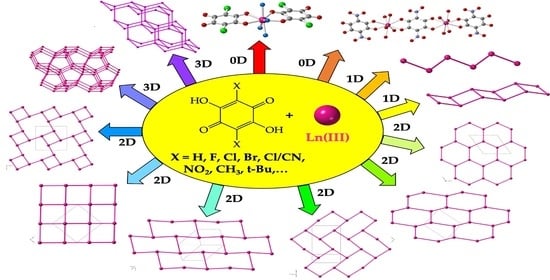
Lanthanoid-Anilato Complexes and Lattices †
Abstract
:1. Introduction
2. Structural Classification
2.1. Discrete (0D) Complexes
| # | CCDC | Structure | Ln | X | Geometry a | L b | Disposition | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | PIQFUT | Monomer | Dy | Cl | SAPR-8 | Tp- | 33 | [19] |
| 2 | DEHQUF | Dimer | Dy | H | PBPY-7 | thf/Cl- | 030/101 | [21] |
| 3 | DEHRAM | Dimer | Y | H | PBPY-7 | thf/Cl- | 030/101 | [21] |
| 4 | DEKTOF | Dimer | Dy | Cl | TDD-8 | Tp- | [20] | |
| 5 | DEKTOF01 | Dimer | Dy | Cl | TDD-8 | Tp- | [22] | |
| 6 | DEKTOF02 | Dimer | Dy | Cl | TDD-8 | Tp- | [19] | |
| 7 | DEKTUL | Dimer | Y | Cl | TDD-8 | Tp- | [20] | |
| 8 | DEKTUL01 | Dimer | Y | Cl | TDD-8 | Tp- | [22] | |
| 9 | DEKVAT | Dimer | Y | Cl | TDD-8 | Tp- | [20] | |
| 10 | DEKVEX | Dimer | Y | H | SAPR-8 | Tp- | [20] | |
| 11 | DEKVIB | Dimer | Y | CH3 | SAPR-8 | Tp- | [20] | |
| 12 | DEKVOH | Dimer | Y | CH3 | TDD-8 | Tp- | [20] | |
| 13 | EDEZAR | Dimer | Gd | NO2 | CSAPR-9 | H2O | 032 | [26] |
| 14 | EDEZEV | Dimer | Tb | NO2 | CSAPR-9 | H2O | 032 | [26] |
| 15 | EDEZIZ | Dimer | Dy | NO2 | CSAPR-9 | H2O | 032 | [26] |
| 16 | EDEZOF | Dimer | Ho | NO2 | CSAPR-9 | H2O | 032 | [26] |
| 17 | EDEZUL | Dimer | Sm | NO2 | CSAPR-9 | H2O | 032 | [26] |
| 18 | JOQSEQ | Dimer | Dy | Br | TDD-8 | Tp- | [23] | |
| 19 | JOQSIU | Dimer | Dy | F | SAPR-8 | Tp- | 33 | [23] |
| 20 | JOQSOA | Dimer | Y | F | SAPR-8 | Tp- | 33 | [23] |
| 21 | JOQSUG | Dimer | Y | Br | TDD-8 | Tp- | [23] | |
| 22 | KOZBEJ | Dimer | Er | Cl | TDD-8 | Tp- | [24] | |
| 23 | KOZBIN | Dimer | Ho | Cl | TDD-8 | Tp- | [24] | |
| 24 | LEPNIG | Dimer | Tb | Cl | TDD-8 | Tp- | [22] | |
| 25 | LEPNIG01 | Dimer | Tb | Cl | TDD-8 | Tp- | [24] | |
| 26 | LEPNOM | Dimer | Gd | Cl | TDD-8 | Tp- | [22] | |
| 27 | LEPNOM01 | Dimer | Gd | Cl | TDD-8 | Tp- | [19] | |
| 28 | OBIBEH | Dimer | Yb | Cl | TDD-8 | Tp- | [25] | |
| 29 | OBIBEH01 | Dimer | Yb | Cl | TDD-8 | Tp- | [24] | |
| 30 | NOQBUT | Dimer-zz | Eu | Cl/CN | CSAPR-9 | H2O | 032 | [27] |
| 31 | NOQGEI | Dimer-zz | Eu/Dy c | Cl/CN | CSAPR-9 | H2O | 032 | [27] |
| 32 | DEKVUN | Tetramer | Y | CH3 | TDD-8 | Tp-/(MeO)4B/MeOH | [20] | |
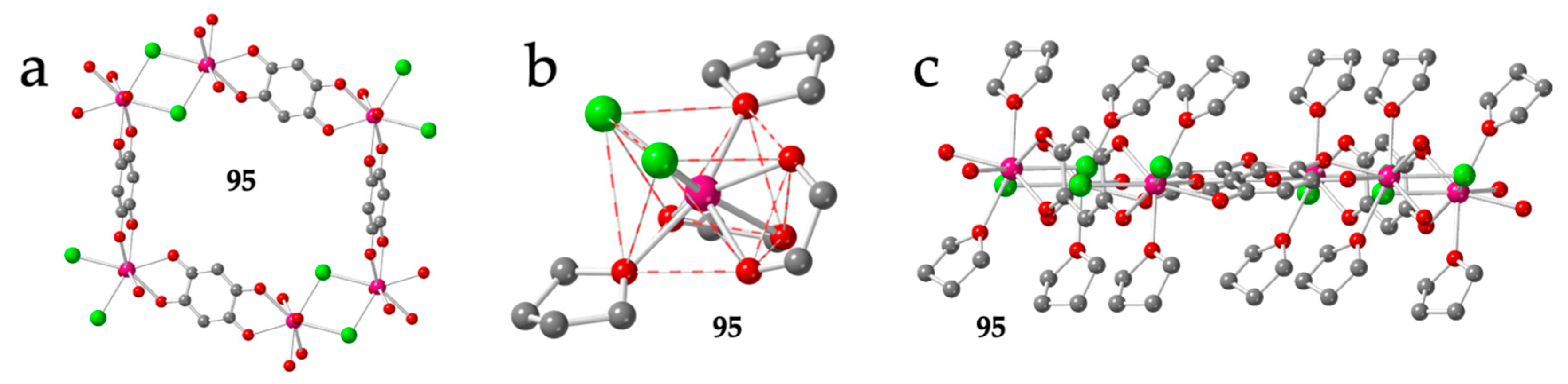
| 33 | MIZZUQ | 1D + mon | Lu | Cl | TDD-8 | H2O | [16] | |
| 34 | NIGNID | 1D-ladder | Er1/Er2 | Cl | BTPR-8/BTPR-8 | hmpa | [28] |
2.2. One Dimensional (1D) Lattices
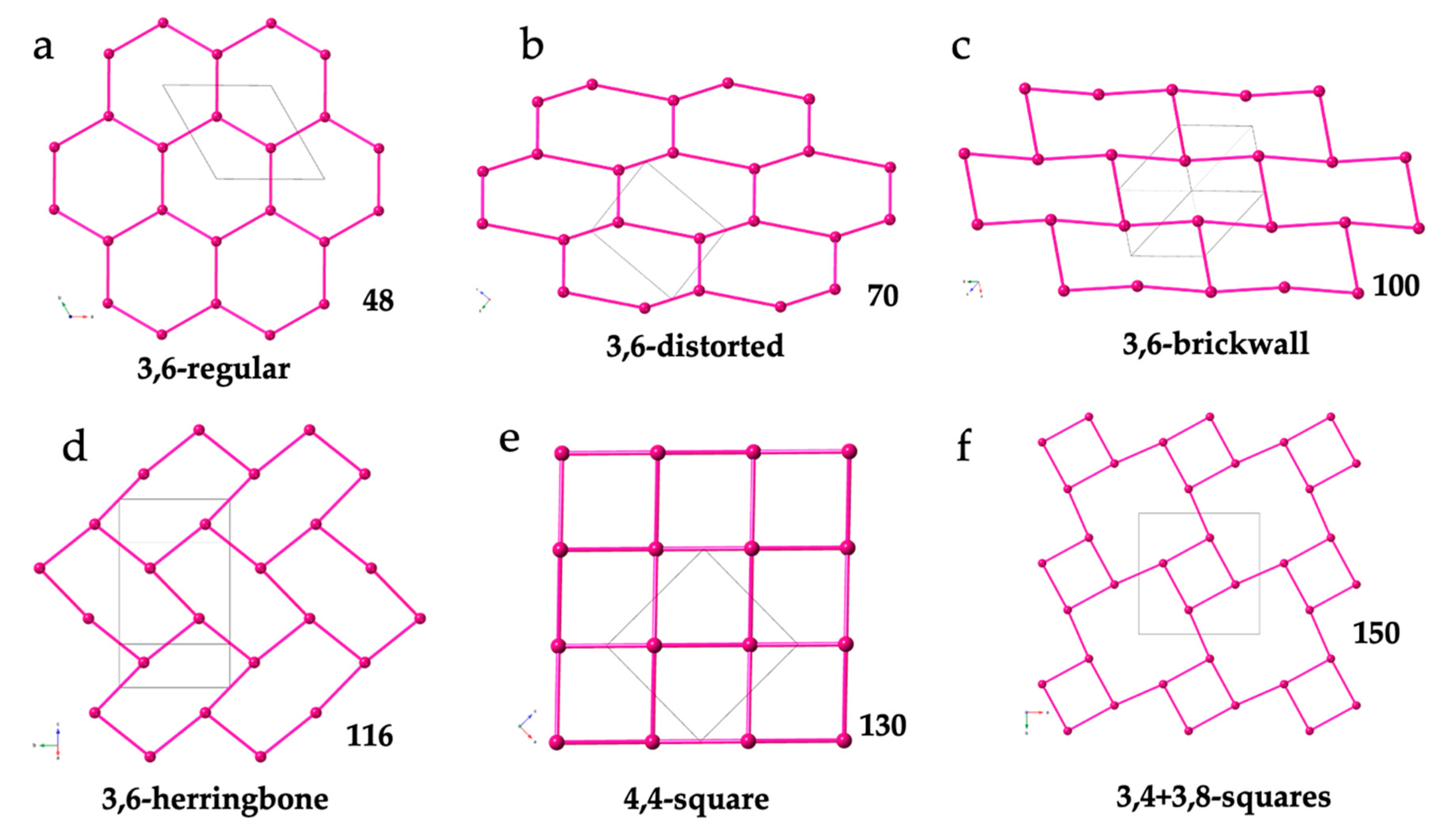
2.3. Two-Dimensional (2D) Lattices
2.3.1. Two-Dimensional Regular Hexagonal Lattices
| # | CCDC | Ln | X | Geometry a | L b | Disposition | α (°) c | Pk d | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 35 | KUYKIZ | Ho | H | TCTPR-9 | H2O | 300 | 107.7 | AL | [43] |
| 36 | MIZXAU | La | H | TCTPR-9 | H2O | 300 | 107.2 | AL | [16] |
| 37 | MIZXEY | Gd | H | TCTPR-9 | H2O | 300 | 107.6 | AL | [16] |
| 38 | MIZXIC | Yb | H | TCTPR-9 | H2O | 300 | 107.8 | AL | [16] |
| 39 | MIZXOI | Lu | H | TCTPR-9 | H2O | 300 | 107.7 | AL | [16] |
| 40 | MIZXUO | Y | H | TCTPR-9 | H2O | 300 | 107.7 | AL | [16] |
| 41 | PIVKAJ | Er | H | TCTPR-9 | H2O | 300 | 107.7 | AL | [44] |
| 42 | ZOTTAD | Ce | H | TCTPR-9 | H2O | 300 | 107.3 | AL | [15] |
| 43 | 1944109 | Pr | H | TCTPR-9 | H2O | 300 | 107.3 | AL | [45] |
| 44 | 1944110 | Nd | H | TCTPR-9 | H2O | 300 | 107.4 | AL | [45] |
| 45 | 1944111 | Sm | H | TCTPR-9 | H2O | 300 | 107.4 | AL | [45] |
| 46 | 1944112 | Eu | H | TCTPR-9 | H2O | 300 | 107.5 | AL | [45] |
| 47 | 1944113 | Tb | H | TCTPR-9 | H2O | 300 | 107.6 | AL | [45] |
| 48 | 1944114 | Dy | H | TCTPR-9 | H2O | 300 | 107.6 | AL | [45] |
| 49 | 1944117 | Tm | H | TCTPR-9 | H2O | 300 | 107.7 | AL | [45] |
| 50 | DIFLEM | Ce | Cl/CN | CSAPR-9 | dmf | 021 | 99.6 | AL | [46] |
| 51 | WOTWIO | Dy | Cl/CN | CSAPR-9 | dmf | 021 | 102.0 | AL | [47] |
| 52 | WOTWOU | Ho | Cl/CN | CSAPR-9 | dmf | 021 | 102.2 | AL | [47] |
| 53 | XIKNOX | Nd | Cl/CN | CSAPR-9 | dmf | 021 | 101.3 | AL | [48] |
| 54 | XIKPAL | Er | Cl/CN | CSAPR-9 | dmf | 021 | 103.0 | AL | [48] |
| 55 | XOYTEN | Dy | Cl/CN | CSAPR-9 | dmf | 021 | 101.8 | AL | [38] |
| 56 | NIGNUP | Er | Cl | CSAPR-9 | fma | 021 | 127.2 | AL | [28] |
2.3.2. Two-Dimensional Distorted Hexagonal Lattices
| CCDC | Ln | X | Geometry a | L b | Disposition | Type | α (°) c | Pk d | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|
| 57 | GEQBAH e | Gd | Cl | TCTPR-9 | H2O | 111 | IIa | 138.0 | AL | [49] |
| 58 | MIZYID | Pr | Cl | TCTPR-9 | H2O | 111 | IIa | 134.9 | AL | [16] |
| 59 | MIZYOJ | Nd | Cl | TCTPR-9 | H2O | IIa | – | AL | [16] | |
| 60 | MIZYUP | Tb | Cl | TCTPR-9 | H2O | 111 | IIa | 135.8 | AL | [16] |
| 61 | MIZZAW e | Ce | Cl | TCTPR-9 | H2O | 111 | IIa | 133.7 | AL | [16] |
| 62 | MIZZAW01 | Ce | Cl | TCTPR-9 | H2O | 111 | IIa | 134.6 | AL | [16] |
| 63 | MIZZEA | Y | Cl | TCTPR-9 | H2O | 111 | IIa | 136.1 | AL | [16] |
| 64 | MIZZIE | Gd | Cl | TCTPR-9 | H2O | 111 | IIb | 136.2 | AL | [16] |
| 65 | MIZZOK | Eu | Cl | TCTPR-9 | H2O | IIb | – | AL | [16] | |
| 66 | NIGNEZ | Er | Cl | TCTPR-9 | H2O | 201 | IIb | 136.6 | AL | [28] |
| 67 | 1944118 | La | Cl | TCTPR-9 | H2O | 111 | IIa | 133.4 | AL | [45] |
| 68 | 1944120 | Sm | Cl | TCTPR-9 | H2O | 111 | IIa | 135.0 | AL | [45] |
| 69 | 1944123 | Dy | Cl | TCTPR-9 | H2O | 111 | IIa | 135.7 | AL | [45] |
| 70 | 1944124 | Ho | Cl | TCTPR-9 | H2O | 111 | IIa | 136.0 | AL | [45] |
| 71 | XAWZUT | Er | Br | TCTPR-9 | H2O | 111 | IIa | 138.4 | AL | [50] |
| 72 | 1565271 | La | Br | TCTPR-9 | H2O | 111 | IIa | 135.0 | AL | [45] |
| 73 | 1565272 | Ce | Br | TCTPR-9 | H2O | 111 | IIa | 136.2 | AL | [45] |
| 74 | 1565273 | Pr | Br | TCTPR-9 | H2O | 111 | IIa | 137.8 | AL | [45] |
| 75 | 1565274 | Nd | Br | TCTPR-9 | H2O | 111 | IIa | 136.9 | AL | [45] |
| 76 | 1565275 | Sm | Br | TCTPR-9 | H2O | 111 | IIa | 137.7 | AL | [45] |
| 77 | 1565276 | Eu | Br | TCTPR-9 | H2O | 111 | IIa | 137.4 | AL | [45] |
| 78 | 1565277 | Gd | Br | TCTPR-9 | H2O | 111 | IIa | 137.7 | AL | [45] |
| 79 | 1565278 | Tb | Br | TCTPR-9 | H2O | 111 | IIa | 138.3 | AL | [45] |
| 80 | 1565279 | Dy | Br | TCTPR-9 | H2O | 111 | IIa | 138.1 | AL | [45] |
| 81 | 1565280 | Ho | Br | TCTPR-9 | H2O | 111 | IIa | 138.3 | AL | [45] |
| 82 | NIDFOY | Tb | Br | TDD-8 | dmso | trans | IIb | 141.0 | AL | [51] |
| 83 | NIDFUE | Dy | Br | TDD-8 | dmso | trans | IIb | 140.7 | AL | [51] |
| 84 | NIDGAL | Ho | Br | TDD-8 | dmso | trans | IIb | 141.4 | AL | [51] |
| 85 | XAXBAC | Er | Br | TDD-8 | dmso | trans | IIb | 140.7 | AL | [50] |
| 86 | NIDGEP | Yb | Br | TDD-8 | dmso | trans | IIb | 141.3 | AL | [51] |
| 87 | DIFLUC | Yb | Cl/CN | TDD-8 | dmso | trans | IIb | 150.0 | AL | [46] |
| 88 | POMTUJ | Yb | Cl/CN | TDD-8 | dmso | trans | IIb | 144.8 | AL | [37] |
| 89 | POMVAR | Yb/Er | Cl/CN | TDD-8 | dmso | trans | IIb | 145.2 | AL | [37] |
| 90 | NIGQIG | Er | Cl | TDD-8 | dmso | trans | IIb | 141.0 | AL | [28] |
| 91 | NIGNOJ | Er | Cl | TDD-8 | dmso | trans | IIb | 142.3 | AL | [28] |
| 92 | KUVBIP | La | t-Bu | TDD-8 | dma | cis | IIb | 138.3 | EC | [52] |
| 93 | KUVBOV | Pr | t-Bu | TDD-8 | dma | cis | IIb | 136.6 | EC | [52] |
| 94 | KUVBUB | Nd | t-Bu | TDD-8 | dma | cis | IIb | 135.8 | EC | [52] |
| 95 | LEBGEG | Gd | H | SAPR-8 | thf/Cl- | 20 | – | 140.8 | AL | [53] |
2.3.3. 2D Rectangular Lattices
| # | CCDC | Ln | X | Geometry a | L b | Disposition | α (°)c | Pk d | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 96 | CAZZAE | Pr | Cl | TCTPR-9 | EtOH | 201 | 162.1 | AL | [13] |
| 97 | GASMUI | Y | Cl | TCTPR-9 | H2O | 201 | 169.9 | AL | [14] |
| 98 | JOGJAT | Er | Cl | CSAPR-9 | H2O | 030 | 166.4 | AL | [54] |
| 99 | 1944126 | Tm | Cl | TCTPR-9 | H2O | 210 | 171.5 | AL | [45] |
| 100 | 1944127 | Yb | Cl | TCTPR-9 | H2O | 210 | 171.3 | AL | [45] |
| 101 | GASMOC | Y | Br | TCTPR-9 | H2O | 210 | 169.5 | AL | [14] |
| 102 | 1565282 | Tm | Br | TCTPR-9 | H2O | 210 | 169.0 | AL | [45] |
| 103 | LUTRIE | Yb | Br | TCTPR-9 | H2O | 210 | 168.8 | AL | [55] |
| 104 | DIFLOW | Pr | Cl/CN | CSAPR-9 | dmso | 030 | 164.0 | EC | [46] |
| 105 | NOQGAE | Eu/Dy | Cl/CN | CSAPR-9 | dmso | 030 | 163.2 | EC | [27] |
| 106 | WOTWUA | Ce | Cl/CN | CSAPR-9 | dmso | 030 | 164.4 | EC | [47] |
| 107 | WOTXAH | Nd | Cl/CN | CSAPR-9 | dmso | 030 | 163.8 | EC | [47] |
| 108 | XOYTUD | Dy | Cl/CN | CSAPR-9 | dmso | 030 | 162.6 | EC | [38] |
| 109 | WOTWEK | Nd | Cl/CN | CSAPR-9 | dmf | 012 | 157.9 | EC | [47] |
| 110 | GEPZUY | Eu | Cl | TCTPR-9 | bipym/H2O | 110/100 | 172.7 | EC | [49] |
| 111 | QOVJUJ | Yb1/Yb2 | Cl/CN | TDD-8/TDD-8 | dmso/dobdc2− | trans | 144.4/165.1 | AL | [56] |
| 112 | QOVJOD | Yb | Cl/CN | TDD-8 | dmso/F4bdc2− | – | 158.4 | AL | [56] |
| # | CCDC | Ln | X | Geometry a | L b | Disposition | α (°) c | Pk d | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 113 | GEQBEL | Gd | Cl | CSAPR-9 | H2O | 030 | 159.9 | EC | [49] |
| 114 | JOGHAR | Ce | Cl | CSAPR-9 | H2O | 030 | 164.5 | AL | [54] |
| 115 | NIGPAX | Er | Cl | CSAPR-9 | dmf | 030 | 160.3 | EC | [28] |
| 116 | NIDLIY | La | Br | CSAPR-9 | dmso | 030 | 165.5 | EC | [51] |
| 117 | NIDLOE | Ce | Br | CSAPR-9 | dmso | 030 | 165.4 | EC | [51] |
| 118 | NIDLUK | Pr | Br | CSAPR-9 | dmso | 030 | 165.5 | EC | [51] |
| 119 | NIDMAR | Nd | Br | CSAPR-9 | dmso | 030 | 164.0 | EC | [51] |
| 120 | NIDMEV | Sm | Br | CSAPR-9 | dmso | 030 | 164.0 | EC | [51] |
| 121 | NIDFEO | Eu | Br | CSAPR-9 | dmso | 030 | 163.8 | EC | [51] |
| 122 | NIDFIS | Gd | Br | CSAPR-9 | dmso | 030 | 163.7 | EC | [51] |
| 123 | NOQBON | Eu/Dy e | Br | CSAPR-9 | dmso | 030 | 163.9 | EC | [27] |
| 124 | XAXBEG | Er | Br | CSAPR-9 | dmf | 030 | 159.0 | EC | [50] |
| 125 | LUTROK | Dy | Br | CSAPR-9 | dmf | 030 | 158.9 | EC | [55] |
| 126 | DIFLIQ | Pr | Cl/CN | CSAPR-9 | dmf | 030 | 170.7 | EC | [46] |
| 127 | XIKNUD | Yb | Cl/CN | CSAPR-9 | dmf | 030 | 167.0 | EC | [48] |
2.3.4. Other 2D (Square) Lattices
| # | CCDC | Structure | Ln | X | Geometry a | A+ | L b | Disposition | α (°) c | Pk d | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 128 | MIZYAV | 3,4 | La | Cl | JSPC-10 | – | H2O | – | 121.3 | EC | [16] |
| 129 | DIFMAJ | 4,4 | Dy | Cl/CN | CSAPR-9 | H3O+ | H2O | 100 | 89.2 | EC | [46] |
| 130 | XOYVAL | 4,4 | Dy | Cl/CN | CSAPR-9 | NEt2H2+ | H2O | 100 | 94.1 | EC | [38] |
| 131 | QOFBAR | 4,4 | Y | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 132 | QOFBOF | 4,4 | Y | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 133 | QOFBUL | 4,4 | Y | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 134 | QOFCAS | 4,4 | Y | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 135 | QOFCEW | 4,4 | Dy | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 136 | QOFNAD | 4,4 | Gd | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 137 | QOFNEH | 4,4 | Yb | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 138 | QOFNOR | 4,4 | Er | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 139 | QOFNUX | 4,4 | Y | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 140 | QOFPAF | 4,4 | Tb | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 141 | QOFPEJ | 4,4 | Lu | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 142 | QOFPIN | 4,4 | Ho | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [57] |
| 143 | WOXTIP | 4,4 | Nd | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [58] |
| 144 | WOXTOV | 4,4 | Eu | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [58] |
| 145 | WOXTUB | 4,4 | Sm | Cl | SAPR-8 | NEt4+ | – | – | 90.0 | EC | [58] |
| 146 | WOXVEN | 4,4 | Ce | Cl | CSAPR-9 | NEt4+ | H2O | 100 | 90.0 | EC | [58] |
| 147 | WOXVIR | 4,4 | La | Cl | CSAPR-9 | NEt4+ | H2O | 100 | 90.0 | EC | [58] |
| 148 | WOXVOX | 4,4 | Nd | Cl | CSAPR-9 | NEt4+ | H2O | 100 | 90.0 | EC | [58] |
| 149 | POMVIZ | (3,4) (3,8) | Er | Cl/CN | CSAPR-9 | dmso | 030 | 175.0 90.0 | AL | [37] | |
| 150 | XOYVEP | (3,4) (3,8) | Dy | Cl/CN | CSAPR-9 | dmso | 030 | 171.8 90.0 | AL | [38] |
2.4. Three Dimensional (3D) Lattices
| # | CCDC | Structure | Ln | X | Geometry a | A+ | L b | Disposition | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 151 | MOBBIO | 3D-diam | Y | Cl | TDD-8 | H3O+ | – | – | [16] |
| 152 | EFOXUV | 3D-diam | Tb | Cl | CSAPR-9 | NMe2H2+ | H2O/dmf/HCOO- | 001/010/010 | [60] |
| 153 | JOGHEV | 3D-diam | Er | Cl | SAPR-8 | DPMP+ | – | – | [54] |
| 154 | JOGHIZ | 3D-diam | Ce | Cl | CSAPR-9 | DPMP+ | H2O | 010 | [54] |
| 155 | JOGHOF | 3D-diam | Ce | Cl | TCTPR-9 | PPh4+ | H2O | 010 | [54] |
| 156 | JOGHUL | 3D-noq | Ce1 Ce2 Ce3 | Cl | CSAPR-9 CSAPR-9 TCTPR-9/MUFF | PPh3Me+ | H2O H2O H2O | 010 001 100 | [54] |
| 157 | JOGJEX | 3D-4,4-c | Er1/Er2 | Cl | CSAPR-9/TDD-8 | PPh3Me+ | H2O/- | 010/- | [54] |
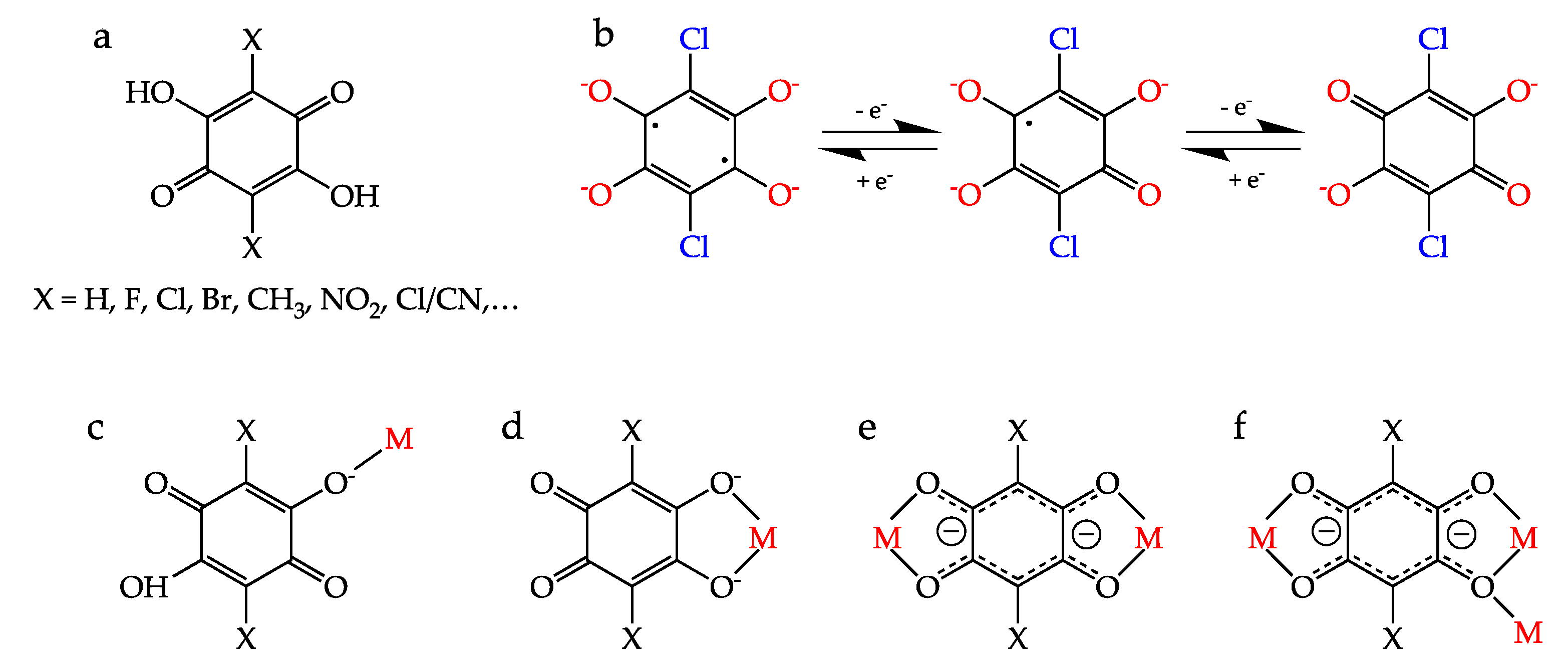
2.5. Anilato-Type Ligands
2.6. Lanthanoid Metal Ions
3. Magnetic Properties
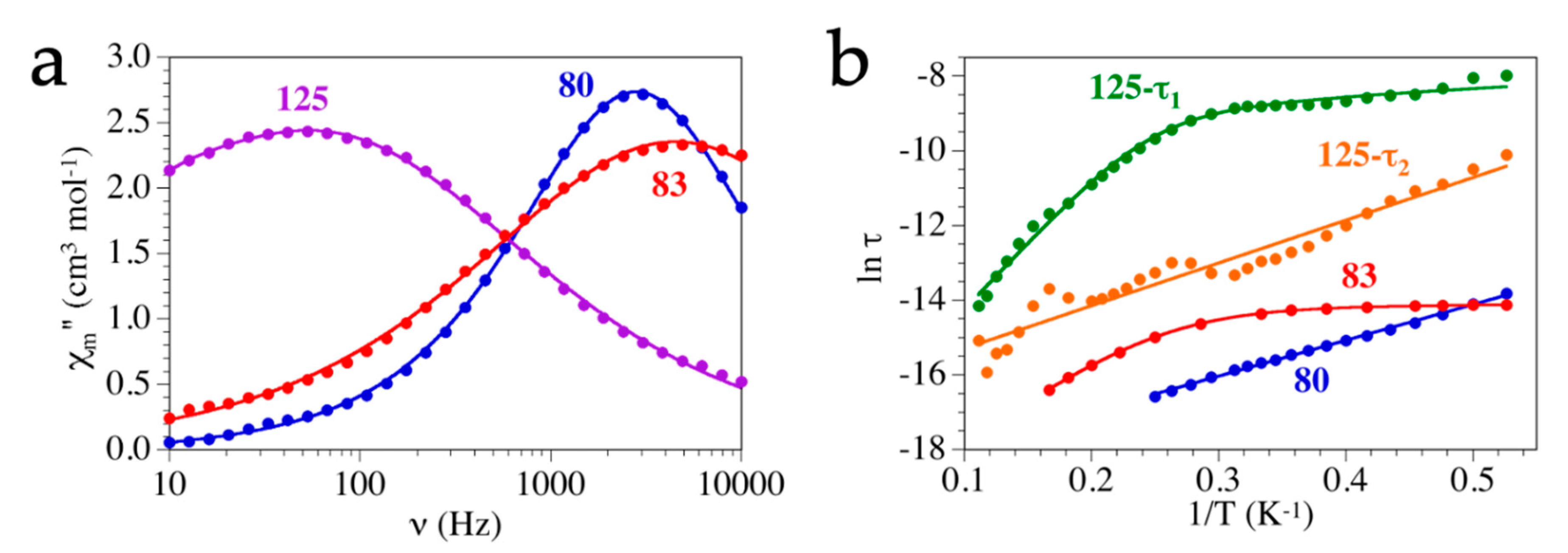
Single-Molecule and Single-Ion Magnets
| # | CCDC | Structure | Ln | X | Geometrya | A+ | L b | H (Oe) | Ueff (K) | Rel. Mec. c | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PIQFUT | Mon. | Dy | Cl | SAPR-8 | – | Tp- | 1500 | 49.4 | R/D | [19] |
| 4 | DEKTOF | Dimer | Dy | Cl | TDD-8 | – | Tp- | 1600 | 24 | O/R | [20] |
| 4′ | - | Dimer | Dy | CH3 | TDD-8 | – | Tp- | 1600 | 47 | O | [20] |
| 5 | DEKTOF01 | Dimer | Dy | Cl | TDD-8 | – | Tp- | 1000 1000 0 | 39 31 e SMM e | O O O | [22] |
| 6 | DEKTOF02 | Dimer | Dy | Cl | TDD-8 | – | Tp- | 950 | 44.7 | R/D | [19] |
| 18 | JOQSEQ | Dimer | Dy | Br | TDD-8 | – | Tp- | 390 0 380 | 7 10.4 e 10.6 e | O/R/D O/R/D O/R/D | [23] |
| 22 | KOZBEJ | Dimer | Er | Cl | TDD-8 | – | Tp- | 1000 | 25.9 | O/R | [24] |
| 24 | LEPNIG | Dimer | Tb | Cl | TDD-8 | – | Tp- | 0 | SMM e | – | [22] |
| 29 | OBIBEH01 | Dimer | Yb | Cl | TDD-8 | – | Tp- | 1000 | 22.3 | O/R | [24] |
| 31 | NOQGEI | Dimer-zz | Eu/Dy d | Cl/CN | CSAPR-9 | – | H2O | 0 | 25.3 | O/D | [27] |
| 80 | 1565279 | 2D-hex | Dy | Br | TCTPR-9 | – | H2O | 1000 | 9.6 | O | [45,55] |
| 83 | NIDFUE | 2D-hex | Dy | Br | TDD-8 | – | dmso | 1000 | 22.8 | O/D/QT | [51,55] |
| 123 | NOQBON | 2D-herr | Eu/Dy d | Br | CSAPR-9 | – | dmso | 0 | 40.9 | O/D | [27] |
| 125 | LUTROK | 2D-herr | Dy | Br | CSAPR-9 | – | dmf | 1000 | 11.4 (FR) 36 (SR) | O O/D | [55] |
| 135 | QOFCEW | 2D-4,4 | Dy | Cl | SAPR-8 | NEt4+ | – | SMM | – | – | [57] |
| 136 | QOFNAD | 2D-4,4 | Gd | Cl | SAPR-8 | NEt4+ | – | SMM | – | – | [57] |
4. Optical Properties
| # | CCDC | Structure | Ln | X | Anilato (nm) | Ln(III) (nm) | Reference |
|---|---|---|---|---|---|---|---|
| 13 | EDEZAR | Dimer | Gd | NO2 | ≈590 | – | [26] |
| 14 | EDEZEV | Dimer | Tb | NO2 | ≈590 | – | [26] |
| 15 | EDEZIZ | Dimer | Dy | NO2 | ≈590 | – | [26] |
| 16 | EDEZOF | Dimer | Ho | NO2 | 596 | 531, 643 | [26] |
| 17 | EDEZUL | Dimer | Sm | NO2 | ≈590 | – | [26] |
| 28 | OBIBEH | Dimer | Yb | Cl | 560 | ≈1000 | [25] |
| 53 | XIKNOX | 2D-hex | Nd | Cl/CN | ≈650 ≈460 a 680–720 b | 900, 1070, 1350 | [48] |
| 54 | XIKPAL | 2D-hex | Er | Cl/CN | ≈650 ≈460 a 680–720 b | ≈1550 | [48] |
| 80 | 1565279 | 2D-hex | Dy | Br | – | ≈500 | [55] |
| 83 | NIDFUE | 2D-hex | Dy | Br | – | ≈500 | [55] |
| 87 | DIFLUC | Distorted 2D-hex | Yb | Cl/CN | ≈700 | 980 | [46] |
| 88 | POMTUJ | Distorted 2D-hex | Yb | Cl/CN | – | 980 | [37] |
| 89 | POMVAR | Distorted 2D-hex | Yb/Er | Cl/CN | – | 980 1530 | [37] |
| 104 | DIFLOW | 2D-brick wall | Pr | Cl/CN | ≈680 | – | [46] |
| 111 | QOVJUJ | 2D-brick wall | Yb | Cl/CN | 650–900 | ≈1000 | [56] |
| 112 | QOVJOD | 2D-brick wall | Yb | Cl/CN | 650–900 | ≈1000 | [56] |
| 125 | LUTROK | 2D-herr | Dy | Br | – | ≈500 | [55] |
| 126 | DIFLIQ | 2D-herringbone | Pr | Cl/CN | ≈680 | – | [46] |
| 127 | XIKNUD | 2D-herringbone | Yb | Cl/CN | ≈650 ≈460 a 680–720 b | ≈980 | [48] |
| 149 | POMVIZ | 2D-(3,4)+(3,8) | Er | Cl/CN | 710 | 1530 | [37] |
5. Gas/Solvent Adsorption/Absorption and Solvent Exchange
6. Redox Studies
| # | CCDC | Structure | Ln | X | Redox processes | Reductant a | Reference |
|---|---|---|---|---|---|---|---|
| 2 | DEHQUF | Dimer | Dy | H | 1 red. | Electrochem/TDAE | [21] |
| 3 | DEHRAM | Dimer | Y | H | 1 red. | Electrochem/TDAE | [21] |
| 4 | DEKTOF | Dimer | Dy | Cl | 2 red./1 oxid. | Electrochem/Co(Cp)2 | [20] |
| 5 | DEKTOF01 | Dimer | Dy | Cl | 2 red. | Electrochem/Co(Cp)2 | [22] |
| 7 | DEKTUL | Dimer | Y | Cl | 2 red./1 oxid. | Electrochem/Co(Cp)2 | [20] |
| 8 | DEKTUL01 | Dimer | Y | Cl | 2 red. | Electrochem/Co(Cp)2 | [22] |
| 9 | DEKVAT | Dimer | Y | Cl | 2 red./1 oxid. | Electrochem/Co(Cp)2 | [20] |
| 11 | DEKVIB | Dimer | Y | CH3 | 2 red./1 oxid. | Electrochem. | [20] |
| 12 | DEKVOH | Dimer | Y | CH3 | 2 red./1 oxid. | Electrochem. | [20] |
| 18 | JOQSEQ | Dimer | Dy | Br | 2 red./1 oxid. | Electrochem/Co(Cp)2 | [23] |
| 21 | JOQSUG | Dimer | Y | Br | 2 red./1 oxid. | Electrochem/Co(Cp)2 | [23] |
| 24 | LEPNIG | Dimer | Tb | Cl | 2 red. | Electrochem/Co(Cp)2 | [22] |
| 26 | LEPNOM | Dimer | Gd | Cl | 2 red. | Electrochem/Co(Cp)2 | [22] |
7. Thin Films
| # | CCDC | Compound | Ln | X | Film thickness | Reference |
|---|---|---|---|---|---|---|
| 42 | ZOTTAD | [Ce2(dhbq)3(H2O)6]·18H2O | Ce | H | 3–4 monolayers | [45] |
| 44 | 1944110 | [Nd2(dhbq)3(H2O)6]·18H2O | Nd | H | 3–4 monolayers | [45] |
| 46 | 1944112 | [Eu2(dhbq)3(H2O)6]·18H2O | Eu | H | 5–6 monolayers | [45] |
| 48 | 1944114 | [Dy2(dhbq)3(H2O)6]·18H2O | Dy | H | 4–5 monolayers | [45] |
| 99 | 1944126 | [Tm2(C6O4Cl2)3(H2O)6]·8H2O | Tm | Cl | 3–4 monolayers | [45] |
| 71 | XAWZUT | [Er2(C6O4Br2)3(H2O)6]·7H2O | Er | Br | 6–7 monolayers | [45] |
| 76 | 1565275 | [Sm2(C6O4Br2)3(H2O)6]·10H2O | Sm | Br | 4–5 monolayers | [45] |
| 53 | XIKNOX | [Nd2(C6O4(CN)Cl)3(dmf)6]·2CH2Cl2 | Nd | Cl/CN | 1–4 monolayers | [48] |
| 54 | XIKPAL | [Er2(C6O4(CN)Cl)3(dmf)6]·2CH2Cl2 | Er | Cl/CN | 1–4 monolayers | [48] |
| 88 | POMTUJ | [Yb2(C6O4(CN)Cl)3(dmso)4]·dmso | Yb | Cl/CN | – | [37] |
| 89 | POMVAR | [YbEr2(C6O4(CN)Cl)3(dmso)4]·dmso | Yb/Er | Cl/CN | – | [37] |
| 111 | QOVJUJ | [Yb4(C6O4(CN)Cl)5(dobdc)(dmso)10]·2dmso a | Yb | Cl/CN | 1-5 monolayers | [56] |
| 112 | QOVJOD | [Yb2(C6O4(CN)Cl)2(F4bdc)(dmso)6] b | Yb | Cl/CN | 1-5 monolayers | [56] |
| 127 | XIKNUD | [Yb2(C6O4(CN)Cl)3(dmf)6] | Yb | Cl/CN | 1–4 monolayers | [48] |
| 149 | POMVIZ | [Er2(C6O4(CN)Cl)3(dmso)6] | Er | Cl/CN | – | [37] |
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fordham, S.; Wang, X.; Bosch, M.; Zhou, H. Lanthanide Metal-Organic Frameworks: Syntheses, Properties and Potential Applications. Struct. Bond. 2015, 163, 1–27. [Google Scholar]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of Water Stable Metal-Organic Frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, W.; Bouwman, E. One-Step Growth of Lanthanoid Metal-Organic Framework (MOF) Films Under Solvothermal Conditions for Temperature Sensing. Chem. Commun. 2016, 52, 6926–6929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, S.; Kawata, S. Coordination Compounds of 1,4-Dihydroxybenzoquinone and its Homologues. Structures and Properties. Coord. Chem. Rev. 2002, 224, 11–34. [Google Scholar] [CrossRef]
- Benmansour, S.; Vallés-García, C.; Gómez-Claramunt, P.; Mínguez Espallargas, G.; Gómez-García, C.J. 2D and 3D Anilato-Based Heterometallic M(I)M(III) Lattices: The Missing Link. Inorg. Chem. 2015, 54, 5410–5418. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Benmansour, S.; Mínguez Espallargas, G.; Clemente-León, M.; Abhervé, A.; Gómez-Claramunt, P.; Coronado, E.; Artizzu, F.; Sessini, E.; Deplano, P.; et al. A Family of Layered Chiral Porous Magnets Exhibiting Tunable Ordering Temperatures. Inorg. Chem. 2013, 52, 10031–10040. [Google Scholar] [CrossRef]
- Jeon, I.; Negru, B.; Duyne, R.P.V.; Harris, T.D. A 2D Semiquinone Radical-Containing Microporous Magnet with Solvent-Induced Switching from Tc = 26 to 80 K. J. Am. Chem. Soc. 2015, 137, 15699–15702. [Google Scholar] [CrossRef]
- Atzori, M.; Artizzu, F.; Sessini, E.; Marchio, L.; Loche, D.; Serpe, A.; Deplano, P.; Concas, G.; Pop, F.; Avarvari, N.; et al. Halogen-Bonding in a New Family of Tris(Haloanilato)Metallate(III) Magnetic Molecular Building Blocks. Dalton Trans. 2014, 43, 7006–7019. [Google Scholar] [CrossRef]
- Benmansour, S.; Gómez-Claramunt, P.; Vallés-García, C.; Mínguez Espallargas, G.; Gómez García, C.J. Key Role of the Cation in the Crystallization of Chiral Tris(Anilato)Metalate Magnetic Anions. Cryst. Growth Des. 2016, 16, 518–526. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Grannas, M.J.; Hudson, T.A.; Hughes, S.A.; Pranoto, N.H.; Robson, R. Synthesis, Structure and Host-Guest Properties of (Et4N)2[SnIVCaII(Chloranilate)4], a New Type of Robust Microporous Coordination Polymer with a 2D Square Grid Structure. Dalton Trans. 2011, 40, 12242–12247. [Google Scholar] [CrossRef]
- Benmansour, S.; Gómez-García, C.J. A Heterobimetallic Anionic 3,6-Connected 2D Coordination Polymer Based on Nitranilate as Ligand. Polymers 2016, 8, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, S.; Saito, Y. Synthesis of Co-Ordination Compounds of High Molecular Weight. Bull. Chem. Soc. Jpn. 1957, 30, 192–193. [Google Scholar] [CrossRef] [Green Version]
- Riley, P.E.; Haddad, S.F.; Raymond, K.N. Preparation of Praseodymium(III) Chloranilate and the Crystal Structures of Pr2(C6Cl2O4)3.8C2H5OH and Na3[C6H2O(OH)(SO3)2].H2O. Inorg. Chem. 1983, 22, 3090–3096. [Google Scholar] [CrossRef]
- Christian, R. Complexes with Substituted 2,5-Dihydroxy-p-Benzoquinones: The Inclusion Compounds [Y(H2O)3]2 (C6Cl2O4)3·6.6H2O and [Y(H2O)3]2 (C6Br2O4)3·6H2O. Mater. Res. Bull. 1987, 22, 1483–1491. [Google Scholar]
- Abrahams, B.F.; Coleiro, J.; Hoskins, B.F.; Robson, R. Gas Hydrate-Like Pentagonal Dodecahedral M2(H2O)18 Cages (M = Lanthanide or Y) in 2,5-Dihydroxybenzoquinone-Derived Coordination Polymers. Chem. Commun. 1996, 603–604. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Coleiro, J.; Ha, K.; Hoskins, B.F.; Orchard, S.D.; Robson, R. Dihydroxybenzoquinone and Chloranilic Acid Derivatives of Rare Earth Metals. J. Chem. Soc. Dalton Trans. 2002, 1586–1594. [Google Scholar] [CrossRef]
- Mercuri, M.L.; Congiu, F.; Concas, G.; Sahadevan, S.A. Recent Advances on Anilato-Based Molecular Materials with Magnetic and/or Conducting Properties. Magnetochemistry 2017, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Benmansour, S.; Gómez-García, C.J. Heterometallic Anilato-Based Layered Magnets. Gen. Chem. 2020, 6, 190033. [Google Scholar] [CrossRef]
- Ishikawa, R.; Michiwaki, S.; Noda, T.; Katoh, K.; Yamashita, M.; Matsubara, K.; Kawata, S. Field-Induced Slow Magnetic Relaxation of Mono- and Dinuclear Dysprosium(III) Complexes Coordinated by a Chloranilate with Different Resonance Forms. Inorganics 2018, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Dunstan, M.A.; Rousset, E.; Boulon, M.; Gable, R.W.; Sorace, L.; Boskovic, C. Slow Magnetisation Relaxation in Tetraoxolene-Bridged Rare Earth Complexes. Dalton Trans. 2017, 46, 13756–13767. [Google Scholar] [CrossRef] [Green Version]
- Moilanen, J.O.; Mansikkamäki, A.; Lahtinen, M.; Guo, F.; Kalenius, E.; Layfield, R.A.; Chibotaru, L.F. Thermal Expansion and Magnetic Properties of Benzoquinone-Bridged Dinuclear Rare-Earth Complexes. Dalton Trans. 2017, 46, 13582–13589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Perfetti, M.; Kern, M.; Hallmen, P.P.; Ungur, L.; Lenz, S.; Ringenberg, M.R.; Frey, W.; Stoll, H.; Rauhut, G.; et al. Exchange Coupling and Single-Molecule Magnetism in Redox-Active Tetraoxolene-Bridged Dilanthanide Complexes. Chem. Sci. 2018, 9, 1221–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, W.R.; Dunstan, M.A.; Gable, R.W.; Phonsri, W.; Murray, K.S.; Mole, R.A.; Boskovic, C. Tetraoxolene-Bridged Rare-Earth Complexes: A Radical-Bridged Dinuclear Dy Single-Molecule Magnet. Dalton Trans. 2019, 48, 15635–15645. [Google Scholar] [CrossRef]
- Ishikawa, R.; Michiwaki, S.; Noda, T.; Katoh, K.; Yamashita, M.; Kawata, S. Series of Chloranilate-Bridged Dinuclear Lanthanide Complexes: Kramers Systems Showing Field-Induced Slow Magnetic Relaxation. Magnetochemistry 2019, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Abdus Subhan, M.; Kawahata, R.; Nakata, H.; Fuyuhiro, A.; Tsukuda, T.; Kaizaki, S. Synthesis, Structure and Spectroscopic Properties of Chloranilate-Bridged 4f–4f Dinuclear Complexes: A Comparative Study of the Emission Properties with Cr-Ln Complexes. Inorg. Chim. Acta 2004, 357, 3139–3146. [Google Scholar] [CrossRef]
- Benmansour, S.; López-Martínez, G.; Canet-Ferrer, J.; Gómez-García, C.J. A Family of Lanthanoid Dimers with Nitroanilato Bridges. Magnetochemistry 2016, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Paredes, A.; Cerezo-Navarrete, C.; Gómez García, C.J.; Benmansour, S. Slow Relaxation in Doped Coordination Polymers and Dimers Based on Lanthanoids and Anilato Ligands. Polyhedron 2019, 170, 476–485. [Google Scholar] [CrossRef]
- Benmansour, S.; Pérez-Herráez, I.; Cerezo-Navarrete, C.; López-Martínez, G.; Martínez Hernandez, C.; Gómez-García, C.J. Solvent-Modulation of the Structure and Dimensionality in Lanthanoid-Anilato Coordination Polymers. Dalton Trans. 2018, 47, 6729–6741. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Bofill, J.M.; Alemany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. SHAPE. 2013. v 2.1. Available online: http://www.ee.ub.edu/index.php?option=com_jdownloads&view=viewcategories&Itemid=529.
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. the Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef]
- Casanova, D.; Cirera, J.; Llunell, M.; Alemany, P.; Avnir, D.; Alvarez, S. Minimal Distortion Pathways in Polyhedral Rearrangements. J. Am. Chem. Soc. 2004, 126, 1755–1763. [Google Scholar] [CrossRef]
- Alvarez, S. Distortion Pathways of Transition Metal Coordination Polyhedra Induced by Chelating Topology. Chem. Rev. 2015, 115, 13447–13483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casanova, D.; Alemany, P.; Bofill, J.M.; Alvarez, S. Shape and Symmetry of Heptacoordinate Transition-Metal Complexes: Structural Trends. Chem. Eur. J. 2003, 9, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number of Vertices: Nine-Coordinate Metal Compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Martínez, A.; Alvarez, S. Stereochemistry of Compounds with Coordination Number Ten. Chem. Eur. J. 2009, 15, 7470–7480. [Google Scholar] [CrossRef] [PubMed]
- Artizzu, F.; Atzori, M.; Liu, J.; Mara, D.; Van Hecke, K.; Van Deun, R. Solution-Processable Yb/Er 2D-Layered Metallorganic Frameworks with High NIR-Emission Quantum Yields. J. Mater. Chem. C 2019, 7, 11207–11214. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Monni, N.; Abhervé, A.; Cosquer, G.; Oggianu, M.; Ennas, G.; Yamashita, M.; Avarvari, N.; Mercuri, M.L. Dysprosium Chlorocyanoanilate-Based 2D-Layered Coordination Polymers. Inorg. Chem. 2019, 58, 13988–13998. [Google Scholar] [CrossRef]
- Weiss, A.; Riegler, E.; Robl, C. Polymeric 2,5-Dihydroxy-1,4-Benzoquinone Transition-Metal Complexes Na2(H2O)24[M2(C6H2O4)3] (M = Mn2+, Cd2+). Z. Naturforsch. B Chem. Sci. 1986, 41, 1501–1505. [Google Scholar] [CrossRef]
- Luo, T.; Liu, Y.; Tsai, H.; Su, C.; Ueng, C.; Lu, K. A Novel Hybrid Supramolecular Network Assembled from Perfect Stacking of an Anionic Inorganic Layer and a Cationic Hydronium-Ion-Mediated Organic Layer. Eur. J. Inorg. Chem. 2004, 4253–4258. [Google Scholar] [CrossRef]
- Diaz-Torres, R.; Alvarez, S. Coordinating Ability of Anions and Solvents Towards Transition Metals and Lanthanides. Dalton Trans. 2011, 40, 10742–10750. [Google Scholar] [CrossRef]
- Alvarez, S. Coordinating Ability of Anions, Solvents, Amino Acids and Gases towards Alkaline and Alkaline-Earth Elements, Transition Metals and Lanthanides. Chem. Eur. J. 2020, 26, 8663. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Ohkoshi, S. Poly[hexa-aqua-tris-[m2-2,5-dihydroxy-1,4-benzoquinonato(2-)] diholmium(III)] Octa-Deca-Hydrate. Acta Cryst. E 2010, 66, m1300. [Google Scholar] [CrossRef] [PubMed]
- Ponjan, N.; Kodchasanthong, K.; Jiajaroen, S.; Chainok, K. Crystal Structure of poly[[hexa-aqua-tris-(m-3,6-di-oxo-cyclo-hexa-1,4-diene-1,4-diolato)dierbium(III)] octa-deca-hydrate]. Acta Cryst. E 2019, 75, 64–67. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, G. Multifunctionality in Molecular Materials Based on Anilato-Type Ligands. PhD. Thesis, University of Valencia, Valencia, Spain, 2017. [Google Scholar]
- Gómez-Claramunt, P.; Benmansour, S.; Hernández-Paredes, A.; Cerezo-Navarrete, C.; Rodríguez-Fernández, C.; Canet-Ferrer, J.; Cantarero, A.; Gómez-García, C.J. Tuning the Structure and Properties of Lanthanoid Coordination Polymers with an Asymmetric Anilato Ligand. Magnetochemistry 2018, 4, 6. [Google Scholar]
- Benmansour, S.; Hernández-Paredes, A.; Gómez-García, C.J. Two-Dimensional Magnetic Coordination Polymers Formed by Lanthanoids and Chlorocyananilato. Magnetochemistry 2018, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Ashoka Sahadevan, S.; Monni, N.; Abhervé, A.; Marongiu, D.; Sarritzu, V.; Sestu, N.; Saba, M.; Mura, A.; Bongiovanni, G.; Cannas, C.; et al. Nanosheets of Two-Dimensional Neutral Coordination Polymers Based on Near-Infrared-Emitting Lanthanides and a Chlorocyananilate Ligand. Chem. Mater. 2018, 30, 6575–6586. [Google Scholar] [CrossRef]
- Zucchi, G.; Thuery, P.; Ephritikhine, M. CSD Communication. 2012. [Google Scholar]
- Benmansour, S.; Pérez-Herráez, I.; López-Martínez, G.; Gómez García, C.J. Solvent-Modulated Structures in Anilato-Based 2D Coordination Polymers. Polyhedron 2017, 135, 17–25. [Google Scholar] [CrossRef]
- Benmansour, S.; Hernández-Paredes, A.; Gómez-García, C.J. Effect of the Lanthanoid-Size on the Structure of a Series of Lanthanoid-Anilato 2-D Lattices. J. Coord. Chem. 2018, 71, 845–863. [Google Scholar] [CrossRef]
- Kharitonov, A.D.; Trofimova, O.Y.; Meshcheryakova, I.N.; Fukin, G.K.; Khrizanforov, M.N.; Budnikova, Y.H.; Bogomyakov, A.S.; Aysin, R.R.; Kovalenko, K.A.; Piskunov, A.V. 2D-metal–organic Coordination Polymers of Lanthanides (La(III), Pr(III) and Nd(III)) with Redox-Active Dioxolene Bridging Ligands. CrystEngComm 2020, 22, 4675–4679. [Google Scholar] [CrossRef]
- Demars, T.; Boltoeva, M.; Vigier, N.; Maynadié, J.; Ravaux, J.; Genre, C.; Meyer, D. From Coordination Polymers to Doped Rare-Earth Oxides. Eur. J. Inorg. Chem. 2012, 2012, 3875–3884. [Google Scholar] [CrossRef]
- Bondaruk, K.; Hua, C. Effect of Counterions on the Formation and Structures of Ce(III) and Er(III) Chloranilate Frameworks. Cryst. Growth Des. 2019, 19, 3338–3347. [Google Scholar] [CrossRef]
- Benmansour, S.; Hernández-Paredes, A.; Mondal, A.; López Martínez, G.; Canet-Ferrer, J.; Konar, S.; Gómez-García, C.J. Slow Relaxation of the Magnetization, Reversible Solvent Exchange and Luminescence in 2D Anilato-Based Frameworks. Chem. Commun. 2020, 56, 9862–9865. [Google Scholar] [CrossRef] [PubMed]
- Ashoka Sahadevan, S.; Monni, N.; Oggianu, M.; Abhervé, A.; Marongiu, D.; Saba, M.; Mura, A.; Bongiovanni, G.; Mameli, V.; Cannas, C.; et al. Heteroleptic NIR-Emitting YbIII/Anilate-Based Neutral Coordination Polymer Nanosheets for Solvent Sensing. ACS Appl. Nano Mater. 2020, 3, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Kingsbury, C.J.; Abrahams, B.F.; Auckett, J.E.; Chevreau, H.; Dharma, A.D.; Duyker, S.; He, Q.; Hua, C.; Hudson, T.A.; Murray, K.S.; et al. Square Grid Metal-Chloranilate Networks as Robust Host Systems for Guest Sorption. Chem. Eur. J. 2019, 25, 5222–5234. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Tay, H.M.; He, Q.; Harris, T.D. A Series of Early Lanthanide Chloranilate Frameworks with a Square Grid Topology. Aust. J. Chem. 2019, 72, 778–785. [Google Scholar] [CrossRef]
- Robson, R. A Net-Based Approach to Coordination Polymers. J. Chem. Soc. Dalton Trans. 2000, 3735–3744. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Li, X.; Zhai, F.; Yan, S.; Liu, N.; Chai, Z.; Xu, Y.; Ouyang, X.; Wang, S. Direct Radiation Detection by a Semiconductive Metal-Organic Framework. J. Am. Chem. Soc. 2019, 141, 8030–8034. [Google Scholar] [CrossRef]
- Schweinfurth, D.; Khusniyarov, M.M.; Bubrin, D.; Hohloch, S.; Su, C.; Sarkar, B. Tuning Spin–Spin Coupling in Quinonoid-Bridged Dicopper(II) Complexes through Rational Bridge Variation. Inorg. Chem. 2013, 52, 10332–10339. [Google Scholar] [CrossRef]
- Liddle, S.T.; van Slageren, J. Improving f-Element Single-Molecule Magnets. Chem. Soc. Rev. 2015, 44, 6655–6669. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.L.; Chen, Y.C.; Tong, M.L. Symmetry Strategies for High Performance Lanthanide-Based Single-Molecule Magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef]
- Goodwin, C.A.P. Blocking Like it’s Hot: A Synthetic Chemists’ Path to High-Temperature Lanthanide Single Molecule Magnets. Dalton Trans. 2020, 49, 14320–14337. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, R.J.; Ho, L.T.A.; Ungur, L.; Korobkov, I.; Chibotaru, L.F.; Murugesu, M. Observation of Unusual Slow-Relaxation of the Magnetisation in a Gd-EDTA Chelate. Dalton Trans. 2015, 44, 20321–20325. [Google Scholar] [CrossRef] [PubMed]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G. Design of Luminescent Lanthanide Complexes: From Molecules to Highly Efficient Photo-Emitting Materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Rehwoldt, R.E.; Chasen, B.; Li, J. 2-chloro-5-cyano-3,6-dihydroxybenzoquinone, a New Analytical Reagent for the Spectrophotometric Determination of Calcium(II). Anal. Chem. 1966, 38, 1018–1019. [Google Scholar] [CrossRef]
- Szostak, M.M.; Kozankiewicz, B.; Lipinski, J. Low-Temperature Photoluminescence of p-Nitroaniline and o-Methyl-p-Nitroaniline Crystals. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1412–1416. [Google Scholar] [CrossRef]
- Atzori, M.; Artizzu, F.; Marchio, L.; Loche, D.; Caneschi, A.; Serpe, A.; Delano, P.; Avarvari, N.; Mercuri, M.L. Switching-on Luminescence in Anilate-Based Molecular Materials. Dalton Trans. 2015, 44, 15786–15802. [Google Scholar] [CrossRef]
- Boskovic, C.; Hay, M. Lanthanoid Complexes as Molecular Materials: The Redox Approach. Chem. Eur. J. 2020. [Google Scholar] [CrossRef]










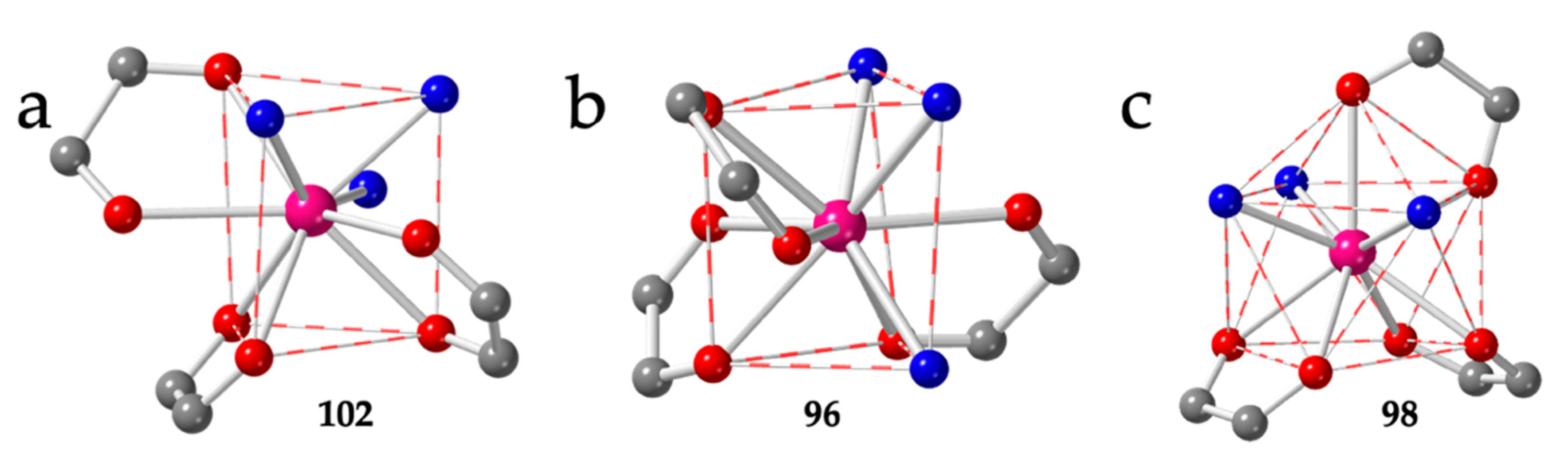





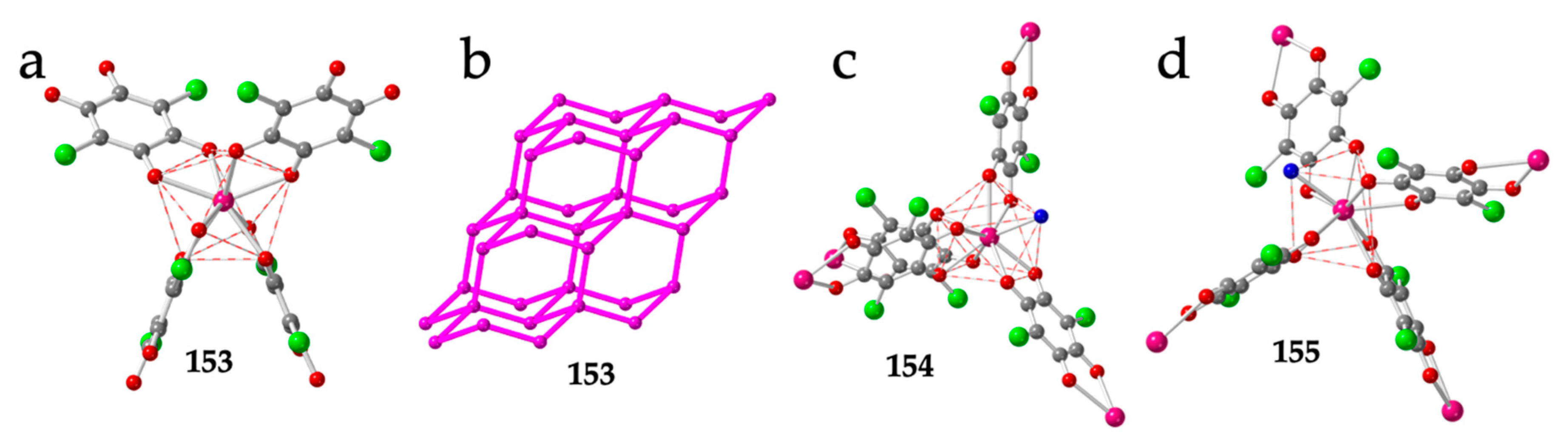
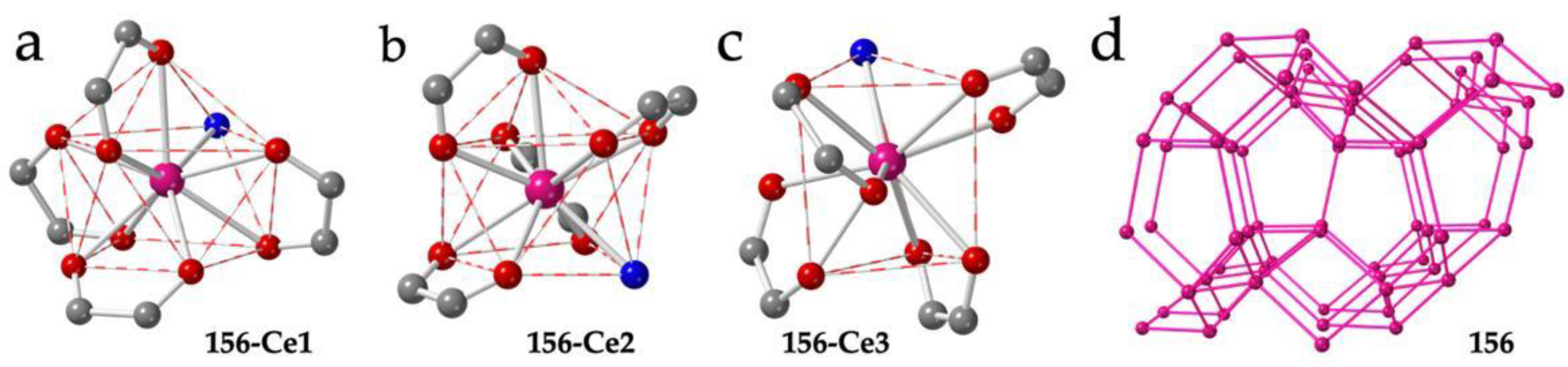
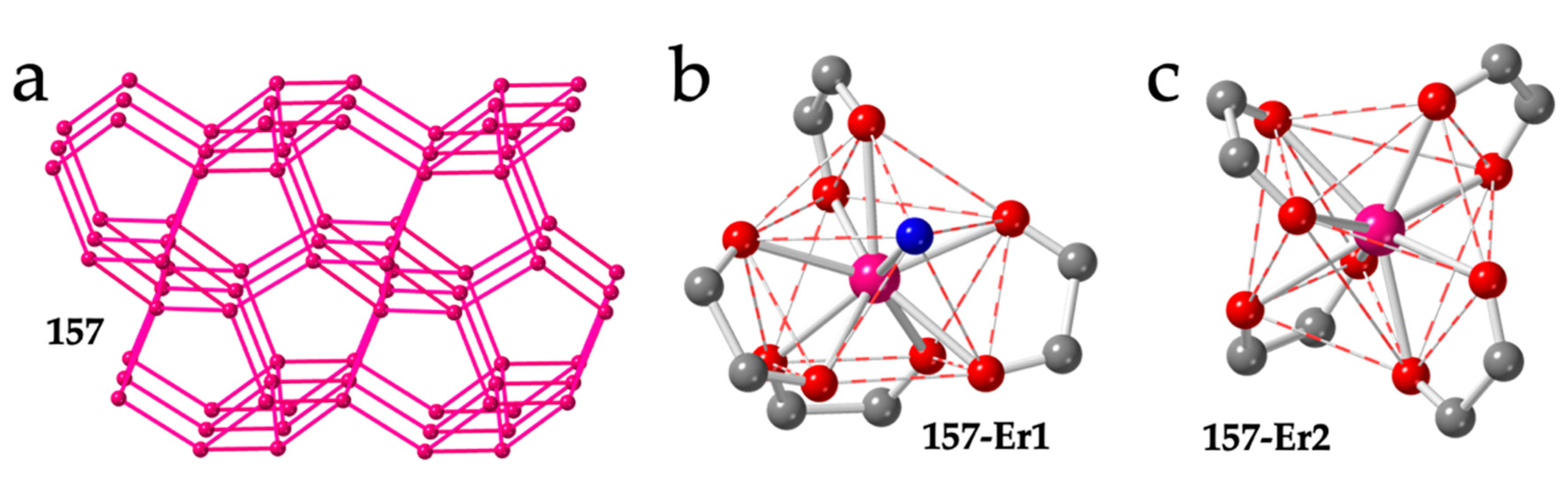





| # | CCDC | Structure | Ln | X | L a | Reference |
|---|---|---|---|---|---|---|
| 2 | DEHQUF | Dimer | Dy | H | thf/Cl- | [21] |
| 3 | DEHRAM | Dimer | Y | H | thf/Cl- | [21] |
| 10 | DEKVEX | Dimer | Y | H | Tp- | [20] |
| 35 | KUYKIZ | 2D-hex | Ho | H | H2O | [43] |
| 36 | MIZXAU | 2D-hex | La | H | H2O | [16] |
| 37 | MIZXEY | 2D-hex | Gd | H | H2O | [16] |
| 38 | MIZXIC | 2D-hex | Yb | H | H2O | [16] |
| 39 | MIZXOI | 2D-hex | Lu | H | H2O | [16] |
| 40 | MIZXUO | 2D-hex | Y | H | H2O | [16] |
| 41 | PIVKAJ | 2D-hex | Er | H | H2O | [44] |
| 42 | ZOTTAD | 2D-hex | Ce | H | H2O | [15] |
| 43 | 1944109 | 2D-hex | Pr | H | H2O | [45] |
| 44 | 1944110 | 2D-hex | Nd | H | H2O | [45] |
| 45 | 1944111 | 2D-hex | Sm | H | H2O | [45] |
| 46 | 1944112 | 2D-hex | Eu | H | H2O | [45] |
| 47 | 1944113 | 2D-hex | Tb | H | H2O | [45] |
| 48 | 1944114 | 2D-hex | Dy | H | H2O | [45] |
| 49 | 1944117 | 2D-hex | Tm | H | H2O | [45] |
| 95 | LEBGEG | 2D-dist.hex | Gd | H | thf/Cl- | [53] |
| # | CCDC | Structure | Ln | X | L a | A+b | Reference |
|---|---|---|---|---|---|---|---|
| 1 | PIQFUT | Monomer | Dy | Cl | Tp- | – | [19] |
| 4 | DEKTOF | Dimer | Dy | Cl | Tp- | – | [20] |
| 5 | DEKTOF01 | Dimer | Dy | Cl | Tp- | – | [22] |
| 6 | DEKTOF02 | Dimer | Dy | Cl | Tp- | – | [19] |
| 7 | DEKTUL | Dimer | Y | Cl | Tp- | – | [20] |
| 8 | DEKTUL01 | Dimer | Y | Cl | Tp- | – | [22] |
| 9 | DEKVAT | Dimer | Y | Cl | Tp- | – | [20] |
| 22 | KOZBEJ | Dimer | Er | Cl | Tp- | – | [24] |
| 23 | KOZBIN | Dimer | Ho | Cl | Tp- | – | [24] |
| 24 | LEPNIG | Dimer | Tb | Cl | Tp- | – | [22] |
| 25 | LEPNIG01 | Dimer | Tb | Cl | Tp- | – | [24] |
| 26 | LEPNOM | Dimer | Gd | Cl | Tp- | – | [22] |
| 27 | LEPNOM01 | Dimer | Gd | Cl | Tp- | – | [19] |
| 28 | OBIBEH | Dimer | Yb | Cl | Tp- | – | [25] |
| 29 | OBIBEH01 | Dimer | Yb | Cl | Tp- | – | [24] |
| 33 | MIZZUQ | 1D + mon | Lu | Cl | H2O | – | [16] |
| 34 | NIGNID | 1D-ladder | Er | Cl | hmpa | – | [28] |
| 56 | NIGNUP | 2D-hex | Er | Cl | fma | – | [28] |
| 57 | GEQBAH | 2D-dist.hex | Gd | Cl | H2O | – | [49] |
| 58 | MIZYID | 2D-dist.hex | Pr | Cl | H2O | – | [16] |
| 59 | MIZYOJ | 2D-dist.hex | Nd | Cl | H2O | – | [16] |
| 60 | MIZYUP | 2D-dist.hex | Tb | Cl | H2O | – | [16] |
| 61 | MIZZAW | 2D-dist.hex | Ce | Cl | H2O | – | [16] |
| 62 | MIZZAW01 | 2D-dist.hex | Ce | Cl | H2O | – | [16] |
| 63 | MIZZEA | 2D-dist.hex | Y | Cl | H2O | – | [16] |
| 64 | MIZZIE | 2D-dist.hex | Gd | Cl | H2O | – | [16] |
| 65 | MIZZOK | 2D-dist.hex | Eu | Cl | H2O | – | [16] |
| 66 | NIGNEZ | 2D-dist.hex | Er | Cl | H2O | – | [28] |
| 67 | 1944118 | 2D-dist.hex | La | Cl | H2O | – | [45] |
| 68 | 1944120 | 2D-dist.hex | Sm | Cl | H2O | – | [45] |
| 69 | 1944123 | 2D-dist.hex | Dy | Cl | H2O | – | [45] |
| 70 | 1944124 | 2D-dist.hex | Ho | Cl | H2O | – | [45] |
| 90 | NIGQIG | 2D-dist.hex | Er | Cl | dmso | – | [28] |
| 91 | NIGNOJ | 2D-dist.hex | Er | Cl | dma | – | [28] |
| 96 | CAZZAE | 2D-brickwall | Pr | Cl | EtOH | – | [13] |
| 97 | GASMUI | 2D-brickwall | Y | Cl | H2O | – | [14] |
| 98 | JOGJAT | 2D-brickwall | Er | Cl | H2O | – | [54] |
| 99 | 1944126 | 2D-brickwall | Tm | Cl | H2O | – | [45] |
| 100 | 1944127 | 2D-brickwall | Yb | Cl | H2O | – | [45] |
| 110 | GEPZUY | 2D-brickwall | Eu | Cl | bipym/H2O | – | [49] |
| 113 | GEQBEL | 2D-herringbone | Gd | Cl | H2O | – | [49] |
| 114 | JOGHAR | 2D-herringbone | Ce | Cl | H2O | – | [54] |
| 115 | NIGPAX | 2D-herringbone | Er | Cl | dmf | – | [28] |
| 128 | MIZYAV | 2D-3,4 | La | Cl | H2O | – | [16] |
| 131 | QOFBAR | 2D-4,4 | Y | Cl | – | NEt4+ | [57] |
| 132 | QOFBOF | 2D-4,4 | Y | Cl | – | NEt4+ | [57] |
| 133 | QOFBUL | 2D-4,4 | Y | Cl | – | NEt4+ | [57] |
| 134 | QOFCAS | 2D-4,4 | Y | Cl | – | NEt4+ | [57] |
| 135 | QOFCEW | 2D-4,4 | Dy | Cl | – | NEt4+ | [57] |
| 136 | QOFNAD | 2D-4,4 | Gd | Cl | – | NEt4+ | [57] |
| 137 | QOFNEH | 2D-4,4 | Yb | Cl | – | NEt4+ | [57] |
| 138 | QOFNOR | 2D-4,4 | Er | Cl | – | NEt4+ | [57] |
| 139 | QOFNUX | 2D-4,4 | Y | Cl | – | NEt4+ | [57] |
| 140 | QOFPAF | 2D-4,4 | Tb | Cl | – | NEt4+ | [57] |
| 141 | QOFPEJ | 2D-4,4 | Lu | Cl | – | NEt4+ | [57] |
| 142 | QOFPIN | 2D-4,4 | Ho | Cl | – | NEt4+ | [57] |
| 143 | WOXTIP | 2D-4,4 | Nd | Cl | – | NEt4+ | [58] |
| 144 | WOXTOV | 2D-4,4 | Eu | Cl | – | NEt4+ | [58] |
| 145 | WOXTUB | 2D-4,4 | Sm | Cl | – | NEt4+ | [58] |
| 146 | WOXVEN | 2D-4,4 | Ce | Cl | H2O | NEt4+ | [58] |
| 147 | WOXVIR | 2D-4,4 | La | Cl | H2O | NEt4+ | [58] |
| 148 | WOXVOX | 2D-4,4 | Nd | Cl | H2O | NEt4+ | [58] |
| 151 | MOBBIO | 3D-diam | Y | Cl | – | H3O+ | [16] |
| 152 | EFOXUV | 3D-diam | Tb | Cl | H2O/dmf/HCOO- | NMe2H2+ | [60] |
| 153 | JOGHEV | 3D-diam | Er | Cl | – | DPMP+ | [54] |
| 154 | JOGHIZ | 3D-diam | Ce | Cl | H2O | DPMP+ | [54] |
| 155 | JOGHOF | 3D-diam | Ce | Cl | H2O | PPh4+ | [54] |
| 156 | JOGHUL | 3D-noq | Ce | Cl | H2O | PPh3Me+ | [54] |
| 157 | JOGJEX | 3D-4,4-c | Er1 | Cl | H2O | PPh3Me+ | [54] |
| # | CCDC | Structure | Ln | X | L a | Reference |
|---|---|---|---|---|---|---|
| 18 | JOQSEQ | Dimer | Dy | Br | Tp- | [23] |
| 21 | JOQSUG | Dimer | Y | Br | Tp- | [23] |
| 71 | XAWZUT | 2D-dist.hex | Er | Br | H2O | [50] |
| 72 | 1565271 | 2D-dist.hex | La | Br | H2O | [45] |
| 73 | 1565272 | 2D-dist.hex | Ce | Br | H2O | [45] |
| 74 | 1565273 | 2D-dist.hex | Pr | Br | H2O | [45] |
| 75 | 1565274 | 2D-dist.hex | Nd | Br | H2O | [45] |
| 76 | 1565275 | 2D-dist.hex | Sm | Br | H2O | [45] |
| 77 | 1565276 | 2D-dist.hex | Eu | Br | H2O | [45] |
| 78 | 1565277 | 2D-dist.hex | Gd | Br | H2O | [45] |
| 79 | 1565278 | 2D-dist.hex | Tb | Br | H2O | [45] |
| 80 | 1565279 | 2D-dist.hex | Dy | Br | H2O | [45] |
| 81 | 1565280 | 2D-dist.hex | Ho | Br | H2O | [45] |
| 82 | NIDFOY | 2D-dist.hex | Tb | Br | dmso | [51] |
| 83 | NIDFUE | 2D-dist.hex | Dy | Br | dmso | [51] |
| 84 | NIDGAL | 2D-dist.hex | Ho | Br | dmso | [51] |
| 85 | XAXBAC | 2D-dist.hex | Er | Br | dmso | [50] |
| 86 | NIDGEP | 2D-dist.hex | Yb | Br | dmso | [51] |
| 101 | GASMOC | 2D-brickwall | Y | Br | H2O | [14] |
| 102 | 1565282 | 2D-brickwall | Tm | Br | H2O | [45] |
| 103 | LUTRIE | 2D-brickwall | Yb | Br | H2O | [55] |
| 116 | NIDLIY | 2D-herringbone | La | Br | dmso | [51] |
| 117 | NIDLOE | 2D-herringbone | Ce | Br | dmso | [51] |
| 118 | NIDLUK | 2D-herringbone | Pr | Br | dmso | [51] |
| 119 | NIDMAR | 2D-herringbone | Nd | Br | dmso | [51] |
| 120 | NIDMEV | 2D-herringbone | Sm | Br | dmso | [51] |
| 121 | NIDFEO | 2D-herringbone | Eu | Br | dmso | [51] |
| 122 | NIDFIS | 2D-herringbone | Gd | Br | dmso | [51] |
| 123 | NOQBON | 2D-herringbone | Eu/Dy | Br | dmso | [27] |
| 124 | XAXBEG | 2D-herringbone | Er | Br | dmf | [50] |
| 125 | LUTROK | 2D-herringbone | Dy | Br | dmf | [55] |
| # | CCDC | Structure | Ln | X | L a | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 30 | NOQBUT | Dimer-zz | Eu | Cl/CN | H2O | – | [27] |
| 31 | NOQGEI | Dimer-zz | Eu/Dy b | Cl/CN | H2O | – | [27] |
| 50 | DIFLEM | 2D-hex | Ce | Cl/CN | dmf | – | [46] |
| 51 | WOTWIO | 2D-hex | Dy | Cl/CN | dmf | – | [47] |
| 52 | WOTWOU | 2D-hex | Ho | Cl/CN | dmf | – | [47] |
| 53 | XIKNOX | 2D-hex | Nd | Cl/CN | dmf | – | [48] |
| 54 | XIKPAL | 2D-hex | Er | Cl/CN | dmf | – | [48] |
| 55 | XOYTEN | 2D-hex | Dy | Cl/CN | dmf | – | [38] |
| 87 | DIFLUC | 2D-dist.hex | Yb | Cl/CN | dmso | – | [46] |
| 88 | POMTUJ | 2D-dist.hex | Yb | Cl/CN | dmso | – | [37] |
| 89 | POMVAR | 2D-dist.hex | Yb/Er | Cl/CN | dmso | – | [37] |
| 104 | DIFLOW | 2D-brickwall | Pr | Cl/CN | dmso | – | [46] |
| 105 | NOQGAE | 2D-brickwall | Eu/Dy | Cl/CN | dmso | – | [27] |
| 106 | WOTWUA | 2D-brickwall | Ce | Cl/CN | dmso | – | [47] |
| 107 | WOTXAH | 2D-brickwall | Nd | Cl/CN | dmso | – | [47] |
| 108 | XOYTUD | 2D-brickwall | Dy | Cl/CN | dmso | – | [38] |
| 109 | WOTWEK | 2D-brickwall | Nd | Cl/CN | dmf | – | [47] |
| 111 | QOVJUJ | 2D-brickwall | Yb1 | Cl/CN | dmso/dobdc2- | – | [56] |
| 112 | QOVJOD | 2D-brickwall | Yb | Cl/CN | dmso/F4bdc2- | – | [56] |
| 126 | DIFLIQ | 2D-herringbone | Pr | Cl/CN | dmf | – | [46] |
| 127 | XIKNUD | 2D-herringbone | Yb | Cl/CN | dmf | – | [48] |
| 129 | DIFMAJ | 2D-4,4 | Dy | Cl/CN | H2O | H3O+ | [46] |
| 130 | XOYVAL | 2D-4,4 | Dy | Cl/CN | H2O | NEt2H2+ | [38] |
| 149 | POMVIZ | 2D-(3,4)+(3,8) | Er | Cl/CN | dmso | – | [37] |
| 150 | XOYVEP | 2D-(3,4)+(3,8) | Dy | Cl/CN | dmso | – | [38] |
| # | CCDC | Structure | Ln | X | L a | Reference |
|---|---|---|---|---|---|---|
| 11 | DEKVIB | Dimer | Y | CH3 | Tp- | [20] |
| 12 | DEKVOH | Dimer | Y | CH3 | Tp- | [20] |
| 32 | DEKVUN | Tetramer | Y | CH3 | Tp-/(MeO)4B/MeOH | [20] |
| 13 | EDEZAR | Dimer | Gd | NO2 | H2O | [26] |
| 14 | EDEZEV | Dimer | Tb | NO2 | H2O | [26] |
| 15 | EDEZIZ | Dimer | Dy | NO2 | H2O | [26] |
| 16 | EDEZOF | Dimer | Ho | NO2 | H2O | [26] |
| 17 | EDEZUL | Dimer | Sm | NO2 | H2O | [26] |
| 19 | JOQSIU | Dimer | Dy | F | Tp- | [23] |
| 20 | JOQSOA | Dimer | Y | F | Tp- | [23] |
| 92 | KUVBIP | 2D-dist.hex | La | t-Bu | dma | [52] |
| 93 | KUVBOV | 2D-dist.hex | Pr | t-Bu | dma | [52] |
| 94 | KUVBUB | 2D-dist.hex | Nd | t-Bu | dma | [52] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 3 | DEHRAM | Dimer | Y | H | thf/Cl- | – | [21] |
| 7 | DEKTUL | Dimer | Y | Cl | Tp- | – | [20] |
| 8 | DEKTUL01 | Dimer | Y | Cl | Tp- | – | [22] |
| 9 | DEKVAT | Dimer | Y | Cl | Tp- | – | [20] |
| 10 | DEKVEX | Dimer | Y | H | Tp- | – | [20] |
| 11 | DEKVIB | Dimer | Y | CH3 | Tp- | – | [20] |
| 12 | DEKVOH | Dimer | Y | CH3 | Tp- | – | [20] |
| 20 | JOQSOA | Dimer | Y | F | Tp- | – | [23] |
| 21 | JOQSUG | Dimer | Y | Br | Tp- | – | [23] |
| 32 | DEKVUN | Tetramer | Y | CH3 | Tp-/(MeO)4B/MeOH | – | [20] |
| 40 | MIZXUO | 2D-hex | Y | H | H2O | – | [16] |
| 63 | MIZZEA | 2D-dist.hex | Y | Cl | H2O | – | [16] |
| 97 | GASMUI | 2D-brickwall | Y | Cl | H2O | – | [14] |
| 101 | GASMOC | 2D-brickwall | Y | Br | H2O | – | [14] |
| 132 | QOFBOF | 2D-4,4 | Y | Cl | – | NEt4+ | [57] |
| 133 | QOFBUL | 2D-4,4 | Y | Cl | – | NEt4+ | [57] |
| 134 | QOFCAS | 2D-4,4 | Y | Cl | – | NEt4+ | [57] |
| 139 | QOFNUX | 2D-4,4 | Y | Cl | – | NEt4+ | [57] |
| 151 | MOBBIO | 3D-diam | Y | Cl | – | H3O+ | [16] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 36 | MIZXAU | 2D-hex | La | H | H2O | – | [16] |
| 67 | 1944118 | 2D-dist.hex | La | Cl | H2O | – | [45] |
| 72 | 1565271 | 2D-dist.hex | La | Br | H2O | – | [45] |
| 92 | KUVBIP | 2D-dist.hex | La | t-Bu | dma | – | [52] |
| 116 | NIDLIY | 2D-herringbone | La | Br | dmso | – | [51] |
| 128 | MIZYAV | 2D-3,4 | La | Cl | H2O | – | [16] |
| 147 | WOXVIR | 2D-4,4 | La | Cl | H2O | NEt4+ | [58] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 42 | ZOTTAD | 2D-hex | Ce | H | H2O | – | [15] |
| 50 | DIFLEM | 2D-hex | Ce | Cl/CN | dmf | – | [46] |
| 61 | MIZZAW | 2D-dist.hex | Ce | Cl | H2O | – | [16] |
| 62 | MIZZAW01 | 2D-dist.hex | Ce | Cl | H2O | – | [16] |
| 73 | 1565272 | 2D-dist.hex | Ce | Br | H2O | – | [45] |
| 106 | WOTWUA | 2D-brickwall | Ce | Cl/CN | dmso | – | [47] |
| 114 | JOGHAR | 2D-herringbone | Ce | Cl | H2O | – | [54] |
| 117 | NIDLOE | 2D-herringbone | Ce | Br | dmso | – | [51] |
| 146 | WOXVEN | 2D-4,4 | Ce | Cl | H2O | NEt4+ | [58] |
| 154 | JOGHIZ | 3D-diam | Ce | Cl | H2O | DPMP+ | [54] |
| 155 | JOGHOF | 3D-diam | Ce | Cl | H2O | PPh4+ | [54] |
| 156 | JOGHUL | 3D-noq | Ce | Cl | H2O | PPh3Me+ | [54] |
| # | CCDC | Structure | Ln | X | L | Reference |
|---|---|---|---|---|---|---|
| 43 | 1944109 | 2D-hex | Pr | H | H2O | [45] |
| 58 | MIZYID | 2D-dist.hex | Pr | Cl | H2O | [16] |
| 74 | 1565273 | 2D-dist.hex | Pr | Br | H2O | [45] |
| 93 | KUVBOV | 2D-dist.hex | Pr | t-Bu | Dma | [52] |
| 96 | CAZZAE | 2D-brickwall | Pr | Cl | EtOH | [13] |
| 104 | DIFLOW | 2D-brickwall | Pr | Cl/CN | dmso | [46] |
| 118 | NIDLUK | 2D-herringbone | Pr | Br | dmso | [51] |
| 126 | DIFLIQ | 2D-herringbone | Pr | Cl/CN | Dmf | [46] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 44 | 1944110 | 2D-hex | Nd | H | H2O | – | [45] |
| 53 | XIKNOX | 2D-hex | Nd | Cl/CN | dmf | – | [48] |
| 59 | MIZYOJ | 2D-dist.hex | Nd | Cl | H2O | – | [16] |
| 75 | 1565274 | 2D-dist.hex | Nd | Br | H2O | – | [45] |
| 94 | KUVBUB | 2D-dist.hex | Nd | t-Bu | dma | – | [52] |
| 107 | WOTXAH | 2D-brickwall | Nd | Cl/CN | dmso | – | [47] |
| 109 | WOTWEK | 2D-brickwall | Nd | Cl/CN | dmf | – | [47] |
| 119 | NIDMAR | 2D-herringbone | Nd | Br | dmso | – | [51] |
| 143 | WOXTIP | 2D-4,4 | Nd | Cl | – | NEt4+ | [58] |
| 148 | WOXVOX | 2D-4,4 | Nd | Cl | H2O | NEt4+ | [58] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 17 | EDEZUL | Dimer | Sm | NO2 | H2O | – | [26] |
| 45 | 1944111 | 2D-hex | Sm | H | H2O | – | [45] |
| 68 | 1944120 | 2D-dist.hex | Sm | Cl | H2O | – | [45] |
| 76 | 1565275 | 2D-dist.hex | Sm | Br | H2O | – | [45] |
| 120 | NIDMEV | 2D-herringbone | Sm | Br | dmso | – | [51] |
| 145 | WOXTUB | 2D-4,4 | Sm | Cl | – | NEt4+ | [58] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 30 | NOQBUT | Dimer-zz | Eu | Cl/CN | H2O | – | [27] |
| 31 | NOQGEI | Dimer-zz | Eu/Dy | Cl/CN | H2O | – | [27] |
| 46 | 1944112 | 2D-hex | Eu | H | H2O | – | [45] |
| 65 | MIZZOK | 2D-dist.hex | Eu | Cl | H2O | – | [16] |
| 77 | 1565276 | 2D-dist.hex | Eu | Br | H2O | – | [45] |
| 105 | NOQGAE | 2D-brickwall | Eu/Dy | Cl/CN | dmso | – | [27] |
| 110 | GEPZUY | 2D-brickwall | Eu | Cl | bipym/H2O | – | [49] |
| 121 | NIDFEO | 2D-herringbone | Eu | Br | dmso | – | [51] |
| 123 | NOQBON | 2D-herringbone | Eu/Dy | Br | dmso | – | [27] |
| 144 | WOXTOV | 2D-4,4 | Eu | Cl | – | NEt4+ | [58] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 13 | EDEZAR | Dimer | Gd | NO2 | H2O | – | [26] |
| 26 | LEPNOM | Dimer | Gd | Cl | Tp- | – | [22] |
| 27 | LEPNOM01 | Dimer | Gd | Cl | Tp- | – | [19] |
| 37 | MIZXEY | 2D-hex | Gd | H | H2O | – | [16] |
| 57 | GEQBAH | 2D-dist.hex | Gd | Cl | H2O | – | [49] |
| 64 | MIZZIE | 2D-dist.hex | Gd | Cl | H2O | – | [16] |
| 78 | 1565277 | 2D-dist.hex | Gd | Br | H2O | – | [45] |
| 95 | LEBGEG | 2D-dist.hex | Gd | H | thf/Cl- | – | [53] |
| 113 | GEQBEL | 2D-herringbone | Gd | Cl | H2O | – | [49] |
| 122 | NIDFIS | 2D-herringbone | Gd | Br | dmso | – | [51] |
| 136 | QOFNAD | 2D-4,4 | Gd | Cl | – | NEt4+ | [57] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 14 | EDEZEV | Dimer | Tb | NO2 | H2O | – | [26] |
| 24 | LEPNIG | Dimer | Tb | Cl | Tp- | – | [22] |
| 25 | LEPNIG01 | Dimer | Tb | Cl | Tp- | – | [24] |
| 47 | 1944113 | 2D-hex | Tb | H | H2O | – | [45] |
| 60 | MIZYUP | 2D-dist.hex | Tb | Cl | H2O | – | [16] |
| 79 | 1565278 | 2D-dist.hex | Tb | Br | H2O | – | [45] |
| 82 | NIDFOY | 2D-dist.hex | Tb | Br | dmso | – | [51] |
| 140 | QOFPAF | 2D-4,4 | Tb | Cl | – | NEt4+ | [57] |
| 152 | EFOXUV | 3D-diam | Tb | Cl | H2O/dmf/HCOO- | NMe2H2+ | [60] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 1 | PIQFUT | Monomer | Dy | Cl | Tp- | – | [19] |
| 2 | DEHQUF | Dimer | Dy | H | thf/Cl- | – | [21] |
| 4 | DEKTOF | Dimer | Dy | Cl | Tp- | – | [20] |
| 5 | DEKTOF01 | Dimer | Dy | Cl | Tp- | – | [22] |
| 6 | DEKTOF02 | Dimer | Dy | Cl | Tp- | – | [19] |
| 15 | EDEZIZ | Dimer | Dy | NO2 | H2O | – | [26] |
| 18 | JOQSEQ | Dimer | Dy | Br | Tp- | – | [23] |
| 19 | JOQSIU | Dimer | Dy | F | Tp- | – | [23] |
| 48 | 1944114 | 2D-hex | Dy | H | H2O | – | [45] |
| 51 | WOTWIO | 2D-hex | Dy | Cl/CN | dmf | – | [47] |
| 55 | XOYTEN | 2D-hex | Dy | Cl/CN | dmf | – | [38] |
| 69 | 1944123 | 2D-dist.hex | Dy | Cl | H2O | – | [45] |
| 80 | 1565279 | 2D-dist.hex | Dy | Br | H2O | – | [45] |
| 83 | NIDFUE | 2D-dist.hex | Dy | Br | dmso | – | [51] |
| 108 | XOYTUD | 2D-brickwall | Dy | Cl/CN | dmso | – | [38] |
| 125 | LUTROK | 2D-herringbone | Dy | Br | dmf | – | [55] |
| 129 | DIFMAJ | 2D-4,4 | Dy | Cl/CN | H2O | H3O+ | [46] |
| 130 | XOYVAL | 2D-4,4 | Dy | Cl/CN | H2O | NEt2H2+ | [38] |
| 135 | QOFCEW | 2D-4,4 | Dy | Cl | – | NEt4+ | [57] |
| 150 | XOYVEP | 2D-(3,4)+(3,8) | Dy | Cl/CN | dmso | – | [38] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 16 | EDEZOF | Dimer | Ho | NO2 | H2O | – | [26] |
| 23 | KOZBIN | Dimer | Ho | Cl | Tp- | – | [24] |
| 35 | KUYKIZ | 2D-hex | Ho | H | H2O | – | [43] |
| 52 | WOTWOU | 2D-hex | Ho | Cl/CN | dmf | – | [47] |
| 70 | 1944124 | 2D-dist.hex | Ho | Cl | H2O | – | [45] |
| 81 | 1565280 | 2D-dist.hex | Ho | Br | H2O | – | [45] |
| 84 | NIDGAL | 2D-dist.hex | Ho | Br | dmso | – | [51] |
| 142 | QOFPIN | 2D-4,4 | Ho | Cl | – | NEt4+ | [57] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 22 | KOZBEJ | Dimer | Er | Cl | Tp- | – | [24] |
| 34 | NIGNID | 1D-ladder | Er | Cl | hmpa | – | [28] |
| 41 | PIVKAJ | 2D-hex | Er | H | H2O | – | [44] |
| 54 | XIKPAL | 2D-hex | Er | Cl/CN | dmf | – | [48] |
| 56 | NIGNUP | 2D-hex | Er | Cl | fma | – | [28] |
| 66 | NIGNEZ | 2D-dist.hex | Er | Cl | H2O | – | [28] |
| 71 | XAWZUT | 2D-dist.hex | Er | Br | H2O | – | [50] |
| 85 | XAXBAC | 2D-dist.hex | Er | Br | dmso | – | [50] |
| 89 | POMVAR | 2D-dist.hex | Yb/Er | Cl/CN | dmso | – | [37] |
| 90 | NIGQIG | 2D-dist.hex | Er | Cl | dmso | – | [28] |
| 91 | NIGNOJ | 2D-dist.hex | Er | Cl | dma | – | [28] |
| 98 | JOGJAT | 2D-brickwall | Er | Cl | H2O | – | [53] |
| 115 | NIGPAX | 2D-herringbone | Er | Cl | dmf | – | [28] |
| 124 | XAXBEG | 2D-herringbone | Er | Br | dmf | – | [50] |
| 138 | QOFNOR | 2D-4,4 | Er | Cl | – | NEt4+ | [57] |
| 149 | POMVIZ | 2D-(3,4)+(3,8) | Er | Cl/CN | dmso | – | [37] |
| 153 | JOGHEV | 3D-diam | Er | Cl | – | DPMP+ | [54] |
| 157 | JOGJEX | 3D-4,4-c | Er | Cl | H2O | PPh3Me+ | [54] |
| # | CCDC | Structure | Ln | X | L | Reference |
|---|---|---|---|---|---|---|
| 49 | 1944117 | 2D-hex | Tm | H | H2O | [45] |
| 99 | 1944126 | 2D-brickwall | Tm | Cl | H2O | [45] |
| 102 | 1565282 | 2D-brickwall | Tm | Br | H2O | [45] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 28 | OBIBEH | Dimer | Yb | Cl | Tp- | – | [25] |
| 29 | OBIBEH01 | Dimer | Yb | Cl | Tp- | – | [24] |
| 38 | MIZXIC | 2D-hex | Yb | H | H2O | – | [16] |
| 86 | NIDGEP | 2D-dist.hex | Yb | Br | dmso | – | [51] |
| 87 | DIFLUC | 2D-dist.hex | Yb | Cl/CN | dmso | – | [46] |
| 88 | POMTUJ | 2D-dist.hex | Yb | Cl/CN | dmso | – | [37] |
| 89 | POMVAR | 2D-dist.hex | Yb/Er | Cl/CN | dmso | – | [37] |
| 100 | 1944127 | 2D-brickwall | Yb | Cl | H2O | – | [45] |
| 103 | LUTRIE | 2D-brickwall | Yb | Br | H2O | – | [55] |
| 111 | QOVJUJ | 2D-brickwall | Yb | Cl/CN | dmso/dobdc2− | – | [56] |
| 112 | QOVJOD | 2D-brickwall | Yb | Cl/CN | dmso/F4bdc2− | – | [56] |
| 127 | XIKNUD | 2D-herringbone | Yb | Cl/CN | dmf | – | [48] |
| 137 | QOFNEH | 2D-4,4 | Yb | Cl | – | NEt4+ | [57] |
| # | CCDC | Structure | Ln | X | L | A+ | Reference |
|---|---|---|---|---|---|---|---|
| 33 | MIZZUQ | 1D + mon | Lu | Cl | H2O | – | [16] |
| 39 | MIZXOI | 2D-hex | Lu | H | H2O | – | [16] |
| 141 | QOFPEJ | 2D-4,4 | Lu | Cl | – | NEt4+ | [57] |
| # | Formula | Ln | Structure | Properties | Reference |
|---|---|---|---|---|---|
| 35–38 41–49 | [Ln2(dhbq)3(H2O)6]·18H2O | La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb | 2D-hexagonal | −H2O/+H2O −H2O/+Guess CO2 adsorption | [45] |
| 58–60 62, 64–70 | [Ln2(C6O4Cl2)3(H2O)6]·nH2O | La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er | Distorted 2D-hex. | −H2O/+H2O −H2O/+Guess CO2 adsorption | [45] |
| 71–81 | [Ln2(C6O4Br2)3(H2O)6]·nH2O | La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er | Distorted 2D-hex. | −H2O/+H2O −H2O/+Guess CO2 adsorption | [45] |
| 80 | [Dy2(C6O4Br2)3(H2O)6]·8H2O | Dy | 2D-brickwall | Solv Exch. | [55] |
| 83 | [Dy2(C6O4Br2)3(dmso)6]·2dmso | Dy | Distorted 2D-hex. | Solv Exch. | [55] |
| 125 | [Dy2(C6O4Br2)3(dmf)6] | Dy | 2D-herringbone | Solv Exch. | [55] |
| 92 | [La2(C6O4(t-Bu)2)3(dma)4] | La | Distorted 2D-hex. | CO2 abs | [52] |
| 93 | [Pr2(C6O4(t-Bu)2)3(dma)4] | Pr | Distorted 2D-hex. | CO2 abs | [52] |
| 94 | [Nd2(C6O4(t-Bu)2)3(dma)4] | Nd | Distorted 2D-hex. | CO2 abs | [52] |
| 99–100 | [Ln2(C6O4Cl2)3(H2O)6]·nH2O | Tm, Yb | 2D-brickwall | −H2O/+H2O −H2O/+Guess | [45] |
| 102–103 | [Ln2(C6O4Br2)3(H2O)6]·nH2O | Tm, Yb | 2D-brickwall | −H2O/+H2O −H2O/+Guess | [45] |
| 131 | (NEt4)[Y(C6O4Cl2)2]·1.4CS2 | Y | 2D-4,4 | 1.4 CS2 abs | [57] |
| 132 | (NEt4)[Y(C6O4Cl2)2] | Y | 2D-4,4 | N2/H2/CO2/CH4 | [57] |
| 133 | (NEt4)[Y(C6O4Cl2)2]·1.9I2 | Y | 2D-4,4 | 1.9 I2 abs | [57] |
| 134 | (NEt4)[Y(C6O4Cl2)2]·0.9Br2 | Y | 2D-4,4 | 0.9 Br2 abs | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benmansour, S.; Gómez-García, C.J. Lanthanoid-Anilato Complexes and Lattices. Magnetochemistry 2020, 6, 71. https://doi.org/10.3390/magnetochemistry6040071
Benmansour S, Gómez-García CJ. Lanthanoid-Anilato Complexes and Lattices. Magnetochemistry. 2020; 6(4):71. https://doi.org/10.3390/magnetochemistry6040071
Chicago/Turabian StyleBenmansour, Samia, and Carlos J. Gómez-García. 2020. "Lanthanoid-Anilato Complexes and Lattices" Magnetochemistry 6, no. 4: 71. https://doi.org/10.3390/magnetochemistry6040071
APA StyleBenmansour, S., & Gómez-García, C. J. (2020). Lanthanoid-Anilato Complexes and Lattices. Magnetochemistry, 6(4), 71. https://doi.org/10.3390/magnetochemistry6040071