Nitronyl Nitroxide Biradical-Based Binuclear Lanthanide Complexes: Structure and Magnetic Properties
Abstract
:1. Introduction
2. Results and Discussion
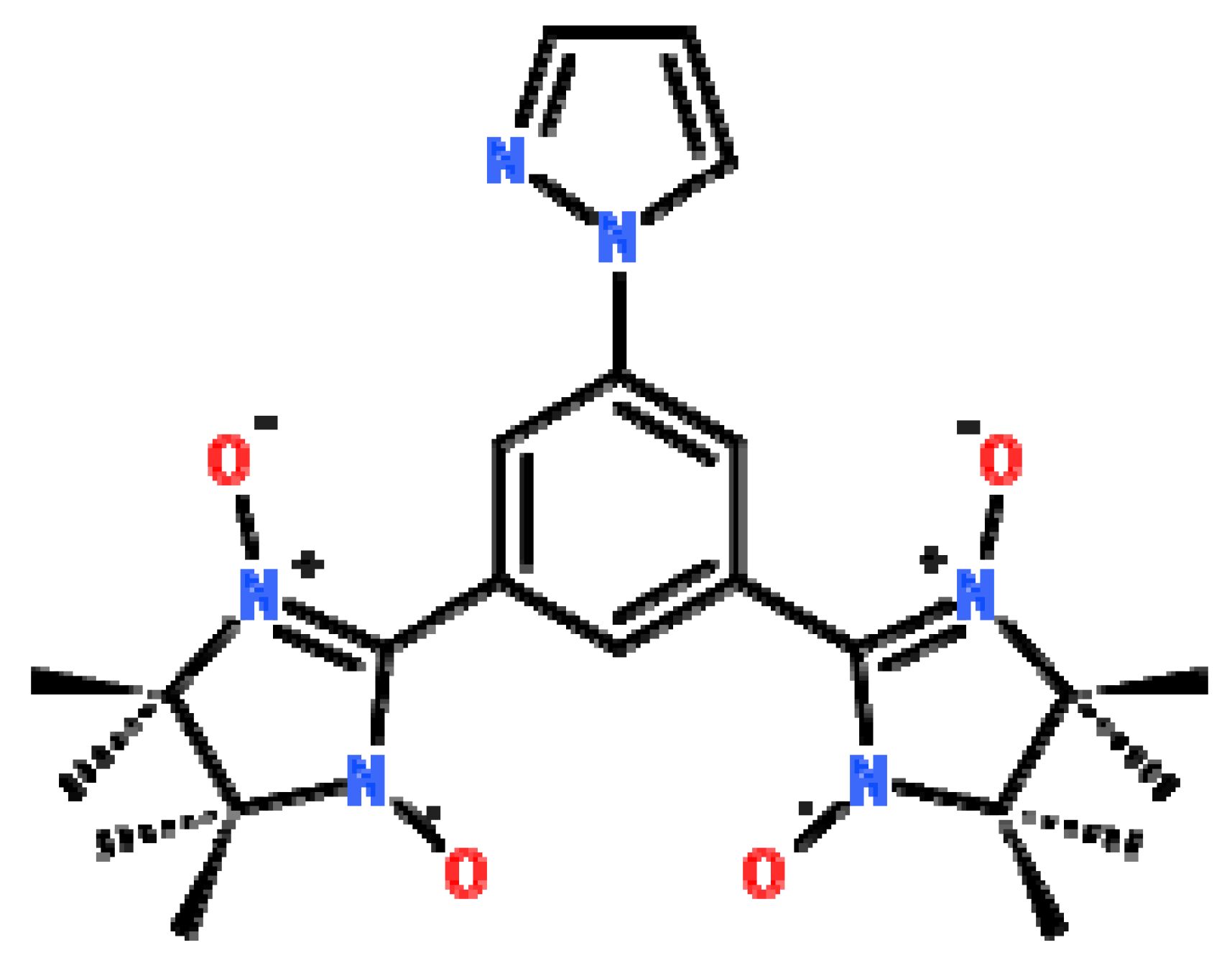
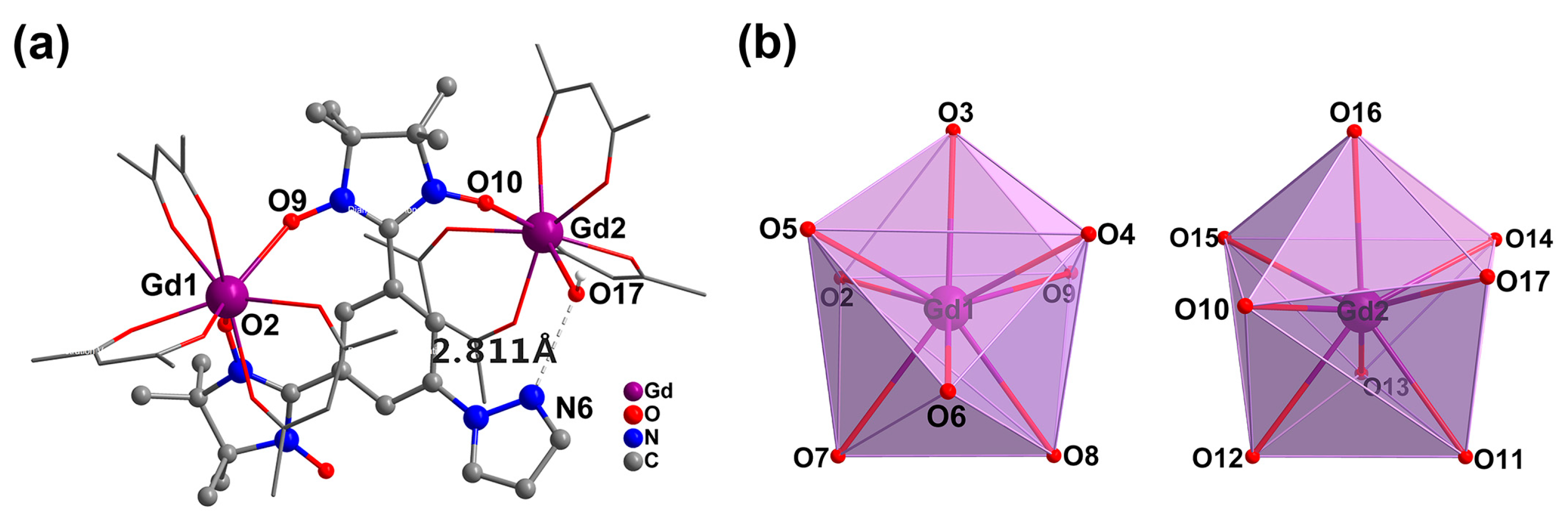
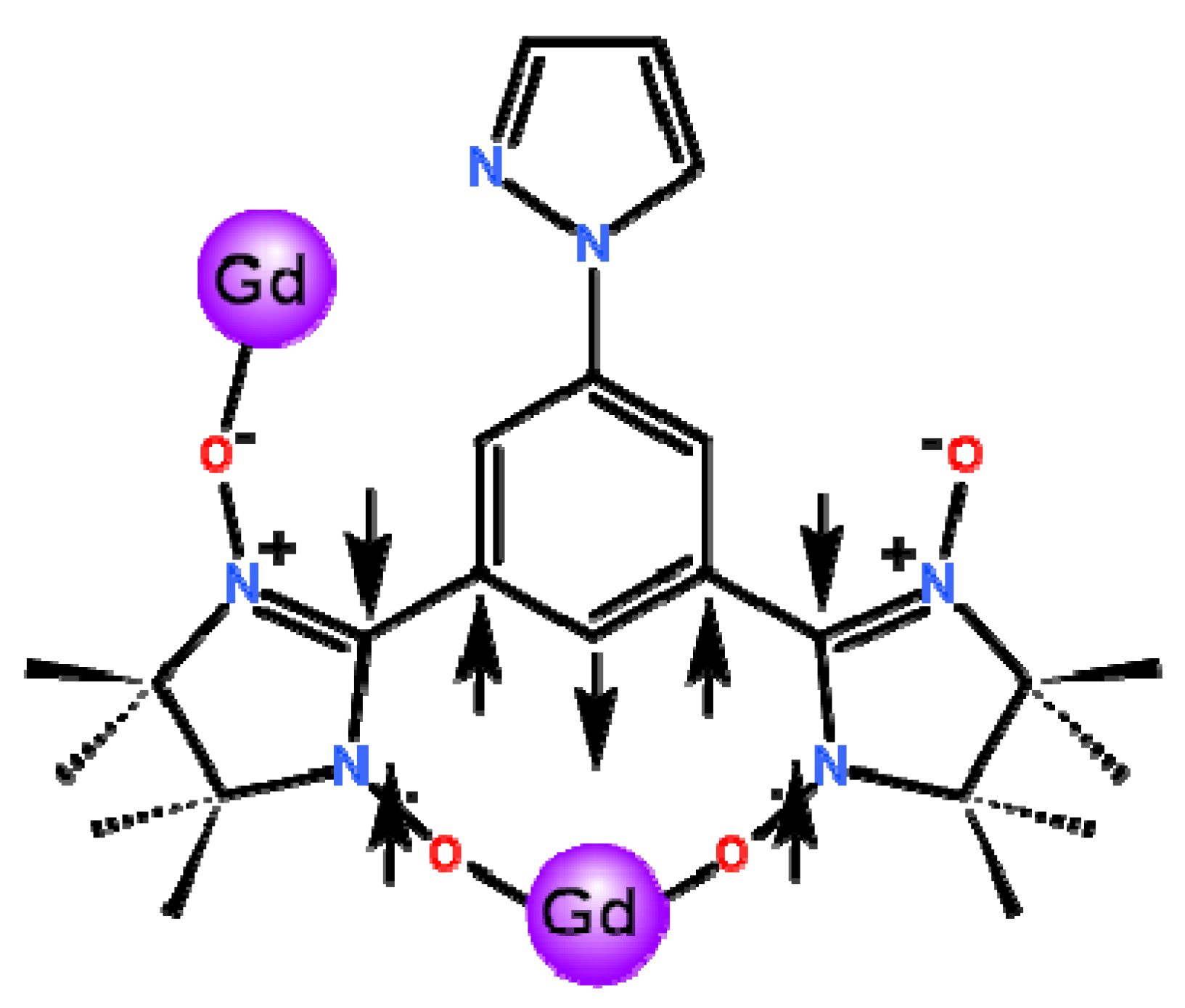
2.1. Synthetic Aspect and Crystal Structures
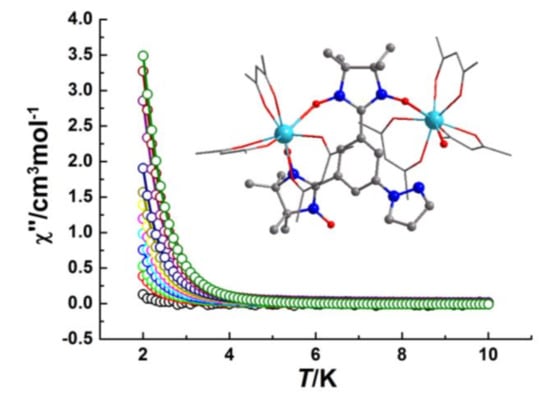
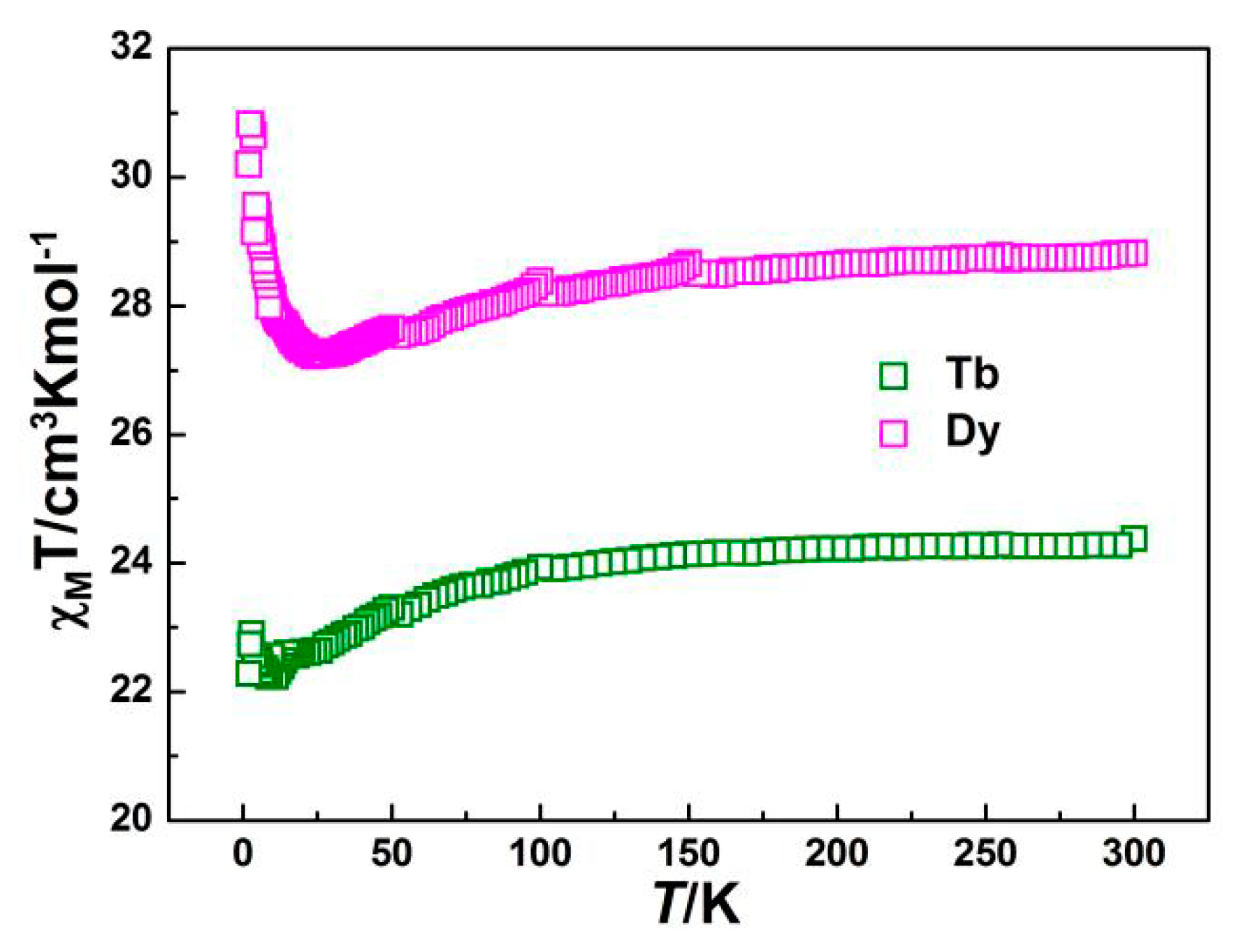
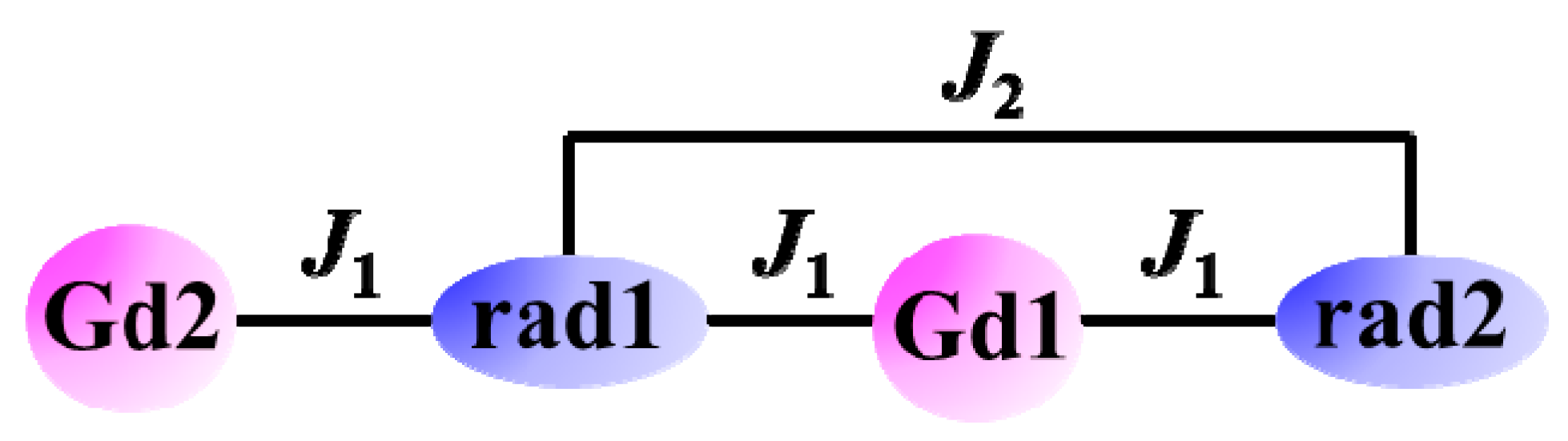
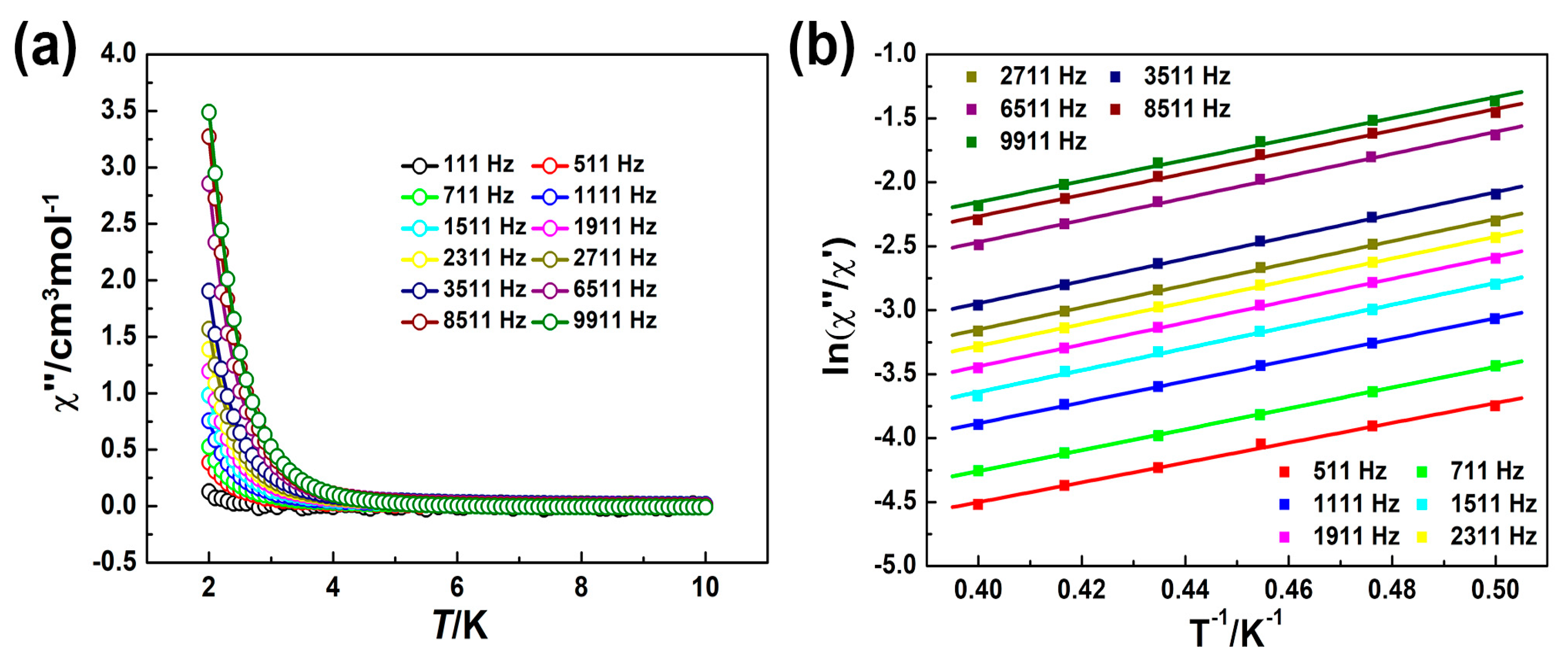
2.2. Magnetic Properties
3. Experimental Section
3.1. Materials and Characterizations
3.2. Preparation of [Ln2(hfac)6(H2O)(NITPhPzbis)]
3.3. X-ray Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demir, S.; Jeon, I.-R.; Long, J.R.; Harris, T.D. Radical Ligand-Containing Single-Molecule Magnets. Coord. Chem. Rev. 2015, 289, 149–176. [Google Scholar] [CrossRef] [Green Version]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide Single-Molecule Magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. A N23−Radical-Bridged Terbium Complex Exhibiting Magnetic Hysteresis at 14 K. J. Am. Chem. Soc. 2011, 133, 14236–14239. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Zadrozny, J.M.; Nippe, M.; Long, J.R. Exchange Coupling and Magnetic Blocking in Bipyrimidyl Radical-Bridged Dilanthanide Complexes. J. Am. Chem. Soc. 2012, 134, 18546–18549. [Google Scholar] [CrossRef] [PubMed]
- Fatila, E.M.; Rouzieres, M.; Jennings, M.C.; Lough, A.J.; Clérac, R.; Preuss, K.E. Fine-Tuning the Single-Molecule Magnet Properties of a [Dy(III)-Radical]2 Pair. J. Am. Chem. Soc. 2013, 135, 9596–9599. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Nippe, M.; Gonzalez, M.I.; Long, J.R. Exchange Coupling and Magnetic Blocking in Dilanthanide Complexes Bridged by the Multi-Electron Redox-Active Ligand 2,3,5,6-Tetra(2-Pyridyl)Pyrazine. Chem. Sci. 2014, 5, 4701–4711. [Google Scholar] [CrossRef]
- Guo, F.-S.; Layfield, R.A. Strong Direct Exchange Coupling and Single-Molecule Magnetism in Indigo-Bridged Lanthanide Dimers. Chem. Commun. 2017, 53, 3130–3133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Perfetti, M.; Kern, M.; Hallmen, P.P.; Ungur, L.; Lenz, S.; Ringenberg, M.R.; Frey, W.; Stoll, H.; Rauhut, G.; et al. Exchange Coupling and Single Molecule Magnetism in Redox-Active Tetraoxolene-Bridged Dilanthanide Complexes. Chem. Sci. 2017, 9, 1221–1230. [Google Scholar] [CrossRef] [Green Version]
- Poneti, G.; Bernot, K.; Bogani, L.; Caneschi, A.; Sessoli, R.; Wernsdorfer, W.; Gatteschi, D. A Rational Approach to the Modulation of the Dynamics of the Magnetisation in a Dysprosium?Nitronyl-Nitroxide Radical Complex. Chem. Commun. 2007, 1807. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. Strong Exchange and Magnetic Blocking in N23−Radical-Bridged Lanthanide Complexes. Nat. Chem. 2011, 3, 538–542. [Google Scholar] [CrossRef]
- Pointillart, F.; Le Guennic, B.; Golhen, S.; Cador, O.; Ouahab, L.S. Low Magnetic Relaxation in Radical Cation Tetrathiaful Valene-Based Lanthanide(III) Dinuclear Complexes. Chem. Commun. 2013, 49, 11632–11634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolinar, B.S.; Gómez-Coca, S.; Alexandropoulos, D.I.; Dunbar, K.R. An Air Stable Radical-Bridged Dysprosium Single Molecule Magnet and Its Neutral Counterpart: Redox Switching of Magnetic Relaxation Dynamics. Chem. Commun. 2017, 53, 2283–2286. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Gonzalez, M.I.; Darago, L.E.; Evans, W.J.; Long, J.R. GIant Coercivity and High Magnetic Blocking Temperatures for N23−Radical-Bridged Dilanthanide Complexes Upon Ligand Dissociation. Nat. Commun. 2017, 8, 2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, C.A.; Darago, L.E.; Gonzalez, M.I.; Demir, S.; Long, J.R. A Trinuclear Radical-Bridged Lanthanide Single-Molecule Magnet. Angew. Chem. Int. Ed. 2017, 56, 10103–10107. [Google Scholar] [CrossRef] [PubMed]
- Dolinar, B.S.; Alexandropoulos, D.I.; Vignesh, K.R.; James, T.; Dunbar, K.R. Lanthanide Triangles Supported by Radical Bridging Ligands. J. Am. Chem. Soc. 2018, 140, 908–911. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.; Prosvirin, A.V.; Zhao, H.; Wernsdorfer, W.; Dunbar, K.R. Heterospin Single-Molecule Magnets Based on Terbium Ions and TCNQF4 Radicals: Interplay between Single-Molecule Magnet and Phonon Bottleneck Phenomena Investigated by Dilution Studies. Chem. Eur. J. 2009, 15, 11390–11400. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.X.; Ma, Y.; Liao, D.Z.; Xu, G.F.; Tang, J.; Wang, C.; Zhou, N.; Yan, S.P.; Cheng, P.; Li, L.C. Four New Lanthanide-Nitronyl Nitroxide (LnIII = PrIII, SmIII, EuIII, TmIII) Complexes and a TbIII Complex Exhibiting Single-Molecule Magnet Behavior. Inorg. Chem. 2009, 48, 8890–8896. [Google Scholar] [CrossRef]
- Pointillart, F.; Bernot, K.; Poneti, G.; Sessoli, R. Crystal Packing Effects on the Magnetic Slow Relaxation of Tb(III)-Nitronyl Nitroxide Radical Cyclic Dinuclear Clusters. Inorg. Chem. 2012, 51, 12218–12229. [Google Scholar] [CrossRef]
- Meihaus, K.R.; Corbey, J.F.; Fang, M.; Ziller, J.W.; Long, J.R.; Evans, W.J. Influence of an Inner-Sphere K+ Ion on the Magnetic Behavior of N23–Radical-Bridged Dilanthanide Complexes Isolated Using an External Magnetic Field. Inorg. Chem. 2014, 53, 3099–3107. [Google Scholar] [CrossRef]
- Chen, P.Y.; Wu, M.Z.; Li, T.; Shi, X.J.; Tian, L.; Liu, Z.Y. Lanthanide Tetranuclear Cage and Mononuclear Cocrystalline Nitronyl Nitroxide Complex with Single-Molecule-Magnet Behavior. Inorg. Chem. 2018, 57, 12466–12470. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Z.; Li, L.; Sutter, J.P. Lanthanide–Nitronyl Nitroxide Chains Derived from Multidentate Nitronyl Nitroxides. Inorg. Chem. 2018, 57, 7507–7511. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Sun, J.; Li, H.; Han, J.; Huang, X.; Li, L. Chain versus Discrete Assembly of Nitronyl Nitroxide Radical-Lanthanide Complexes: Regulating Magnetization Dynamics by Modifying Coordination Symmetry. Cryst. Growth Des. 2020, 20, 3785–3794. [Google Scholar] [CrossRef]
- Wang, K.; Qi, D.; Wang, H.; Cao, W.; Li, W.; Liu, T.; Duan, C.; Jiang, J. Binuclear Phthalocyanine-Based Sandwich-Type Rare Earth Complexes: Unprecedented Two π-Bridged Biradical-Metal Integrated SMMs. Chem. Eur. J. 2013, 19, 11162–11166. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Damjanović, M.; Katoh, K.; Kitagawa, Y.; Yasuda, N.; Lan, Y.; Wernsdorfer, W.; Breedlove, B.K.; Enders, M.; Yamashita, M. Comparison of the Magnetic Anisotropy and Spin Relaxation Phenomenon of Dinuclear Terbium(III) Phthalocyaninato Single-Molecule Magnets Using the Geometric Spin Arrangement. J. Am. Chem. Soc. 2018, 140, 2995–3007. [Google Scholar] [CrossRef]
- Bernot, K.; Pointillart, F.; Rosa, P.; Etienne, M.; Sessoli, R.; Gatteschi, D. Single Molecule Magnet Behaviour in Robust Dysprosium–Biradical Complexes. Chem. Commun. 2010, 46, 6458. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, T.; Tian, L.; Liu, Z.-Y.; Wang, X.G. A Family of Rare Earth Complexes with Chelating Furan Biradicals: Syntheses, Structures and Magnetic Properties. RSC Adv. 2015, 5, 74864–74873. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Yang, M.; Sun, Z.; Xie, J.; Ma, Y.; Li, L. Functionalized Nitronyl Nitroxide Biradical Bridged One-Dimensional Lanthanide Chains: Slow Magnetic Relaxation in the Tb and Dy Analogues. New J. Chem. 2017, 41, 10181–10188. [Google Scholar] [CrossRef]
- Xi, L.; Li, H.; Sun, J.; Ma, Y.; Tang, J.; Li, L. Designing Multicoordinating Nitronyl Nitroxide Radical Toward Multinuclear Lanthanide Aggregates. Inorg. Chem. 2019, 59, 443–451. [Google Scholar] [CrossRef]
- Catala, L.; Le Moigne, J.; Kyritsakas, N.; Rey, P.; Novoa, J.J.; Turek, P. Towards a Better Understanding of the Magnetic Interactions withinm-Phenyleneα-Nitronyl Imino Nitroxide Based Biradicals. Chem. Eur. J. 2001, 7, 2466–2480. [Google Scholar] [CrossRef]
- Luneau, D.; Laugier, J.; Rey, P.; Ulrich, G.; Ziessel, R.; Legoll, P.; Drillon, M. Synthesis, Coordination and Magnetic Properties of a Novel Family of Stable Chelate Based Biradicals: Molecular Structure of a 2,2′-Bipyridine N-Oxide N-Oxyl Biradical and Its Copper(II) Complex. J. Chem. Soc. Chem. Commun. 1994, 6, 741. [Google Scholar] [CrossRef]
- Caneschi, A.; Chiesi, P.; David, L.; Ferraro, F.; Gatteschi, D.; Sessoli, R. Crystal Structure and Magnetic Properties of Two Nitronyl Nitroxide Biradicals and of Their Copper(II) Complexes. Inorg. Chem. 1993, 32, 1445–1453. [Google Scholar] [CrossRef]
- Casanova, D.; Llunell, M.; Alemany, P.; Álvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE 2.1; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Chilton, N.F.; Anderson, R.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-Coupled Polynucleard-And f-Block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.A.; Nelyubina, Y.V.; Kats, S.V.; Penkova, L.V.; Efimov, N.N.; Dmitrienko, A.O.; Vologzhanina, A.V.; Belov, A.S.; Voloshin, Y.Z.; Novikov, V.V. Polymorphism in a Cobalt-Based Single-Ion Magnet Tuning Its Barrier to Magnetization Relaxation. J. Phys. Chem. Lett. 2016, 7, 4111–4116. [Google Scholar] [CrossRef]
- Benelli, C.; Caneschi, A.; Gatteschi, D.; Guillou, O.; Pardi, L. Synthesis, Crystal Structure, and Magnetic Properties of Tetranuclear Complexes Containing Exchange-Coupled Dilanthanide-Dicopper (Lanthanide = Gadolinium, Dysprosium) Species. Inorg. Chem. 1990, 29, 1750–1755. [Google Scholar] [CrossRef]
- Andruh, M.; Ramade, I.; Codjovi, E.; Guillou, O.; Kahn, O.; Trombe, J.C. Crystal Structure and Magnetic Properties of [Ln2Cu4] Hexanuclear Clusters (Where Ln = Trivalent Lanthanide). Mechanism of the Gadolinium(III)-Copper(II) Magnetic Interaction. J. Am. Chem. Soc. 1993, 115, 1822–1829. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Li, L.C.; Liao, D.Z.; Jiang, Z.H.; Yan, S.P.; Fang, R.B. Synthesis and Properties of the Complexes of Lanthanides with Nitronylnitroxidebiradical. Chin. J. Chem. 2000, 18, 561–564. [Google Scholar]
- Benelli, C.; Caneschi, A.; Gatteschi, D.; Pardi, L.; Rey, P. Linear-Chain Gadolinium(III) Nitronyl Nitroxide Complexes with Dominant Next-Nearest-Neighbor Magnetic Interactions. Inorg. Chem. 1990, 29, 4223–4228. [Google Scholar] [CrossRef]
- Tian, L.; Sun, Y.-Q.; Na, B.; Cheng, P. A Family of Homologous Heterospin Complexes Based on Lanthanides and Biradical Ligands. Eur. J. Inorg. Chem. 2013, 2013, 4329–4335. [Google Scholar] [CrossRef]
- Gupta, T.; Rajeshkumar, T.; Rajaraman, G. Magnetic Exchange in {Gd(III)-Radical Complexes: Method Assessment, Mechanism of Coupling and Magneto-Structural Correlations. Phys. Chem. Chem. Phys. 2014, 16, 14568–14577. [Google Scholar] [CrossRef]
- Ke, H.; Xu, G.F.; Guo, Y.N.; Gamez, P.; Beavers, C.M.; Teat, S.J.; Tang, J. A Linear Tetranucleardysprosium(III) Compound Showing Single-Molecule Magnet Behavior. Chem. Commun. 2010, 46, 6057–6059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Zhao, L.; Lin, H.; Tang, J.; Li, G. Butterfly-Shaped Pentanuclear Dysprosium Single-Molecule Magnets. Chem. Eur. J. 2013, 19, 13235–13241. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Montigaud, V.; Cador, O.; Wu, J.; Zhao, L.; Li, X.-L.; Guo, M.; Le Guennic, B.; Tang, J. Lanthanide(III) Hexanuclear Circular Helicates: Slow Magnetic Relaxation, Toroidal Arrangement of Magnetic Moments, and Magnetocaloric Effects. Inorg. Chem. 2019, 58, 11903–11911. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Zhang, D.; Zhu, D.-B. Field-Induced Single-Ion Magnets Based on Enantiopure Chiral β-Diketonate Ligands. Inorg. Chem. 2013, 52, 8933–8940. [Google Scholar] [CrossRef]
- Bartolomé, J.; Filoti, G.; Kuncser, V.; Schinteie, G.; Meracre, V.; Anson, C.E.; Powell, A.K.; Prodius, D.; Turta, C. Magnetostructural Correlations in the Tetranuclear Series of {Fe3LnO2} Butterfly Core Clusters: Magnetic and Mössbauer Spectroscopic Study. Phys. Rev. B. 2009, 80, 014430. [Google Scholar] [CrossRef]
- Luis, F.; Bartolomé, J.; Fernandez, J.F.; Tejada, J.; Hernández, J.M.; Zhang, X.X.; Ziolo, R. Thermally Activated and Field-Tuned Tunneling in Mn12Ac Studied by Ac Magnetic Susceptibility. Phys. Rev. B 1997, 55, 11448–11456. [Google Scholar] [CrossRef] [Green Version]
- Gatteschi, D.; Sessoli, R. Quantum Tunneling of Magnetization and Related Phenomena in Molecular Materials. Angew. Chem. Int. Ed. 2003, 42, 268–297. [Google Scholar] [CrossRef]
- Chilton, N.F.; Collison, D.; McInnes, E.J.L.; Winpenny, R.E.P.; Soncini, A. An Electrostatic Model for the Determination of Magnetic Anisotropy in Dysprosium Complexes. Nat. Commun. 2013, 4, 2551. [Google Scholar] [CrossRef]
- Ruamps, R.; Maurice, R.; de Graaf, C.; Guihery, N. Interplay between Local Anisotropies in Binuclear Complexes. Inorg. Chem. 2014, 53, 4508–4516. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Pineda, E.; Chilton, N.F.; Marx, R.; Dörfel, M.; Sells, D.O.; Neugebauer, P.; Jiang, S.-D.; Collison, D.; Van Slageren, J.; McInnes, E.J.L.; et al. Direct Measurement of Dysprosium(III) Dysprosium(III) Interactions in a Single-Molecule Magnet. Nat. Commun. 2014, 5, 5243. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Zheng, Y.; Lin, W.-Q.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Tong, M.-L. Switching the Anisotropy Barrier of a Single-Ion Magnet by Symmetry Change From Quasi-D5h to Quasi-Oh. Chem. Sci. 2013, 4, 3310–3316. [Google Scholar] [CrossRef]
- Bernot, K.; Bogani, L.; Sessoli, R.; Gatteschi, D. [TmIII(hfac)3(NITPhOPh)]∞: A New Member of a Lanthanide-Based Single Chain Magnets Family. Inorg. Chim. Acta 2007, 360, 3807–3812. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, L.C.; Liao, D.Z. Slow Magnetic Relaxation in Lanthanide Complexes with Chelating Nitrony lnitroxide Radical. Inorg. Chem. 2010, 49, 4735–4737. [Google Scholar] [CrossRef] [PubMed]
- Ullman, E.F.; Call, L.; Osiecki, J.H. Stable Free Radicals. VIII. New Imino, Amidino, and Carbamoy lnitroxides. J. Org. Chem. 1970, 35, 3623–3631. [Google Scholar] [CrossRef]
- Ullman, E.F.; Osiecki, J.H.; Boocock, D.G.B.; Darcy, R. Stable Free Radicals. X. Nitronylnitroxidemonoradicals and Biradicals as Possible Small Molecule Spin Labels. J. Am. Chem. Soc. 1972, 94, 7049–7059. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: Weinheim, Germany, 1993. [Google Scholar]
- Sheldrick, G.M. SHELXS-2014, Program for Structure Solution; Universität of Göttingen: Gottingen, Germany, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXL-2014, Program for Structure Refinement; Universität of Göttingen: Göttingen, Germany, 2014. [Google Scholar]



| Complex | 1 Gd | 2 Tb | 3 Dy |
|---|---|---|---|
| Formula | C53H38F36Gd2N6O17 | C53H38F36Tb2N6O17 | C53H38F36Dy2N6O17 |
| M, g·mol−1 | 2029.39 | 2032.73 | 2039.89 |
| T/K | 113(2) | 113(2) | 113(2) |
| Crystal system | triclinic | triclinic | triclinic |
| Space group | Pī | Pī | Pī |
| a/Å | 11.976(2) | 11.997(2) | 11.997(2) |
| b/Å | 13.060(3) | 13.050(3) | 13.009(3) |
| c/Å | 26.438(5) | 26.470(5) | 26.471(5) |
| α/deg | 78.39(3) | 78.04(3) | 78.07(3) |
| β/deg | 77.93(3) | 77.70(3) | 77.76(3) |
| γ/deg | 64.89(3) | 64.80(3) | 64.83(3) |
| V/Å3 | 3632.1(16) | 3631.8(16) | 3622.2(16) |
| Z | 2 | 2 | 2 |
| Dcalcd/g·cm–3 | 1.856 | 1.859 | 1.870 |
| μ/mm−1 | 1.969 | 2.090 | 2.206 |
| θ/deg | 1.588–25.009 | 1.895–25.009 | 1.588–25.009 |
| F(000) | 1972 | 1976 | 1980 |
| Reflns collected | 27,871 | 30,362 | 34,633 |
| Unique reflns/Rint | 12,651/0.0717 | 12,514/0.0512 | 12,738/0.0711 |
| GOF (F2) | 1.078 | 1.034 | 1.019 |
| R1/wR2 (I > 2σ(I)) | 0.0717/0.1782 | 0.0522/0.1416 | 0.0571/0.1538 |
| R1/wR2 (all data) | 0.0921/0.2202 | 0.0632/0.1509 | 0.0737/0.1752 |
| Complex | 1 Gd | 2 Tb | 3 Dy |
|---|---|---|---|
| Ln–Orad | 2.383(8) 2.472(7) 2.454(7) | 2.365(5) 2.466(4) 2.437(4) | 2.352(6) 2.460(5) 2.429(5) |
| Ln–Ohfac Ln–OH2O | 2.343(9)–2.412(7) 2.378(7) | 2.323(5)–2.399(5) 2.373(4) | 2.306(6)–2.390(5) 2.364(5) |
| Orad–Ln–Orad | 82.0(3) | 82.34(16) | 82.0(2) |
| Orad–Ln–OH2O | 77.7(2) | 77.91(15) | 77.69(17) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, L.; Han, J.; Huang, X.; Li, L. Nitronyl Nitroxide Biradical-Based Binuclear Lanthanide Complexes: Structure and Magnetic Properties. Magnetochemistry 2020, 6, 48. https://doi.org/10.3390/magnetochemistry6040048
Xi L, Han J, Huang X, Li L. Nitronyl Nitroxide Biradical-Based Binuclear Lanthanide Complexes: Structure and Magnetic Properties. Magnetochemistry. 2020; 6(4):48. https://doi.org/10.3390/magnetochemistry6040048
Chicago/Turabian StyleXi, Lu, Jing Han, Xiaohui Huang, and Licun Li. 2020. "Nitronyl Nitroxide Biradical-Based Binuclear Lanthanide Complexes: Structure and Magnetic Properties" Magnetochemistry 6, no. 4: 48. https://doi.org/10.3390/magnetochemistry6040048
APA StyleXi, L., Han, J., Huang, X., & Li, L. (2020). Nitronyl Nitroxide Biradical-Based Binuclear Lanthanide Complexes: Structure and Magnetic Properties. Magnetochemistry, 6(4), 48. https://doi.org/10.3390/magnetochemistry6040048