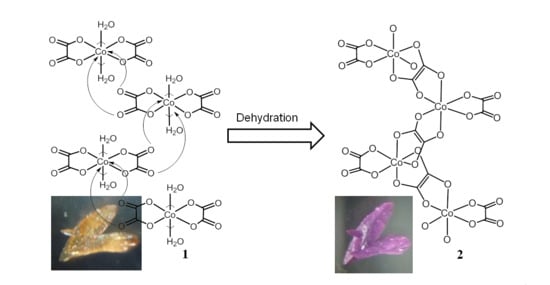
Crystal-to-Crystal Transformation from K2[Co(C2O4)2(H2O)2]·4H2O to K2[Co(?-C2O4)(C2O4)]
Abstract
:1. Introduction
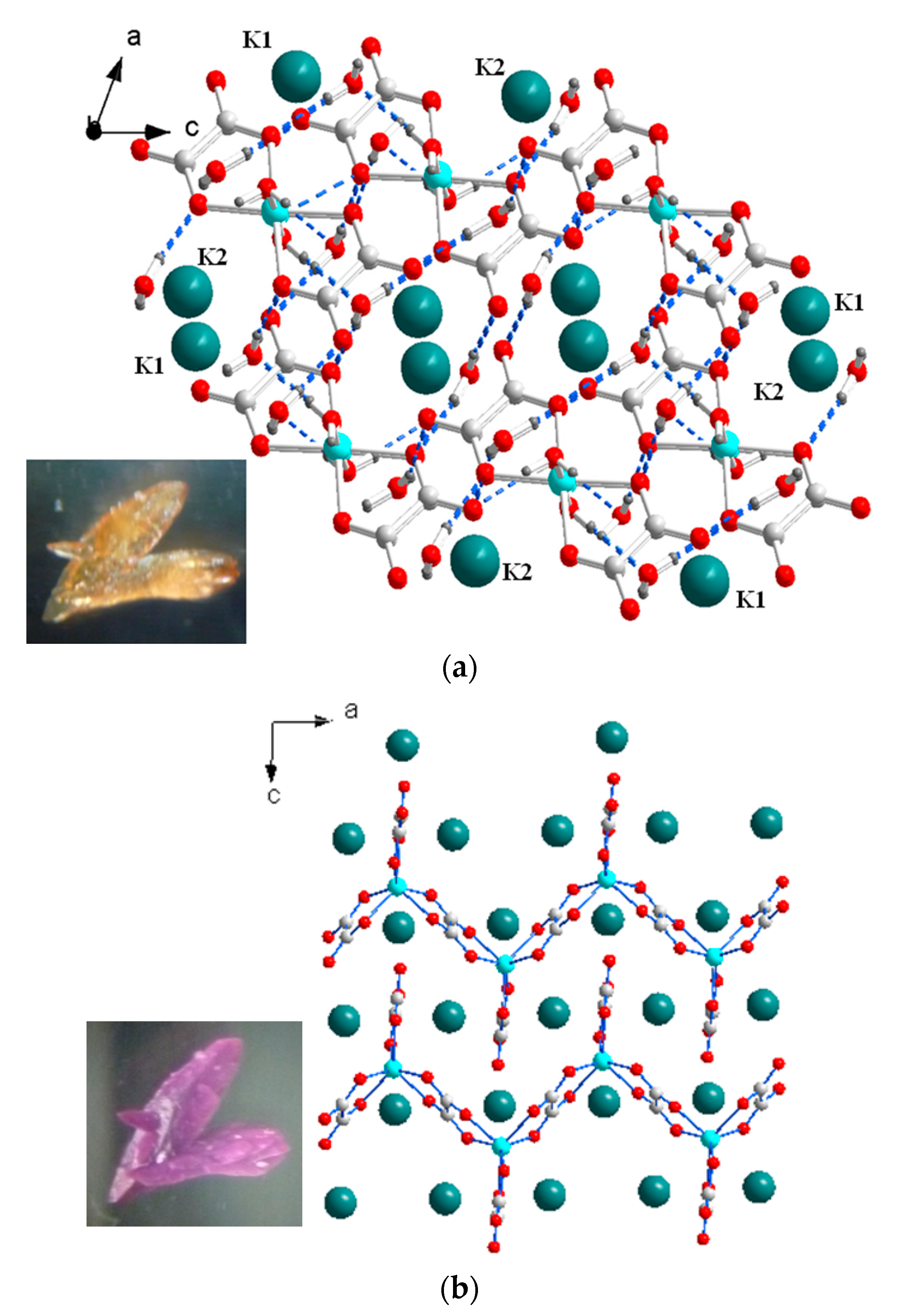
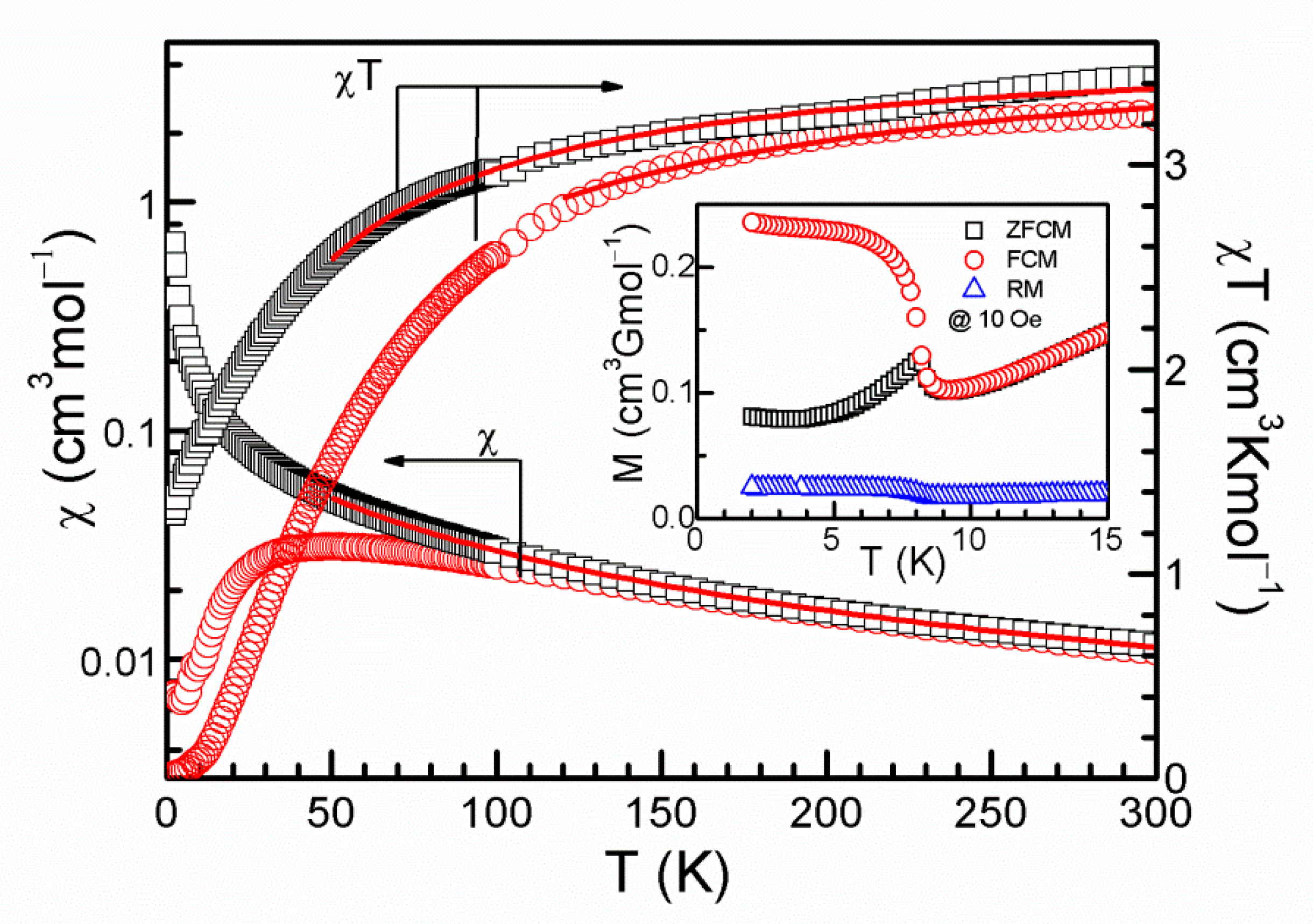
2. Experiment and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, S.H.; Olmstead, M.M.; Balch, A.L. Molecular accordion: Vapoluminescence and molecular flexibilityin the orange and green luminescent crystals of the dimer, Au2(μ-bis-(diphenylphosphino)ethane)2Br2. J. Am. Chem. Soc. 2011, 133, 10229–10238. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Schoenecker, B.; Mu, P.; Carson, C.G.; Karra, J.R.; Cai, Y.; Walton, K.S. A porous flexible homochiral SrSi2 array of single-stranded helical nanotubes exhibiting single-crystal-to-single-crystal oxidation transformation. Angew. Chem. Int. Ed. 2011, 50, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.B.; Audhya, A.; Bhattacharya, S.; Abtab, S.M.T.; Bhattacherya, K.; Chaudhury, M. Single crystal-to-single crystal irreversible transformation from a discrete Vanadium(V)-Alcoholate to an Aldehydic-Vanadium(IV) oligomer. J. Am. Chem. Soc. 2010, 132, 15842–15845. [Google Scholar] [CrossRef]
- Huang, Z.; White, P.S.; Brookhart, M. Ligand exchanges and selective catalytic hydrogenation in molecular single crystals. Nature 2010, 465, 598–601. [Google Scholar] [CrossRef]
- Kawasaki, T.; Hakoda, Y.; Mineki, H.; Suzuki, K.; Soai, K. Generation of absolute controlled crystal chirality by the removal of crystal water from achiral crystal of nucleobase cytosine. J. Am. Chem. Soc. 2010, 132, 2874–2875. [Google Scholar] [CrossRef]
- Allan, P.K.; Xiao, B.; Teat, S.J.; Knight, J.W.; Morris, R.E. In situ single-crystal diffraction studies of the structural transition of metal-organic framework copper 5-sulfoisophthalate, Cu-SIP-3. J. Am. Chem. Soc. 2010, 132, 3605–3611. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal-Organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Kaneko, W.; Ohba, M.; Kitagawa, S. A flexible coordination polymer crystal providing reversible structural and magnetic conversions. J. Am. Chem. Soc. 2007, 129, 13706–13712. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Kanegawa, S.; Sato, O. Reversible single-crystal-to-single-crystal transformation from achiral antiferromagnetic hexanuclears to a chiral ferrimagnetic double zigzag chain. J. Am. Chem. Soc. 2009, 131, 7942–7943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, T.; Kanegawa, S.; Sato, O. Interconversion between a nonporous nanocluster and a microporous coordination polymer showing selective gas adsorption. J. Am. Chem. Soc. 2010, 132, 912–913. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, Y.; Zhang, B.; Zhu, D. Crystal-to-Crystal transformation from antiferromagnetic chains into a ferromagnetic diamondoid framework. J. Am. Chem. Soc. 2009, 131, 6934–6935. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, D.; Zhang, Y. Crystal-to-Crystal transformation from a mononuclear compound in a hydrogen-bonded three-dimensional framework to a layered coordination polymer. Chem. Eur. J. 2010, 16, 9994–9997. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, Y.; Zhang, B.; Zhu, D. Crystal-to-crystal transformation from a chain compound to a layered coordination polymer. Dalton Trans. 2016, 45, 89–92. [Google Scholar] [CrossRef]
- Mathoniere, C.; Carling, S.G.; Yusheng, D.; Day, P. Molecular-based Mixed Valency Ferrimagnets (XR4)FeIIFeIII(C2O4)3 (X = N, P; R = n-propyle, n-butyl, phenyl): Anomalous negative magnetisation in the tetra-n-butylammonium derivative. J. Chem. Soc. Chem. Commun. 1994, 1551–1552. [Google Scholar] [CrossRef]
- Clemente-Leon, M.; Coronado, E.; Marti-Gastaldo, C.; Romero, F.M. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef]
- Hernandez-Molina, M.; Lloret, F.; Ruiz-Perez, C.; Julve, M. Weak ferromagnetism in chiral 3-dimensional oxalate-bridged Cobalt(II) compounds. crystal structure of [Co(bpy)3][Co2Ox3]ClO4. Inorg. Chem. 1998, 37, 4131–4135. [Google Scholar] [CrossRef]
- Hursthouse, M.B.; Light, M.E.; Price, D.J. One-dimensional magnetism in new anhydrous iron and cobalt ternary oxalates with rare trigonal prismatic metal coordination. Angew. Chem. Int. Ed. 2004, 43, 472–475. [Google Scholar] [CrossRef]
- Coronado, E.; Galan-Mascaros, J.R.; Marti-Gastalo, C. Single chain magnets based on the oxalate ligand. J. Am. Chem. Soc. 2008, 130, 14987–14989. [Google Scholar] [CrossRef]
- Glerup, J.; Goodson, P.A.; Hodgson, D.J.; Michelsen, K. Magnetic Exchange through Oxalate Bridges: Synthesis and Characterization of (μ-Oxalato)dimetal (II) Complexes of Magnanese, Iron, Cobalt, Nickel, Copper and Zinc. Inorg. Chem. 1995, 34, 6255–6264. [Google Scholar] [CrossRef]
- Garcia-Couceiro, U.; Castillo, O.; Luque, A. A new hydrated phase of cobalt(II) oxalate: Crystal structure, thermal behavior and magnetic properties of {[Co(-Ox)(H2O)2]·2H2O}n. Inorg. Chim. Acta 2004, 357, 339–344. [Google Scholar] [CrossRef]
- Duan, Z.; Zhang, Y.; Zhang, B.; Zhu, D. Co(C2O4)(HO(CH2)3OH): An antiferromagnetic neutral zigzag chain compound showing long-range-ordering of spin canting. Inorg. Chem. 2008, 47, 9152–9154. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, Y.; Zhang, B.; Pratt, F. Two homometallic antiferromagnets based on oxalato-bridged honeycomb assemblies: (A)2[MII2C2O4)3] (A = ammonium salt derived from diethylenetriamine; MII = Fe2+, Co2+). Inorg. Chem. 2009, 48, 2140–2146. [Google Scholar] [CrossRef]
- Desplanches, C.; Ruiz, E.; Rodrigue-Fortea, A.; Alvarez, S. Exchange coupling of transition-metal ions through hydrogen bonding: A theoretical investigation. J. Am. Chem. Soc. 2002, 124, 5197–5205. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, Y.; Chang, G.; Wang, Z.; Zhu, D. Crystal-to-Crystal Transformation from K2[Co(C2O4)2(H2O)2]·4H2O to K2[Co(?-C2O4)(C2O4)]. Magnetochemistry 2021, 7, 77. https://doi.org/10.3390/magnetochemistry7060077
Zhang B, Zhang Y, Chang G, Wang Z, Zhu D. Crystal-to-Crystal Transformation from K2[Co(C2O4)2(H2O)2]·4H2O to K2[Co(?-C2O4)(C2O4)]. Magnetochemistry. 2021; 7(6):77. https://doi.org/10.3390/magnetochemistry7060077
Chicago/Turabian StyleZhang, Bin, Yan Zhang, Guangcai Chang, Zheming Wang, and Daoben Zhu. 2021. "Crystal-to-Crystal Transformation from K2[Co(C2O4)2(H2O)2]·4H2O to K2[Co(?-C2O4)(C2O4)]" Magnetochemistry 7, no. 6: 77. https://doi.org/10.3390/magnetochemistry7060077
APA StyleZhang, B., Zhang, Y., Chang, G., Wang, Z., & Zhu, D. (2021). Crystal-to-Crystal Transformation from K2[Co(C2O4)2(H2O)2]·4H2O to K2[Co(?-C2O4)(C2O4)]. Magnetochemistry, 7(6), 77. https://doi.org/10.3390/magnetochemistry7060077