Core Size and Interface Impact on the Exchange Bias of Cobalt/Cobalt Oxide Nanostructures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Samples
- (i)
- For the l-series, the precipitate was calcined in air at 300 °C to yield Co3O4 (as identified by XRD). Then, Co3O4 was used to synthesize Co nanostructures by reduction in extra pure hydrogen gas (99.993%) at 900 °C using a controlled-atmosphere tube furnace for 30 min. Finally, the resulting Co particles were oxidized in pure oxygen gas at 900 °C for different time durations, namely 15, 60, 300, and 540 min, resulting in the samples that are denoted as l(15), l(60), l(300), and l(540), respectively.
- (ii)
- For the s-series, the Co(OH)2 precursor was not calcined in air but directly reduced in hydrogen at (only) 300 °C, which allowed the formation of metallic Co particles, and subsequently oxidized also at the relatively low temperature of 300 °C for different treatment durations, resulting in the samples we label as s(10), s(20), s(40), and s(80), where the numbers give the duration of the oxidation treatment in minutes.
2.2. Structural and Magnetic Characterization Methods
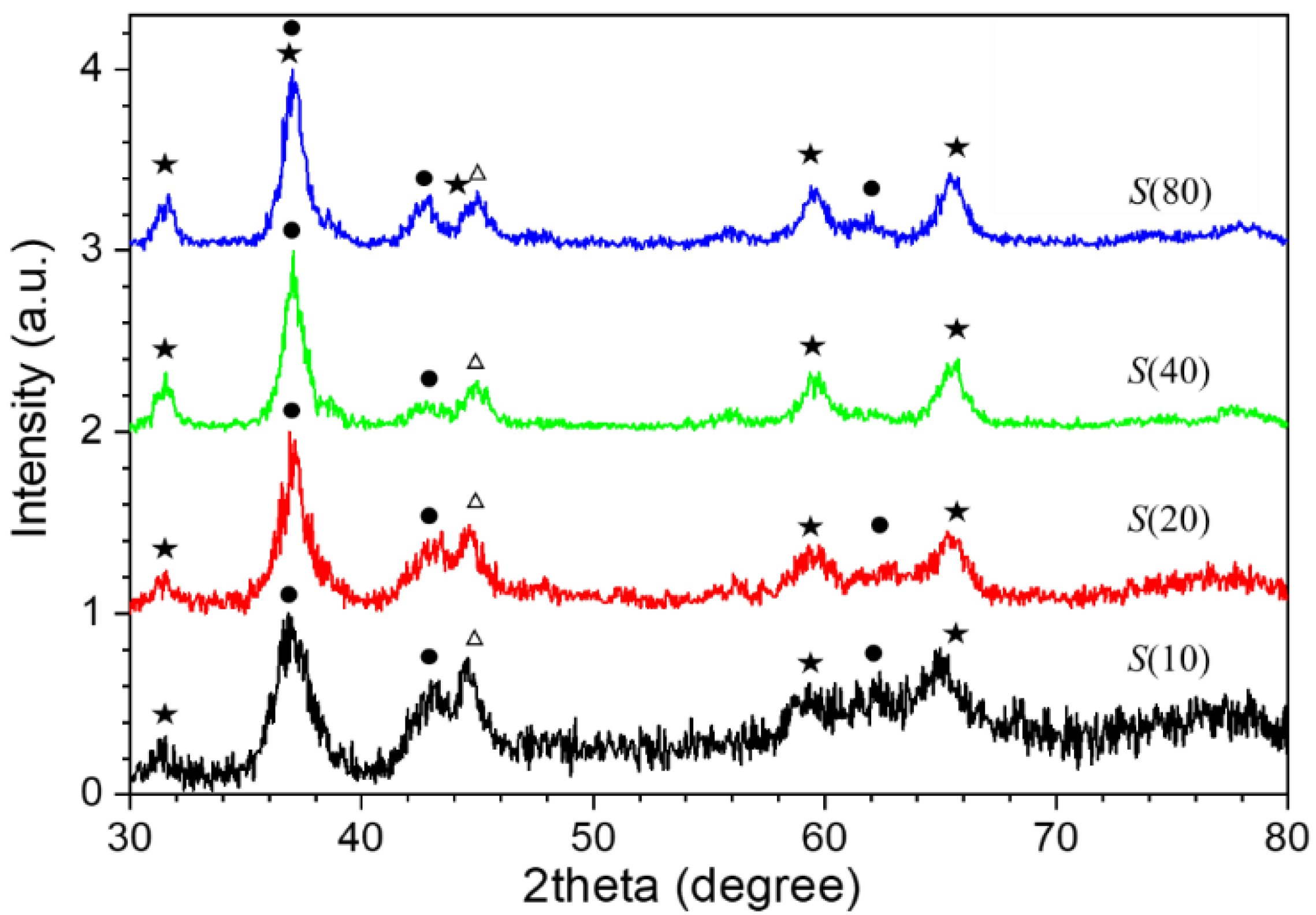
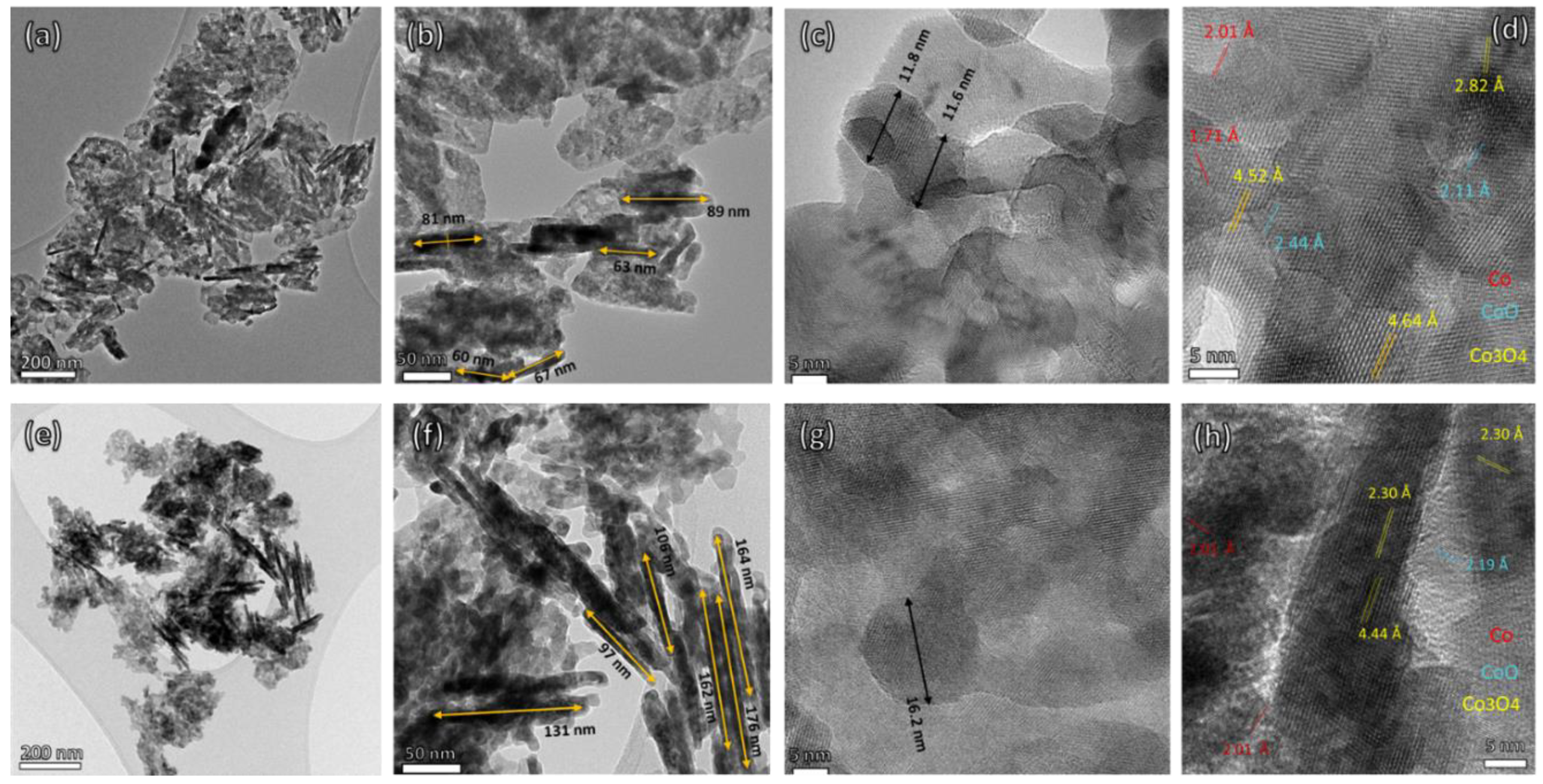
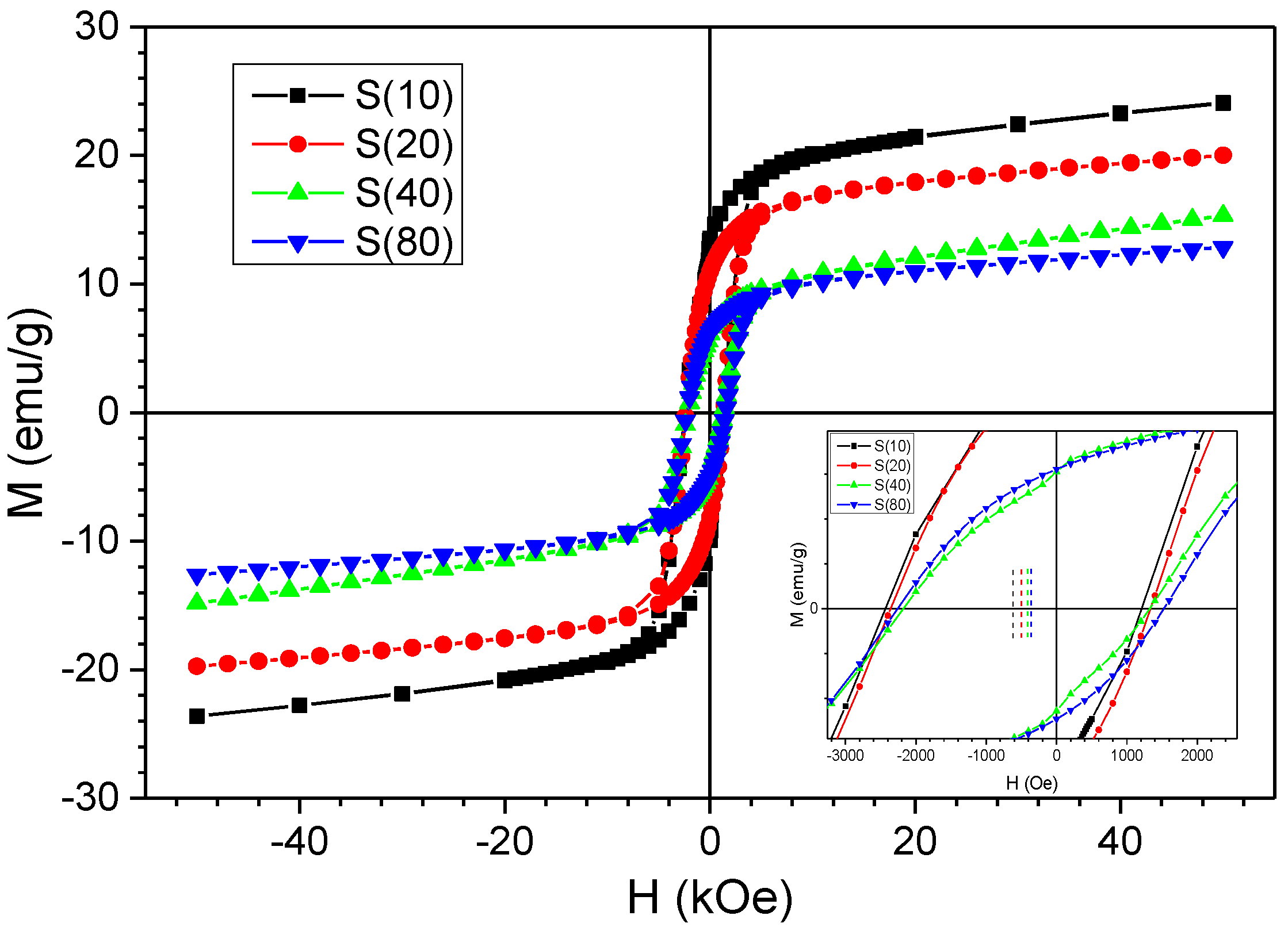
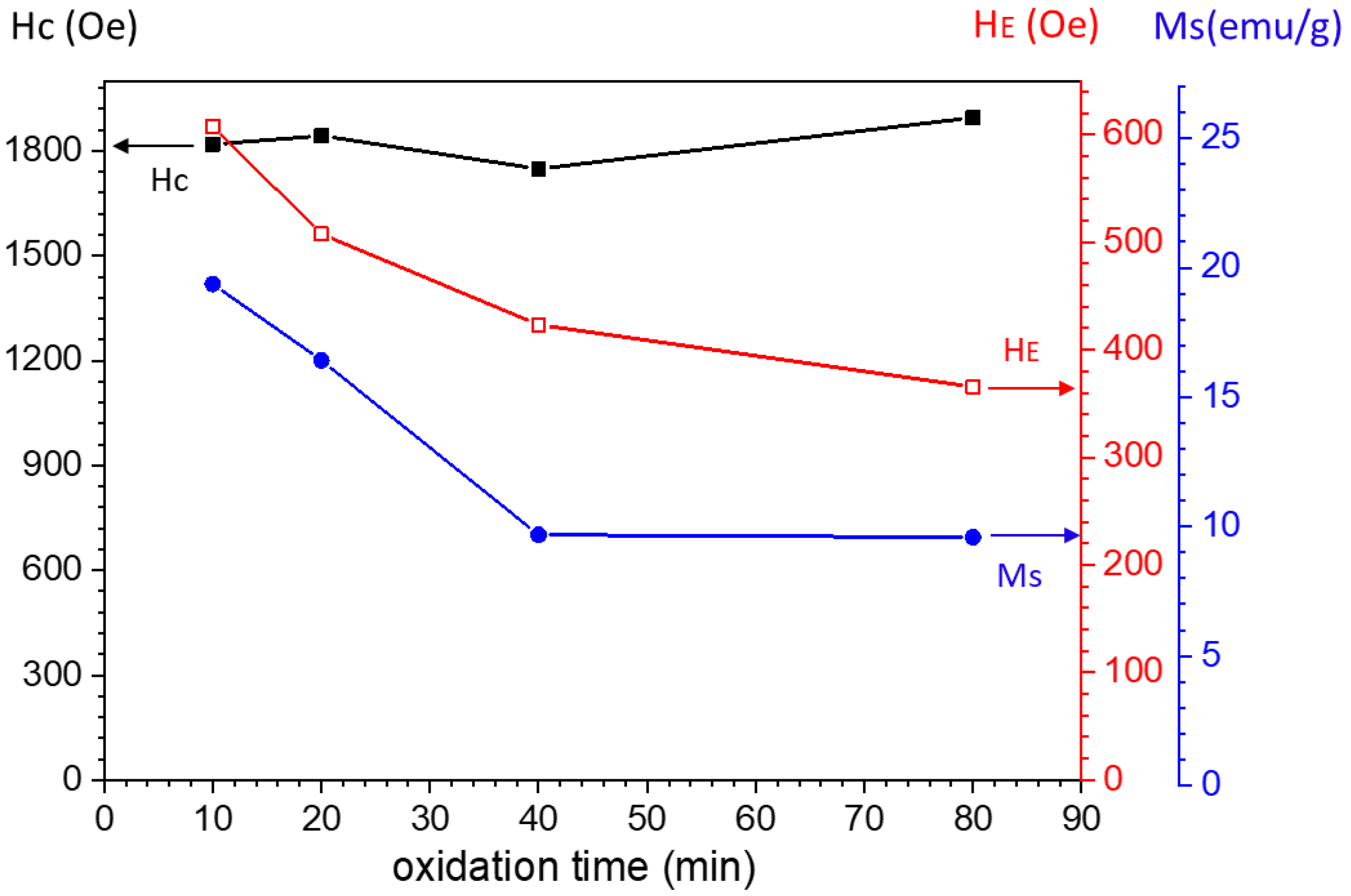
3. Result and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nogués, J.; Sort, J.; Langlais, V.; Skumryev, V.; Suriñach, S.; Muñoz, J.S.; Baró, M.D. Exchange bias in nanostructures. Phys. Rep. 2005, 422, 65–117. [Google Scholar] [CrossRef]
- López-Ortega, A.; Estrader, M.; Salazar-Alvarez, G.; Roca, A.G.; Nogués, J. Applications of exchange coupled bi-magnetic hard/soft and soft/hard magnetic core/shell nanoparticles. Phys. Rep. 2015, 553, 1–32. [Google Scholar] [CrossRef] [Green Version]
- Vatansever, Z.D.; Vatansever, E. Tunning exchange bias in inverted antiferromagnetic/ferromagnetic core/shell nanoparticles by binary alloy shells. Phys. Lett. A 2018, 382, 2901–2907. [Google Scholar] [CrossRef]
- González, J.A.; Andrés, J.P.; López Antón, R.; De Toro, J.A.; Normile, P.S.; Muniz, P.; Riveiro, J.M.; Nogués, J. Maximizing Exchange Bias in Co/CoO Core/Shell Nanoparticles by Lattice Matching between the Shell and the Embedding Matrix. Chem. Mater. 2017, 29, 5200–5206. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Reethu, K.; Thanveer, T.; Myint, M.T.Z.; Al-Harthi, S.H. Effect of shell thickness on the exchange bias blocking temperature and coercivity in Co-CoO core-shell nanoparticles. J. Appl. Phys. 2017, 122, 063902. [Google Scholar] [CrossRef]
- Obaidat, I.M.; Nayek, C.; Manna, K.; Bhattacharjee, G.; Al-Omari, I.A.; Gismelseed, A. Investigating exchange bias and coercivity in Fe3O4–γ-Fe2O3 core–shell nanoparticles of fixed core diameter and variable shell thicknesses. Nanomaterials 2017, 7, 415. [Google Scholar] [CrossRef] [Green Version]
- Nogués, J.; Schuller, I.K. Exchange Bias. J. Magn. Magn. Mater. 1999, 192, 203–232. [Google Scholar] [CrossRef]
- Giri, S.; Patra, M.; Majumdar, S. Exchange bias effect in alloys and compounds. J. Phys. Condens. Matter 2011, 23, 073201. [Google Scholar] [CrossRef]
- Eftaxias, E.; Trohidou, K.N. Numerical study of the exchange bias effects in magnetic nanoparticles with core/shell morphology. Phys. Rev. B 2005, 71, 134406. [Google Scholar] [CrossRef]
- Ghoshani, M.; Mozaffari, M.; Al-nabhani, A. Influence of milling time on the structural and exchange bias of CoO and Co–Co oxide nanocomposite systems. Ceram. Int. 2021, 47, 5133–5144. [Google Scholar] [CrossRef]
- González, J.A.; Andrés, J.P.; De Toro, J.A.; Muñiz, P.; Muñoz, T.; Crisan, O.; Binns, C.; Riveiro, J.M. Co–CoO nanoparticles prepared by reactive gas-phase aggregation. J. Nanopart. Res. 2009, 11, 2105–2111. [Google Scholar] [CrossRef]
- Riveiro, J.M.; De Toro, J.A.; Andrés, J.P.; González, J.A.; Muñoz, T. Exchange-bias stabilization of the magnetic nanoparticles in a granular alloy grown by reactive sputtering. Appl. Phys. Lett. 2005, 86, 172503. [Google Scholar] [CrossRef]
- Salazar, J.S.; Perez, L.; De Abril, O.; Phuoc, L.T.; Ihiawakrim, D.; Vazquez, M.; Greneche, J.; Begin-colin, S.; Pourroy, G. Magnetic Iron Oxide Nanoparticles in 10–40 nm Range: Composition in Terms of Magnetite/Maghemite Ratio and Effect on the Magnetic Properties. Chem. Mater. 2011, 23, 1379–1386. [Google Scholar] [CrossRef]
- Falk, R.B.; Hooper, G.D. Elongated Iron-Cobalt: Ferrite, a New, Lightweight, Permanent Magnet Material. J. Appl. Phys. 1961, 32, 190–192. [Google Scholar] [CrossRef]
- Dimitrov, D.V.; van Ek, J.; Li, Y.F.; Xiao, J.Q. Enhanced magnetic stability in spin valves with synthetic antiferromagnet. J. Appl. Phys. 2000, 87, 6427–6429. [Google Scholar] [CrossRef]
- Kools, J.C.S. Exchange-biased spin-valves for magnetic storage. IEEE Trans. Magn. 1996, 32, 3165–3184. [Google Scholar] [CrossRef]
- Gurney, B.A.; Speriosu, V.S.; Wilhoit, D.R.; Lefakis, H.; Fontana, R.E.; Heim, D.E.; Dovek, M. Can spin valves be reliably deposited for magnetic recording applications? J. Appl. Phys. 1997, 81, 3998–4003. [Google Scholar] [CrossRef]
- Estrader, M.; López-Ortega, A.; Estradé, S.; Golosovsky, I.V.; Salazar-Alvarez, G.; Vasilakaki, M.; Trohidou, K.N.; Varela, M.; Stanley, D.C.; Sinko, M.; et al. Robust antiferromagnetic coupling in hard-soft bi-magnetic core/shell nanoparticles. Nat. Commun. 2013, 4, 2960. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Bertoni, G.; De Julián Fernández, C.; Sangregorio, C. Topotaxial Phase Transformation in Cobalt Doped Iron Oxide Core/Shell Hard Magnetic Nanoparticles. Chem. Mater. 2017, 29, 1279–1289. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Silvestri, N.; Fernandez-Cabada, T.; Marinaro, F.; Fernandes, S.; Fiorito, S.; Miscuglio, M.; Serantes, D.; Ruta, S.; Livesey, K.; et al. Exploiting unique alignment of cobalt-ferrite nanoparticles, mild hyperthermia, and controlled intrinsic cobalt toxicity for cancer therapy. Adv. Mater. 2020, 91, 017203. [Google Scholar] [CrossRef]
- Sharrock, M.P. Recent advances in metal particulate recording media: Toward the ultimate particle. IEEE Trans. Magn. 2000, 36, 2420–2425. [Google Scholar] [CrossRef]
- Goh, C.K.; Yuan, Z.M.; Liu, B. Magnetization reversal in enclosed composite pattern media structure. J. Appl. Phys. 2009, 105, 083920. [Google Scholar] [CrossRef]
- De Toro, J.A.; Marques, D.P.; Muñiz, P.; Skumryev, V.; Sort, J.; Givord, D.; Nogués, J. High Temperature Magnetic Stabilization of Cobalt Nanoparticles by an Antiferromagnetic Proximity Effect. Phys. Rev. Lett. 2015, 115, 057201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Normile, P.S.; De Toro, J.A.; Muñoz, T.; González, J.A.; Andrés, J.P.; Muñiz, P.; Galindo, R.E.; Riveiro, J.M. Influence of spacer layer morphology on the exchange-bias properties of reactively sputtered Co/Ag multilayers. Phys. Rev. B 2007, 76, 104430. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Takano, K. Exchange Anisoropy-a Review. J. Magn. Magn. Mater. 1999, 200, 552–570. [Google Scholar] [CrossRef]
- Baltz, V.; Sort, J.; Rodmacq, B.; Dieny, B.; Landis, S. Thermal activation effects on the exchange bias in ferromagnetic- antiferromagnetic nanostructures. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 72, 104419. [Google Scholar] [CrossRef] [Green Version]
- Feygenson, M.; Yiu, Y.; Kou, A.; Kim, K.S.; Aronson, M.C. Controlling the exchange bias field in Co core/CoO shell nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 81, 195445. [Google Scholar] [CrossRef]
- Stiles, M.D.; McMichael, R.D. Coercivity in exchange-bias bilayers. Phys. Rev. B Condens. Matter Mater. Phys. 2001, 63, 064405. [Google Scholar] [CrossRef]
- Nascimento, V.P.; Passamani, E.C.; Alvarenga, A.D.; Biondo, A.; Pelegrini, F.; Saitovitch, E.B. Bottom and top AF/FM interfaces of NiFe/FeMn/NiFe trilayers. Appl. Surf. Sci. 2008, 254, 2114–2119. [Google Scholar] [CrossRef]
- Kovylina, M.; Del Muro, M.G.; Konstantinović, Z.; Varela, M.; Iglesias, O.; Labarta, A.; Batlle, X. Controlling exchange bias in Co-CoOx nanoparticles by oxygen content. Nanotechnology 2009, 20, 175702. [Google Scholar] [CrossRef]
- Iglesias, Ò.; Labarta, A.; Batlle, X. Exchange Bias Phenomenology and Models of Core / Shell Nanoparticles. J. Nanosci. Nanotechnol. 2008, 8, 2761–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Patra, M.; Majumdar, S.; Giri, S. Exchange bias effect at the irregular interfaces between Co and CoO nanostructures. J. Alloys Compd. 2009, 488, 27–30. [Google Scholar] [CrossRef]
- Tracy, J.B.; Weiss, D.N.; Dinega, D.P.; Bawendi, M.G. Exchange biasing and magnetic properties of partially and fully oxidized colloidal cobalt nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 72, 064404. [Google Scholar] [CrossRef]
- Tracy, J.B.; Bawendi, M.G. Defects in CoO in oxidized cobalt nanoparticles dominate exchange biasing and exhibit anomalous magnetic properties. Phys. Rev. B Condens. Matter Mater. Phys. 2006, 74, 184434. [Google Scholar] [CrossRef]
- Antón, R.L.; González, J.A.; Andrés, J.P.; Normile, P.S.; Canales-Vázquez, J.; Muñiz, P.; Riveiro, J.M.; De Toro, J.A. Exchange bias optimization by controlled oxidation of cobalt nanoparticle films prepared by sputter gas aggregation. Nanomaterials 2017, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Antón, R.L.; González, J.A.; Andrés, J.P.; Canales-Vázquez, J.; De Toro, J.A.; Riveiro, J.M. High-vacuum annealing reduction of Co/CoO nanoparticles. Nanotechnology 2014, 25, 105702. [Google Scholar] [CrossRef]
- Bhowmik, R.N.; Nagarajan, R.; Ranganathan, R. Magnetic enhancement in antiferromagnetic nanoparticle of CoRh2O4. Phys. Rev. B 2004, 69, 054430. [Google Scholar] [CrossRef]
- Sundaresan, A.; Bhargavi, R.; Rangarajan, N.; Siddesh, U.; Rao, C.N.R. Ferromagnetism as a universal feature of nanoparticles of the otherwise nonmagnetic oxides. Phys. Rev. B 2006, 74, 161306. [Google Scholar] [CrossRef]
- Kisan, B.; Kumar, J.; Saravanan, P.; Perumal, A. Defect induced ferromagnetism in NiO nanocrystals: Insight from experimental and DFT+U study. Physica B 2020, 593, 412319. [Google Scholar] [CrossRef]
- Dutta, D.P.; Sharma, G.; Manna, P.K.; Tyagi, A.K.; Yusuf, S.M. Room temperature ferromagnetism in CoO nanoparticles obtained from sonochemically synthesized precursors. Nanotechnology 2008, 19, 245609. [Google Scholar] [CrossRef]
- Roca, A.G.; Golosovsky, I.V.; Winkler, E.; López-Ortega, A.; Estrader, M.; Zysler, R.D.; Baró, M.D.; Nogués, J. Unravelling the Elusive Antiferromagnetic Order in Wurtzite and Zinc Blende CoO Polymorph Nanoparticles. Small 2018, 14, 1703963. [Google Scholar] [CrossRef]
- Skumryev, V.; Stoyanov, S.; Zhang, Y.; Hadjipanayis, G.; Givord, D.; Nogués, J. Beating the superparamagnetic limit with exchange bias. Nature 2003, 423, 850–853. [Google Scholar] [CrossRef]
- Morrish, A.H. The Physical Principles of Magnetism; IEEE Press Classic Reissue: Holmdel, NJ, USA, 2001; ISBN 9780470546581. [Google Scholar]
- Zhu, H.T.; Luo, J.; Liang, J.K.; Rao, G.H.; Li, J.B.; Zhang, J.Y.; Du, Z.M. Synthesis and magnetic properties of antiferromagnetic Co3O4 nanoparticles. Physica B 2008, 403, 3141–3145. [Google Scholar] [CrossRef]
- He, L.; Chen, C. Finite size effect on Néel temperature with Co3O4 nanoparticles. J. Appl. Phys. 2007, 102, 103911. [Google Scholar] [CrossRef] [Green Version]
- Dobrynin, A.N.; Levlev, D.N.; Temst, K.; Lievens, P.; Margueritat, J. Critical size for exchange bias in ferromagnetic-antiferromagnetic particles. Appl. Phys. Lett. 2005, 87, 012501. [Google Scholar] [CrossRef] [Green Version]
- Inderhees, S.E.; Borchers, J.A.; Green, K.S.; Kim, M.S.; Sun, K.; Strycker, G.L.; Aronson, M.C. Manipulating the Magnetic Structure of Co Core/CoO Shell Nanoparticles: Implications for Controlling the Exchange Bias. Phys. Rev. Lett. 2008, 101, 117202. [Google Scholar] [CrossRef]
- You, B.; Wang, Y.; Zhao, Y.; Sun, L.; Sheng, W.; Pan, M.; Du, J.; Hu, A.; Lu, M. Exchange bias in Co/Co3O4 bilayers. J. Appl. Phys. 2003, 93, 6587–6589. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Cao, Y.; Lu, M.; Yang, J. Properties of exchange biased Co/Co3O4 bilayer films. J. Alloys Compd. 2008, 450, 128–130. [Google Scholar] [CrossRef]
- Mishra, S.R.; Dubenko, I.; Khan, M.; Young, T.; Ganegoda, H.; Ali, N.; Marasinghe, G.K. Exchange-Coupled FeNi–X (X = CuO, NiO, and CoO) Nanocomposites Prepared Via Ball Milling. IEEE Trans. Magn. 2006, 42, 2808–2811. [Google Scholar] [CrossRef]
- Ohldag, H.; Scholl, A.; Nolting, F.; Arnholz, E.; Maat, S.; Young, A.T.; Carey, M.; Stöhr, J. Correlation between Exchange Bias and Pinned Interfacial Spins. Phys. Rev. Lett. 2003, 91, 17203. [Google Scholar] [CrossRef] [Green Version]
- Fitzsimmons, M.R.; Kirby, B.J.; Roy, S.; Li, Z.; Roshchin, I.V.; Sinha, S.K.; Schuller, I.K. Pinned magnetization in the antiferromagnet and ferromagnet of an exchange bias system. Phys. Rev. B 2007, 75, 214412. [Google Scholar] [CrossRef]

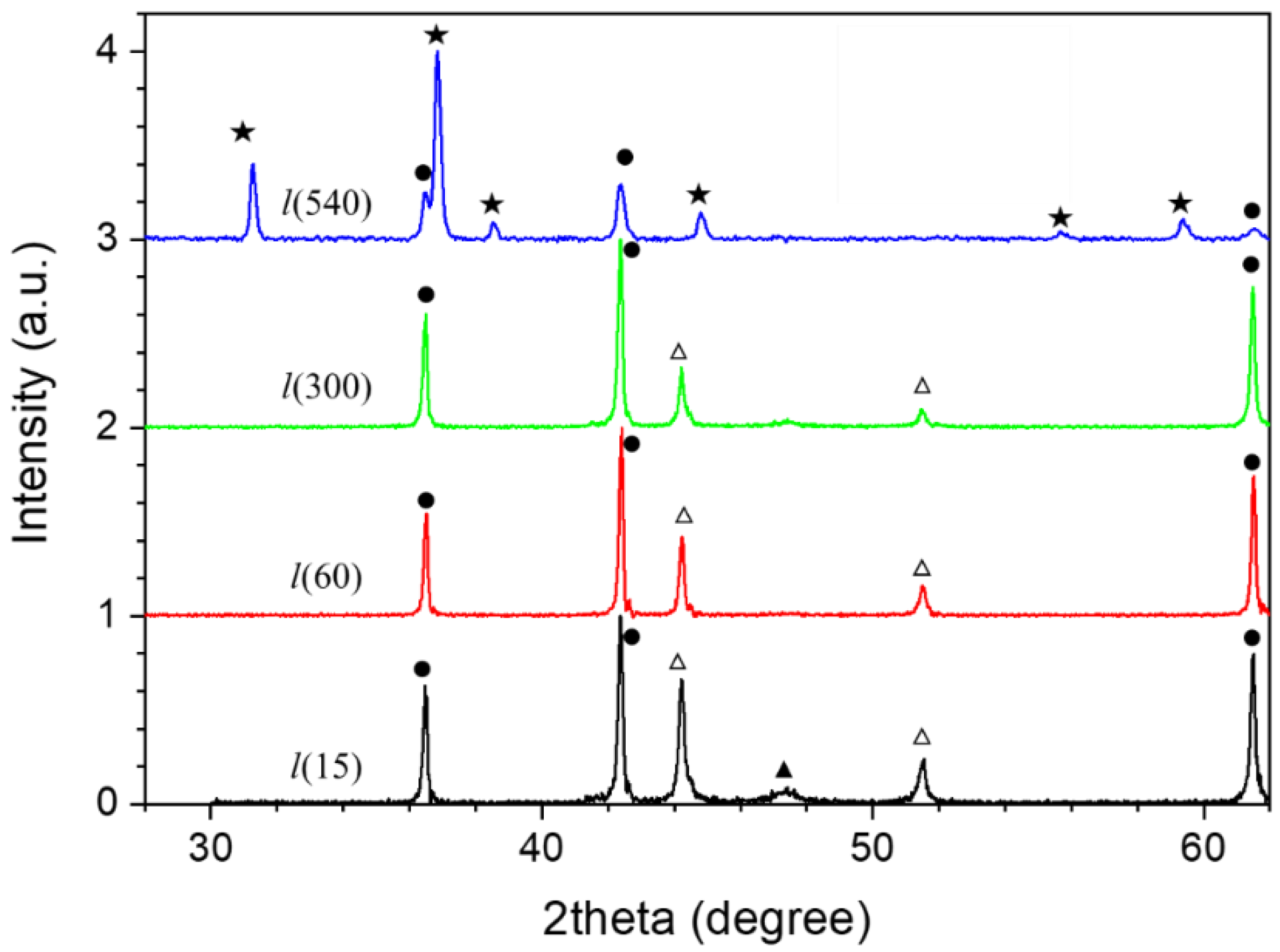
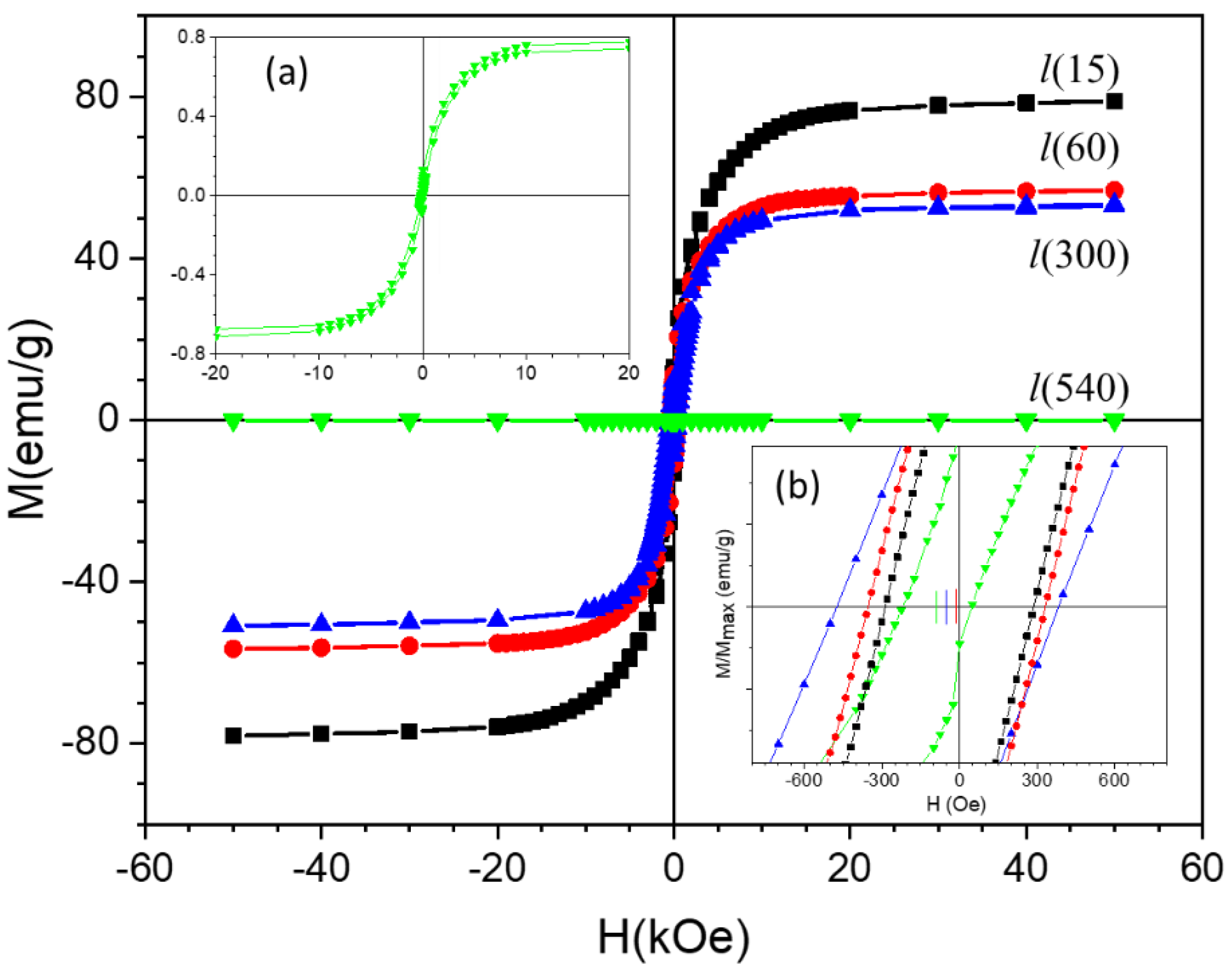
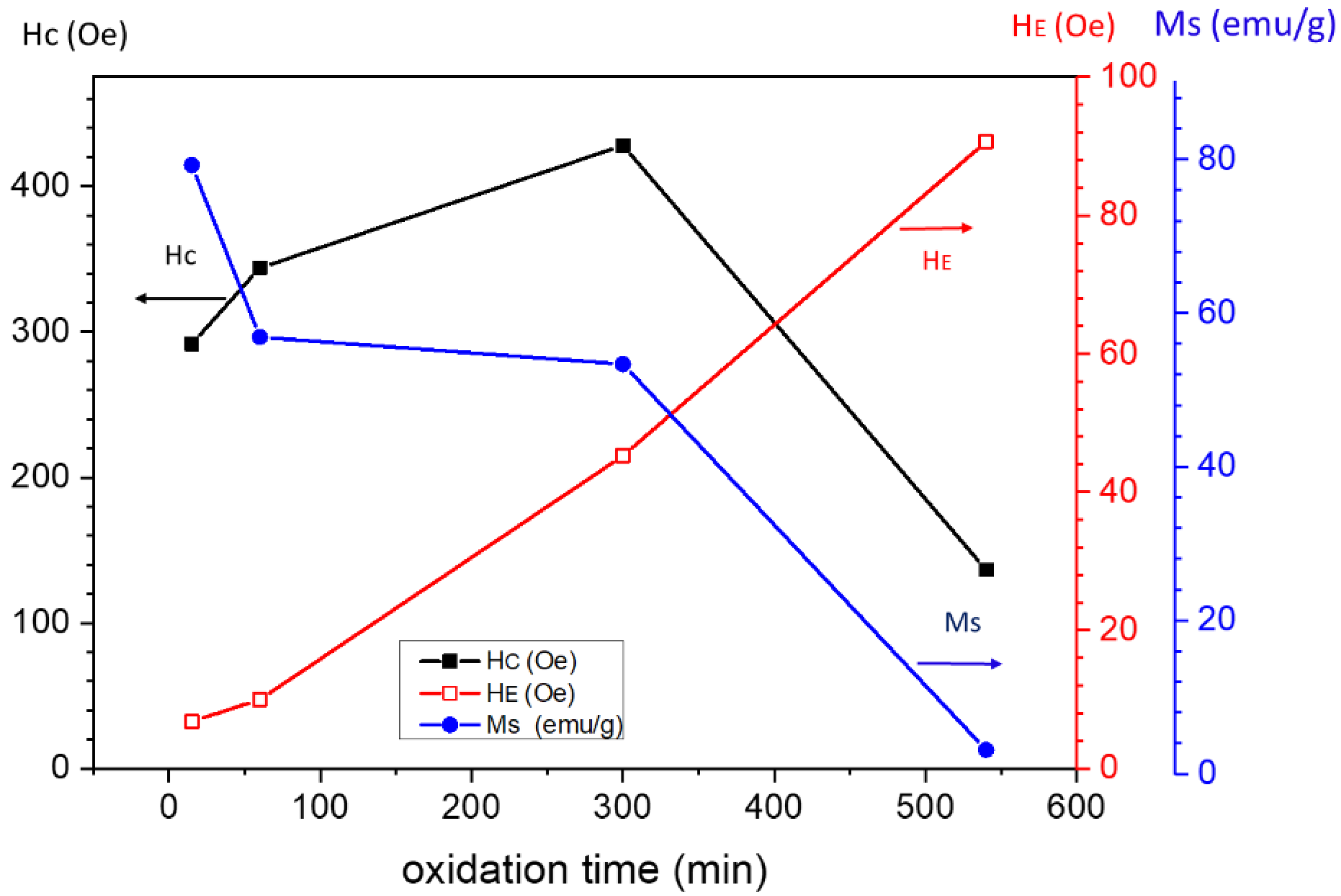
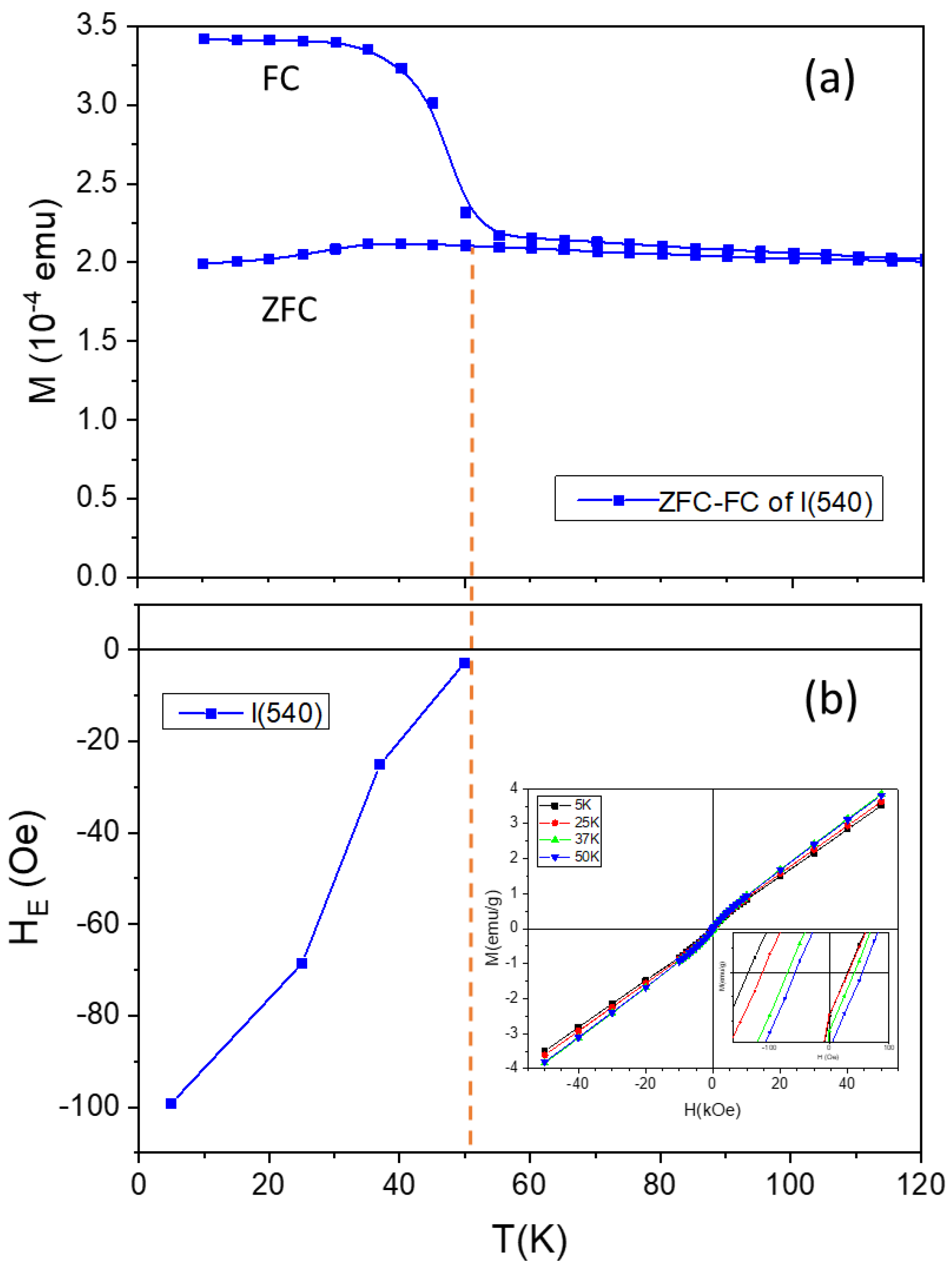

| Sample | Co-Oxide Percentage (1-(MS/MS,bulk)) ×100 | Co Percentage (MS/MS,bulk) ×100 | Co-Oxide Percentage (XRD) | Co Percentage (XRD) | Co-Oxide Crystallite Size (nm) | Co Crystallite Size (nm) |
|---|---|---|---|---|---|---|
| l(15) | 51.9 | 48.1 | 56.4 | 43.6 | 89 | 92 |
| l(60) | 65.5 | 34.5 | 73.3 | 26.7 | 94 | 74 |
| l(300) | 67.7 | 32.3 | 75.9 | 24.1 | 107 | 70 |
| l(540) | 98 | 2 | 100 | 0 | 65 | 0 |
| Sample | Co-Oxide Percentage (1-(MS/MS,bulk)) × 100 | Co Percentage (MS/MS,bulk) ×100 | Co-Oxide Percentage (XRD) | Co Percentage (XRD) | Co-Oxide Crystallite Size (nm) | Co Crystallite Size (nm) |
|---|---|---|---|---|---|---|
| s(10) | 87.9 | 12.1 | 73 | 27 | 8 | 13 |
| s(20) | 89.7 | 10.3 | 77 | 23 | 14.5 | 10 |
| s(40) | 93.9 | 6.1 | 81 | 19 | 14 | 9.7 |
| s(80) | 94 | 6 | 82 | 18 | 13 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghoshani, M.; Mozaafari, M.; Normile, P.S.; De Toro, J.A.; Al-Nabhani, A. Core Size and Interface Impact on the Exchange Bias of Cobalt/Cobalt Oxide Nanostructures. Magnetochemistry 2021, 7, 40. https://doi.org/10.3390/magnetochemistry7030040
Ghoshani M, Mozaafari M, Normile PS, De Toro JA, Al-Nabhani A. Core Size and Interface Impact on the Exchange Bias of Cobalt/Cobalt Oxide Nanostructures. Magnetochemistry. 2021; 7(3):40. https://doi.org/10.3390/magnetochemistry7030040
Chicago/Turabian StyleGhoshani, Maral, Morteza Mozaafari, Peter S. Normile, Jose A. De Toro, and Abdulrahman Al-Nabhani. 2021. "Core Size and Interface Impact on the Exchange Bias of Cobalt/Cobalt Oxide Nanostructures" Magnetochemistry 7, no. 3: 40. https://doi.org/10.3390/magnetochemistry7030040
APA StyleGhoshani, M., Mozaafari, M., Normile, P. S., De Toro, J. A., & Al-Nabhani, A. (2021). Core Size and Interface Impact on the Exchange Bias of Cobalt/Cobalt Oxide Nanostructures. Magnetochemistry, 7(3), 40. https://doi.org/10.3390/magnetochemistry7030040






