Magnetic Switching in Vapochromic Oxalato-Bridged 2D Copper(II)-Pyrazole Compounds for Biogenic Amine Sensing †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations of 1 and Its Methylamine Adsorbates 2 and 3
2.2.1. {Cu(ox)(4-Hmpz)·1/3H2O}n (1)
2.2.2. {Cu(ox)(4-Hmpz)·3MeNH2·1/3H2O}n (2)
2.2.3. {Cu(ox)(4-Hmpz)·MeNH2·1/3H2O}n (3)
2.3. Vapor Adsorption Studies
2.4. Physical Techniques
2.5. X-ray Crystallographic Data Collection and Structure Refinement
3. Results and Discussion
3.1. Synthesis and General Physicochemical Characterization of 1–3
3.2. Description of the Structure of 1
3.3. Sorption Properties
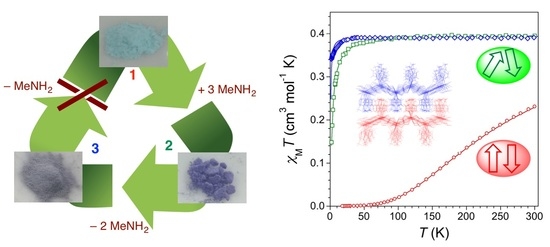
3.4. Magnetic Properties of 1–3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ouahab, L. (Ed.) Multifunctional Molecular Materials; Pan Standford Publishing: Singapore, 2013. [Google Scholar]
- Sieklucka, B.; Pinkowicz, D. (Eds.) Molecular Magnetic Materials: Concepts and Applications; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Day, P. New transparent ferromagnets. Acc. Chem. Res. 1979, 12, 236–243. [Google Scholar] [CrossRef]
- Day, P.; Kurmoo, M. Molecular magnetic semiconductors, metals and superconductors: BEDT-TTF salts with magnetic anions. J. Mater. Chem. 1997, 7, 1291–1295. [Google Scholar] [CrossRef]
- Alivisatos, P.; Barbara, P.F.; Castleman, A.W.; Chang, J.; Dixon, D.A.; Klein, M.L.; McLendon, G.L.; Miller, J.S.; Ratner, M.A.; Rossky, P.J.; et al. From molecules to materials: Current trends and future directions. Adv. Mater. 1998, 10, 1297–1336. [Google Scholar] [CrossRef]
- Dujardin, E.; Mann, S. Morphosynthesis of molecular magnetic materials. Adv. Mater. 2004, 16, 1125–1129. [Google Scholar] [CrossRef]
- Coronado, E.; Day, P. Magnetic molecular conductors. Chem. Rev. 2004, 104, 5419–5448. [Google Scholar] [CrossRef]
- Coronado, E.; Gatteschi, D. Trends and challenges in molecule-based magnetic materials. J. Mater. Chem. 2006, 16, 2513–2515. [Google Scholar]
- Pinkowicz, D.; Czarnecki, B.; Reczynski, M.; Arczinski, M. Multifunctionality in molecular magnetism. Sci. Prog. 2015, 98, 346–378. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Kepert, C.J. Advanced functional properties in nanoporous coordination framework materials. Chem. Commun. 2006, 695–700. [Google Scholar] [CrossRef]
- Maspoch, D.; Ruiz-Molina, D.; Veciana, J. Old materials with new tricks: Multifunctional open-framework materials. Chem. Soc. Rev. 2007, 36, 770–818. [Google Scholar] [CrossRef]
- Pardo, E.; Ruiz-García, R.; Cano, J.; Ottenwaelder, X.; Lescouëzec, R.; Journaux, Y.; Lloret, F.; Julve, M. Ligand design for multidimensional magnetic materials: A metallosupramolecular perspective. Dalton Trans. 2008, 2780–2805. [Google Scholar] [CrossRef]
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef]
- Dul, M.-C.; Pardo, E.; Lescouëzec, R.; Journaux, Y.; Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Julve, M.; Lloret, F.; Cangussu, D.; et al. Supramolecular coordination chemistry of aromatic polyoxalamide ligands: A metallosupramolecular approach toward functional magnetic materials. Coord. Chem. Rev. 2010, 254, 2281–2296. [Google Scholar] [CrossRef] [Green Version]
- Dechambenoit, P.; Long, J.R. Microporous magnets. Chem. Soc. Rev. 2011, 40, 3249–3265. [Google Scholar] [CrossRef]
- Muñoz, M.C.; Real, J.A. Thermo-, piezo-, phot- and chemo-switchable spin crossover iron(II)-metallocyanate based coordination polymers. Coord. Chem. Rev. 2011, 255, 2068–2093. [Google Scholar] [CrossRef]
- Coronado, E.; Mínguez-Espallargas, G. Dynamic magnetic MOFs. Chem. Soc. Rev. 2013, 42, 1525–1539. [Google Scholar] [CrossRef]
- Grancha, T.; Ferrando-Soria, J.; Castellano, M.; Julve, M.; Pasán, J.; Armentano, D.; Pardo, E. Oxamato-based coordination polymers: Recent advances in multifunctional magnetic materials. Chem. Commun. 2014, 50, 7569–7585. [Google Scholar] [CrossRef]
- Coronado, E.; Mínguez-Espallargas, G. magnetic functionalities in MOFs: From the framework to the pore. Chem. Soc. Rev. 2018, 47, 533–557. [Google Scholar]
- Thorarinsdottir, A.E.; Harris, T.D. Metal-organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Kumar, R.; Arya, S.K.; Nair, M.; Malhotra, B.D.; Bhansali, S. Organic-inorganic hybrid nanocomposite-based gas sensors for environmental monitoring. Chem. Rev. 2015, 115, 4571–4606. [Google Scholar] [CrossRef]
- Kumar, P.; Deep, A.; Kim, K.-H.; Brown, R.J.C. Coordination polymers: Opportunities and challenges for monitoring volatile organic compounds. Prog. Polym. Sci. 2015, 45, 102–118. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical sensors based on metal-organic frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kim, K.-H.; Kumar, P.; Jeon, B.-H.; Kim, J.-C. Functional hybrid nanostructure materials: Advanced strategies for sensing applications toward volatile organic compounds. Coord. Chem. Rev. 2017, 342, 80–105. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.-C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Rice, A.M.; Martin, C.R.; Shustova, N.B. Photochemistry and photophysics of MOFs: Steps towards MOF-based sensing enhancements. Chem. Soc. Rev. 2018, 47, 4710–4728. [Google Scholar] [CrossRef]
- Wang, H.; Lusting, W.P.; Li, J. Sensing and capture of toxic and hazardous gases and vapors by metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 4729–4756. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Van Der Voort, P. Luminescent lanthanide MOFs: A unique platform for chemical sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [Green Version]
- Rasheed, T.; Nabeel, F. Luminescent metal-organic frameworks as potential sensory materials for various environmental toxic agents. Coord. Chem. Rev. 2019, 401, 213065. [Google Scholar] [CrossRef]
- Wang, P.-L.; Xie, L.-H.; Joseph, E.A.; Li, J.-R.; Su, X.-O.; Zhou, H.-C. Metal-organic frameworks for food safety. Chem. Rev. 2019, 119, 10638–10690. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Wang, Z.; Qin, L.; Fu, Y.; Li, B.; Zhang, M.; Liu, S.; Li, L.; Yi, H.; Liu, X.; et al. Metal-organic frameworks as burgeoning materials for the capture and sensing of indoor VOCs and radon gases. Coord. Chem. Rev. 2021, 427, 213565. [Google Scholar] [CrossRef]
- Ohba, M.; Yoneda, K.; Agustí, G.; Muñoz, M.C.; Gaspar, A.B.; Real, J.A.; Yamasaki, M.; Ando, H.; Nakao, Y.; Sakaki, S.; et al. Bidirectional chemo-switching of spin state in a microporous framework. Angew. Chem. Int. Ed. 2009, 48, 4767–4771. [Google Scholar] [CrossRef] [PubMed]
- Agustí, G.; Ohtani, R.; Yoneda, K.; Gaspar, A.B.; Ohba, M.; Sánchez-Royo, J.F.; Muñoz, M.C.; Kitagawa, S.; Real, J.A. Oxidative addition of halogens on open metal sites in a microporous spin-crossover coordination polymer. Angew. Chem. Int. Ed. 2009, 48, 8944–8947. [Google Scholar] [CrossRef]
- Ohba, M.; Yoneda, K.; Kitagawa, S. Guest-responsive porous magnetic frameworks using polycyanometallates. CrystEngComm 2010, 12, 159–165. [Google Scholar] [CrossRef]
- Ohtani, R.; Yoneda, K.; Furukawa, S.; Horike, N.; Kitagawa, S.; Gaspar, A.B.; Muñoz, M.C.; Real, J.A.; Ohba, M. Precise control and consecutive modulation of spin transition temperature using chemical migration in porous coordination polymers. J. Am. Chem. Soc. 2011, 133, 8600–8605. [Google Scholar] [CrossRef]
- Coronado, E.; Giménez-Marqués, M.; Mínguez-Espallargas, G.; Brammer, L. Tuning the magneto-structural properties of non-porous coordination polymers by HCl chemisorption. Nat. Commun. 2012, 3, 1827. [Google Scholar] [CrossRef] [Green Version]
- Ferrando-Soria, J.; Ruiz-García, R.; Cano, J.; Stiriba, S.-E.; Vallejo, J.; Castro, I.; Julve, M.; Lloret, F.; Amorós, P.; Pasán, J.; et al. Reversible solvatomagnetic switching in a spongelike manganese(II)-copper(II) open framework with a pillared square/octagonal layer architecture. Chem. Eur. J. 2012, 18, 1608–1617. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Serra-Crespo, P.; de Lange, M.; Gascon, J.; Kapteijn, F.; Julve, M.; Cano, J.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; et al. Selective gas and vapor sorption and magnetic sensing by an isoreticular mixed-metal-organic framework. J. Am. Chem. Soc. 2012, 134, 15301–15304. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Soria, J.; Khajavi, H.; Serra-Crespo, P.; Gascon, J.; Kapteijn, F.; Julve, M.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; Journaux, Y.; et al. Highly selective chemical sensing in a luminiscent nanoporous magnet. Adv. Mater. 2012, 24, 5625–5629. [Google Scholar] [CrossRef]
- Vallejo, J.; Fortea-Pérez, F.R.; Pardo, E.; Benmansour, S.; Castro, I.; Krzystek, J.; Armentano, D.; Cano, J. Guest-dependent single-ion magnet behaviour in a cobalt(II) metal-organic framework. Chem. Sci. 2016, 7, 2286–2293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Wang, D.; Zhu, L.; Zhai, F.; Weng, L.; Sun, J.; Ling, Y.; Chen, Z.; Zhou, Y. HCl chemisorption-induced drastic magneto-structural transformation in a layered cobalt-phosphonotriazolate coordination polymer. Dalton Trans. 2016, 45, 10510–10513. [Google Scholar] [CrossRef] [PubMed]
- Perlepes, P.; Oyarzabal, I.; Mailman, A.; Yquel, M.; Platunov, M.; Dovgaliuk, I.; Rouzières, M.; Négrier, P.; Mondieig, D.; Suturina, E.A.; et al. Metal-organic magnets with large coercivity and ordering temperatures up to 242 °C. Science 2020, 370, 587–592. [Google Scholar] [CrossRef]
- Turo-Cortes, R.; Bartual-Murgui, C.; Castells-Gil, J.; Muñoz, M.C.; Martí-Gastaldo, C.; Real, J.A. Reversible guest-induced gate-opening with multiplex spin crossover responses in two-dimensional Hofmann clathrates. Chem. Sci. 2020, 11, 11224–11234. [Google Scholar] [CrossRef]
- Piñeiro-López, L.; Valverde-Muñoz, F.-J.; Trzop, E.; Muñoz, M.C.; Seredyuk, M.; Castells-Gil, J.; da Silva, I.; Martí-Gastaldo, C.; Collet, E.; Real, J.A. Guest induced reversible on-off switching of elastic frustration in a 3D spin crossover coordination polymer with room temperature hysteretic behaviour. Chem. Sci. 2021, 12, 1317–1326. [Google Scholar] [CrossRef]
- Cingolani, A.; Galli, S.; Masciocchi, N.; Pandolfo, L.; Pettinari, C.; Sironi, A. Sorption-desorption behavior of bispirazolato-copper(II) 1D coordination polymers. J. Am. Chem. Soc. 2005, 127, 6144–6145. [Google Scholar] [CrossRef]
- Qiu, L.-G.; Li, Z.-Q.; Wu, Y.; Wang, W.; Xu, T.; Jiang, X. Facile synthesis of nanocrystals of a microporous metal-organic framework by an ultrasonic method and selective sensing of orgonoamines. Chem. Commun. 2008, 3642–3644. [Google Scholar] [CrossRef] [PubMed]
- Bencini, A.; Casarin, M.; Forrer, D.; Franco, L.; Garau, F.; Masciocchi, N.; Pandolfo, L.; Pettinari, C.; Ruzzi, M.; Vittadini, A. Magnetic properties and vapochromic reversible guest-induced transformation in a bispyrazolato copper(II) polymer: An experimental and dispersion-corrected density functional theory study. Inorg. Chem. 2009, 48, 4044–4051. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Zhu, G.; Hewitt, I.J.; Sun, F.; Qiu, S. Synthesis of a metal-organic framework film by direct conversion technique for VOCs sensing. Dalton Trans. 2009, 3009–3013. [Google Scholar] [CrossRef]
- Chen, J.; Yi, F.-Y.; Yu, H.; Jiao, S.; Pang, G.; Sun, Z.-M. Fast response and highly selective sensing of amine vapors using a luminiscent coordination polymer. Chem. Commun. 2014, 50, 10506–10509. [Google Scholar] [CrossRef]
- Mallick, A.; Garai, B.; Addicoat, M.A.; St. Petkov, P.; Heine, T.; Banerjee, R. Solid state organic amine detection in a photochromic porous metal-organic framework. Chem. Sci. 2015, 6, 1420–1425. [Google Scholar] [CrossRef] [Green Version]
- Tan, B.; Chen, C.; Cai, L.-X.; Zhang, Y.-J.; Huang, X.-Y.; Zhang, J. Introduction of Lewis acidic and redox-active sites into a porous framework for ammonia capture with visual color response. Inorg. Chem. 2015, 54, 3456–3461. [Google Scholar] [CrossRef]
- Chen, C.; Cai, L.-X.; Tan, B.; Zhang, Y.-J.; Yang, X.-D.; Zhang, J. Ammonia detection by using flexible Lewis acidic sites in luminiscent porous frameworks constructed from a bipyridinium derivative. Chem. Commun. 2015, 51, 8189–8192. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.G.; Sheberla, D.; Liu, S.F.; Swager, T.M.; Dinca, M. Cu3(hexaiminotriphenylene)2: An electrically conductive 2D metal-organic framework for chemiresistive sensing. Angew. Chem. Int. Ed. 2015, 54, 4349–4352. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yan, B. A novel fluorescence probe for sensing organic amine vapors from a Eu3+ β-diketonate functionalized bio-MOF-1 hybrid system. J. Mater. Chem. C 2015, 3, 7038–7044. [Google Scholar] [CrossRef]
- Takahashi, A.; Tanaka, H.; Parajuli, D.; Nakamura, T.; Minami, K.; Sugiyama, Y.; Hakuta, Y.; Ohkoshi, S.; Kawamoto, T. Historical pigment exhibiting ammonia gas capture beyond standard adsorbents with adsorption sites of two kinds. J. Am. Chem. Soc. 2016, 138, 6376–6379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-S.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. Fluorescent aromatic tag-functionalized MOFs for highly selective sensing of metal ions and small organic molecules. Inorg. Chem. 2016, 5, 2261–2273. [Google Scholar] [CrossRef]
- Yang, N.-N.; Sun, W.; Xi, F.-G.; Sui, Q.; Chen, L.-J.; Gao, E.-Q. Postsynthetic N-methylation making a metal-organic framework responsive to alkylamines. Chem. Commun. 2017, 53, 1747–1750. [Google Scholar] [CrossRef]
- Świtlicka-Olszewska, A.; Machura, B.; Mroziński, J.; Kalińska, N.; Kruszynski, R.; Penkala, M. Effect of N-donor ancillary ligands on structural and magnetic properties of oxalate copper(II) complexes. New J. Chem. 2014, 38, 1611–1626. [Google Scholar] [CrossRef] [Green Version]
- Calatayud, M.L.; Orts-Arroyo, M.; Julve, M.; Lloret, F.; Marino, N.; De Munno, G.; Ruiz-García, R.; Castro, I. Magneto-structural correlations in asymmetric oxalato-bridged dicopper(II) complexes with polymethyl-substituted pyrazole ligands. J. Coord. Chem. 2017, 71, 657–674. [Google Scholar] [CrossRef]
- Castro, I.; Calatayud, M.L.; Orts-Arroyo, M.; Moliner, N.; Marino, N.; Lloret, F.; Ruiz-García, R.; De Munno, G.; Julve, M. Ferro- and Antiferromagnetic Interactions in Oxalato-Centered Inverse Hexanuclear and Chain Copper(II) Complexes with Pyrazole Derivatives. Molecules 2021, 26, 2792. [Google Scholar] [CrossRef]
- Bruker. SMART; Data Collection Software (Version 4.050); Siemens Analytical Instruments Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Bruker. SAINT; (Version 7.68A); Bruker AXS Inc.: Madison, WI, USA, 2009. [Google Scholar]
- Bruker. SADABS; (Version 2008/1); Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Diamond Software; Version 4.6.4 (2021); Crystal Impact Kreuzherrenstr: Bonn, Germany, 2021.
- Zeroka, D.; Jensen, J.O. Infrared spectra of some isotopomers of methylamine and the methylammonium ion: A theoretical study. J. Mol. Struct. 1998, 425, 181–192. [Google Scholar] [CrossRef]
- Cavalca, L.; Villa, A.C.; Manfredotti, A.G.; Tomlinson, A.A.G. Crystal, molecular, and electronic structure of catena- µ-oxalato-ammine-copper(II). J. Chem. Soc. Dalton Trans. 1972, 3, 391–395. [Google Scholar] [CrossRef]
- PubChem Database. CID Numbers: 6329 (Methylamine), 674 (Dimethylamine), and 1146 (Trimethylamine); National Center for Biotechnology Information (NCBI): Bethesda, MD, USA.
- Hall, J.W.; Marsh, W.E.; Weller, R.R.; Hatfield, W.E. Exchange coupling in the alternating-chain compounds catena-di-µ-chloro-bis(4-methylpyridine)copper(II), catena-di-µ-bromo-bis(N-methylimidazole)copper(II), catena-[hexadione bis(thiosemicarbazonato)]copper(II), and catena-[octanedione bis(thiosemicarbazonato)]copper(II). Inorg. Chem. 1981, 20, 1033–1037. [Google Scholar]
- Girerd, J.J.; Kahn, O.; Verdaguer, M. Orbital reversal in (oxalato)copper(II) linear chains. Inorg. Chem. 1980, 19, 274–276. [Google Scholar] [CrossRef]
- Julve, M.; Verdaguer, M.; Gleizes, A.; Philoche-Levisalles, M.; Kahn, O. Design of µ-oxalato copper(II) binuclear complexes exhibiting expected magnetic properties. Inorg. Chem. 1984, 23, 3808–3818. [Google Scholar] [CrossRef]
- Journaux, Y.; Sletten, J.; Kahn, O. Tunable interactions in µ-oxamido copper(II) binuclear complexes. Inorg. Chem. 1985, 24, 4063–4069. [Google Scholar] [CrossRef]







| Formula | C6H6.67CuN2O4.33 |
| Fw | 239.66 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a/Å | 9.9554(5) |
| b/Å | 9.3037(4) |
| c/Å | 25.2695(12) |
| β/° | 94.670(3) |
| V/Å3 | 2332.74(19) |
| Z | 12 |
| Dc/g cm−3 | 2.047 |
| T/K | 90(2) |
| μ/mm−1 | 3.949 |
| F(000) | 1444 |
| Refl. Collected | 22,317 |
| Refl. indep. [Rint] | 3947 [0.0592] |
| Refl. obs. [I > 2σ(I)] | 2956 |
| Goodness-of-fit on F2 | 1.079 |
| R1a [I > 2σ(I)] (all) | 0.0497 (0.0690) |
| wR2b [I > 2σ(I)] (all) | 0.1309 (0.1422) |
| Δρmax, min/e Å−3 | 0.973 and −0.432 |
Cu(1) environment | Cu(2) environment | Cu(3) environment | |||
| Cu(1)-N(1) | 1.985(4) | Cu(2)-N(3) | 1.983(4) | Cu(3)-N(5) | 1.982(4) |
| Cu(1)-O(1) | 1.969(3) | Cu(2)-O(6) | 1.961(3) | Cu(3)-O(8d) | 1.968(3) |
| Cu(1)-O(3) | 1.984(3) | Cu(2)-O(10) | 1.998(3) | Cu(3)-O(11) | 1.993(3) |
| Cu(1)-O(4b) | 2.010(3) | Cu(2)-O(9) | 1.978(3) | Cu(3)-O(12) | 1.991(3) |
| Cu(1)-O(5) | 2.388(3) | Cu(2)-O(7c) | 2.561(3) | Cu(3)-O(2) | 2.527(3) |
| Cu(1)-O(2a) | 2.428(3) | Cu(2)-O(5) | 2.317(3) | Cu(3)-O(7d) | 2.287(3) |
| O(1)-Cu(1)-N(1) | 97.70(15) | O(6)-Cu(2)-N(3) | 93.96(15) | O(8d)-Cu(3)-N(5) | 95.18(15) |
| O(1)-Cu(1)-O(2a) | 75.08(12) | O(6)-Cu(2)-O(5) | 78.54(12) | O(8d)-Cu(3)-O(7d) | 79.09(12) |
| O(1)-Cu(1)-O(5) | 94.19(12) | O(6)-Cu(2)-O(7c) | 88.96(12) | O(8d)-Cu(3)-O(2) | 91.07(12) |
| O(1)-Cu(1)-O(3) | 90.90(13) | O(6)-Cu(2)-O(9) | 91.76(13) | O(8d)-Cu(3)-O(12) | 89.94(13) |
| O(1)-Cu(1)-O(4b) | 172.64(13) | O(6)-Cu(2)-O(10) | 176.00(13) | O(8d)-Cu(3)-O(11) | 173.95(13) |
| O(3)-Cu(1)-N(1) | 170.77(15) | O(9)-Cu(2)-N(3) | 173.60(14) | O(12)-Cu(3)-N(5) | 174.88(14) |
| O(3)-Cu(1)-O(5) | 88.91(12) | O(9)-Cu(2)-O(7c) | 83.13(11) | O(12)-Cu(3)-O(2) | 87.89(11) |
| O(3)-Cu(1)-O(2a) | 86.76(12) | O(9)-Cu(2)-O(5) | 86.99(12) | O(12)-Cu(3)-O(7d) | 91.32(12) |
| O(3)-Cu(1)-O(4b) | 83.28(13) | O(9)-Cu(2)-O(10) | 84.32(13) | O(11)-Cu(3)-O(12) | 84.10(13) |
| N(1)-Cu(1)-O(5) | 93.76(14) | N(3)-Cu(2)-O(7c) | 94.10(13) | N(5)-Cu(3)-O(2) | 91.81(14) |
| N(1)-Cu(1)-O(2a) | 92.22(14) | N(3)-Cu(2)-O(5) | 96.95(14) | N(5)-Cu(3)-O(7d) | 89.84(14) |
| N(1)-Cu(1)-O(4b) | 87.87(15) | N(3)-Cu(2)-O(10) | 90.00(14) | N(5)-Cu(3)-O(11) | 90.78(15) |
| O(4b)-Cu(1)-O(2a) | 100.00(12) | O(10)-Cu(2)-O(5) | 100.36(12) | O(11)-Cu(3)-O(7d) | 102.05(12) |
| O(4b)-Cu(1)-O(5) | 90.22(12) | O(10)-Cu(2)-O(7c) | 91.39(12) | O(11)-Cu(3)-O(2) | 87.66(11) |
| O(5)-Cu(1)-O(2a) | 168.34(11) | O(7c)-Cu(2)-O(5) | 163.82(11) | O(2)-Cu(3)-O(7d) | 170.13(11) |
| Intrachain Cu-(μ-ox)-Cu | Intrachain Cu-(μ4-ox)-Cu | Interchain shortest Cu⋯Cu | |||
| Cu(1)⋯Cu(1b) | 5.2216(13) | Cu(1)⋯Cu(1a) | 5.6913(13) | Cu(1)⋯Cu(2) | 4.1148(9) |
| Cu(2)⋯Cu(3) | 5.1824(9) | Cu(2)⋯Cu(3e) | 5.5166(9) | Cu(1a)⋯Cu(3) | 4.3324(9) |
| D-H⋯A | d(D-H) | d(H⋯A) | d(D⋯A) | <(DHA) |
|---|---|---|---|---|
| N(2)-H(2)⋯O(1w) | 0.88 | 1.8 | 2.662(5) | 166.1 |
| O(1w)-H(1w1)⋯O(12) | 0.956(10) | 1.923(19) | 2.828(5) | 157(4) |
| O(1w)-H(1w2)⋯O(3a) | 0.958(10) | 1.796(12) | 2.752(5) | 176(4) |
| N(4)-H(4)⋯O(11f) | 0.88 | 1.99 | 2.800(5) | 152.1 |
| N(6)-H(6)⋯N(1a) | 0.88 | 2.46 | 3.296(6) | 158.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, N.; Calatayud, M.L.; Orts-Arroyo, M.; Pascual-Álvarez, A.; Moliner, N.; Julve, M.; Lloret, F.; De Munno, G.; Ruiz-García, R.; Castro, I. Magnetic Switching in Vapochromic Oxalato-Bridged 2D Copper(II)-Pyrazole Compounds for Biogenic Amine Sensing. Magnetochemistry 2021, 7, 65. https://doi.org/10.3390/magnetochemistry7050065
Marino N, Calatayud ML, Orts-Arroyo M, Pascual-Álvarez A, Moliner N, Julve M, Lloret F, De Munno G, Ruiz-García R, Castro I. Magnetic Switching in Vapochromic Oxalato-Bridged 2D Copper(II)-Pyrazole Compounds for Biogenic Amine Sensing. Magnetochemistry. 2021; 7(5):65. https://doi.org/10.3390/magnetochemistry7050065
Chicago/Turabian StyleMarino, Nadia, María Luisa Calatayud, Marta Orts-Arroyo, Alejandro Pascual-Álvarez, Nicolás Moliner, Miguel Julve, Francesc Lloret, Giovanni De Munno, Rafael Ruiz-García, and Isabel Castro. 2021. "Magnetic Switching in Vapochromic Oxalato-Bridged 2D Copper(II)-Pyrazole Compounds for Biogenic Amine Sensing" Magnetochemistry 7, no. 5: 65. https://doi.org/10.3390/magnetochemistry7050065
APA StyleMarino, N., Calatayud, M. L., Orts-Arroyo, M., Pascual-Álvarez, A., Moliner, N., Julve, M., Lloret, F., De Munno, G., Ruiz-García, R., & Castro, I. (2021). Magnetic Switching in Vapochromic Oxalato-Bridged 2D Copper(II)-Pyrazole Compounds for Biogenic Amine Sensing. Magnetochemistry, 7(5), 65. https://doi.org/10.3390/magnetochemistry7050065