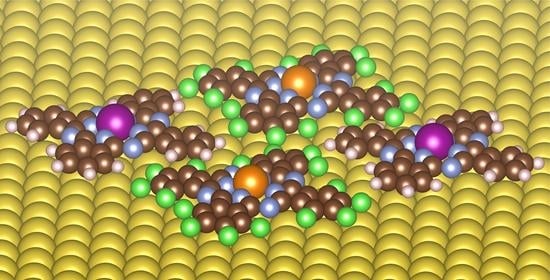
Self-Assembly and Magnetic Order of Bi-Molecular 2D Spin Lattices of M(II,III) Phthalocyanines on Au(111)
Abstract
:1. Introduction
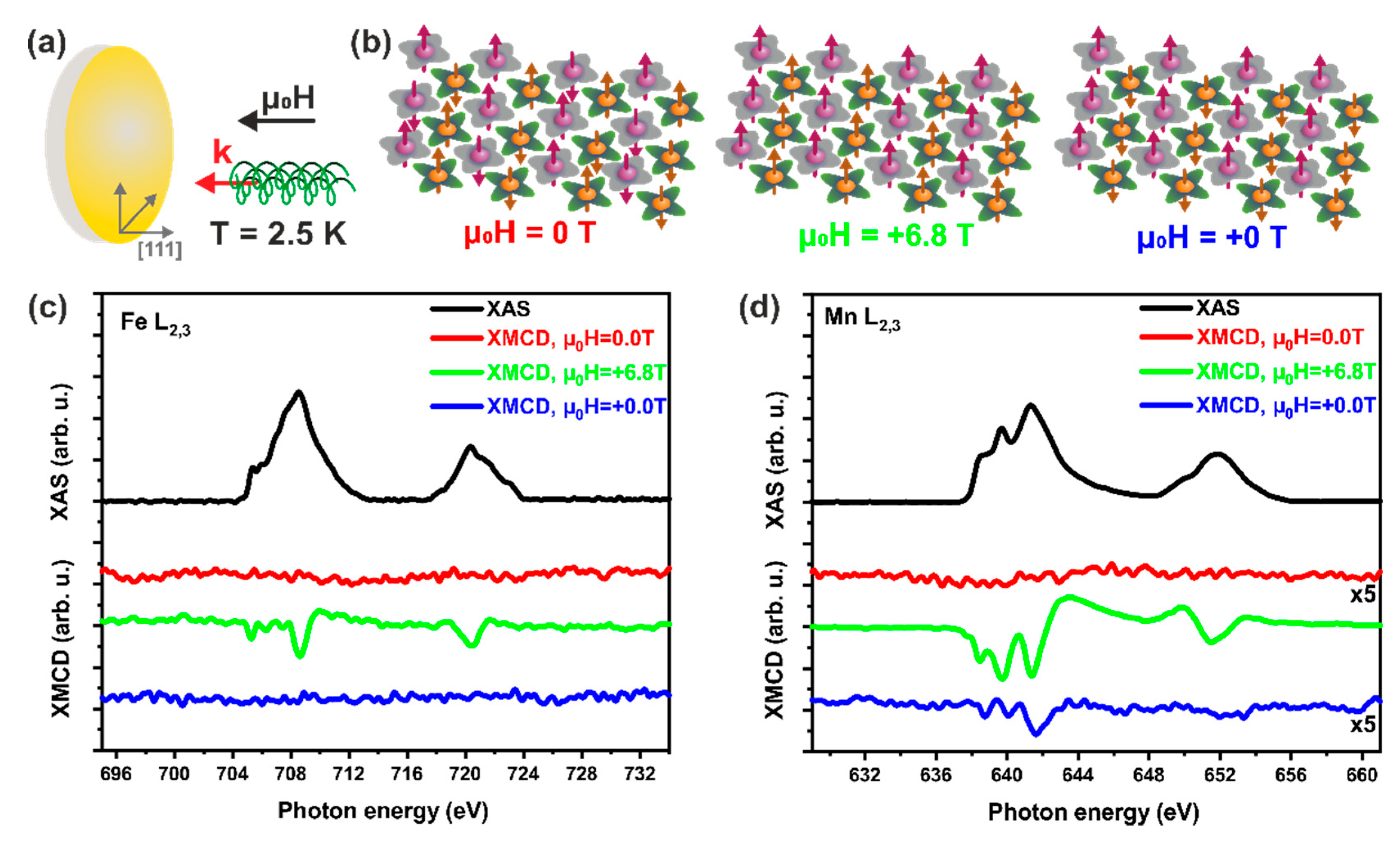
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vardeny, Z.V.; Wohlgenannt, M. World Scientific Reference on Spin in Organics; World Scientific Publishing: Singapore, 2018; Volume 1–4, ISBN 978-981-323-015-6. [Google Scholar]
- Cuevas, J.C.; Scheer, E. Molecular Electronics; World Scientific Series in Nanoscience and Nanotechnology; World Scientific Publishing: Singapore, 2010; Volume 1, ISBN 978-981-4282-58-1. [Google Scholar]
- Petty, M.C. Molecular Electronics: From Principles to Practice; Wiley: Hoboken, NJ, USA, 2008; ISBN 9781282123083. [Google Scholar]
- Herrer, L.; Martín, S.; Cea, P. Nanofabrication Techniques in Large-Area Molecular Electronic Devices. Appl. Sci. 2020, 10, 6064. [Google Scholar] [CrossRef]
- Girovsky, J.; Nowakowski, J.; Ali, M.E.; Baljozovic, M.; Rossmann, H.R.; Nijs, T.; Aeby, E.A.; Nowakowska, S.; Siewert, D.; Srivastava, G.; et al. Long-Range Ferrimagnetic Order in a Two-Dimensional Supramolecular Kondo Lattice. Nat. Commun. 2017, 8, 15388. [Google Scholar] [CrossRef]
- Hua, M.; Xia, B.; Wang, M.; Li, E.; Liu, J.; Wu, T.; Wang, Y.; Li, R.; Ding, H.; Hu, J.; et al. Highly Degenerate Ground States in a Frustrated Antiferromagnetic Kagome Lattice in a Two-Dimensional Metal-Organic Framework. J. Phys. Chem. Lett. 2021, 12, 3733–3739. [Google Scholar] [CrossRef]
- Goiri, E.; Borghetti, P.; El-Sayed, A.; Ortega, J.E.; Oteyza, D.G. de Multi-Component Organic Layers on Metal Substrates. Adv. Mater. 2016, 28, 1340–1368. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Vázquez de Parga, A.L.; Gallego, J.M. Electronic, Structural and Chemical Effects of Charge-Transfer at Organic/Inorganic Interfaces. Surf. Sci. Rep. 2017, 72, 105–145. [Google Scholar] [CrossRef]
- Kuch, W.; Bernien, M. Controlling the Magnetism of Adsorbed Metal-Organic Molecules. J. Phys. Condens Matter 2017, 29, 023001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballav, N.; Wäckerlin, C.; Siewert, D.; Oppeneer, P.M.; Jung, T.A. Emergence of On-Surface Magnetochemistry. J. Phys. Chem. Lett. 2013, 4, 2303–2311. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.H.; Chang, Y.H.; Kim, N.-Y.; Kim, H.; Lee, S.-H.; Choi, M.-S.; Kim, Y.-H.; Kahng, S.-J. Tuning and Sensing Spin Interactions in Co-Porphyrin/Au with NH3 and NO2 Binding. Phys. Rev. B 2019, 100, 245406. [Google Scholar] [CrossRef]
- Tsukahara, N.; Minamitani, E.; Kim, Y.; Kawai, M.; Takagi, N. Controlling Orbital-Selective Kondo Effects in a Single Molecule through Coordination Chemistry. J. Chem. Phys. 2014, 141, 054702. [Google Scholar] [CrossRef]
- Tsukahara, N.; Shiraki, S.; Itou, S.; Ohta, N.; Takagi, N.; Kawai, M. Evolution of Kondo Resonance from a Single Impurity Molecule to the Two-Dimensional Lattice. Phys. Rev. Lett. 2011, 106. [Google Scholar] [CrossRef]
- Tsukahara, N.; Noto, K.; Ohara, M.; Shiraki, S.; Takagi, N.; Takata, Y.; Miyawaki, J.; Taguchi, M.; Chainani, A.; Shin, S.; et al. Adsorption-Induced Switching of Magnetic Anisotropy in a Single Iron(II) Phthalocyanine Molecule on an Oxidized Cu(110) Surface. Phys. Rev. Lett. 2009, 102. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, L.; Zhang, L.; Xiao, W.; Fei, X.; Chen, H.; Du, S.; Ernst, K.-H.; Gao, H.-J. Reversible Achiral-to-Chiral Switching of Single Mn-Phthalocyanine Molecules by Thermal Hydrogenation and Inelastic Electron Tunneling Dehydrogenation. ACS Nano 2014, 8, 2246–2251. [Google Scholar] [CrossRef]
- Stróżecka, A.; Soriano, M.; Pascual, J.I.; Palacios, J.J. Reversible Change of the Spin State in a Manganese Phthalocyanine by Coordination of CO Molecule. Phys. Rev. Lett. 2012, 109, 147202. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.W.; Yang, K.; Xiao, W.D.; Jiang, Y.H.; Song, B.Q.; Du, S.X.; Gao, H.-J. Selective Adsorption of Metal-Phthalocyanine on Au(111) Surface with Hydrogen Atoms. Appl. Phys. Lett. 2013, 103, 023110. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, Z.; Zhong, J.Q.; Yuan, K.D.; Shen, Q.; Xu, L.L.; Niu, T.C.; Gu, C.D.; Wright, C.A.; Tadich, A.; et al. Single-Molecule Imaging of Activated Nitrogen Adsorption on Individual Manganese Phthalocyanine. Nano Lett. 2015, 15, 3181–3188. [Google Scholar] [CrossRef]
- Liu, L.; Yang, K.; Jiang, Y.; Song, B.; Xiao, W.; Li, L.; Zhou, H.; Wang, Y.; Du, S.; Ouyang, M.; et al. Reversible Single Spin Control of Individual Magnetic Molecule by Hydrogen Atom Adsorption. Sci. Rep. 2013, 3, 1210. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Lohr, T.G.; Pignedoli, C.A.; Liu, J.; Berger, R.; Urgel, J.I.; Müllen, K.; Feng, X.; Ruffieux, P.; Fasel, R. Tailoring Bond Topologies in Open-Shell Graphene Nanostructures. ACS Nano 2018, 12, 11917–11927. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Beyer, D.; Eimre, K.; Kezilebieke, S.; Berger, R.; Gröning, O.; Pignedoli, C.A.; Müllen, K.; Liljeroth, P.; Ruffieux, P.; et al. Topological Frustration Induces Unconventional Magnetism in a Nanographene. Nat. Nanotechnol. 2020, 15, 22–28. [Google Scholar] [CrossRef]
- Mishra, S.; Beyer, D.; Berger, R.; Liu, J.; Gröning, O.; Urgel, J.I.; Müllen, K.; Ruffieux, P.; Feng, X.; Fasel, R. Topological Defect-Induced Magnetism in a Nanographene. J. Am. Chem. Soc. 2020, 142, 1147–1152. [Google Scholar] [CrossRef]
- Mishra, S.; Beyer, D.; Eimre, K.; Liu, J.; Berger, R.; Gröning, O.; Pignedoli, C.A.; Müllen, K.; Fasel, R.; Feng, X.; et al. Synthesis and Characterization of π-Extended Triangulene. J. Am. Chem. Soc. 2019, 141, 10621–10625. [Google Scholar] [CrossRef]
- Mishra, S.; Beyer, D.; Eimre, K.; Ortiz, R.; Fernández-Rossier, J.; Berger, R.; Gröning, O.; Pignedoli, C.A.; Fasel, R.; Feng, X.; et al. Collective All-Carbon Magnetism in Triangulene Dimers. Angew. Chem. Int. Ed. 2020, 59, 12041–12047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Yao, X.; Chen, Q.; Eimre, K.; Gröning, O.; Ortiz, R.; Di Giovannantonio, M.; Sancho-García, J.C.; Fernández-Rossier, J.; Pignedoli, C.A.; et al. Large Magnetic Exchange Coupling in Rhombus-Shaped Nanographenes with Zigzag Periphery. Nat. Chem. 2021, 13, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Catarina, G.; Wu, F.; Ortiz, R.; Jacob, D.; Eimre, K.; Ma, J.; Pignedoli, C.A.; Feng, X.; Ruffieux, P.; et al. Observation of Fractional Edge Excitations in Nanographene Spin Chains. arXiv 2021, arXiv:2105.09102. [Google Scholar]
- Leznoff, C.C.; Lever, A.B.P. Phthalocyanines: Properties and Applications. Volume 1; VCH: New York, NY, USA; Weinheim, Germany; Cambridge, UK, 1989; ISBN 0-89573-753-1. [Google Scholar]
- Leznoff, C.C.; Lever, A.B.P. Phthalocyanines: Properties and Applications. Volume 2; VCH: New York, NY, USA; Weinheim, Germany; Cambridge, UK, 1993; ISBN 1-56081-544-2. [Google Scholar]
- Leznoff, C.C.; Lever, A.B.P. Phthalocyanines: Properties and Applications. Volume 3; VCH: New York, NY, USA; Weinheim, Germany; Cambridge, UK, 1993; ISBN 1-56081-638-4. [Google Scholar]
- Leznoff, C.C.; Lever, A.B.P. Phthalocyanines: Properties and Applications. Volume 4; VCH: New York, NY, USA; Weinheim, Germany; Cambridge, UK, 1996; ISBN 1-56081-916-2. [Google Scholar]
- Jiang, J. (Ed.) Functional Phthalocyanine Molecular Materials; Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2010; Volume 135, ISBN 978-3-642-04751-0. [Google Scholar]
- McKeown, N.B. Phthalocyanine Materials: Synthesis, Structure, and Function; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1998; ISBN 978-0-521-49623-0. [Google Scholar]
- Rusanova, J.; Pilkington, M.; Decurtins, S. A Novel Fully Conjugated Phenanthroline-Appended Phthalocyanine: Synthesis and Characterisation. Chem. Commun. 2002, 2236–2237. [Google Scholar] [CrossRef] [PubMed]
- Loosli, C.; Jia, C.; Liu, S.-X.; Haas, M.; Dias, M.; Levillain, E.; Neels, A.; Labat, G.; Hauser, A.; Decurtins, S. Synthesis and Electrochemical and Photophysical Studies of Tetrathiafulvalene-Annulated Phthalocyanines. J. Org. Chem. 2005, 70, 4988–4992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottfried, J.M. Surface Chemistry of Porphyrins and Phthalocyanines. Surf. Sci. Rep. 2015, 70, 259–379. [Google Scholar] [CrossRef]
- Otsuki, J. STM Studies on Porphyrins. Coord. Chem. Rev. 2010, 254, 2311–2341. [Google Scholar] [CrossRef]
- Komeda, T.; Katoh, K.; Yamashita, M. Double-Decker Phthalocyanine Complex: Scanning Tunneling Microscopy Study of Film Formation and Spin Properties. Prog. Surf. Sci. 2014, 89, 127–160. [Google Scholar] [CrossRef]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-Assembly as a Key Player for Materials Nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [Green Version]
- Auwärter, W.; Écija, D.; Klappenberger, F.; Barth, J.V. Porphyrins at Interfaces. Nat. Chem. 2015, 7, 105–120. [Google Scholar] [CrossRef]
- Wintjes, N.; Lobo-Checa, J.; Hornung, J.; Samuely, T.; Diederich, F.; Jung, T.A. Two-Dimensional Phase Behavior of a Bimolecular Porphyrin System at the Solid-Vacuum Interface. J. Am. Chem. Soc. 2010, 132, 7306–7311. [Google Scholar] [CrossRef] [PubMed]
- Patera, L.L.; Liu, X.; Mosso, N.; Decurtins, S.; Liu, S.-X.; Repp, J. Crystallization of a Two-Dimensional Hydrogen-Bonded Molecular Assembly: Evolution of the Local Structure Resolved by Atomic Force Microscopy. Angew. Chem. Int. Ed. 2017, 56, 10786–10790. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, L.M.A.; Saywell, A.; Fontes, G.N.; Staniec, P.A.; Goretzki, G.; Phillips, A.G.; Champness, N.R.; Beton, P.H. Functionalized Supramolecular Nanoporous Arrays for Surface Templating. Chem. A Eur. J. 2008, 14, 7600–7607. [Google Scholar] [CrossRef]
- Perdigão, L.M.A.; Staniec, P.A.; Champness, N.R.; Beton, P.H. Entrapment of Decanethiol in a Hydrogen-Bonded Bimolecular Template. Langmuir 2009, 25, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Yang, Y.; Zhang, P.; Zhong, D.; Fuchs, H.; Wang, Y.; Chi, L. Building Chessboard-like Supramolecular Structures on Au(111) Surfaces. Nanotechnology 2015, 26, 385601. [Google Scholar] [CrossRef] [PubMed]
- Kocić, N.; Liu, X.; Chen, S.; Decurtins, S.; Krejčí, O.; Jelínek, P.; Repp, J.; Liu, S.-X. Control of Reactivity and Regioselectivity for On-Surface Dehydrogenative Aryl–Aryl Bond Formation. J. Am. Chem. Soc. 2016, 138, 5585–5593. [Google Scholar] [CrossRef] [PubMed]
- Wäckerlin, C.; Chylarecka, D.; Kleibert, A.; Müller, K.; Iacovita, C.; Nolting, F.; Jung, T.A.; Ballav, N. Controlling Spins in Adsorbed Molecules by a Chemical Switch. Nat. Commun. 2010, 1, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wäckerlin, C.; Tarafder, K.; Siewert, D.; Girovsky, J.; Hählen, T.; Iacovita, C.; Kleibert, A.; Nolting, F.; Jung, T.A.; Oppeneer, P.M.; et al. On-Surface Coordination Chemistry of Planar Molecular Spin Systems: Novel Magnetochemical Effects Induced by Axial Ligands. Chem. Sci. 2012, 3, 3154–3160. [Google Scholar] [CrossRef]
- Wäckerlin, C.; Tarafder, K.; Girovsky, J.; Nowakowski, J.; Hählen, T.; Shchyrba, A.; Siewert, D.; Kleibert, A.; Nolting, F.; Oppeneer, P.M.; et al. Ammonia Coordination Introducing a Magnetic Moment in an On-Surface Low-Spin Porphyrin. Angew. Chem. Int. Ed. 2013, 52, 4568–4571. [Google Scholar] [CrossRef] [Green Version]
- Suto, K.; Yoshimoto, S.; Itaya, K. Two-Dimensional Self-Organization of Phthalocyanine and Porphyrin: Dependence on the Crystallographic Orientation of Au. J. Am. Chem. Soc. 2003, 125, 14976–14977. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Honda, Y.; Ito, O.; Itaya, K. Supramolecular Pattern of Fullerene on 2D Bimolecular “Chessboard” Consisting of Bottom-up Assembly of Porphyrin and Phthalocyanine Molecules. J. Am. Chem. Soc. 2008, 130, 1085–1092. [Google Scholar] [CrossRef]
- Sabik, A.; Mazur, P.; Gołek, F.; Trembulowicz, A.; Antczak, G. Phthalocyanine Arrangements on Ag(100): From Pure Overlayers of CoPc and F16CuPc to Bimolecular Heterostructure. J. Chem. Phys. 2018, 149, 144702. [Google Scholar] [CrossRef]
- Sabik, A.; Trembulowicz, A.; Mazur, P.; Antczak, G. STM Studies of the Bimolecular Layer of CoPc and F16CuPc on Ag(100) with Non-Equal Composition. Surf. Sci. 2020, 693, 121534. [Google Scholar] [CrossRef]
- Sabik, A.; Trembułowicz, A.; Antczak, G. Self-Organization of CoPc-F16CuPc Mixture with Non-Equal Composition on Ag(100): From Sub-Monolayer to Monolayer Coverage. Surf. Sci. 2021, 705, 121764. [Google Scholar] [CrossRef]
- Wäckerlin, A.; Fatayer, S.; Nijs, T.; Nowakowska, S.; Mousavi, S.F.; Popova, O.; Ahsan, A.; Jung, T.A.; Wäckerlin, C. Molecular Chessboard Assemblies Sorted by Site-Specific Interactions of Out-of-Plane d-Orbitals with a Semimetal Template. Nano Lett. 2017, 17, 1956–1962. [Google Scholar] [CrossRef]
- Wäckerlin, C.; Nowakowski, J.; Liu, S.-X.; Jaggi, M.; Siewert, D.; Girovsky, J.; Shchyrba, A.; Hählen, T.; Kleibert, A.; Oppeneer, P.M.; et al. Two-Dimensional Supramolecular Electron Spin Arrays. Adv. Mater. 2013, 25, 2404–2408. [Google Scholar] [CrossRef] [PubMed]
- Mermin, N.D.; Wagner, H. Absence of Ferromagnetism or Antiferromagnetism in One- or Two-Dimensional Isotropic Heisenberg Models. Phys. Rev. Lett. 1966, 17, 1133–1136. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Du, S.X.; Gao, H.-J. Binding Configuration, Electronic Structure, and Magnetic Properties of Metal Phthalocyanines on a Au(111) Surface Studied with Ab Initio Calculations. Phys. Rev. B 2011, 84, 125446. [Google Scholar] [CrossRef] [Green Version]
- Bartolomé, J.; Monton, C.; Schuller, I.K. Magnetism of Metal Phthalocyanines. In Molecular Magnets; Bartolomé, J., Luis, F., Fernández, J.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 221–245. ISBN 978-3-642-40608-9. [Google Scholar]
- Scardamaglia, M.; Struzzi, C.; Lizzit, S.; Dalmiglio, M.; Lacovig, P.; Baraldi, A.; Mariani, C.; Betti, M.G. Energetics and Hierarchical Interactions of Metal–Phthalocyanines Adsorbed on Graphene/Ir(111). Langmuir 2013, 29, 10440–10447. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.V.; Brune, H.; Ertl, G.; Behm, R.J. Scanning Tunneling Microscopy Observations on the Reconstructed Au(111) Surface: Atomic Structure, Long-Range Superstructure, Rotational Domains, and Surface Defects. Phys. Rev. B 1990, 42, 9307–9318. [Google Scholar] [CrossRef] [Green Version]
- Wahl, P.; Simon, P.; Diekhöner, L.; Stepanyuk, V.S.; Bruno, P.; Schneider, M.A.; Kern, K. Exchange Interaction between Single Magnetic Adatoms. Phys. Rev. Lett. 2007, 98. [Google Scholar] [CrossRef] [Green Version]
- Fischer, B.; Klein, M.W. Magnetic and Nonmagnetic Impurities in Two-Dimensional Metals. Phys. Rev. B 1975, 11, 2025–2029. [Google Scholar] [CrossRef]
- Henk, J.; Hoesch, M.; Osterwalder, J.; Ernst, A.; Bruno, P. Spin–Orbit Coupling in the L-Gap Surface States of Au(111): Spin-Resolved Photoemission Experiments and First-Principles Calculations. J. Phys. Condens. Matter 2004, 16, 7581–7597. [Google Scholar] [CrossRef]
- Ryu, H.; Song, I.; Kim, B.; Cho, S.; Soltani, S.; Kim, T.; Hoesch, M.; Kim, C.H.; Kim, C. Photon Energy Dependent Circular Dichroism in Angle-Resolved Photoemission from Au(111) Surface States. Phys. Rev. B 2017, 95. [Google Scholar] [CrossRef] [Green Version]
- Sachs, S.; Schwalb, C.H.; Marks, M.; Schöll, A.; Reinert, F.; Umbach, E.; Höfer, U. Electronic Structure at the Perylene-Tetracarboxylic Acid Dianhydride/Ag(111) Interface Studied with Two-Photon Photoelectron Spectroscopy. J. Chem. Phys. 2009, 131, 144701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwalb, C.H.; Sachs, S.; Marks, M.; Schöll, A.; Reinert, F.; Umbach, E.; Höfer, U. Electron Lifetime in a Shockley-Type Metal-Organic Interface State. Phys. Rev. Lett. 2008, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziroff, J.; Gold, P.; Bendounan, A.; Forster, F.; Reinert, F. Adsorption Energy and Geometry of Physisorbed Organic Molecules on Au(111) Probed by Surface-State Photoemission. Surf. Sci. 2009, 603, 354–358. [Google Scholar] [CrossRef]
- Nicoara, N.; Román, E.; Gómez-Rodríguez, J.M.; Martín-Gago, J.A.; Méndez, J. Scanning Tunneling and Photoemission Spectroscopies at the PTCDA/Au(111) Interface. Org. Electron. 2006, 7, 287–294. [Google Scholar] [CrossRef]
- Zaitsev, N.L.; Nechaev, I.A.; Echenique, P.M.; Chulkov, E.V. Transformation of the Ag(111) Surface State Due to Molecule-Surface Interaction with Ordered Organic Molecular Monolayers. Phys. Rev. B 2012, 85. [Google Scholar] [CrossRef] [Green Version]
- Tamai, A.; Seitsonen, A.P.; Baumberger, F.; Hengsberger, M.; Shen, Z.-X.; Greber, T.; Osterwalder, J. Electronic Structure at the C 60/Metal Interface: An Angle-Resolved Photoemission and First-Principles Study. Phys. Rev. B 2008, 77. [Google Scholar] [CrossRef] [Green Version]
- Caputo, M.; Panighel, M.; Lisi, S.; Khalil, L.; Santo, G.D.; Papalazarou, E.; Hruban, A.; Konczykowski, M.; Krusin-Elbaum, L.; Aliev, Z.S.; et al. Manipulating the Topological Interface by Molecular Adsorbates: Adsorption of Co-Phthalocyanine on Bi2Se3. Nano Lett. 2016, 16, 3409–3414. [Google Scholar] [CrossRef]
- Scheybal, A.; Müller, K.; Bertschinger, R.; Wahl, M.; Bendounan, A.; Aebi, P.; Jung, T.A. Modification of the Cu(110) Shockley Surface State by an Adsorbed Pentacene Monolayer. Phys. Rev. B 2009, 79. [Google Scholar] [CrossRef] [Green Version]
- Bendounan, A.; Aït-Ouazzou, S. Role of the Shockley State in Doping of Organic Molecule Monolayer. J. Phys. Chem. C 2016, 120, 11456–11464. [Google Scholar] [CrossRef]
- Stepanow, S.; Miedema, P.S.; Mugarza, A.; Ceballos, G.; Moras, P.; Cezar, J.C.; Carbone, C.; de Groot, F.M.F.; Gambardella, P. Mixed-Valence Behavior and Strong Correlation Effects of Metal Phthalocyanines Adsorbed on Metals. Phys. Rev. B 2011, 83. [Google Scholar] [CrossRef] [Green Version]
- Krzystek, J.; Pardi, L.A.; Brunel, L.-C.; Goldberg, D.P.; Hoffman, B.M.; Licoccia, S.; Telser, J. High-Frequency and -Field Electron Paramagnetic Resonance of High-Spin Manganese(III) in Tetrapyrrole Complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 1113–1127. [Google Scholar] [CrossRef]
- Hieringer, W.; Flechtner, K.; Kretschmann, A.; Seufert, K.; Auwärter, W.; Barth, J.V.; Görling, A.; Steinrück, H.-P.; Gottfried, J.M. The Surface Trans Effect: Influence of Axial Ligands on the Surface Chemical Bonds of Adsorbed Metalloporphyrins. J. Am. Chem. Soc. 2011, 133, 6206–6222. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.G.; Twigg, M.V. Fluorinated Iron Phthalocyanine. Inorg. Chem. 1969, 8, 2018–2019. [Google Scholar] [CrossRef]
- Piamonteze, C.; Flechsig, U.; Rusponi, S.; Dreiser, J.; Heidler, J.; Schmidt, M.; Wetter, R.; Calvi, M.; Schmidt, T.; Pruchova, H.; et al. X-Treme Beamline at SLS: X-Ray Magnetic Circular and Linear Dichroism at High Field and Low Temperature. J. Synchrotron Rad. 2012, 19, 661–674. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular-Dynamics Simulation of the Liquid-Metal--Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Dudarev, S.L.; Botton, G.A.; Savrasov, S.Y.; Humphreys, C.J.; Sutton, A.P. Electron-Energy-Loss Spectra and the Structural Stability of Nickel Oxide: An LSDA+U Study. Phys. Rev. B 1998, 57, 1505–1509. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Xu, N.; Liu, J.-C.; Tang, G.; Geng, W.-T. VASPKIT: A User-Friendly Interface Facilitating High-Throughput Computing and Analysis Using VASP Code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baljozović, M.; Liu, X.; Popova, O.; Girovsky, J.; Nowakowski, J.; Rossmann, H.; Nijs, T.; Moradi, M.; Mousavi, S.F.; Plumb, N.C.; et al. Self-Assembly and Magnetic Order of Bi-Molecular 2D Spin Lattices of M(II,III) Phthalocyanines on Au(111). Magnetochemistry 2021, 7, 119. https://doi.org/10.3390/magnetochemistry7080119
Baljozović M, Liu X, Popova O, Girovsky J, Nowakowski J, Rossmann H, Nijs T, Moradi M, Mousavi SF, Plumb NC, et al. Self-Assembly and Magnetic Order of Bi-Molecular 2D Spin Lattices of M(II,III) Phthalocyanines on Au(111). Magnetochemistry. 2021; 7(8):119. https://doi.org/10.3390/magnetochemistry7080119
Chicago/Turabian StyleBaljozović, Miloš, Xunshan Liu, Olha Popova, Jan Girovsky, Jan Nowakowski, Harald Rossmann, Thomas Nijs, Mina Moradi, S. Fatemeh Mousavi, Nicholas C. Plumb, and et al. 2021. "Self-Assembly and Magnetic Order of Bi-Molecular 2D Spin Lattices of M(II,III) Phthalocyanines on Au(111)" Magnetochemistry 7, no. 8: 119. https://doi.org/10.3390/magnetochemistry7080119
APA StyleBaljozović, M., Liu, X., Popova, O., Girovsky, J., Nowakowski, J., Rossmann, H., Nijs, T., Moradi, M., Mousavi, S. F., Plumb, N. C., Radović, M., Ballav, N., Dreiser, J., Decurtins, S., Pašti, I. A., Skorodumova, N. V., Liu, S.-X., & Jung, T. A. (2021). Self-Assembly and Magnetic Order of Bi-Molecular 2D Spin Lattices of M(II,III) Phthalocyanines on Au(111). Magnetochemistry, 7(8), 119. https://doi.org/10.3390/magnetochemistry7080119