Effect of the Alkaline Metal Ion on the Crystal Structure and Magnetic Properties of Heterometallic GdIII-VIV Complexes Based on Cyclobutane-1,1-Dicarboxylate Anions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
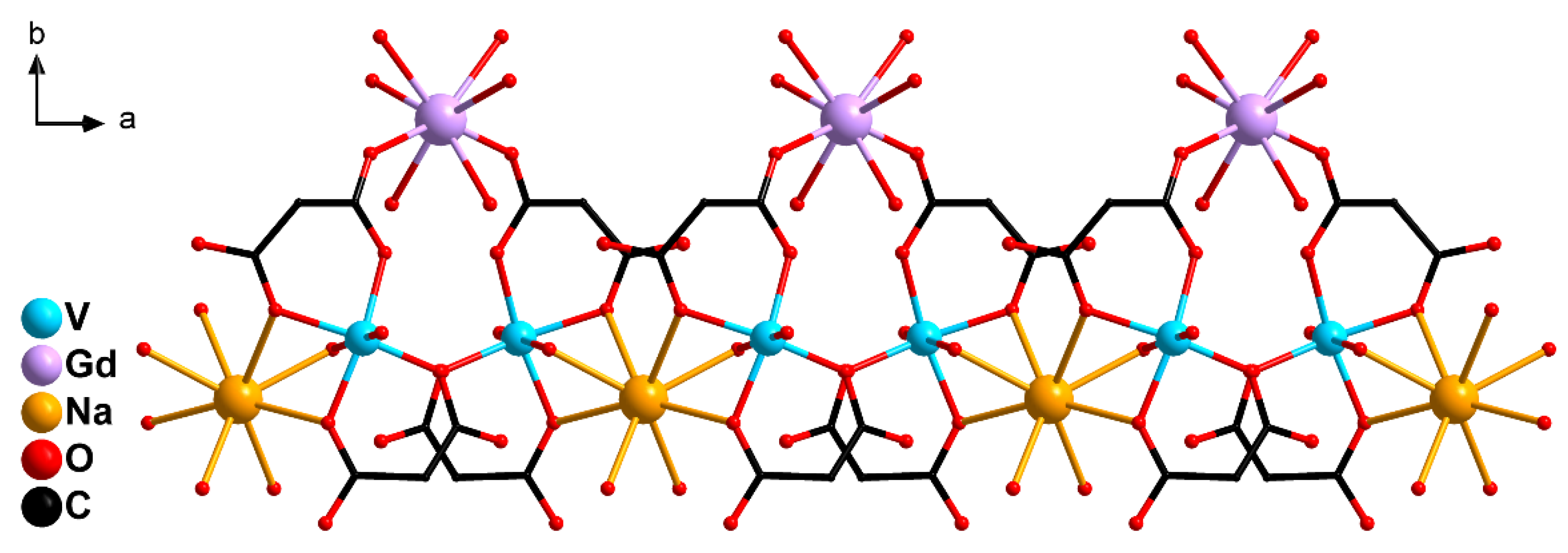
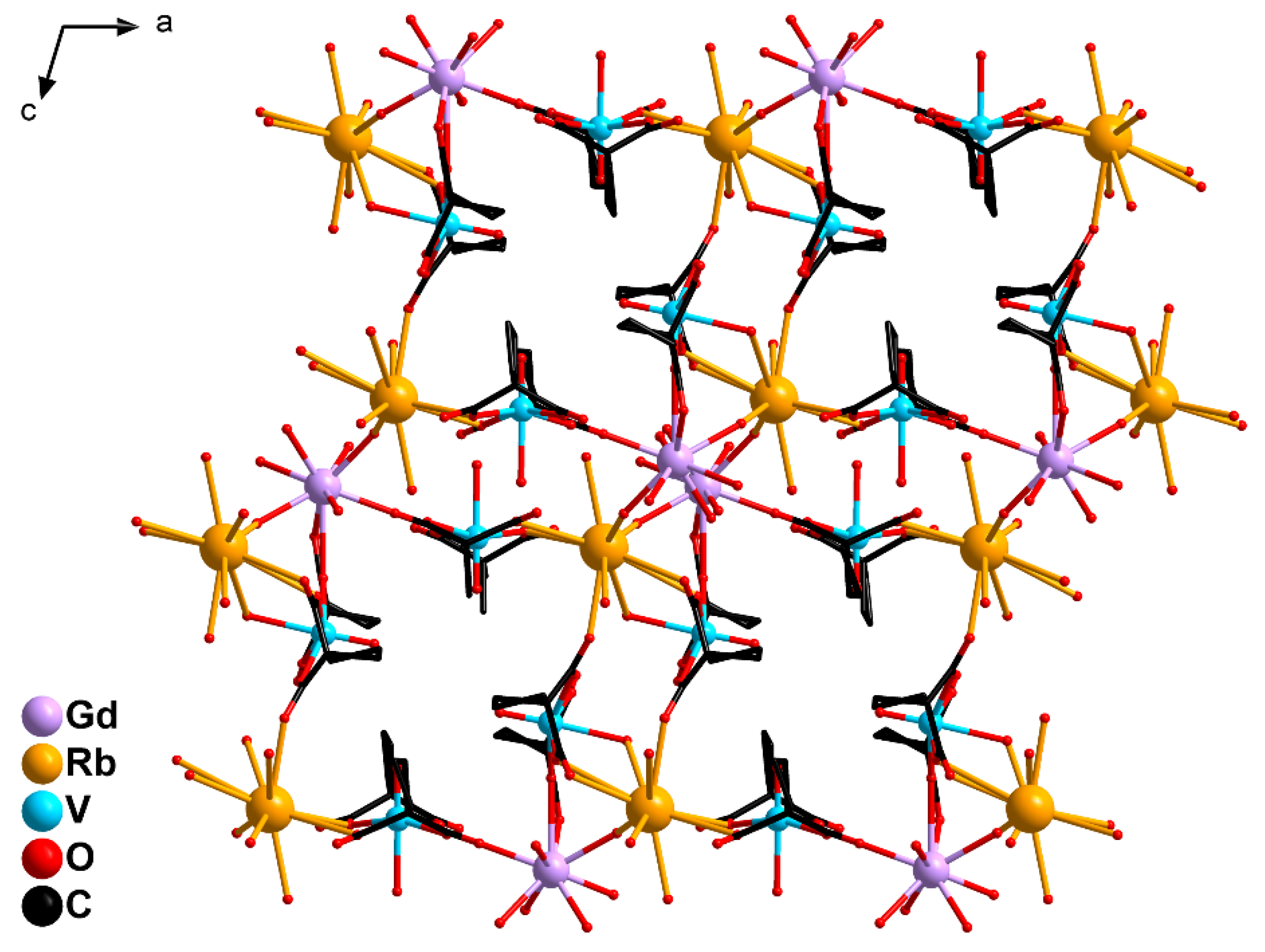
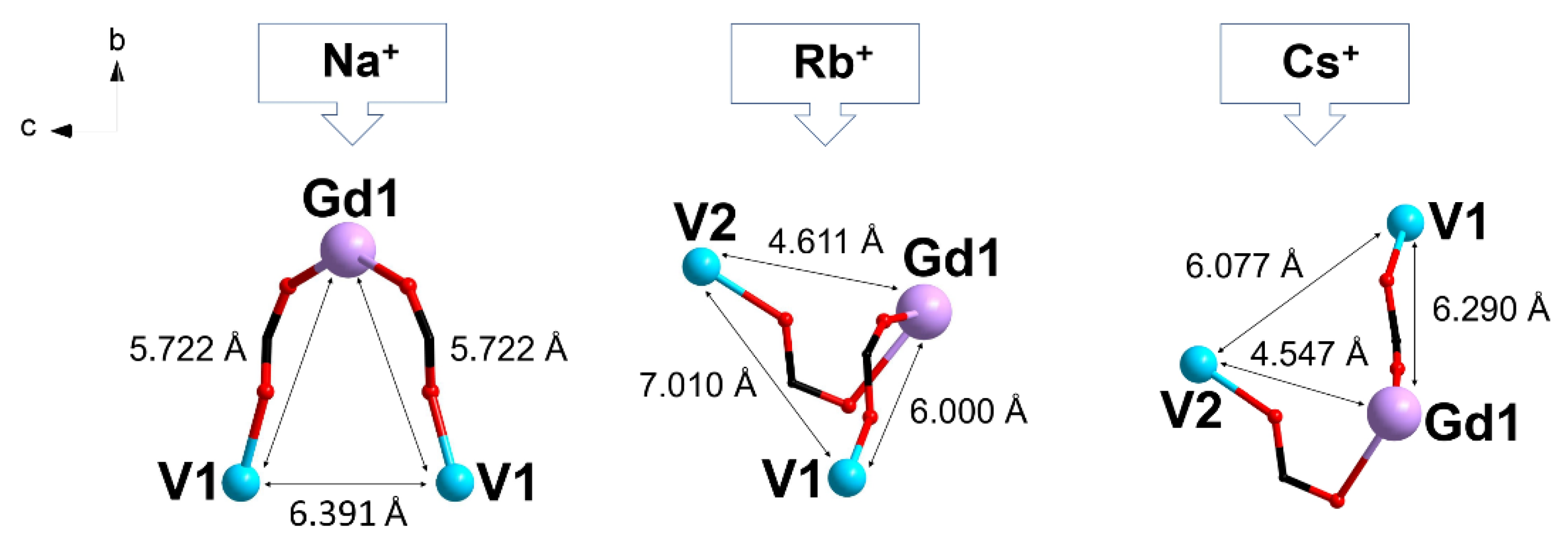
2.2. Crystal Structure Description
2.3. EPR Spectroscopy
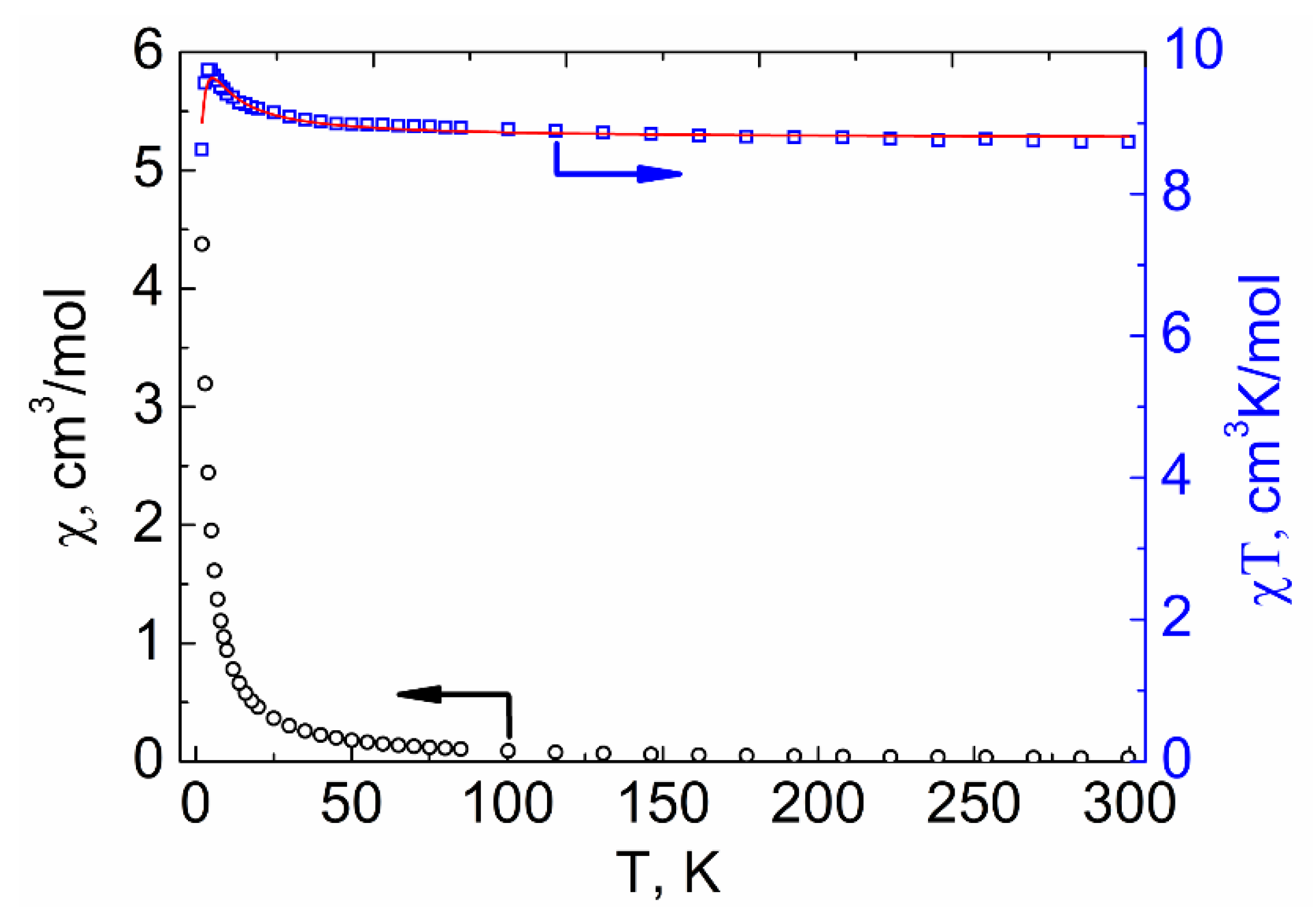
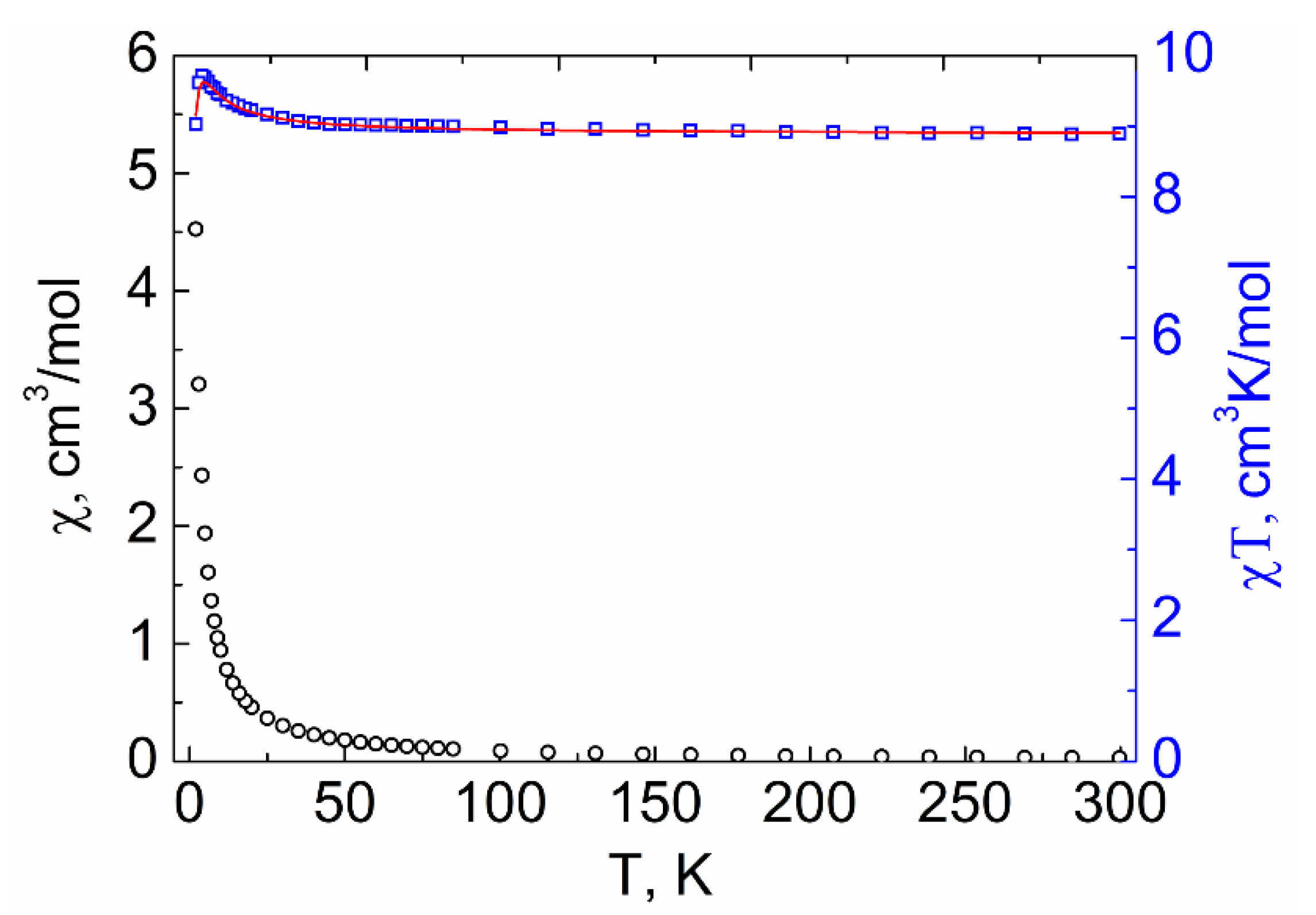
2.4. Magnetic Properties
2.4.1. DC Susceptibility Measurements
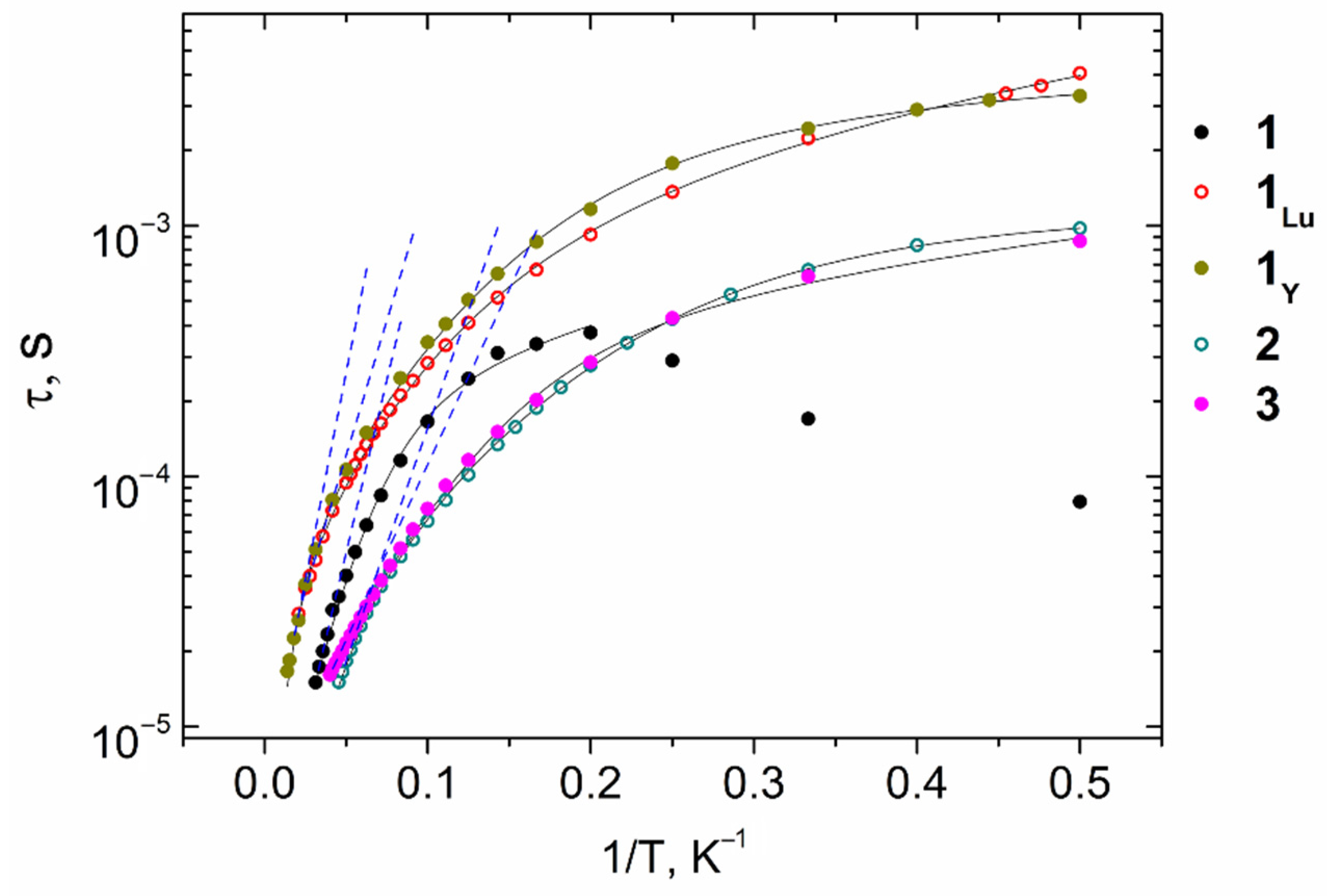
2.4.2. AC Susceptibility Measurements
3. Materials and Methods
3.1. Synthesis
3.1.1. General Details
3.1.2. Synthetic Procedure
3.2. Single Crystal X-ray Diffraction
3.3. Powder X-ray Diffraction
3.4. EPR Spectroscopy
3.5. Magnetic Measurements
3.6. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Wang, L.; Xu, C.; Yang, H.; Chen, W.; Gao, G.; Liu, W. Anion-induced 3d-4f luminescent coordination clusters: Structural characteristics and chemical fixation of CO2 under mild conditions. Dalton Trans. 2018, 47, 7159–7165. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zou, J.-Y.; You, S.-Y.; Cui, H.-M.; Zeng, G.-P.; Cui, J.-Z. Tuning the luminescence of two 3d-4f metal-organic frameworks for the fast response and highly selective detection of aniline. Dalton Trans. 2017, 46, 16432–16438. [Google Scholar] [CrossRef]
- Dey, A.; Acharya, J.; Chandrasekhar, V. Heterometallic 3d-4f Complexes as Single—Molecule Magnets. Chem. Asian J. 2019, 14, 4433–4453. [Google Scholar] [CrossRef]
- Dey, A.; Bag, P.; Kalita, P.; Chandrasekhar, V. Heterometallic CuII-LnIII complexes: Single molecule magnets and magnetic refrigerants. Coord. Chem. Rev. 2021, 432, 213707. [Google Scholar] [CrossRef]
- Yin, J.-J.; Lu, T.-Q.; Chen, C.; Zhuang, G.-L.; Zheng, J.; Zheng, X.-Y.; Shao, F. Magnetocaloric Effect and Slow Magnetic Relaxation on Two Dimensional Layered 3d-4f Cluster-Based Metal-Organic Frameworks. Cryst. Growth Des. 2020, 20, 4005–4012. [Google Scholar] [CrossRef]
- Cremades, E.; Gómez-Coca, S.; Aravena, D.; Alvarez, S.; Ruiz, E. Theoretical Study of Exchange Coupling in 3d-Gd Complexes: Large Magnetocaloric Effect Systems. J. Am. Chem. Soc. 2012, 134, 10532–10542. [Google Scholar] [CrossRef]
- Lin, Q.; Li, J.; Dong, Y.; Zhou, G.; Song, Y.; Xu, Y. Lantern-shaped 3d–4f high-nuclearity clusters with magnetocaloric effect. Dalton Trans. 2017, 46, 9745–9749. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-M.; Zhang, D.-Q.; Hao, X.; Zhu, D.-B. Assembly of chiral 3d–4f wheel-like cluster complexes with achiral ligands: Single—molecule magnetic behavior and magnetocaloric effect. Inorg. Chem. Front. 2020, 7, 3340–3351. [Google Scholar] [CrossRef]
- Oyarzabal, I.; Echenique-Errandonea, E.; San Sebastián, E.; Rodríguez-Diéguez, A.; Seco, J.M.; Colacio, E. Synthesis, Structural Features and Physical Properties of a Family of Triply Bridged Dinuclear 3d-4f Complexes. Magnetochemistry 2021, 7, 22. [Google Scholar] [CrossRef]
- Griffiths, K.; Kostakis, G.E. Transformative 3d-4f coordination cluster carriers. Dalton Trans. 2018, 47, 12011–12034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.; Griffiths, K.; Anson, C.E.; Powell, A.K.; Kostakis, G.E. A tetranuclear CuII2DyIII2 coordination cluster as a Suzuki (C–C) coupling reaction promoter. Dalton Trans. 2018, 47, 17202–17205. [Google Scholar] [CrossRef]
- Cancino, P.; Santibañez, L.; Stevens, C.; Fuentealba, P.; Audebrand, N.; Aravena, D.; Torres, J.; Martinez, S.; Kremere, C.; Spodine, E. Influence of the channel size of isostructural 3d-4f MOFs on the catalytic aerobic oxidation of cycloalkenes. New J. Chem. 2019, 43, 11057–11064. [Google Scholar] [CrossRef]
- Dermitzaki, D.; Psycharis, V.; Sanakis, Y.; Stamatatos, T.C.; Pissas, M.; Raptopoulou, C.P. Extending the family of heptanuclear heterometallic Cu5Ln2 (Ln = Gd, Tb, Dy) complexes: Synthesis, crystal structures, magnetic and magnetocaloric studies. Polyhedron 2019, 169, 135–143. [Google Scholar] [CrossRef]
- Zhang, H.-G.; Yang, H.; Sun, L.; Li, D.-C.; Dou, J.-M. A New Family of 2D Coordination Polymers Based on 3d–4f 15—Metallacrown-5 Units: Synthesis, Structure, and Single-Molecule Magnet Behavior. Eur. J. Inorg. Chem. 2019, 2019, 2839–2843. [Google Scholar] [CrossRef]
- Worrell, A.; Sun, D.; Mayans, J.; Lampropoulos, C.; Escuer, A.; Stamatatos, T.C. Oximato-Based Ligands in 3d/4f-Metal Cluster Chemistry: A Family of {Cu3Ln} Complexes with a “Propeller”-like Topology and Single-Molecule Magnetic Behavior. Inorg. Chem. 2018, 57, 13944–13952. [Google Scholar] [CrossRef]
- Zhao, X.; Li, G.; Ma, J.; Liu, W. Two Octanuclear {Cu4Ln4} (Ln = Dy or Tb) Complexes with a Butterfly-Shaped Unit Exhibiting Zero-Field Single-Molecule Magnet Behavior. Inorg. Chem. 2020, 59, 2328–2336. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.Y.; Chen, P.Y.; Wu, M.Z.; Tian, L.; Liu, Z.Y. Synthesis of a series of hetero-multi-spin Ln2Cu3 complexes based on a methyl-pyrazole nitronyl nitroxide radical with slow magnetic relaxation behaviors. Dalton Trans. 2019, 48, 9187–9193. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Maity, S.; Mayans, J.; Ghosh, A. Family of Isomeric CuII-LnIII (Ln = Gd, Tb, and Dy) Complexes Presenting Field-Induced Slow Relaxation of Magnetization Only for the Members Containing GdIII. Inorg. Chem. 2021, 60, 438–448. [Google Scholar] [CrossRef]
- Liu, J.-L.; Lin, W.-Q.; Chen, Y.-C.; Gómez-Coca, S.; Aravena, D.; Ruiz, E.; Leng, J.-D.; Tong, M.-L. CuII-GdIII Cryogenic Magnetic Refrigerants and Cu8Dy9 Single-Molecule Magnet Generated by In Situ Reactions of Picolinaldehyde and Acetylpyridine: Experimental and Theoretical Study. Chem. Eur. J. 2013, 19, 17567–17577. [Google Scholar] [CrossRef]
- Singh, M.K.; Rajeshkumar, T.; Kumar, R.; Singh, S.K.; Rajaraman, G. Role of (1,3) {Cu-Cu} Interaction on the Magneto-Caloric Effect of Trinuclear {CuII-GdIII-CuII} Complexes: Combined DFT and Experimental Studies. Inorg. Chem. 2018, 57, 1846–1858. [Google Scholar] [CrossRef] [PubMed]
- Alexandropoulos, D.I.; Poole, K.M.; Cunha-Silva, L.; Sheikh, J.A.; Wernsdorfer, W.; Christou, G.; Stamatatos, T.C. A family of ‘windmill’-like {Cu6Ln12} complexes exhibiting single-molecule magnetism behavior and large magnetic entropy changes. Chem. Commun. 2017, 53, 4266–4269. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Das, S.; Palacios, M.A.; Colacio, E.; Chandrasekhar, V. Single-Molecule Magnet and Magnetothermal Properties of Two-Dimensional Polymers Containing Heterometallic [Cu5Ln2] (Ln = GdIII and DyIII) Motifs. Eur. J. Inorg. Chem. 2018, 2018, 1645–1654. [Google Scholar] [CrossRef]
- Alexandropoulos, D.I.; Cunha-Silva, L.; Tang, J.; Stamatatos, T.C. Heterometallic Cu/Ln cluster chemistry: Ferromagnetically-coupled {Cu4Ln2} complexes exhibiting single-molecule magnetism and magnetocaloric properties. Dalton Trans. 2018, 47, 11934–11941. [Google Scholar] [CrossRef] [PubMed]
- Kettles, F.J.; Milway, V.A.; Tuna, F.; Valiente, R.; Thomas, L.H.; Wernsdorfer, W.; Ochsenbein, S.T.; Murrie, M. Exchange Interactions at the Origin of Slow Relaxation of the Magnetization in {TbCu3} and {DyCu3} Single-Molecule Magnets. Inorg. Chem. 2014, 53, 8970–8978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.-J.; Zhang, J.-C.; Jiang, Y.-X.; Tao, J.-X.; Wang, W.-Y.; Huang, X.-C. Two-dimensional heterometallic CuIILnIII (Ln = Tb and Dy) coordination polymers bridged by dicyanamides showing slow magnetic relaxation behavior. CrystEngComm 2019, 21, 5145–5151. [Google Scholar] [CrossRef]
- Chen, Y.; Long, Q.-Q.; Hu, Z.-B.; Wang, H.-S.; Huang, Z.-Y.; Chen, W.; Song, Y.; Zhang, Z.-C.; Yang, F.-J. Synthesis, crystal structures and magnetic properties of a series of pentanuclear heterometallic [CuII3LnIII2] (Ln = Ho, Dy, and Gd) complexes containing mixed organic ligands. New J. Chem. 2019, 43, 8101–8108. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dupuis, A.; Laurent, J.-P. An original heterodinuclear VO2+, Gd3+ complex with a nonet ground state. J. Chem. Soc. Dalton Trans. 1998, 735–736. [Google Scholar] [CrossRef]
- Costes, J.-P.; Dahan, F.; Donnadieu, B.; Garcia-Tojal, J.; Laurent, J.-P. Versatility of the Nature of the Magnetic Gadolinium(III)–Vanadium(IV) Interaction—Structure and Magnetic Properties of Two Heterobinuclear [Gd, V(O)] Complexes. Eur. J. Inorg. Chem. 2001, 2001, 363–365. [Google Scholar] [CrossRef]
- Costes, J.-P.; Shova, S.; Clemente-Juan, J.M.; Suet, N. The first example of a hetero-tetranuclear [(VO)Gd]2 complex: Synthesis, crystal structure and magnetic properties of [VOLGd(hfa)2CH3OH]2·2CH3OH·2(CH3)2CO. Dalton Trans. 2005, 17, 2830–2832. [Google Scholar] [CrossRef]
- Shi, W.; Chen, X.-Y.; Zhao, Y.-N.; Zhao, B.; Cheng, P.; Yu, A.; Song, H.-B.; Wang, H.-G.; Liao, D.-Z.; Yan, S.-P.; et al. Synthesis, Crystal Structures, and Properties of Oxovanadium(IV)–Lanthanide(III) Heteronuclear Complexes. Chem. Eur. J. 2005, 11, 5031–5039. [Google Scholar] [CrossRef]
- Rocha, J.; Almeida Paz, F.A.; Shi, F.-N.; Ferreira, R.A.S.; Trindade, T.; Carlos, L.D. Photoluminescent Porous Modular Lanthanide–Vanadium–Organic Frameworks. Eur. J. Inorg. Chem. 2009, 2009, 4931–4945. [Google Scholar] [CrossRef]
- Ishida, T.; Watanabe, R.; Fujiwara, K.; Okazawa, A.; Kojima, N.; Tanaka, G.; Yoshii, S.; Nojiri, H. Exchange coupling in TbCu and DyCu single-molecule magnets and related lanthanide and vanadium analogs. Dalton Trans. 2012, 41, 13609–13619. [Google Scholar] [CrossRef] [PubMed]
- Hickson, J.R.; Horsewill, S.J.; Bamforth, C.; McGuire, J.; Wilson, C.; Sproules, S.; Farnaby, J.H. The modular synthesis of rare earth-transition metal heterobimetallic complexes utilizing a redox-active ligand. Dalton Trans. 2018, 47, 10692–10701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.; Guha, A.K. Supramolecular assembly of binuclear [V2O3(NTA)2]4− unit with [Ce(H2O)9]3+: Synthesis, structure, luminescent, magnetic properties and TD-DFT calculation. Polyhedron 2017, 137, 1–9. [Google Scholar] [CrossRef]
- Shi, W.; Chen, X.-Y.; Zhao, B.; Yu, A.; Song, H.-B.; Cheng, P.; Wang, H.-G.; Liao, D.-Z.; Yan, S.-P. Construction of 3d-4f Mixed-Metal Complexes Based on a Binuclear Oxovanadium Unit: Synthesis, Crystal Structure, EPR, and Magnetic Properties. Inorg. Chem. 2006, 45, 3949–3957. [Google Scholar] [CrossRef]
- Orendáč, M.; Sedláková, L.; Čižmár, E.; Orendáčová, A.; Feher, A.; Zvyagin, S.A.; Wosnitza, J.; Zhu, W.H.; Wang, Z.M.; Gao, S. Spin relaxation and resonant phonon trapping in [Gd2(fum)3(H2O)4]3H2O. Phys. Rev. 2010, B81, 214410. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.J.; Cardona-Serra, S.; Schlegel, C.; Moro, F.; Alonso, P.J.; Prima-García, H.; Clemente-Juan, J.M.; Evangelisti, M.; Gaita-Ariño, A.; Sesé, J.; et al. Gd-Based Single-Ion Magnets with Tunable Magnetic Anisotropy: Molecular Design of Spin Qubits. Phys. Rev. Lett. 2012, 108, 247213. [Google Scholar] [CrossRef] [Green Version]
- Rossin, A.; Giambastiani, G.; Peruzzini, M.; Sessoli, R. Amine-Templated Polymeric Lanthanide Formates: Synthesis, Characterization, and Applications in Luminescence and Magnetism. Inorg. Chem. 2012, 51, 6962–6968. [Google Scholar] [CrossRef] [PubMed]
- Arauzo, A.; Lazarescu, A.; Shova, S.; Bartolomé, E.; Cases, R.; Luzón, J.; Bartolomé, J.; Turta, C. Structural and magnetic properties of some lanthanide (Ln = Eu(III), Gd(III) and Nd(III)) cyanoacetate polymers: Field-induced slow magnetic relaxation in the Gd and Nd substitutions. Dalton Trans. 2014, 43, 12342–12356. [Google Scholar] [CrossRef]
- Khalfaoui, O.; Beghidja, A.; Long, J.; Boussadia, A.; Beghidja, C.; Guari, Y.; Larionova, J. Cinnamic acid derivative rare-earth dinuclear complexes and one-dimensional architectures: Synthesis, characterization and magnetic properties. Dalton Trans. 2017, 46, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Girginova, P.I.; Pereira, L.C.J.; Coutinho, J.T.; Santos, I.C.; Almeida, M. Slow magnetic relaxation in lanthanide ladder type coordination polymers. Dalton Trans. 2014, 43, 1897–1905. [Google Scholar] [CrossRef]
- Holmberg, R.J.; Ho, L.T.A.; Ungur, L.; Korobkov, I.; Chibotaru, L.F.; Murugesu, M. Observation of unusual slow-relaxation of the magnetisation in a Gd-EDTA chelate. Dalton Trans. 2015, 44, 20321–20325. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Cosquer, G.; Izuogu, D.C.; Ohtsu, H.; Kawano, M.; Lan, Y.; Wernsdorfer, W.; Nojiri, H.; Breedlove, B.K.; Yamashita, M. Field-Induced Slow Magnetic Relaxation of GdIII Complex with a Pt−Gd Heterometallic Bond. Chem. Eur. J. 2017, 23, 4551–4556. [Google Scholar] [CrossRef]
- Handzlik, G.; Magott, M.; Arczyński, M.; Sheveleva, A.M.; Tuna, F.; Sarewicz, M.; Osyczka, A.; Rams, M.; Vieru, V.; Chibotaru, L.F.; et al. Magnetization Dynamics and Coherent Spin Manipulation of a Propeller Gd(III) Complex with the Smallest Helicene Ligand. J. Phys. Chem. Lett. 2020, 11, 1508–1515. [Google Scholar] [CrossRef]
- Bazhina, E.S.; Aleksandrov, G.G.; Kiskin, M.A.; Korlyukov, A.A.; Efimov, N.N.; Bogomyakov, A.S.; Starikova, A.A.; Mironov, V.S.; Ugolkova, E.A.; Minin, V.V.; et al. The First Series of Heterometallic LnIII-VIV Complexes Based on Substituted Malonic Acid Anions: Synthesis, Structure and Magnetic Properties. Eur. J. Inorg. Chem. 2018, 2018, 5075–5090. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE v.2.1, Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Universitat de Barcelona: Barselona, Spain, 2013. [Google Scholar]
- Casanova, D.; Llunell, M.; Alemany, P.; Alvarez, S. The Rich Stereochemistry of Eight-Vertex Polyhedra: A Continuous Shape Measures Study. Chem. Eur. J. 2005, 11, 1479–1494. [Google Scholar] [CrossRef] [PubMed]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164. [Google Scholar] [CrossRef]
- Atzori, M.; Chiesa, A.; Morra, E.; Chiesa, M.; Sorace, L.; Carretta, S.; Sessoli, R. A two-qubit molecular architecture for electron-mediated nuclear quantum simulation. Chem. Sci. 2018, 9, 6183–6192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atzori, M.; Tesi, L.; Benci, S.; Lunghi, A.; Righini, R.; Taschin, A.; Torre, R.; Sorace, L.; Sessoli, R. Spin Dynamics and Low Energy Vibrations: Insights from Vanadyl-Based Potential Molecular Qubits. J. Am. Chem. Soc. 2017, 139, 4338–4341. [Google Scholar] [CrossRef] [Green Version]
- Atzori, M.; Morra, E.; Tesi, L.; Albino, A.; Chiesa, M.; Sorace, L.; Sessoli, R. Quantum Coherence Times Enhancement in Vanadium(IV)-based Potential Molecular Qubits: The Key Role of the Vanadyl Moiety. J. Am. Chem. Soc. 2016, 138, 11234–11244. [Google Scholar] [CrossRef]
- Atzori, M.; Tesi, L.; Morra, E.; Chiesa, M.; Sorace, L.; Sessoli, R. Room-Temperature Quantum Coherence and Rabi Oscillations in Vanadyl Phthalocyanine: Toward Multifunctional Molecular Spin Qubits. J. Am. Chem. Soc. 2016, 138, 2154–2157. [Google Scholar] [CrossRef] [PubMed]
- Atzori, M.; Benci, S.; Morra, E.; Tesi, L.; Chiesa, M.; Torre, R.; Sorace, L.; Sessoli, R. Structural Effects on the Spin Dynamics of Potential Molecular Qubit. Inorg. Chem. 2018, 57, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Tesi, L.; Lunghi, A.; Atzori, M.; Lucaccini, E.; Sorace, L.; Tottia, F.; Sessoli, R. Giant spin–phonon bottleneck effects in evaporable vanadyl-based molecules with long spin coherence. Dalton Trans. 2016, 45, 16635–16643. [Google Scholar] [CrossRef]
- Kurganskii, I.V.; Bazhina, E.S.; Korlyukov, A.A.; Babeshkin, K.A.; Efimov, N.N.; Kiskin, M.A.; Veber, S.L.; Sidorov, A.A.; Eremenko, I.L.; Fedin, M.V. Mapping Magnetic Properties and Relaxation in Vanadium(IV) Complexes with Lanthanides by Electron Paramagnetic Resonance. Molecules 2019, 24, 4582. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Yamashita, S.; Yasuda, N.; Kitagawa, Y.; Breedlove, B.K.; Nakazawa, Y.; Yamashita, M. Control of the Spin Dynamics of Single-Molecule Magnets by using a Quasi One-Dimensional Arrangement. Angew. Chem. Int. Ed. 2018, 57, 9262–9267. [Google Scholar] [CrossRef] [PubMed]
- SMART (Control) and SAINT (Integration) Software, Version 5.0; Bruker AXS, Inc.: Madison, WI, USA, 1997.
- Sheldrick, G.M. SADABS, Program for Scaling and Correction of Area Detector Data; Göttingen University: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 (Revision E.01); Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Douglas, M.; Kroll, N.M. Quantum electrodynamical corrections to the fine structure of helium. Ann. Phys. 1974, 82, 89–155. [Google Scholar] [CrossRef]
- Hess, B.A. Applicability of the no-pair equation with free-particle projection operators to atomic and molecular structure calculations. Phys. Rev. A 1985, 32, 756–763. [Google Scholar] [CrossRef] [PubMed]
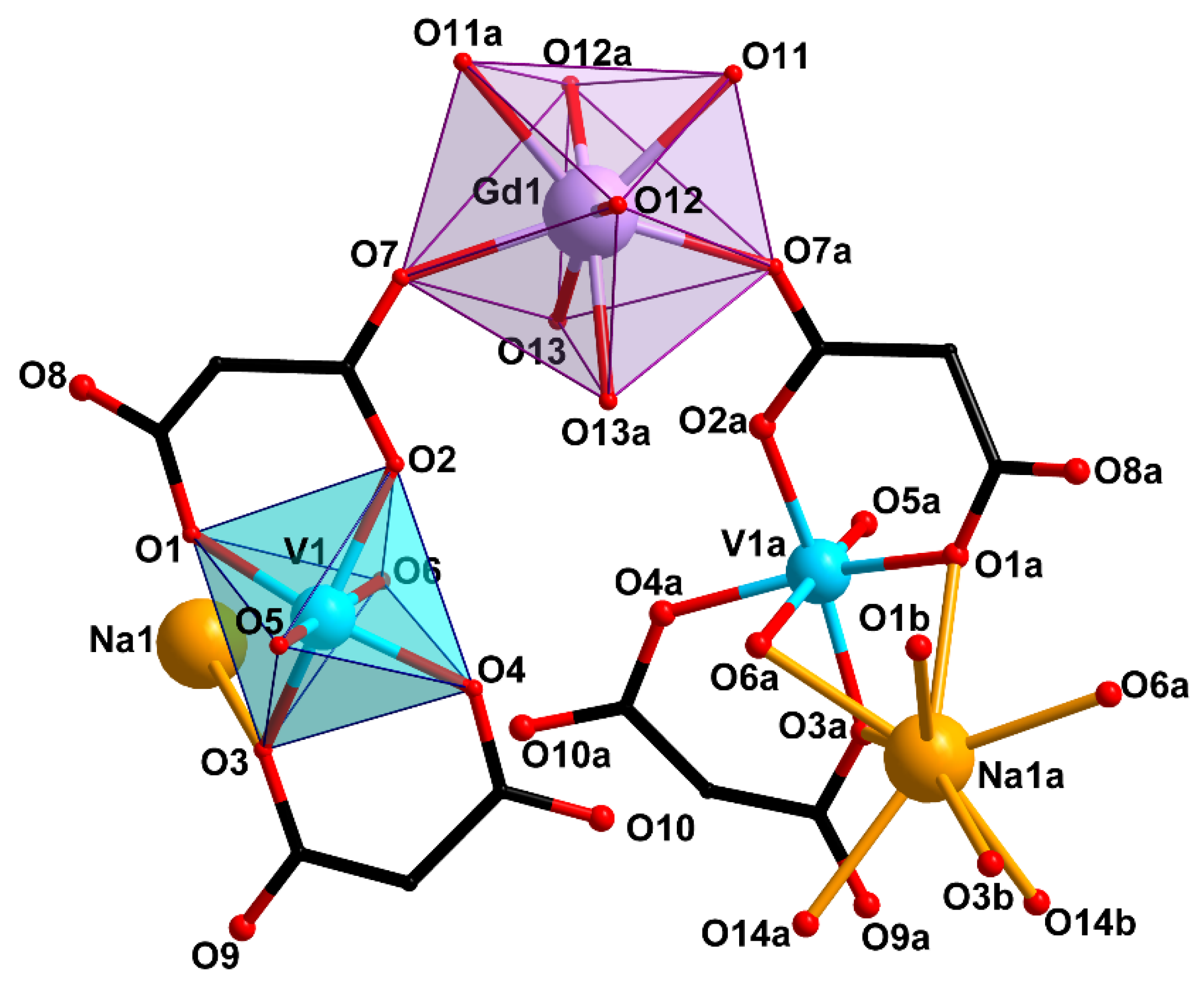
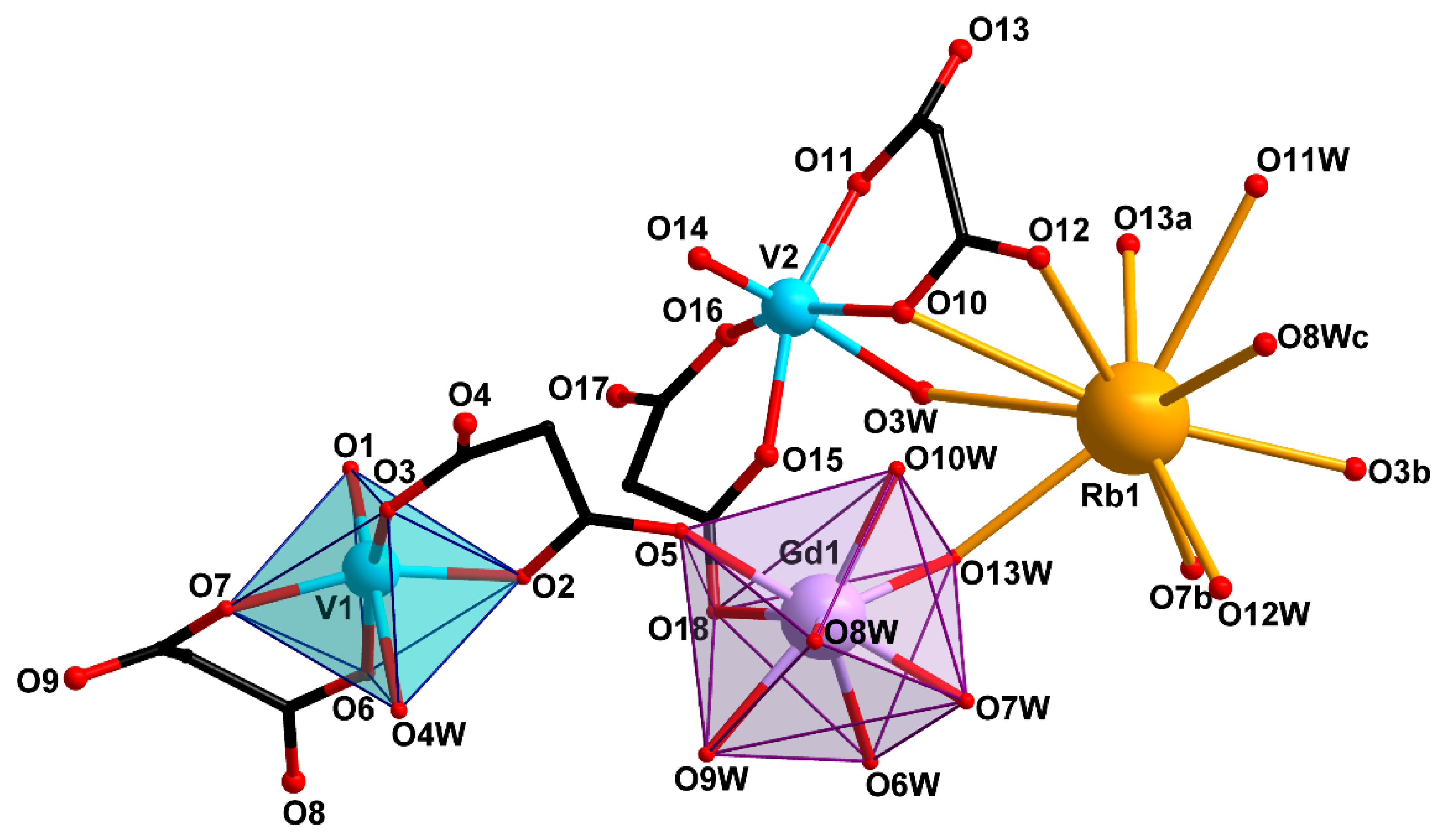




| Bond | d, Å | ||
|---|---|---|---|
| 1 (M = Na) | 2 (M = Rb) | 3 (M = Cs) | |
| V=O | 1.597(2) | 1.584(2), 1.589(3) | 1.581(4), 1.589(4) |
| V–O(cbdc) | 1.963(2)–2.021(2) | 1.984(2)–2.019(2) | 1.983(4)–2.017(3) |
| V–O(H2O) | 2.300(2) | 2.238(2), 2.417(2) | 2.251(4), 2.369(4) |
| Gd–O(cbdc) | 2.379(2) | 2.363(2), 2.454(2) | 2.326(3), 2.414(3) |
| Gd–O(H2O) | 2.372(2)–2.409(2) | 2.331(2)–2.491(2) | 2.383(4)–2.478(3) |
| M–O(cbdc) | 2.536(2), 2.631(2) | 2.843(2)–3.371(2) | 3.111(3)–3.611(4) |
| M–O(H2O) | 2.417(2), 2.445(2) | 2.845(2)–3.499(3) | 3.163(4)–3.470(4) |
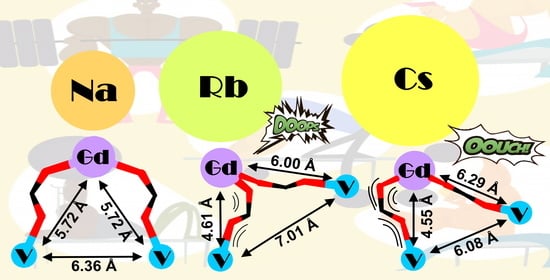
| Complex | Distance V···Gd, Å | Exchange Parameter JGdV, cm−1 | Ref. |
|---|---|---|---|
| {[Gd(H2O)7(VO)2(TTHA)][(VO)2(TTHA)]0.5}·8.5H2O, H6TTHA = triethylenetetraaminehexaacetic acid | 6.099 | −0.21 | [30] |
| [VO(L)Gd(hfa)2(MeOH)]2·2MeOH·2Me2CO, H3L = 2-hydroxy-N-{[(2-hydroxyphenyl)methylene]amino}-2-methylpropyl) benzamide | 6.280 | +0.46 | [29] |
| [L1(VO)Gd(H2O)(NO3)3]·H2O, H2L1 = N,N′-bis(3-methoxysalicylidene)-1,2-diamino-2-methylpropane | 3.5191 | +1.5 | [27] |
| [L2(VO)(Me2CO)Gd(NO3)3], H2L2 = N,N′-bis(3-methoxysalicylidene)-1,3-diamino-2,2′-dimethylpropane | 3.5043 | −2.6 | [28] |
| [NaGd(VO)2(cbdc)4(H2O)10]n (1) | 5.722 | +0.163 ± 0.008 | This work |
| {[RbGd(VO)2(cbdc)4(H2O)10]·2.5H2O}n (2) | 6.000 (V1) 4.611 (V2) | +0.989 ± 0.028 −0.089 ± 0.008 | This work |
| {[CsGd(VO)2(cbdc)4(H2O)11]·5H2O}2 (3) | 6.290 (V1) 4.547 (V2) | +0.656 ± 0.009 −0.050 ± 0.004 | This work |
| Compound (Optimal dc-Field, Oe) | Orbach | Orbach + Direct | Orbach + Raman + QTM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δeff/kB, K | τ0, s | Δeff/kB, K | τ0, s | Adirect, K−1Oe−4s−1 | Δeff/kB, K | τ0, s | CRaman, s−1K−n_Raman | n_Raman | B, s−1 | |
| 1 (5000) | 63 | 2∙10−6 | 60 | 3∙10−6 | 8.0∙10−13 | - | - | - | - | - |
| 2 (2500) | 43 | 2∙10−6 | - | - | - | 15 | 4.3∙10−5 | 44 | 2.32 | 794 |
| 3 (2500) | 32 | 4∙10−6 | 27 | 8∙10−6 | 1.43∙10−11 | - | - | - | - | - |
| Compound (Optimal dc-Field, Oe) | Orbach | Orbach + Raman | Orbach + Raman + QTM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Δeff/kB, K | τ0, s | Δeff/kB, K | τ0, s | CRaman, s−1K−n_Raman | n_Raman | Δeff/kB, K | τ0, s | CRaman, s−1K−n_Raman | n_Raman | B, s−1 | |
| 1Y (5000) | 76 | 5.81∙10−6 | - | - | - | - | 22 | 9∙10−5 | 26 | 1.8 | 207 |
| 1Lu (2500) | 50 | 1.0∙10−5 | 26 | 9∙10−5 | 89 | 1.50 | - | - | - | - | - |
| Parameter | 1 | 2 | 3 |
|---|---|---|---|
| Empirical formula | C24H40GdNaO28V2 | C24H49GdO30.5RbV2 | C24H56CsGdO34V2 |
| T/K | 150(2) | 120 | 120 |
| Formula weight (g·mol−1) | 1058.68 | 1170.23 | 1280.72 |
| Crystal system | monoclinic | monoclinic | triclinic |
| Space group | C2/c | C2/c | P |
| a (Å) | 9.0936 (2) | 23.375 (2) | 12.0203 (14) |
| b (Å) | 24.7129 (5) | 13.2166 (12) | 13.3110 (15) |
| c (Å) | 17.1366 (4) | 26.305 (2) | 14.3630 (17) |
| α (deg) | 90 | 90 | 110.948 (2) |
| β (deg) | 104.7320 (10) | 106.853 (2) | 92.627 (2) |
| γ (deg) | 90 | 90 | 97.051 (2) |
| V (Å3) | 3724.49 (14) | 7777.7 (12) | 2120.0 (4) |
| Z | 4 | 8 | 2 |
| Dcalc (g·cm−3) | 1.888 | 1.999 | 2.006 |
| θmin–θmax (deg) | 2.366 | 3.507 | 2.934 |
| μ (mm−1) | 2.46–30.51 | 2.23–33.72 | 2.18–30.41 |
| Number of measured reflections | 20,747 | 52,901 | 20,664 |
| Number of reflections with I > 2σ (I) | 4643 | 9629 | 6790 |
| Rint | 0.0339 | 0.0588 | 0.0488 |
| GooF | 1.045 | 1.024 | 1.037 |
| R1[a], wR2[b] (I > 2σ (I)) | 0.0276, 0.0756 | 0.0367, 0.0774 | 0.0377, 0.0824 |
| R1[a], wR2[b] (all data) | 0.0303, 0.0773 | 0.0513, 0.0839 | 0.0507, 0.0900 |
| Tmin/Tmax | 0.718/0.833 | 0.429/0.526 | 0.533/0.746 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bazhina, E.S.; Korlyukov, A.A.; Voronina, J.K.; Babeshkin, K.A.; Ugolkova, E.A.; Efimov, N.N.; Fedin, M.V.; Kiskin, M.A.; Eremenko, I.L. Effect of the Alkaline Metal Ion on the Crystal Structure and Magnetic Properties of Heterometallic GdIII-VIV Complexes Based on Cyclobutane-1,1-Dicarboxylate Anions. Magnetochemistry 2021, 7, 82. https://doi.org/10.3390/magnetochemistry7060082
Bazhina ES, Korlyukov AA, Voronina JK, Babeshkin KA, Ugolkova EA, Efimov NN, Fedin MV, Kiskin MA, Eremenko IL. Effect of the Alkaline Metal Ion on the Crystal Structure and Magnetic Properties of Heterometallic GdIII-VIV Complexes Based on Cyclobutane-1,1-Dicarboxylate Anions. Magnetochemistry. 2021; 7(6):82. https://doi.org/10.3390/magnetochemistry7060082
Chicago/Turabian StyleBazhina, Evgeniya S., Alexander A. Korlyukov, Julia K. Voronina, Konstantin A. Babeshkin, Elena A. Ugolkova, Nikolay N. Efimov, Matvey V. Fedin, Mikhail A. Kiskin, and Igor L. Eremenko. 2021. "Effect of the Alkaline Metal Ion on the Crystal Structure and Magnetic Properties of Heterometallic GdIII-VIV Complexes Based on Cyclobutane-1,1-Dicarboxylate Anions" Magnetochemistry 7, no. 6: 82. https://doi.org/10.3390/magnetochemistry7060082
APA StyleBazhina, E. S., Korlyukov, A. A., Voronina, J. K., Babeshkin, K. A., Ugolkova, E. A., Efimov, N. N., Fedin, M. V., Kiskin, M. A., & Eremenko, I. L. (2021). Effect of the Alkaline Metal Ion on the Crystal Structure and Magnetic Properties of Heterometallic GdIII-VIV Complexes Based on Cyclobutane-1,1-Dicarboxylate Anions. Magnetochemistry, 7(6), 82. https://doi.org/10.3390/magnetochemistry7060082