Translational Hurdles with Magnetic Nanoparticles and Current Clinical Scenario in Hyperthermia Applications
Abstract
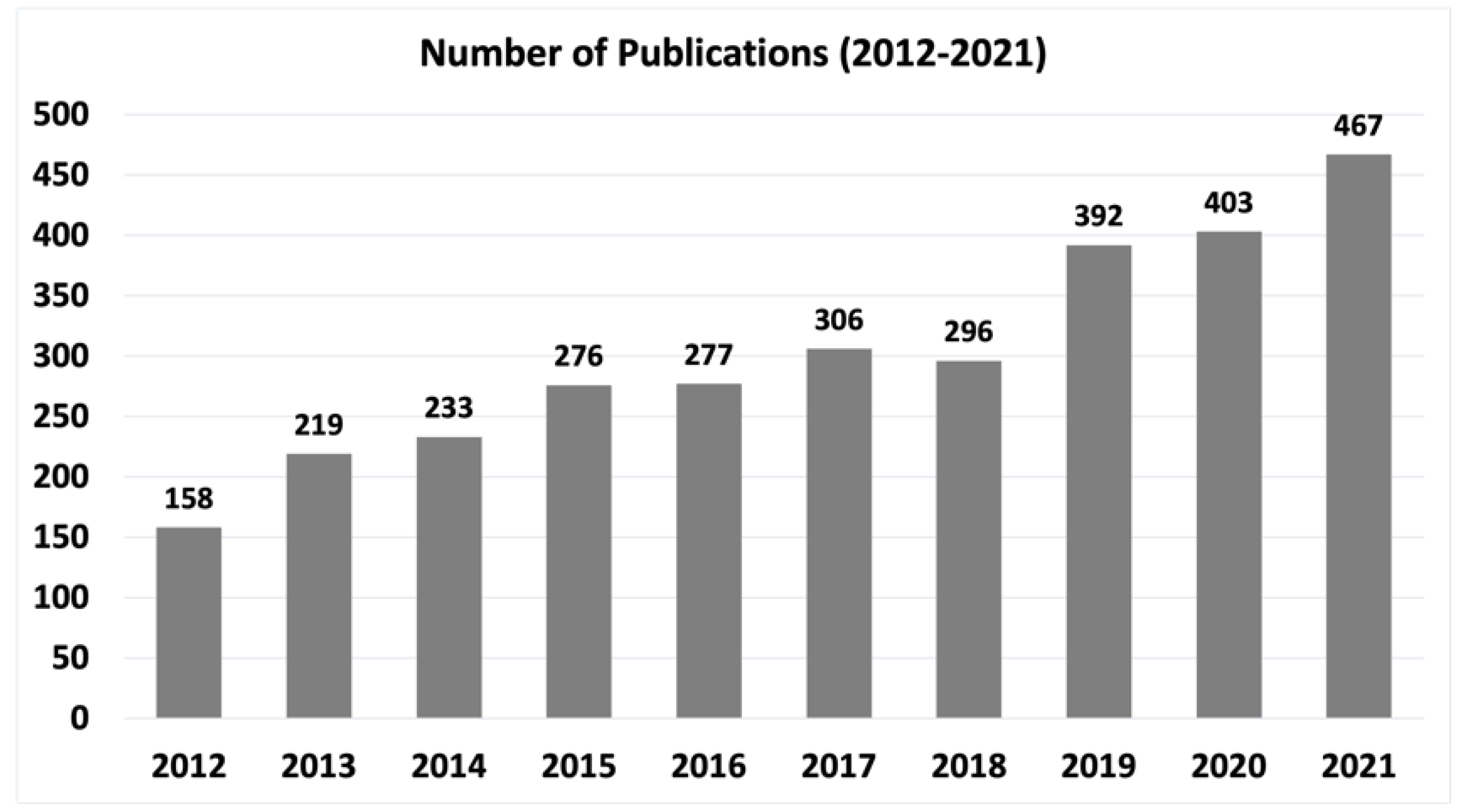
:1. Introduction
2. Technical Challenges
- A specific absorption rate (SAR) is the key indicator for evaluating the efficiency of any magnetic fluid. One hypothesis suggests that an SAR close to 1000 W g−1 at a fluid concentration of 5 mg mL−1 may be adequate. It has to be noted that the SAR depends upon intrinsic and extrinsic properties of the fluid [13,14,15,16]. Higher heating efficiency would be highly desirable as it would reduce the number of nanoparticles, field strength and frequency required to induce significant heating [17].
- Protein corona engulfment by macrophages is one of the leading causes of failure in clinical settings. It has to be noted that the aggregations of MNPs form large clusters, which are easily detected by the reticuloendothelial system (RES) of the host body [18].
- On-site delivery of the particles to overcome technical limitations will be discussed. Ideally, a high concentration of MNPs (and subsequent high heating effect) should be localized at the tumour and not accumulate in healthy tissues. In reality, this is seldom the case [3]. Still, the low availability of MNPs and fluid diffusion into the surrounding tissue is observed.
- Irrespective of the injection procedure, applied MNPs uniformly designed with cell-specific identification fractions (i.e., antibodies, proteins) somehow make their way to off-target excretory organs (spleen, liver, or kidneys). As validated in preclinical animal trials and MFH clinical settings, this results in side effects such as secondary heat damage to healthy tissues [19].
- Another major technical challenge is the lack of control in the real-time monitoring of temperature rise during treatment. This is because MNPs are heated in a non-uniform manner, depending on their location in the tumour [7]. Randomly oriented accumulation of magnetic fluid within the tumour allows for comparatively significant temperature variations during the application of MFH as measured by temperature measurement using invasive thermal sensors.
- Patients withstand lower magnetic field strengths during therapy. However, higher magnetic field values have been reported to cause pain in the perineal area or groin, burning sensations, and increased skin irritation due to the development of hot zones [20].
- In addition, official studies on the actual achieved clinical temperature of the tumour cells measured by invasive thermometry differ considerably by several °C, evaluated by the temperature expected by the bioheat transfer equation resulting from the treatment outcomes.
- The lack of standard measurement protocols, the standard composition of fluid concentration and different reported SAR/SLP values create lots of ambiguity [14].
3. Proposed Criteria to Maximize the Efficiency of MFH
- The first and foremost criteria are to generate maximum temperature and SAR/SLP values with a low quantity of fluid at biologically benign field (f, H) values to minimize the potential side effects [7].
- Secondly, NPs should possess high size uniformity and zeta potential values to favour homogeneous heat dissipation inside the tumour. For this, a robust and straightforward synthesis method should be developed to produce bulk and homogenous synthesis of MNPs [21].
- Designing the core–shell structure can be a vital strategy to increase SAR/SLP values with a low quantity of fluid at biologically benign field (f, H) values to minimize the potential side effects.
- Additionally, magneto-tactic bacteria (MTB), metal-doped spinel ferrite, magnetic–plasmonic multifunctional nanohybrids optically active in the NIR region, and self-controlled MFH, have also been studied with outstanding outcomes.
- A more relevant organoid model should be designed for better performance tracking, preventing rapid and more accurate therapeutics and avoiding later stage failure.
4. Feature Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Singh, G.; Volochaev, M.; Sokolov, A.; Rodionova, V.; Peddis, D. Tunable magnetic properties of Ni-doped CoFe2O4 nanoparticles prepared by the sol–gel citrate self-combustion method. J. Magn. Magn. Mater. 2019, 476, 387–391. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Chaurasia, A.K.; Pawar, S.H. Cancer cell extinction through a magnetic fluid hyperthermia treatment produced by superparamagnetic Co–Zn ferrite nanoparticles. RSC Adv. 2015, 5, 47225–47234. [Google Scholar] [CrossRef]
- Bohara, R.A. Introduction and Types of Hybrid Nanostructures for Medical Applications. In Hybrid Nanostructures for Cancer Theranostics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–16. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Du, X.; Zhao, F.; Xu, B. Magnetic nanoparticles for the manipulation of proteins and cells. Chem. Soc. Rev. 2012, 41, 2912–2942. [Google Scholar] [CrossRef]
- Qingling, F.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. Part A 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Predescu, A.M.; Matei, E.; Berbecaru, A.C.; Pantilimon, C.; Drăgan, C.; Vidu, R.; Predescu, C.; Kuncser, V. Synthesis and characterization of dextran-coated iron oxide nanoparticles. R. Soc. Open Sci. 2018, 5, 171525. [Google Scholar] [CrossRef] [Green Version]
- Wust, P.; Gneveckow, U.; Johannsen, M.; Böhmer, D.; Henkel, T.; Kahmann, F.; Sehouli, J.; Felix, R.; Ricke, J.; Jordan, A. Magnetic nanoparticles for interstitial thermotherapy—Feasibility, tolerance and achieved temperatures. Int. J. Hyperth. 2006, 22, 673–685. [Google Scholar] [CrossRef]
- Maier-HauffFrank, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Etemadi, H.; Plieger, P.G. Magnetic Fluid Hyperthermia Based on Magnetic Nanoparticles: Physical Characteristics, Historical Perspective, Clinical Trials, Technological Challenges, and Recent Advances. Adv. Ther. 2020, 3, 2000061. [Google Scholar] [CrossRef]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; Waldöfner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef] [Green Version]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef]
- Bohara, R.A.; Throat, N.D.; Mulla, N.A.; Pawar, S.H. Surface-Modified Cobalt Ferrite Nanoparticles for Rapid Capture, Detection, and Removal of Pathogens: A Potential Material for Water Purification. Appl. Biochem. Biotechnol. 2017, 182, 598–608. [Google Scholar] [CrossRef]
- Niculaes, D.; Lak, A.; Anyfantis, G.C.; Marras, S.; Laslett, O.; Avugadda, S.K.; Cassani, M.; Serantes, D.; Hovorka, O.; Chantrell, R.; et al. Asymmetric Assembling of Iron Oxide Nanocubes for Improving Magnetic Hyperthermia Performance. ACS Nano 2017, 11, 12121–12133. [Google Scholar] [CrossRef]
- Lanier, O.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int. J. Hyperth. 2019, 36, 686–700. [Google Scholar] [CrossRef]
- Chang, D.; Lim, M.; Goos, J.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Bohara, R.A.; Singh, P. Multiple Myeloma: Role of Magnetic Nanoparticles. In Magnetic Nanoheterostructures; Springer: Cham, Swizerland, 2020; pp. 479–494. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Thorat, N.D.; Bohara, R.A.; Yadav, H.M.; Tofail, S.A.M. Multi-modal MR imaging and magnetic hyperthermia study of Gd doped Fe3O4 nanoparticles for integrative cancer therapy. RSC Adv. 2016, 6, 94967–94975. [Google Scholar] [CrossRef]
- Thorat, N.D.; Lemine, O.M.; Bohara, R.A.; Omri, K.; El Mir, L.; Tofail, S.A.M. Superparamagnetic iron oxide nanocargoes for combined cancer thermotherapy and MRI applications. Phys. Chem. Chem. Phys. 2016, 18, 21331–21339. [Google Scholar] [CrossRef]
| Advantage | Improvement Required |
|---|---|
| As theranostics | Improvement in large-scale synthesis techniques |
| Improved Biocompatibility and Biodegradation | Development of simple functionalization techniques with the use of biodegradable materials |
| Targeting ability | The detailed understanding of immune interaction is required |
| Manipulated by Magnetic Field | Batch after Batch reproducibility |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohara, R.A.; Leporatti, S. Translational Hurdles with Magnetic Nanoparticles and Current Clinical Scenario in Hyperthermia Applications. Magnetochemistry 2022, 8, 123. https://doi.org/10.3390/magnetochemistry8100123
Bohara RA, Leporatti S. Translational Hurdles with Magnetic Nanoparticles and Current Clinical Scenario in Hyperthermia Applications. Magnetochemistry. 2022; 8(10):123. https://doi.org/10.3390/magnetochemistry8100123
Chicago/Turabian StyleBohara, Raghvendra A., and Stefano Leporatti. 2022. "Translational Hurdles with Magnetic Nanoparticles and Current Clinical Scenario in Hyperthermia Applications" Magnetochemistry 8, no. 10: 123. https://doi.org/10.3390/magnetochemistry8100123
APA StyleBohara, R. A., & Leporatti, S. (2022). Translational Hurdles with Magnetic Nanoparticles and Current Clinical Scenario in Hyperthermia Applications. Magnetochemistry, 8(10), 123. https://doi.org/10.3390/magnetochemistry8100123








