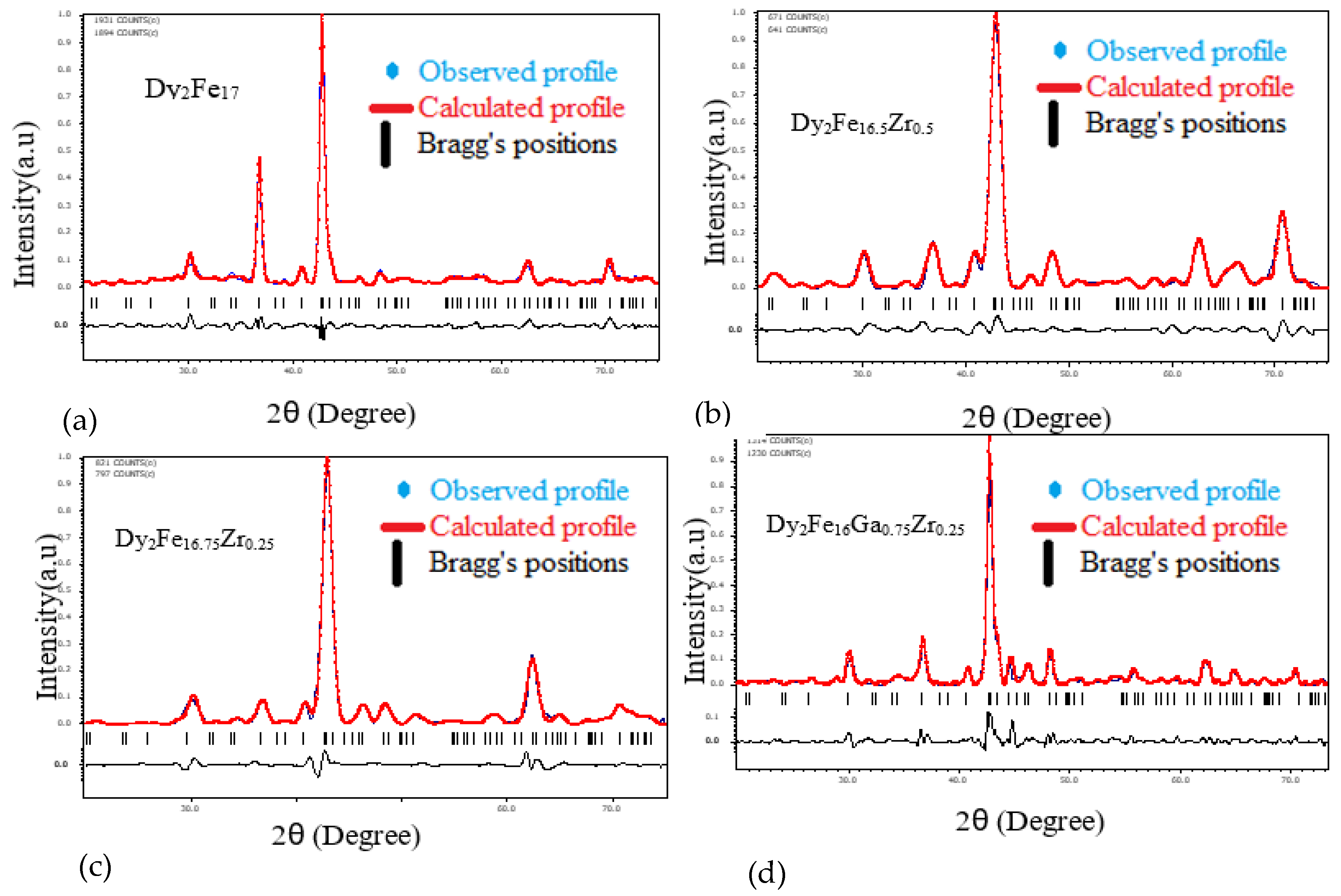
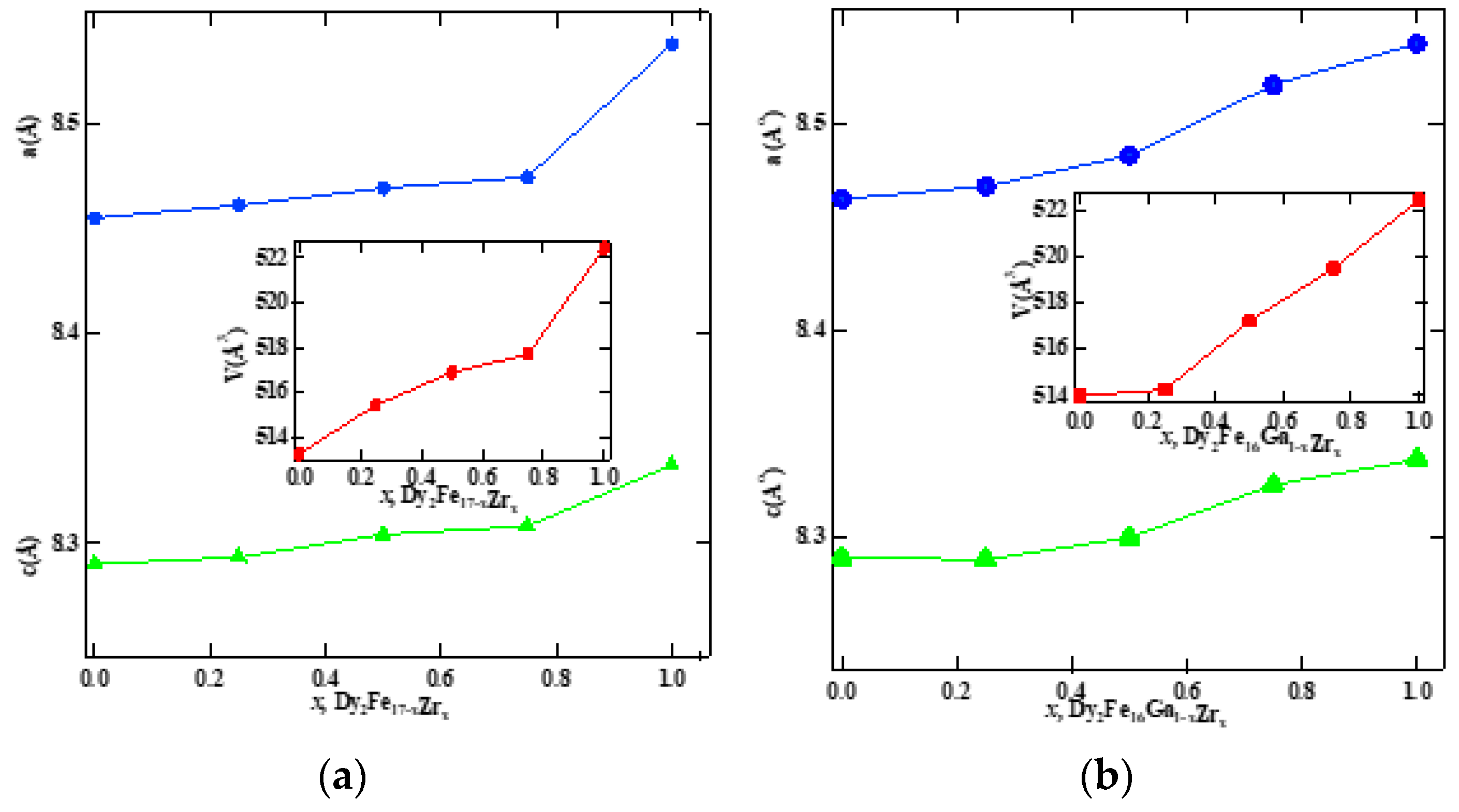
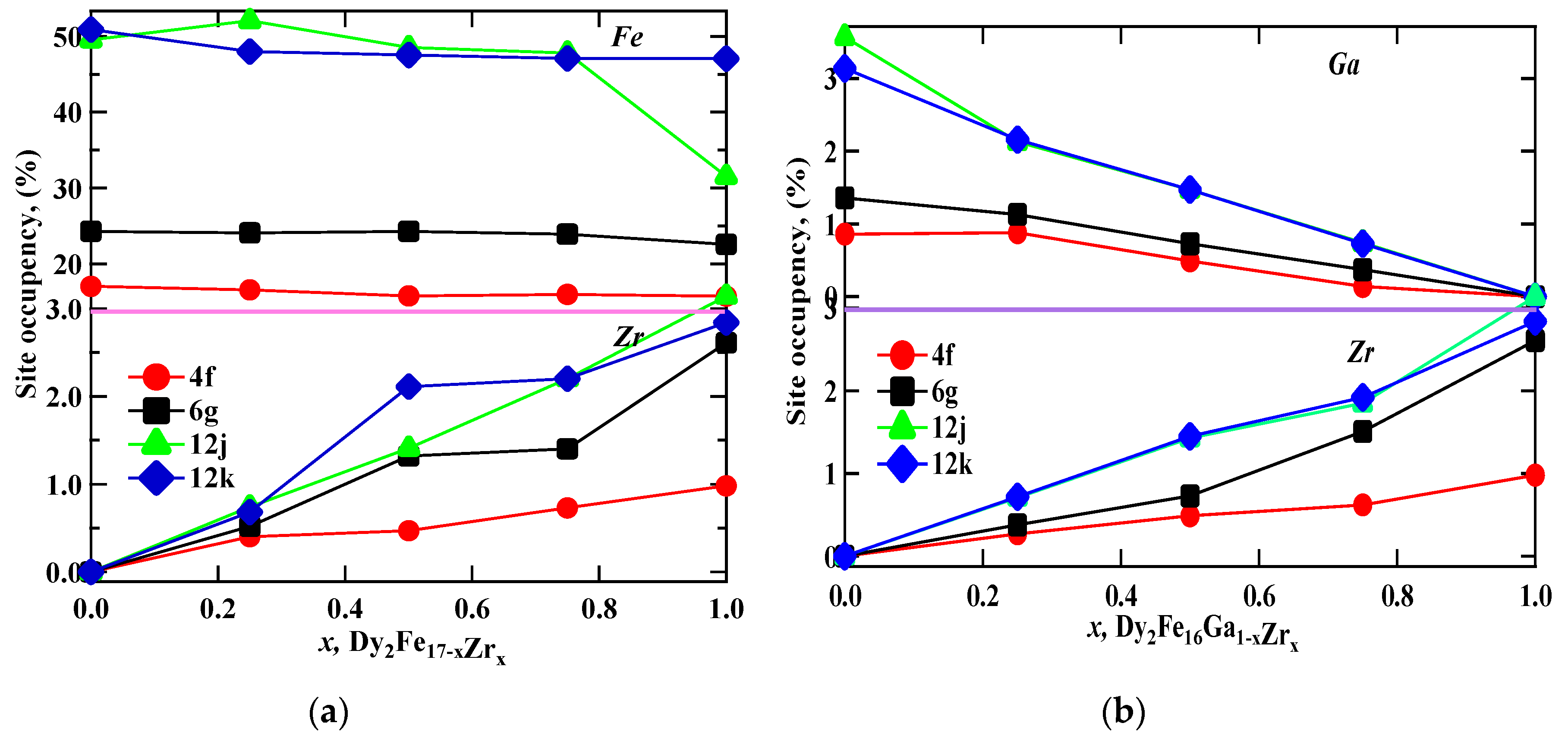
Figure 1 represent the representative Rietveld refinement profiles of Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx (x = 0.00, 0.25, 0.50, 0.75, 1.00). The Rietveld [25] fittings reveal a nice matching of the observed and calculated profiles for both systems. A small additional phase DyFe3 was observed for x = 1 Zr content during refinement. During the refinement process, the scale, structural parameters, lattice parameters, peak shift, preferred orientation, background profile functions, thermal parameters, and surface roughness were refined until the observed XRD profile matched well with the calculated profile. The initial crystal structure parameters were used as given by Liao et al. [27]. In the hexagonal setting, Dy was fixed at the 2b (0, 0, 0.25) and 2d (0.333, 0.667, 0.75) sites, and Fe was fixed at the 4f (0.333, 0.667, 0.105), 6g (0.5, 0, 0), 12j (0.333, 0.969, 0.25), and 12k (0.167, 0.333, 0.985) sites. The profile was constructed using a pseudo-Voigt function. Profile asymmetry was introduced by employing the multi-term Simpson rule integration devised by Howard [28]. A surface roughness correction was also applied using the Pitschke, Hermann, and Matter [29] model. Table 1 shows the refined structural parameters for both Dy2Fe16−xZrx and Dy2Fe16Ga1−xZrx. From Table 1, the refined results confirmed that the unit cell volume (Å3) increased with x-content. The plots for lattice parameters a and c and unit cell volume (V) are shown in Figure 2a,b. This increase in volume may have occurred because of a bigger atom Ga (rionic = 0.62(3) Å) and Zr (rionic = 0.84(3) Å) [30] sitting at the smaller iron (rionic = 0.55(3) Å) atom site. The increasing lattice parameters confirmed that both Zr and Ga addition to the host lattice was done properly. Table 2 represents the site occupancy table for Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx, which shows that the Zr atom occupied all 4f, 6g, 12j, and 12k sites, but the 12j and 12k sites were mostly impacted by the Zr substitution (Figure 3a). The site occupancies of Zr and Ga for Dy2Fe16Ga1−xZrx are plotted in Figure 3b, and the values are presented in Table 2. From these results, both Zr and Ga atoms prefer to be in 12j and 12k sites with a minimum affinity for 4f and 6g sites [5,31], and the increase in Zr occupancy and the decrease in Ga occupancy at 12j and 12k site show that the Zr atom was replaced by the Ga atom in Dy2Fe16Ga1−xZrx.
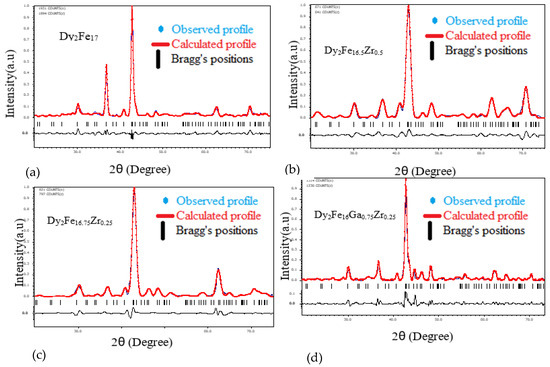
Figure 1.
Rietveld refinement profile for (a) Dy2Fe17, (b) Dy2Fe16.5Zr0.5, (c) Dy2Fe16.25Zr0.75 and (d) Dy2Fe16Ga0. Zr0.25.

Table 1.
Lattice parameters and unit cell volume of Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx obtained from Rietveld refinements.
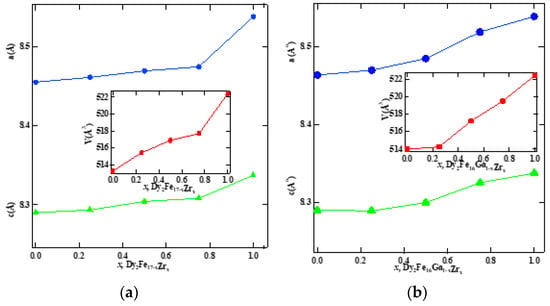
Figure 2.
Lattice parameters a and c and unit cell volume of (a) Dy2Fe17−xZrx and (b) Dy2Fe16Ga1−xZrx as a function of Zr content.

Table 2.
The site occupancy table for Dy2Fe17−xZrx, and Dy2Fe16Ga1−xZrx.
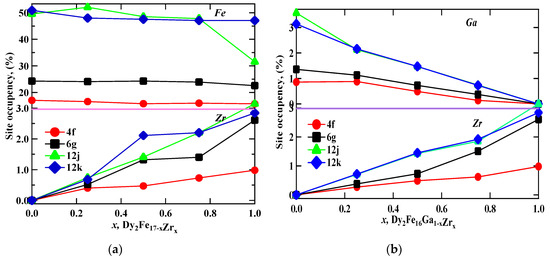
Figure 3.
The percentage occupancy of (a) Fe and Zr at iron sites in Dy2Fe17−xZrx, and (b) Fe and Zr at Ga sites in Dy2Fe16Ga1−xZrx as a function of Zr content.
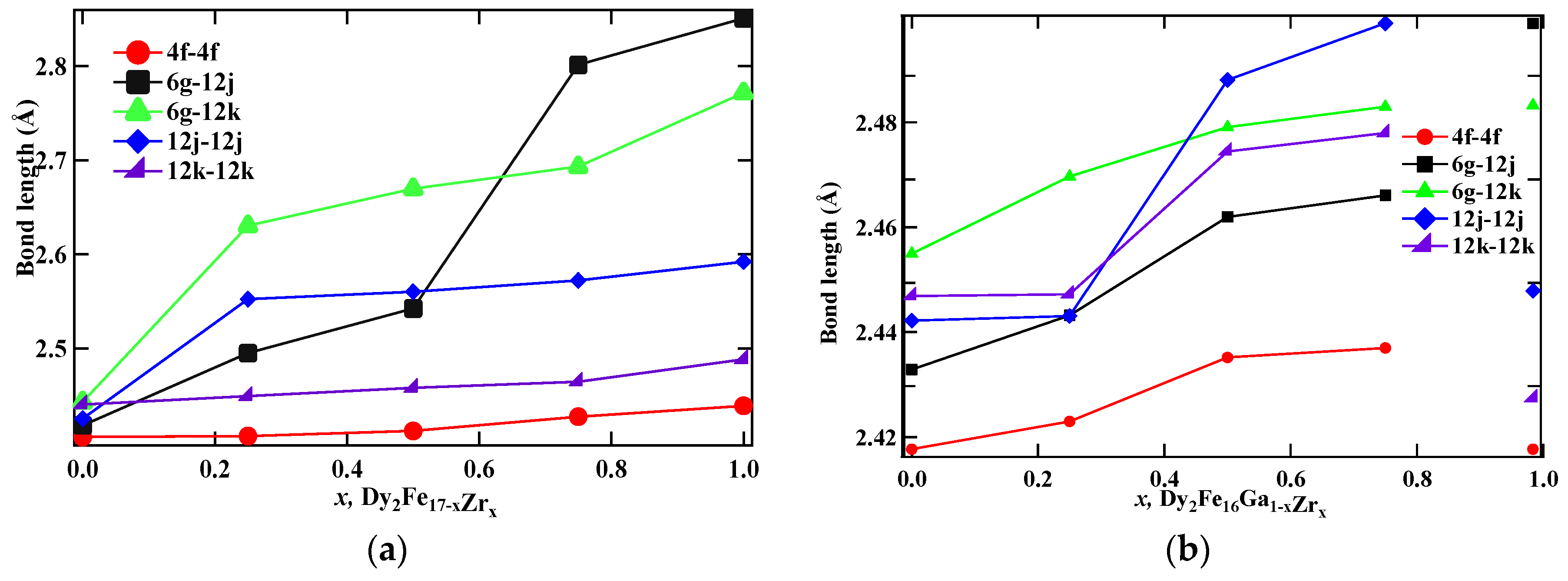
Table 3 represents the distance between the Fe–Fe neighbor sites, Ms, and Tc for Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx, and the values are plotted in Figure 4a,b. It can be observed from Table 3 that the average bond distance between each site changes with the concentration of both Zr and Ga (4f–4f ≈ 2.42 Å; 12k–12k ≈ 2.46 Å; 12j–12j ≈ 2.52 Å). The 12j–12j sites show that both Zr and Ga prefer to stay in the same sites.

Table 3.
Distance between the Fe–Fe neighbor sites, Ms, and Tc for Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx.
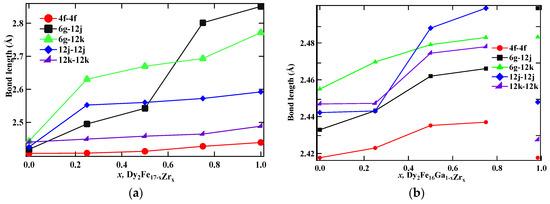
Figure 4.
Dependence of bond lengths on x of (a) Dy2Fe17−xZrx, and (b) Dy2Fe16Ga1−xZrx.
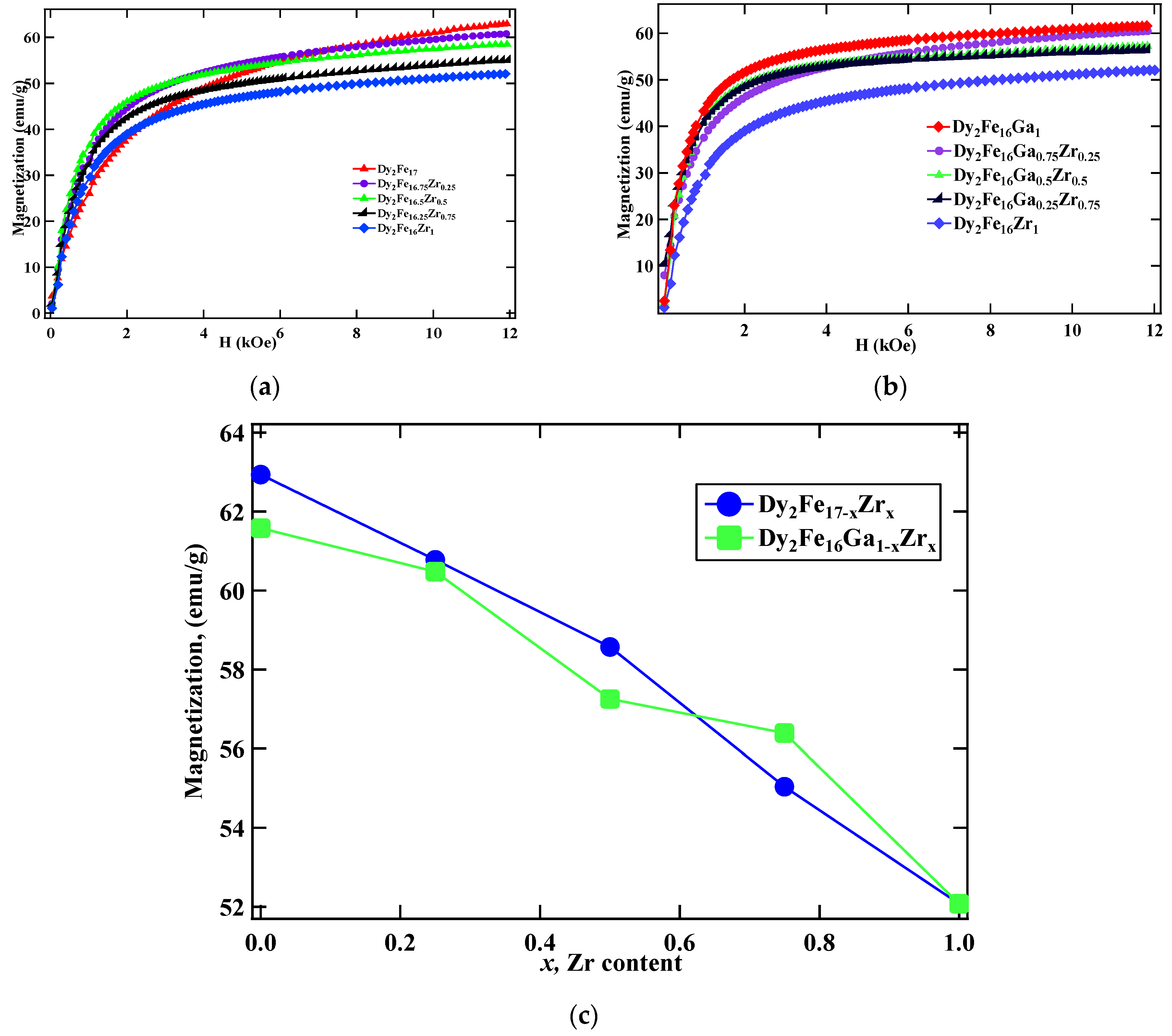
The room temperature magnetic properties obtained from the M vs. H measurement for Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx are plotted in Figure 5a,b, respectively. The M vs. H plot shows that the saturation magnetization (Ms) decreases with an increase in the Zr substitution in both Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx. To determine the saturation magnetization, the “law of approach” was used, which describes the relationship between magnetization M on the applied magnetic field for H greater than the coercive field Hc. The magnetization near Ms can be written as [11,32], where M is the magnetization, H is the applied magnetic field, and MS is the saturation magnetization attained at high field. The field-induced increase in the spontaneous magnetization of the domains is represented by the term κH. This term is very small at a temperature well below the Curie temperature and could be neglected. The term “a” is generally interpreted as due to microstress and ignored in the high-field region, and “b” is interpreted as due to crystal anisotropy. Where magneto-crystalline is a dominant term, a plot of M vs. 1/H2 in the high-field region gives a straight line, the intercept of which (with the M-axis) gives the Ms and the slope of which gives the magneto-crystalline anisotropy constant. The decrease in saturation magnetization in Dy2Fe17−xZrx with the increase in Zr content is attributed to the substitution of non-magnetic Zr atom for the Fe atom. This decrease in the saturation magnetization is the result of the dilution effect, i.e., replacing the Fe atom (2.2 μB) with non-magnetic Zr atom. A similar reduction in Ms was reported earlier for Dy2Fe17−xGax [18], Ce2Fe17−xGax [33], Sm2Fe17−xGax [34], and Dy2Fe17−xNbx [35] compounds. From Figure 5c, it is observed that the saturation magnetization decreases linearly from 62.94 emu/g to 52.07 emu/g per Zr atom for Dy2Fe17−xZrx; however, magnetization decreases from 61.58 emu/g to 52.07 emu/g for Dy2Fe16Ga1−xZrx. The dropdown of magnetization in both cases could be due to the reduction of the magnetic moment of iron upon Zr substitution, as well as the band filling effect coming from four valence electrons from Zr while doping.
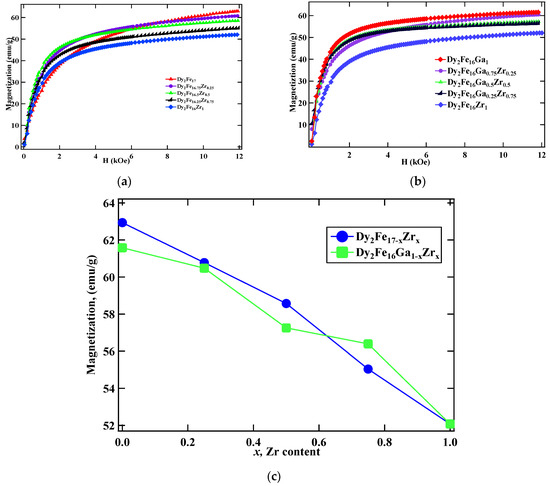
Figure 5.
Room temperature (RT) M vs. H plot of (a) Dy2Fe17−xZrx and (b) Dy2Fe16Ga1−xZrx; (c) magnetization (Ms) vs. x for Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx.
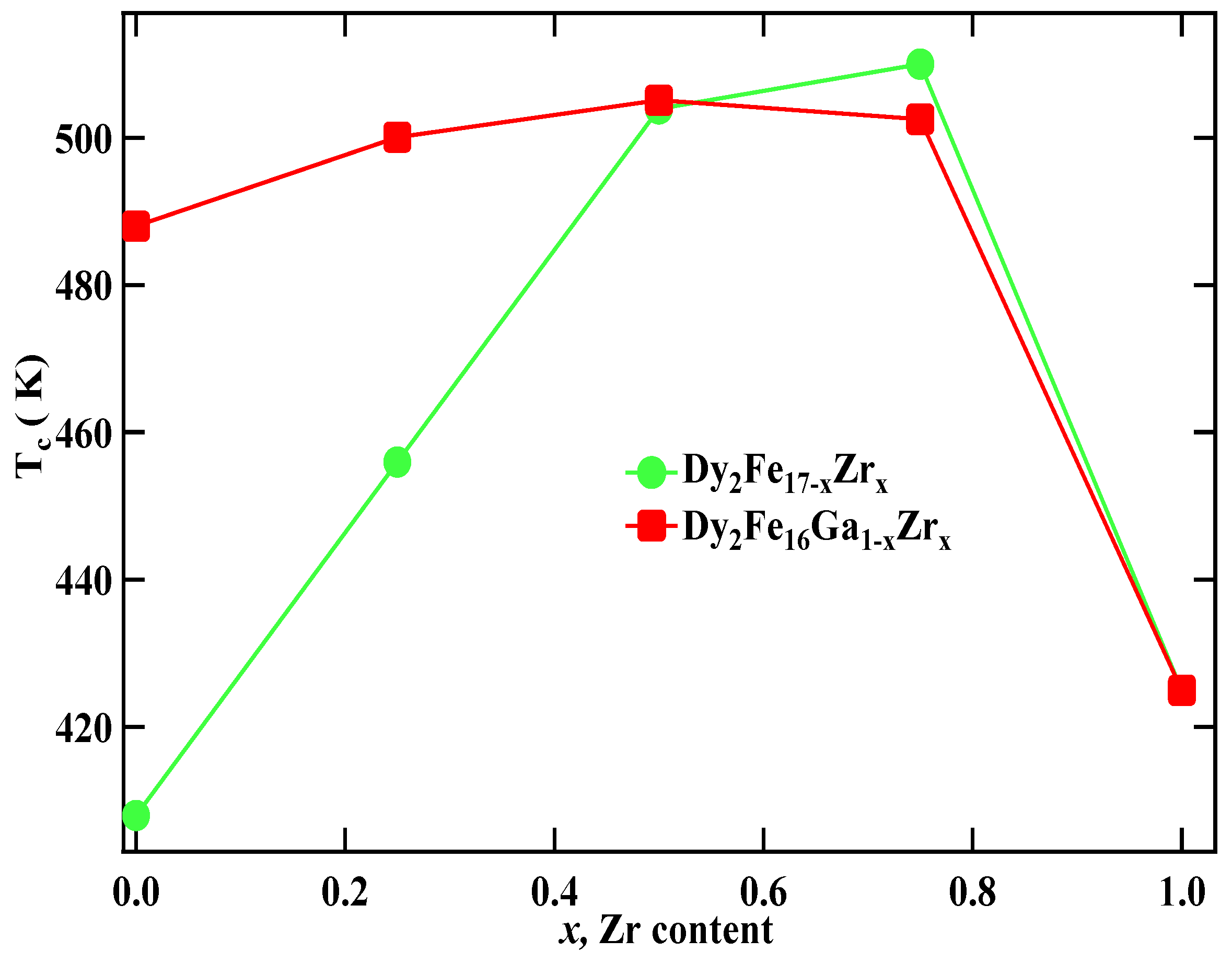
The Curie temperature (Tc) of Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx as a function of Zr content is shown in Figure 6, and corresponding data are listed in Table 3. It is observed that, for Dy2Fe17−xZrx, Curie temperature increases from 408 K (x = 0.00) to a maximum of 510 K (x = 0.75) and then decreases to 425 K (x = 1.00). The achieved Curie temperature of Dy2Fe16.25Zr0.75 is 102 K higher than that of Dy2Fe17. It is observed from Figure 4a,b that the bond length increases with the increase in the Zr content. In general, the Curie temperature in rare-earth intermetallic is due to three kinds of exchange interactions, namely, the 3d–3d exchange interactions, i.e., between the magnetic moment of the Fe sub-lattice (JFeFe), 4f–4f exchange interaction, i.e., the interaction between the magnetic moment within the R sub-lattice (JRR), and the inter sub-lattice 3d–4f exchange interaction (JRFe). It is reported that the Curie temperature (Tc) increases with an increase in JFeFe [5]. Thus, the increase or decrease in Tc in the R2Fe17 intermetallic may be due to the increase or decrease in the interaction parameter JFeFe. The interactions between the rare-earth spins (4f–4f) are assumed to be weak and negligible in comparison with the other two types of interactions. The low TC observed in parent Dy2Fe17 compound is believed to be due to the short Fe–Fe interatomic distances found at the 4f(6c) sites in the hexagonal (rhombohedral) structure which couple antiferromagnetically [36], since their separation is less than 2.45 Å (Figure 4a,b), needed for ferromagnetic ordering [37]. In the compounds with hexagonal structure the Fe(4f)–Fe(4f) interactions are strongly negative, whereas the Fe(6g)–Fe(12j), Fe(6g)–Fe(12k), and Fe(12k)–Fe(12k) interactions are weakly negative. Thus, the increase in Tc is considered due to the increase in strength of the Fe–Fe exchange coupling that occurs from the increase in Fe–Fe bond lengths for each R2Fe17 compound. The strength of Fe–Fe exchange interaction highly depends on interatomic Fe–Fe distance [38,39]. Accordingly, the exchange interactions between iron atoms situated at distances smaller (greater) than 2.45–2.50 Å are negative (positive). In the R2Fe17, the majority of Fe–Fe distances favor the negative interaction [18]. The negative exchange interaction can be reduced either by volume expansion or by reducing the number of Fe–Fe pairs with negative exchange interactions. It is noted that the increase in Tc was reported earlier with Ga substitution (x = 1) [18]; however, the simultaneous substation of non-magnetic Ga and Zr atoms enhances the Curie temperature of the intermetallic without significantly lowering the saturation magnetization. It is found that, for Dy2Fe16Ga1−xZrx, Curie temperature increases slowly from 488 K (x = 0.00) to a maximum of 505.1 K (x = 0.50) and then decreases to 425 K (x = 1.00). The maximum Tc observed in Dy2Fe16Ga0.5Zr0.5 (505.1 K) is 17.1 K greater than that of Dy2Fe16Ga1 and 97 K higher than that of Dy2Fe17. Overall co-substitution of Zr and Ga shows a greater degree of enhancement in Tc over the single substation.
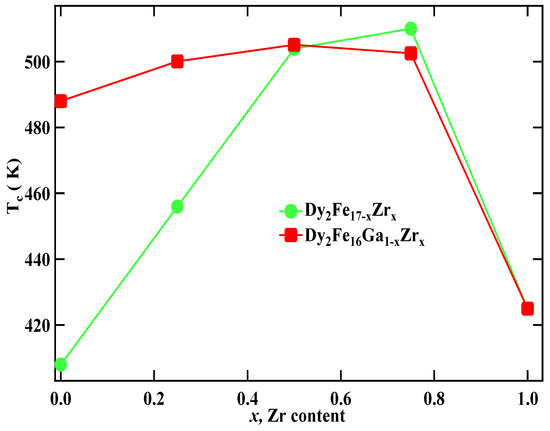
Figure 6.
Comparative study for the Tc vs. x for Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx.
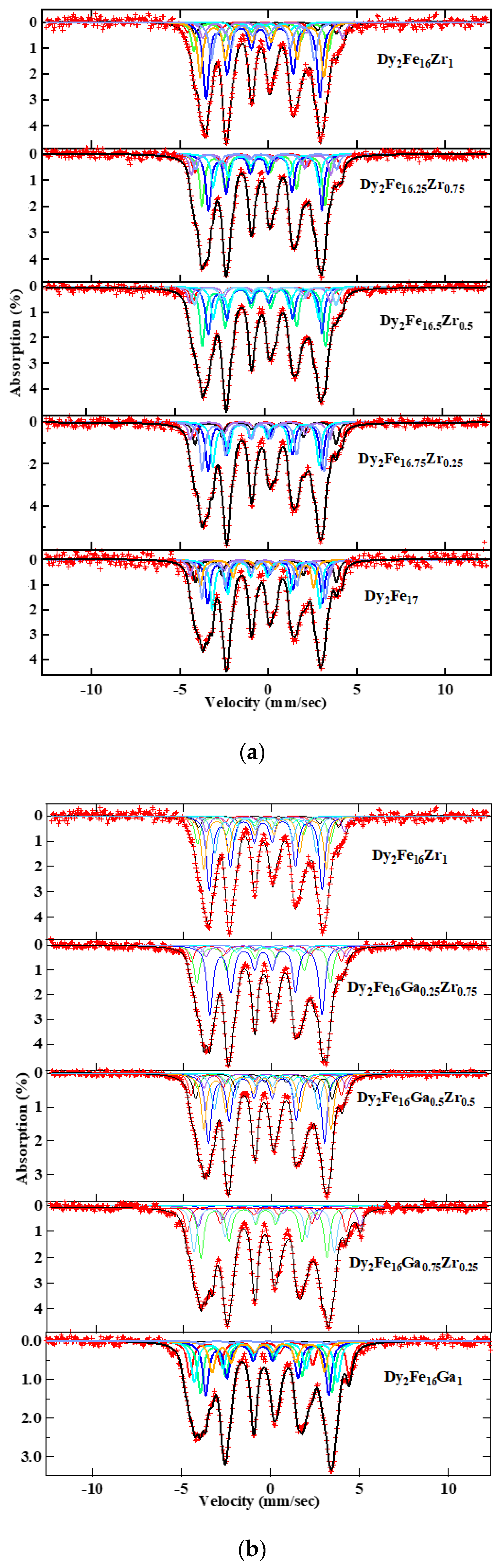
The Mössbauer spectra are plotted in Figure 7a,b for Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx (x = 0.00, 0.25, 0.50, 0.75, 1.00) respectively. The resulting spectra were fitted using the WMOSS program. The hyperfine field (Bhf), isomer shift (δ or IS), and quadrupole splitting (∆ or QS), as well as the percentage of area occupied by the different sites, were extracted from the fit. Since the Dy2Fe17 compounds have a basal magnetization, eight magnetic sextets are required to fit their Mössbauer spectra. The fitted data shown in Figure 7a,b were determined with eight magnetic sextets assigned to 4f, 6g, 12j, and 12k sites in Dy2Fe17. Doublets were used at x = 1 Zr content for the additional phase paramagnetic phase (DyFe3) observed during Mössbauer fitting. While fitting Mossbauer spectra, the relative areas of 4f and 4e sites were adjusted, and the relative areas of 6g4, 6g2, 12j8, 12j4, 12k8, and 12k4 were constrained in the ratio 4:2:8:4:8:4 [40].
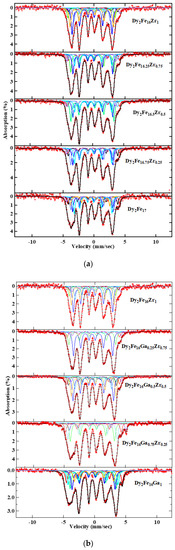
Figure 7.
RT Mössbauer spectra for (a) Dy2Fe17−xZrx, and (b) Dy2Fe16Ga1−xZrx.
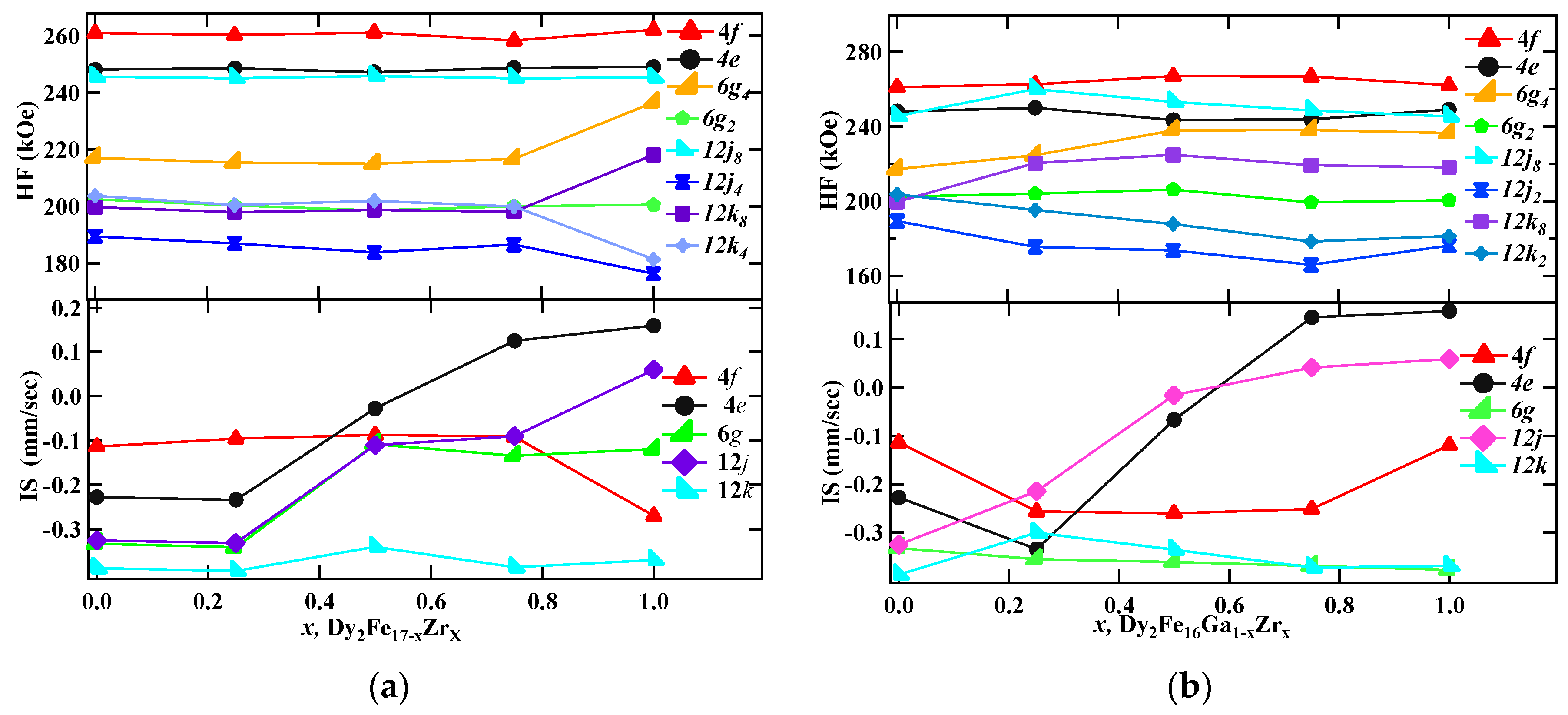
The hyperfine parameters derived from the fitting are listed in Table 4, and the hyperfine field (HF) and isomer shifts (IS) are plotted in Figure 8a,b. There exists a direct correlation between hyperfine field values of a site with its near neighbor (NN) iron sites. In the case of the Th2Ni17 structure, the 12k site has nine NN Fe sites (one (4f), two (6g), four (12j), two (12k)), the 12j site has 10 NN Fe sites (two (4f), two (6g), two (12j), four (12k)), the 6g site has 10 NN Fe sites (two (4f), zero (6g), four (12j), four (12k)), and the 4f site has 11 NN Fe sites (one (4f), three (6g), six (12j), three (12k)). Following the NN distribution, the observed HF values are in the order 4f(6c) > 12j(18f) > 6g(9d) > 12k(18h), which is similar to the sequence observed in other R2Fe17 compounds [40,41]. It is obvious that the 4f(6c) site has the maximum hyperfine field, since it has the maximum number of Fe nearest neighbors, whereas the 18h(12k) site has the minimum number of Fe neighbors and, consequently, has the lowest HF value. Although 6g(9d) and 12j(18f) sites have the same number of Fe neighbors, the former has comparatively smaller Fe–Fe distances and, hence, a larger hyperfine field. The hyperfine field (Bhf) decreases with increasing Zr content. The decrease in the hyperfine fields could be from the decrease in magnetic moments of iron in Dy2Fe17−xZrx. It is found that 9.23% of total area is occupied by the DyFe3 paramagnetic phase for x = 1 Zr content. This observation supports the formation of an additional paramagnetic phase DyFe3 as confirmed from the XRD Rietveld refinement.

Table 4.
The RT hyperfine parameters, HF (kOe), IS (mm/s), QS (mm/s), and area (%) of the Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx.
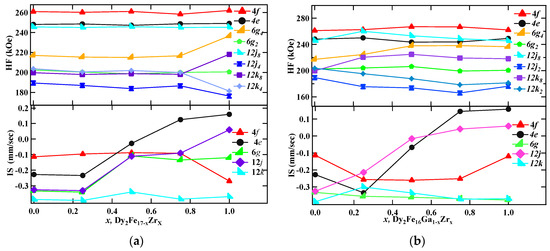
Figure 8.
RT Mössbauer hyperfine parameters plots for (a) Dy2Fe17−xZrx, and (b) Dy2Fe16Ga1−xZrx.
On average, the isomer shifts (IS) of all sites increase with the increase in Zr content. The increase in the isomer shift (IS) could be related to the volume expansion resulting from the Zr substitution for Fe. The volume expansion lowers the s-electron density at the Fe nuclei, which leads to an increase in isomer shift (IS). At higher Zr content (x = 1.00), a decrease in isomer shift (IS) was observed. This decrease in the isomer shift may be due to the combined effect of volume expansion and the increase in electron density. The decrease in hyperfine fields on Dy2Fe16Ga1−xZrx is attributed to the decrease in magnetization because of the competition between the positive effect on magnetic moments due to the bond length and the negative effect caused by magnetic dilution due to the non-magnetic atom substitution. The average isomer shift (IS) of all sites increases with increasing Zr content except for the 12k site.
4. Conclusions
The effects of substitution of Ga and Zr in intermetallic compounds Dy2Fe17 prepared via arc melting were carefully studied. The intermetallic compounds were α-Fe free and found to crystallize in hexagonal the Th2Ni17 type structure. Paramagnetic phase DyFe3 was observed for x = 1 and confirmed using Mössbauer spectroscopy. From the Rietveld refinement, it was observed that most Zr atoms occupied 12j and 12k sites of Fe for Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx intermetallic compounds. Lattice parameters a and c and unit cell volume increased with the substitution of Zr and Ga. The Fe–Fe bond length increased with Zr substitution. However, the maximum change in Fe–Fe bond length was observed for 6g–12j, 6g–12k, and 12j–12j sites. This change in bond length was found to be affected by the strength of Fe–Fe exchange interaction. The maximum Tc was observed in Dy2Fe16Ga0.5Zr0.5 (505.1 K) for a double substitution of Ga and Zr; however, for a single substitution, maximum Tc was observed at 510 K in Dy2Fe16.25Zr0.75. This increase in the Curie temperature was attributed to an enhancement in Fe–Fe positive exchange interaction. The saturation magnetization, Curie temperature, and Fe hyperfine fields were remarkably affected by Zr substitution. The hyperfine field values decreased for both Dy2Fe17−xZrx and Dy2Fe16Ga1−xZrx with Zr doping due to the decrease in the magnetic moment of Fe atoms. The isomer shift (IS) showed an increase in nature due to the increase in volume effect. The Co-doped 2:17 intermetallics with improved Tc and Ms ensures their potential use in high-temperature permanent magnet applications.
Author Contributions
Conceptualization, J.N.D. and S.R.M.; methodology, visualization, validation, formal analysis, investigation, data curation writing—original draft preparation, J.N.D.; resources J.N.D. and S.R.M.; writing review and editing, J.N.D., K.S.S.A., S.R.M. and D.N.; software J.N.D., S.R.M. and K.S.S.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding NSF-CMMI (Grant #: 1029780) and Journal APC was funded by MDPI.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Strnat, K.J. Chapter 2—Rare-earth-cobalt permanent magnets. In Handbook of Ferromagnetic Materials; Elsevier B.V.: Amsterdam, The Netherlands, 1988; Volume 4, pp. 131–209. [Google Scholar]
- Oesterreicher, H.; McNeely, D. Studies on compounds DyFe3, Dy6Fe23 and Dy2Fe17 with al substitution for Fe I: Structural investigations. J. Less Common Met. 1977, 53, 235–243. [Google Scholar] [CrossRef]
- Pszczola, J.; Zukrowski, J.; Suwalski, J.; Kucharski, Z.; Lukasiak, M. The 161Dy and 57Fe Mössbauer studies of the Dy2Fe17−yAlycompounds. J. Magn. Magn. Mater. 1983, 40, 197. [Google Scholar] [CrossRef]
- Pfranger, R.; Plusa, D.; Szymura, S.; Wyslocki, B. Domain Structures and anisotropy constant in the compound Dy2Fe17. J. Magn. Magn. Mater. 1980, 21, 43. [Google Scholar] [CrossRef]
- Rao, K.V.S.R.; Ehrenberg, H.; Markandeyulu, G.; Varadaraju, U.V.; Venkatesan, M.; Suresh, K.G.; Murthy, V.S.; Schmidt, P.C.; Fuess, H. On the Structural and Magnetic Properties of R2Fe17—x (A, T)x (R = Rare- Earth; A = Al, Si, Ga; T = Transition Metal) Compounds. Phys. Stat. Sol. A 2002, 189, 373–388. [Google Scholar] [CrossRef]
- Plugaru, N.; Rubin, J.; Bartolome, J.; Piquer, C.; Artigas, M. Magnetic properties of RFe11.3W0.7 (R = Dy, Ho, Er, and Lu): On the R−Fe exchange interaction in the R(Fe,T)12 class of compounds. Phys. Rev. B 2002, 65, 134419. [Google Scholar] [CrossRef]
- Zinkevich, M.; Mattern, N.; Bacher, I.; Puerta, S. Formation, crystal structure and magnetic properties of (1:12) compounds in the Fe-Gd-Mo-Zr system. J. Alloys Compd. 2002, 336, 320–328. [Google Scholar] [CrossRef]
- Chen, Z.; Hadjipanayis, G.C. Effects of Cr substitution on the formation, structure and magnetic properties of Sm 2(Fe,Cr)17Cx alloys. IEEE Trans. Magn. 1997, 33, 3856–3858. [Google Scholar] [CrossRef]
- Kataoka, M.; Satoh, T.; Otsuki, E. Structure and magnetic properties of Sm3 (Fe, V) 29 NX. J. Appl. Phys. 1999, 85, 4675–4677. [Google Scholar] [CrossRef]
- Gebel, B.; Kubis, M.; Müller, K. Permanent magnets prepared from Sm10.5Fe88.5Zr1.0Ny without homogenization. J. Magn. Magn. Mater. 1997, 174, L1–L4. [Google Scholar] [CrossRef]
- Dahal, J.N.; Ali, K.S.; Mishra, S.R.; Alam, J. Structural, Magnetic, and Mössbauer Studies of Transition Metal-Doped Gd2Fe16Ga0.5TM0.5 Intermetallic Compounds (TM = Cr, Mn, Co, Ni, Cu, and Zn). Magnetochemistry 2018, 4, 54. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Sun, H. Improved magnetic properties by treatment of iron-based rare earth intermetallic compounds in ammonia. J. Magn. Magn. Mater. 1990, 87, L251–L254. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Sun, H.; Otani, Y.; Hurley, D.P.F. Gas-phase carbonation of R2Fe17; R = Y, Sm. J. Magn. Magn. Mater. 1991, 98, 76. [Google Scholar] [CrossRef]
- Zhong, X.P.; Radwanski, R.J.; de B-er, F.R.; Jacobs, T.H.; Buschow, K.H.J. Magnetic and crystallographic characteristics of rare-earth ternary carbides derived from R2Fe17 compounds. J. Magn. Magn. Mater. 1990, 86, 333. [Google Scholar] [CrossRef]
- Buschow, K.H.J.; Coehoorn, R.; de Waard, D.B.d.K.; Jacobs, T.H. Structure and magnetic properties of R2Fe17Nx compounds. J. Magn. Magn. Mater. 1990, 92, L35–L38. [Google Scholar] [CrossRef]
- Wang, Z.; Dunlap, R.A. Effects of Al substitutions on the magnetic anisotropy of Sm2Fe17 compounds. J. Phys. Condens. Matter 1993, 5, 1407–1414. [Google Scholar] [CrossRef]
- Yelon, W.B.; Xie, H. Neutron diffraction and Mössbauer effect study of several Nd2Fe17−xAlxsolid solutions. J. Appl. Phys. 1993, 73, 6029. [Google Scholar] [CrossRef]
- Shen, B.-G.; Cheng, Z.-H.; Gong, H.-Y.; Liang, B.; Yan, Q.-W.; Zhan, W.-S. Magnetic anisotropy of Dy2Fe17−xGax compounds. Solid State Commun. 1995, 95, 813–816. [Google Scholar] [CrossRef]
- Dahal, J.N.; Neupane, D.; Poudel, T.P. Synthesis and magnetic properties of 4: 1 hard-soft SrFe12O19-La1-xSrxMnO3 nanocomposite prepared by auto-combustion method. AIP Adv. 2019, 9, 075308. [Google Scholar] [CrossRef]
- Gubbens, P.M.; Moolenaar, A.A.; Boender, G.J.; Kraan, A.M.V.D.; Jacobs, T.H.; Buschow, K.H.J. 166Er and 57Fe Mössbauer effect in Er2Fe17Nx. J. Magn. Magn. Mater. 1991, 97, 69–72. [Google Scholar] [CrossRef]
- Betancourt, I.; Davies, H. Influence of Zr and Nb dopant additions on the microstructure and magnetic properties of nanocomposite RE2(Fe,Co)14B/α(Fe,Co) (RE = Nd–Pr) alloys. J. Magn. Magn. Mater. 2003, 261, 328–336. [Google Scholar] [CrossRef]
- Long, G.J.; Marasinghe, G.K.; Mishra, S.; Pringle, O.A.; Hu, Z.; Yelon, W.B.; Middleton, D.P.; Buschow, K.H.J.; Grandjean, F. A Magnetic, Neutron Diffraction, and Mössbauer Spectral Study of the Nd2Fe17−xAlx Solid Solutions. J. Appl. Phys. 1994, 76, 5383–5384. [Google Scholar] [CrossRef]
- Isnard, O.; Miraglia, S.; Soubeyroux, J.L.; Fruchart, D. Neutron powder diffraction study of R2Fe17Hx compounds with R = Pr and Nd. Solid State Commun. 1992, 81, 13–19. [Google Scholar] [CrossRef]
- Coey, J.M.D. New permanent magnets; manganese compounds. J. Phys. Condens. Matter. 2014, 26, 064211. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Cryst. 1969, 2, 65. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. 2014, 229, 345. [Google Scholar] [CrossRef]
- Liao, L.X.; Altounican, Z.; Ryan, D.H. Cobalt site preferences in iron rare-earth-based compounds. Phys. Rev. B 1993, 47, 11230. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.J. The approximation of asymmetric neutron powder diffraction peaks by sums of Gaussians. J. Appl. Crystallogr. 1982, 15, 15615. [Google Scholar] [CrossRef]
- Pitschke, W.; Hermann, H.; Mattern, N. The influence of surface roughness on diffracted X-ray intensities in Bragg–Brentano geometry and its effect on the structure determination by means of Rietveld analysis. Powder Diff. 1993, 8, 74. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmad, R.; Rashid, M.S.; Hassan, M.; Hussnain, A.; Khan, M.A.; Haq, M.E.u.; Shafique, M.A.; Riaz, S. Structural and magnetic studies on Zr doped ZnO diluted magnetic semiconductor. Curr. Appl. Phys. 2014, 14, 176–181. [Google Scholar] [CrossRef]
- Yan-ming, H.; Qi-wei, Y.; Pan-lin, Z.; Xiang-dong, S.; Fang-wei, W.; Bao-gen, S. Magnetic properties of Dy2Fe17−xCrx and Er2Fe17−xCrx (x = 0–3) compounds. Acta Phys. Sin. 1997, 6, 440. [Google Scholar]
- Chikazumi, S.; Graham, C.D. Physics of Ferromagnetism, 2nd ed.; Oxford University Press: Oxford, UK, 2009; p. 94. [Google Scholar]
- Long, G.J.; Mishra, S.R.; Pringle, O.A.; Hu, W.B.Y.Z.; Grandjean, F.; Middleton, D.P.; Buschow, K.H.J. A magnetic, neutron diffraction, and Mossbauer spectral study of the Ce2Fe17−xGax solid solutions. J. Magn. Magn. Mater. 1997, 176, 217–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Budnick, J.I.; Hines, W.A.; Shen, B.G.; Cheng, Z. Effect of Ga substitution on the Sm sublattice anisotropy in Sm2Fe17−xGax (0 <x <8). J. Appl. Phys. 1999, 85, 4663–4665. [Google Scholar]
- Rai, K.; Ali, K.S.S.; Mishra, S.R.; Khanra, S.; Ghosh, K. A structural, magnetic, and Mössbauer study of the Dy2Fe17−xZrx solid solutions. J. Magn. Magn. Mater. 2014, 353, 51–56. [Google Scholar] [CrossRef]
- Bara, J.J.; Pedziwiatr, A.T.; Zarek, W. Investigations of crystal and magnetic properties of Dy-Fe intermetallic compounds. J. Magn. Magn. Mater. 1982, 27, 168–174. [Google Scholar] [CrossRef]
- Valeanu, M.; Plugaru, N.; Burzo, E. Effect of nitrogenation on the magnetic properties of Y2Fe17−xMx compounds, with M = Al, Ga or Si. Solid State Commun. 1994, 89, 519. [Google Scholar] [CrossRef]
- Neél, L. Propriétés magnétiques de l’état métallique et énergie d’interaction entre atoms magnétiques. Ann. Phys. 1936, 5, 232. [Google Scholar] [CrossRef]
- Li, Z.W.; Morris, A.H. Negative exchange interactions and Curie temperatures for Sm2Fe17 and Sm2Fe17Ny. Phys. Rev. B 1997, 55, 3670. [Google Scholar] [CrossRef]
- Long, G.J.; Isnard, O.; Grandjean, F. A Mossbauer spectral study of the magnetic properties of Ho2Fe17 and Ho2Fe17D3.8. J. Appl. Phys. 2002, 91, 1423. [Google Scholar] [CrossRef]
- Grandjean, F.; Isnard, O.; Long, G.J. Magnetic and Mossbauer spectral evidence for the suppression of the magnetic spin reorientation in Tm2Fe17 by deuterium. Phys. Rev. B 2002, 65, 064429. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).