Spin Crossover in Bipyridine Derivative Bridged One-Dimensional Iron(III) Coordination Polymer
Abstract
:1. Introduction
2. Results and Discussion
2.1. Syntheses and General Characterization
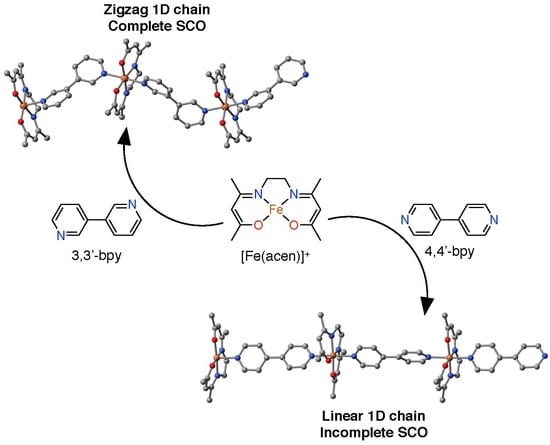
2.2. Structural Descriptions
2.3. Continuous-Wave X-Band Electron Paramagnetic Spectroscopy
2.4. Magnetic Properties
2.5. 57Fe Mössßauer Spectroscopy
3. Experimental Section
3.1. Material and Methods
3.2. Synthesis of [Fe(acen)(3,3′-bpy)][BPh4] (1)
3.3. Synthesis of [NEt3H][Fe(acen)(4,4′-bpy)][BPh4]2·0.5(4,4′-bpy) (2)
3.4. Single Crystal X-ray Crystallography
3.5. Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sato, O. Optically Switchable Molecular Solids: Photoinduced Spin-Crossover, Photochromism, and Photoinduced Magnetization. Acc. Chem. Res. 2003, 36, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Goodwin, H.A. Spin Crossover in Transition Metal Compounds I–III, Topics in Current Chemistry; Springer: Berlin, Germany, 2004. [Google Scholar]
- Murray, K.S. Advances in polynuclear iron(II), iron(III) and cobalt(II) spin-crossover compounds. Eur. J. Inorg. Chem. 2008, 2008, 3101–3121. [Google Scholar] [CrossRef]
- Gamez, P.; Costa, J.S.; Quesada, M.; Aromí, G. Iron Spin-Crossover compounds: From fundamental studies to practical applications. Dalton Trans. 2009, 38, 7845–7853. [Google Scholar] [CrossRef] [PubMed]
- Halcrow, M.A. Structure:function relationships in molecular spin-crossover complexes. Chem. Soc. Rev. 2011, 40, 4119–4142. [Google Scholar] [CrossRef]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [Green Version]
- Halcrow, M.A. Spin-Crossover Materials: Properties and Applications, 1st ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013. [Google Scholar]
- Halcrow, M.A. Spin-crossover Compounds with Wide Thermal Hysteresis. Chem. Lett. 2014, 43, 1178–1188. [Google Scholar] [CrossRef]
- Brooker, S. Spin crossover with thermal hysteresis: Practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef] [Green Version]
- Sato, O. Dynamic molecular crystals with switchable physical properties. Nat. Chem. 2016, 8, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-S.; Liu, T. Manipulating Spin Transition to Achieve Switchable Multifunctions. Acc. Chem. Res. 2019, 52, 1369–1379. [Google Scholar] [CrossRef]
- Kahn, O.; Martinez, C.J. Spin-Transition Polymers: From Molecular Materials toward Memory Devices. Science 1998, 279, 44–48. [Google Scholar] [CrossRef]
- Gaspar, A.B.; Ksenofontov, V.; Seredyuk, M.; Gütlich, P. Multifunctionality in spin crossover materials. Coord. Chem. Rev. 2005, 249, 2661–2676. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, H.J.; Gural’skiy, I.A.; Quintero, C.M.; Tricard, S.; Salmon, L.; Molnár, G.; Bousseksou, A. Molecular actuators driven by cooperative spin-state switching. Nat. Commun. 2013, 4, 2607. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, A.B.; Seredyuk, M. Spin crossover in soft matter. Coord. Chem. Rev. 2014, 268, 41–58. [Google Scholar] [CrossRef]
- Gentili, D.; Demitri, N.; Schäfer, B.; Liscio, F.; Bergenti, I.; Ruani, G.; Ruben, M.; Cavallini, M. Multi-modal sensing in spin crossover compounds. J. Mater. Chem. C 2015, 3, 7836–7844. [Google Scholar] [CrossRef] [Green Version]
- Lefter, C.; Davesne, V.; Salmon, L.; Molnár, G.; Demont, P.; Rotaru, A.; Bousseksou, A. Charge Transport and Electrical Properties of Spin Crossover Materials: Towards Nanoelectronic and Spintronic Devices. Magnetochemistry 2016, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.S.; Ruben, M. Emerging trends in spin crossover (SCO) based functional materials and devices. Coord. Chem. Rev. 2017, 346, 176–205. [Google Scholar] [CrossRef]
- Armand, F.; Badoux, C.; Bonville, P.; Ruaudel-Teixier, A.; Kahn, O. Langmuir-Blodgett Films of Spin Transition Iron(II) Metalloorganic Polymers. 1. Iron(II) Complexes of Octadecyl-1,2,4-triazole. Langmuir 1995, 11, 3467–3472. [Google Scholar] [CrossRef]
- Cobo, S.; Molnár, G.; Real, J.A.; Bousseksou, A. Multilayer Sequential Assembly of Thin Films That Display Room-Temperature Spin Crossover with Hysteresis. Angew. Chem. Int. Ed. 2006, 45, 5786–5789. [Google Scholar] [CrossRef]
- Molnár, G.; Cobo, S.; Real, J.A.; Carcenac, F.; Daran, E.; Vieu, C.; Bousseksou, A. A Combined Top-Down/Bottom-Up Approach for the Nanoscale Patterning of Spin-Crossover Coordination Polymers. Adv. Mater. 2007, 19, 2163–2167. [Google Scholar] [CrossRef]
- Agustí, G.; Cobo, S.; Gaspar, A.B.; Molnár, G.; Moussa, N.O.; Szilágyi, P.Á.; Pálfi, V.; Vieu, C.; Carmen Muñoz, M.; Real, J.A.; et al. Thermal and Light-Induced Spin Crossover Phenomena in New 3D Hofmann-Like Microporous Metalorganic Frameworks Produced as Bulk Materials and Nanopatterned Thin Films. Chem. Mater. 2008, 20, 6721–6732. [Google Scholar] [CrossRef]
- Cavallini, M.; Bergenti, I.; Milita, S.; Ruani, G.; Salitros, I.; Qu, Z.-R.; Chandrasekar, R.; Ruben, M. Micro- and Nanopatterning of Spin-Transition Compounds into Logical Structures. Angew. Chem. Int. Ed. 2008, 47, 8596–8600. [Google Scholar] [CrossRef]
- Cavallini, M. Inhomogeneous thin deposits: A strategy to exploit their functionality. J. Mater. Chem. 2009, 19, 6085–6092. [Google Scholar] [CrossRef]
- Cavallini, M.; Bergenti, I.; Milita, S.; Kengne, J.C.; Gentili, D.; Ruani, G.; Salitros, I.; Meded, V.; Ruben, M. Thin Deposits and Patterning of Room-Temperature-Switchable One-Dimensional Spin-Crossover Compounds. Langmuir 2011, 27, 4076–4081. [Google Scholar] [CrossRef] [PubMed]
- Nihei, M.; Shiga, T.; Maeda, Y.; Oshio, H. Spin crossover iron(III) complexes. Coord. Chem. Rev. 2007, 251, 2606–2621. [Google Scholar] [CrossRef]
- Harding, D.J.; Harding, P.; Phonsri, W. Spin crossover in iron(III) complexes. Coord. Chem. Rev. 2016, 313, 38–61. [Google Scholar] [CrossRef]
- Gerloch, M.; Lewis, J.; Mabbs, F.E.; Richards, A. The preparation and magnetic properties of some Schiff base–iron(III) halide complexes. J. Chem. Soc. A. 1968, 112–116. [Google Scholar] [CrossRef]
- Nishida, U.; Oshio, S.; Kida, S. Synthesis and Magnetic Properties of Iron(III) Complexes with Several Quadridentate Schiff Bases. Bull. Chem. Soc. Jpn. 1977, 50, 119–122. [Google Scholar] [CrossRef]
- McCusker, J.K.; Rheingold, A.L.; Hendrickson, D.N. Studies of Laser-Initiated 5T2 → 1A1 Intersystem Crossing in Spin-Crossover Complexes: Empirical Correlations between Activation Parameters and Ligand Structure. Inorg. Chem. 1996, 35, 2100–2112. [Google Scholar] [CrossRef]
- Nishida, Y.; Kino, K.; Kida, S. Crystal structures of low- and high-spin iron(III) complexes with quadridentate Schiff bases. J. Chem. Soc. Dalton Trans. 1987, 1157–1161. [Google Scholar] [CrossRef]
- Wang, X.; Pennington, W.T.; Ankers, D.L.; Fanning, J.C. Comparative crystal structure examination of some iron(III) quadridentate schiff base complexes. Polyhedron 1992, 11, 2253–2264. [Google Scholar] [CrossRef]
- Maeda, Y.; Oshio, H.; Toriumi, K.; Takashima, Y. Crystal structures, mössbauer spectra and magnetic properties of two iron(III) spin-crossover complexes. J. Chem. Soc. Dalton Trans. 1991, 1227–1235. [Google Scholar] [CrossRef]
- Imatomi, S.; Kitashima, R.; Hamamastu, T.; Okeda, M.; Ogawa, Y.; Matsumoto, N. One-dimensional Polynuclear Spin-crossover Iron(III) Complex Axially Bridged by 1,3-Bis(4-pyridyl)propane. Chem. Lett. 2006, 35, 502–503. [Google Scholar] [CrossRef]
- Imatomi, S.; Hashimoto, S.; Matsumoto, N. Inter- and intrachain spin-transition processes in one-dimensional polynuclear iron(III) complexes of N,N′-ethylenebis(acetylacetonylideneimine) bridged by 1,3-bis(4-pyridyl)propane and 1,4-bis(imidazolyl)butane. Eur. J. Inorg. Chem. 2009, 721–726. [Google Scholar] [CrossRef]
- Ross, T.M.; Neville, S.M.; Innes, D.S.; Turner, D.R.; Moubaraki, B.; Murray, K.S. Spin crossover in iron(III) Schiff-base 1-D chain complexes. Dalton Trans. 2010, 39, 149–159. [Google Scholar] [CrossRef]
- Okuhata, M.; Funasako, Y.; Takahashi, K.; Mochida, T. A spin-crossover ionic liquid from the cationic iron(iii) Schiff base complex. Chem. Commun. 2013, 49, 7662–7664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzystek, J.; Ozarowski, A.; Telser, J. Multi-frequency, high-field EPR as a powerful tool to accurately determine zero-field splitting in high-spin transition metal coordination complexes. Coord. Chem. Rev. 2006, 250, 2308–2324. [Google Scholar] [CrossRef]
- Nishida, Y.; Oshio, S.; Kida, S. ESR spectra of low-spin iron(III) complexes with several quadridentate schiff bases. Inorganica Chim. Acta 1977, 23, 59–61. [Google Scholar] [CrossRef]
- Shongwe, M.S.; Al-Rashdi, B.A.; Adams, H.; Morris, M.J.; Mikuriya, M.; Hearne, G.R. Thermally Induced Two-Step, Two-Site Incomplete 6A1 ↔ 2T2 Crossover in a Mononuclear Iron(III) Phenolate−Pyridyl Schiff-Base Complex: A Rare Crystallographic Observation of the Coexistence of Pure S = 5/2 and 1/2 Metal Centers in the Asymmetric Unit. Inorg. Chem. 2007, 46, 9558–9568. [Google Scholar] [CrossRef]
- Tang, J.; Sánchez Costa, J.; Smulders, S.; Molnár, G.; Bousseksou, A.; Teat, S.J.; Li, Y.; van Albada, G.A.; Gamez, P.; Reedijk, J. Two-Step Spin-Transition Iron(III) Compound with a Wide [High Spin-Low Spin] Plateau. Inorg. Chem. 2009, 48, 2128–2135. [Google Scholar] [CrossRef] [PubMed]
- Shongwe, M.S.; Al-Rahbi, S.H.; Al-Azani, M.A.; Al-Muharbi, A.A.; Al-Mjeni, F.; Matoga, D.; Gismelseed, A.; Al-Omari, I.A.; Yousif, A.; Adams, H.; et al. Coordination versatility of tridentate pyridyl aroylhydrazones towards iron: Tracking down the elusive aroylhydrazono-based ferric spin-crossover molecular materials. Dalton Trans. 2012, 41, 2500–2514. [Google Scholar] [CrossRef] [PubMed]
- Domracheva, N.E.; Pyataev, A.V.; Vorobeva, V.E.; Zueva, E.M. Detailed EPR Study of Spin Crossover Dendrimeric Iron(III) Complex. J. Phys. Chem. B 2013, 117, 7833–7842. [Google Scholar] [CrossRef] [PubMed]
- Pogány, L.; Brachňaková, B.; Moncol, J.; Pavlik, J.; Nemec, I.; Trávníček, Z.; Mazúr, M.; Bučinský, L.; Suchánek, L.; Šalitroš, I. Impact of Substituent Variation on the Presence of Thermal Spin Crossover in a Series of Mononuclear Iron(III) Schiff Base Complexes with Terminal Pseudohalido Co-ligands. Chem. Eur. J. 2018, 24, 5191–5203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dey, B.; Roy, S.; Titiš, J.; Boča, R.; Bera, S.P.; Mondal, A.; Konar, S. Above Room Temperature Spin Transition in Thermally Stable Mononuclear Fe(III) Complexes. Inorg. Chem. 2019, 58, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, S.; Kühne, I.A.; Kelly, C.T.; Barker, A.; Salley, D.; Müller-Bunz, H.; Powell, A.K.; Morgan, G.G. Anion Influence on Spin State in Two Novel Fe(III) Compounds: [Fe(5F-sal2333)]X. Crystals 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Aasa, R. Powder Line Shapes in the Electron Paramagnetic Resonance Spectra of High-Spin Ferric Complexes. J. Chem. Phys. 1970, 52, 3919–3930. [Google Scholar] [CrossRef]
- Maeda, Y.; Ohshio, H.; Takashima, Y. Rapid Electronic Relaxation Phenomenon in the Transition between 6A1 and 2T2 states. Chem. Lett. 1982, 11, 943–946. [Google Scholar] [CrossRef] [Green Version]
- Ohshio, H.; Maeda, Y.; Takashima, Y. Rapid Electronic Relaxation Phenomenon in a 2T ⇄ 6A Spin-Equilibrium System. Inorg. Chem. 1983, 22, 2684–2689. [Google Scholar] [CrossRef]
- Slichter, C.P.; Drickamer, H.G. Pressure-Induced Electronic Changes in Compounds of Iron. J. Chem. Phys. 1972, 56, 2142–2160. [Google Scholar] [CrossRef]
- Sorai, M.; Seki, S. Phonon coupled cooperative low-spin 1A1high-spin 5T2 transition in [Fe(phen)2(NCS)2] and [Fe(phen)2(NCSe)2] crystals. J. Phys. Chem. Solids 1974, 35, 555–570. [Google Scholar] [CrossRef]
- Nakamoto, T.; Tan, Z.-C.; Sorai, M. Heat Capacity of the Spin Crossover Complex [Fe(2-pic)3]Cl2·MeOH: A Spin Crossover Phenomenon with Weak Cooperativity in the Solid State. Inorg. Chem. 2001, 40, 3805–3809. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Bill, E.; Trautwein, A.X. Mössbauer Spectroscopy and Transition Metal Chemistry, Fundamentals and Applications; Springer: Berlin, Germany, 2011. [Google Scholar]
- CrystalClear-SM; Version 1.4.0 SP1; Rigaku and Rigaku/MSC: The Woodlands, TX, USA, 2008.
- CrystalStructure; Version 4.2.2; Rigaku and Rigaku/MSC: The Woodlands, TX, USA, 2017.
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G.; Spagna, R. SIR2011: A new package for crystal structure determination and refinement. J. Appl. Crystallogr. 2012, 45, 357–361. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Prescher, C.; McCammon, C.; Dubrovinsky, L. MossA: A program for analyzing energy-domain Mössbauer spectra from conventional and synchrotron sources. J. Appl. Crystallogr. 2012, 45, 329–331. [Google Scholar] [CrossRef]





| 1 (100 K) | 1 (300 K) | 2 (100 K) | 2 (300 K) | |
|---|---|---|---|---|
| Bond distances | ||||
| Fe1–N1 | 1.911(2) | 2.025(2) | 1.890(3) | 1.912(3) |
| Fe1–N2 | 1.921(2) | 2.039(2) | 1.912(3) | 1.922(2) |
| Fe1–N3 | 2.048(2) | 2.199(2) | 2.004(3) | 2.036(3) |
| Fe1–N4 i | 2.049(2) | 2.188(2) | 1.998(4) | 2.035(2) |
| Fe1–O1 | 1.912(1) | 1.929(1) | 1.906(2) | 1.904(2) |
| Fe1–O2 | 1.901(1) | 1.921(2) | 1.896(2) | 1.894(2) |
| Bond angles | ||||
| N1–Fe1–N2 | 85.51(7) | 81.91(7) | 85.1(1) | 84.7(1) |
| N1–Fe1–N3 | 88.33(6) | 94.49(6) | 88.2(1) | 88.9(1) |
| N1–Fe1–N4 i | 94.10(6) | 88.98(6) | 94.1(1) | 93.8(1) |
| N2–Fe1–N3 | 93.62(6) | 90.58(6) | 93.4(1) | 92.5(1) |
| N2–Fe1–N4 i | 90.62(6) | 94.72(7) | 89.3(1) | 90.0(1) |
| N1–Fe1–O1 | 94.51(6) | 90.77(6) | 93.8(1) | 93.23(9) |
| N2–Fe1–O2 | 93.77(6) | 90.15(7) | 94.4(1) | 93.89(9) |
| N3–Fe1–O1 | 91.50(6) | 84.23(6) | 89.1(9) | 89.79(8) |
| N3–Fe1–O2 | 87.87(6) | 88.94(6) | 86.9(9) | 87.18(8) |
| N4 i–Fe1–O1 | 84.27(6) | 90.88(6) | 88.2(9) | 87.82(9) |
| N4 i–Fe1–O2 | 89.76(6) | 88.30(6) | 90.8(9) | 90.19(9) |
| O1–Fe1–O2 | 86.56(6) | 97.47(6) | 86.9(9) | 88.30(8) |
| N3–Fe1–N4 i | 175.27(6) | 174.03(6) | 176.6(1) | 176.50(9) |
| N1–Fe1–O2 | 176.08(5) | 171.36(7) | 175.1(1) | 175.8(1) |
| N2–Fe1–O1 | 174.88(6) | 170.68(7) | 177.3(1) | 176.9(1) |
| Σ | 35.82(6) | 36.70(6) | 32.8(1) | 26.92(9) |
| Complex 2 | ΔFe–Oequatorial | ΔFe–Nequatorial | ΔFe–Naxial | Reference |
|---|---|---|---|---|
| [Fe(acen)(3,4-Me2py)2][BPh4] | 0.024 | 0.141 | 0.150 | [34] |
| [Fe(acen)(bpyp)][BPh4] | 0.020 | 0.138 | 0.165 | [35,36] |
| [Fe(acen)(bimb)][BPh4] | 0.030 | 0.117 | 0.116 | [36] |
| 1 | 0.019 | 0.116 | 0.146 | this work |
| 2 | 0.002 | 0.016 | 0.034 | this work |
| Complex 1 | g2 | ζ (cm–1) | ΔE (cm−1) | C | Reference |
|---|---|---|---|---|---|
| [Fe(acen)(4-Mepy)][BPh4] | 2.14 | 360 | 652 | 76 | [50] |
| [Fe(acen)(3,4-Me2py)][BPh4] | 2.13 | 430 | 528 | 151 | [50] |
| [Fe(acen)(bpyp)][BPh4] | 2.14 | 163 | 536 | 76 | [49,50] |
| 1 | 2.14 | 150(5) | 675(7) | 125(4) | this work |
| 2 | 2.14 | 334(4) | 1431(11) | 311(9) | this work |
| Complex 1 | ΔH (kJ mol−1) | ΔS (J K–1 mol−1) | T1/2 (K) 2 | Reference |
|---|---|---|---|---|
| [Fe(acen)(4-Mepy)][BPh4] | 12.06 | 50.21 | 240 | [50] |
| [Fe(acen)(3,4-Me2py)][BPh4] | 11.25 | 50.21 | 224 | [50] |
| [Fe(acen)(bpyp)][BPh4] | 8.88 | 46.02 | 193 | [49,50] |
| 1 | 8.97(3) | 42.25(14) | 212 | this work |
| 2 | 19.37(2) | 50.42(5) | 384 | this work |
| Complex 1 | Type of SCO 2 | T1/2 (K) 3 | Conformation 4 | Reference |
|---|---|---|---|---|
| discrete mononuclear system | ||||
| [Fe(acen)(Him)2][BPh4] | n.a. | n.a. | envelope | [30,32,40] |
| [Fe(acen)(py)2][BPh4] | n.a. | n.a. | n.a. | [30] |
| [Fe(acen)(4-NH2py)2][ClO4] | n.a. | n.a. | n.a. | [30,40] |
| [Fe(acen)(3-Mepy)2][ClO4] | n.a. | n.a. | n.a. | [30] |
| [Fe(acen)(4-Mepy)2][BPh4] | g, c | 240 | n.a. | [30,40,50] |
| [Fe(acen)(3,4-Me2py)2][BPh4] | g, c | 224 | meso | [34,40,50] |
| [Fe(acen)(1-Buim)2][PF6] | g, ic | n.a | envelope | [38] |
| [Fe(acen)(1-Buim)2][Tf2N] | g, ic | n.a | n.a. | [38] |
| discrete dinuclear system | ||||
| (tvpH)[{Fe(acen)}2(μ-tvp)(tvp)(tvpH)][BPh4]4·1.5(MeOH) | g, ic | n.a | envelope | [37] |
| 1D chain system | ||||
| [Fe(acen)(bpe)][BPh4] | g, ic | n.a | n.a. | [37] |
| [Fe(acen)(bpdh)][BPh4]·(MeOH) | g, ic | n.a | n.a. | [37] |
| [Fe(acen)(bix)][BPh4]·(MeOH) | g, ic | n.a | n.a. | [37] |
| [Fe(acen)(pin)][BPh4]·3(MeOH) | a, c | 135 | n.a. | [37] |
| [Fe(acen)(bpyp)][BPh4] | g, c | 193 | meso | [35,36,37,49,50] |
| [Fe(acen)(bimb)][BPh4] | g, ic | ~296 | meso | [36] |
| 1 | g, c | 212 | meso | this work |
| 2 | g, ic | 384 | envelope | this work |
| T (K) | δ (mm s−1) | ΔEQ (mm s−1) | Γ (mm s−1) |
|---|---|---|---|
| 300 | 0.342(10) | 1.677(20) | 0.633(31) |
| 250 | 0.302(18) | 1.850(35) | 0.697(50) |
| 200 | 0.285(12) | 2.310(24) | 0.832(40) |
| 175 | 0.240(8) | 2.655(16) | 0.790(27) |
| 150 | 0.238(6) | 2.781(12) | 0.454(19) |
| 100 | 0.237(7) | 2.853(4) | 0.357(7) |
| 8.5 | 0.235(2) | 2.905(4) | 0.285(6) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishikawa, R.; Noda, T.; Ueno, S.; Okubo, T.; Yamakawa, H.; Sakamoto, K.-i.; Kawata, S. Spin Crossover in Bipyridine Derivative Bridged One-Dimensional Iron(III) Coordination Polymer. Magnetochemistry 2020, 6, 29. https://doi.org/10.3390/magnetochemistry6030029
Ishikawa R, Noda T, Ueno S, Okubo T, Yamakawa H, Sakamoto K-i, Kawata S. Spin Crossover in Bipyridine Derivative Bridged One-Dimensional Iron(III) Coordination Polymer. Magnetochemistry. 2020; 6(3):29. https://doi.org/10.3390/magnetochemistry6030029
Chicago/Turabian StyleIshikawa, Ryuta, Takeshi Noda, Shunya Ueno, Takashi Okubo, Hirofumi Yamakawa, Ken-ichi Sakamoto, and Satoshi Kawata. 2020. "Spin Crossover in Bipyridine Derivative Bridged One-Dimensional Iron(III) Coordination Polymer" Magnetochemistry 6, no. 3: 29. https://doi.org/10.3390/magnetochemistry6030029
APA StyleIshikawa, R., Noda, T., Ueno, S., Okubo, T., Yamakawa, H., Sakamoto, K.-i., & Kawata, S. (2020). Spin Crossover in Bipyridine Derivative Bridged One-Dimensional Iron(III) Coordination Polymer. Magnetochemistry, 6(3), 29. https://doi.org/10.3390/magnetochemistry6030029