Coercivity and Exchange Bias in Ti-Doped Maghemite Nanoparticles
Abstract
:1. Introduction
2. Experimental Methods
2.1. Synthesis of Ti-Doped Maghemite Nanoparticles
2.2. Structure Analysis
2.3. Magnetic Measurements
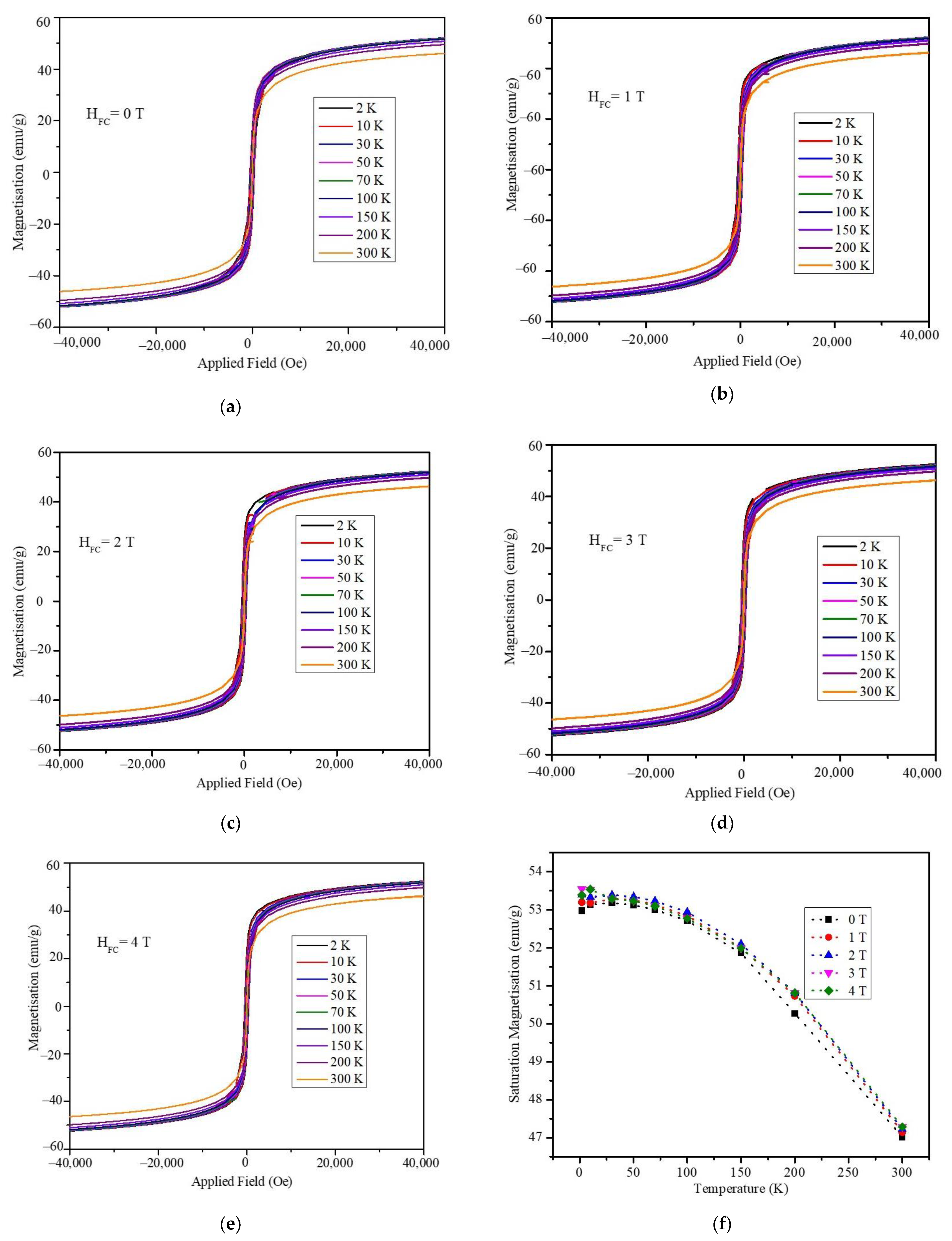
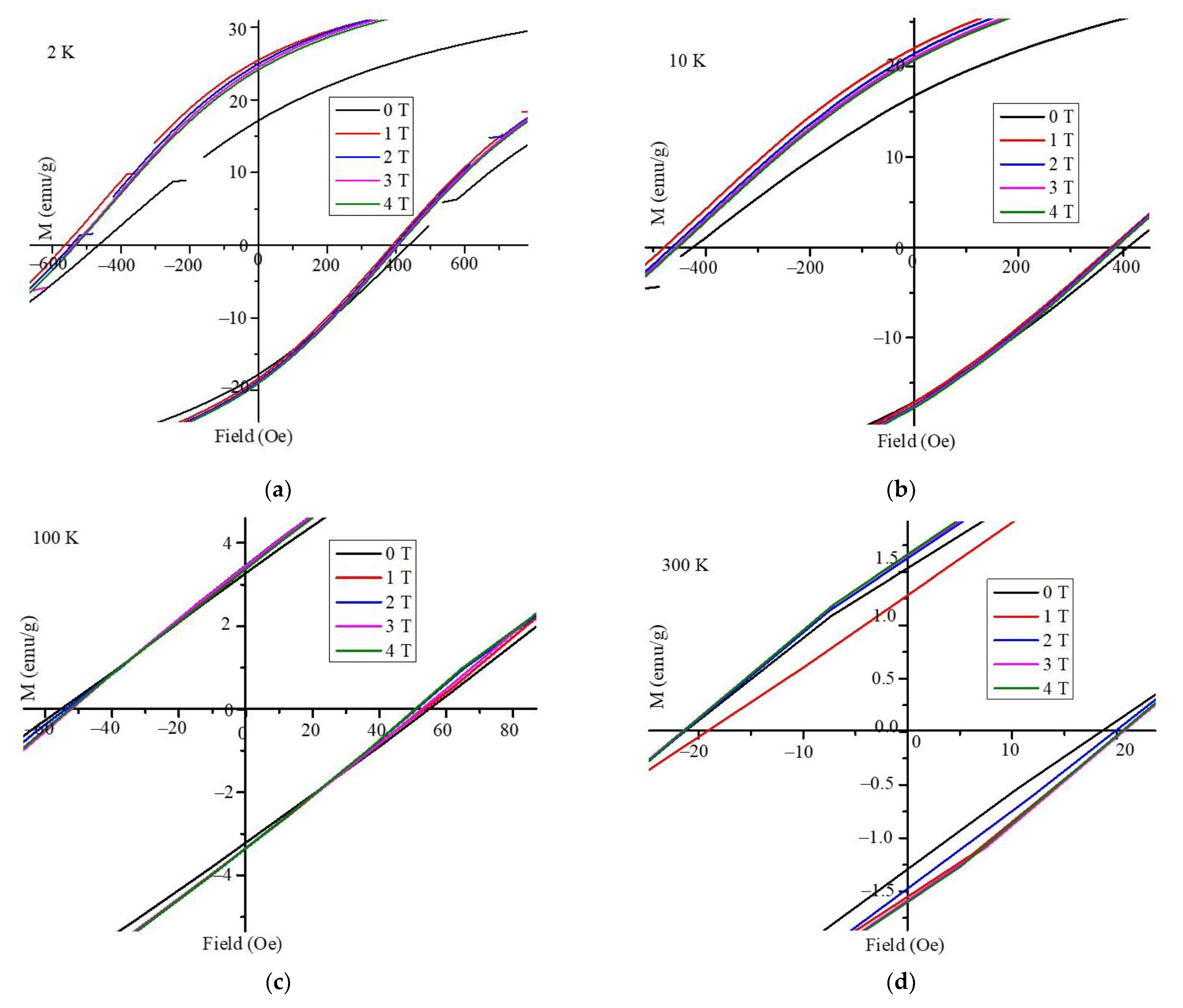
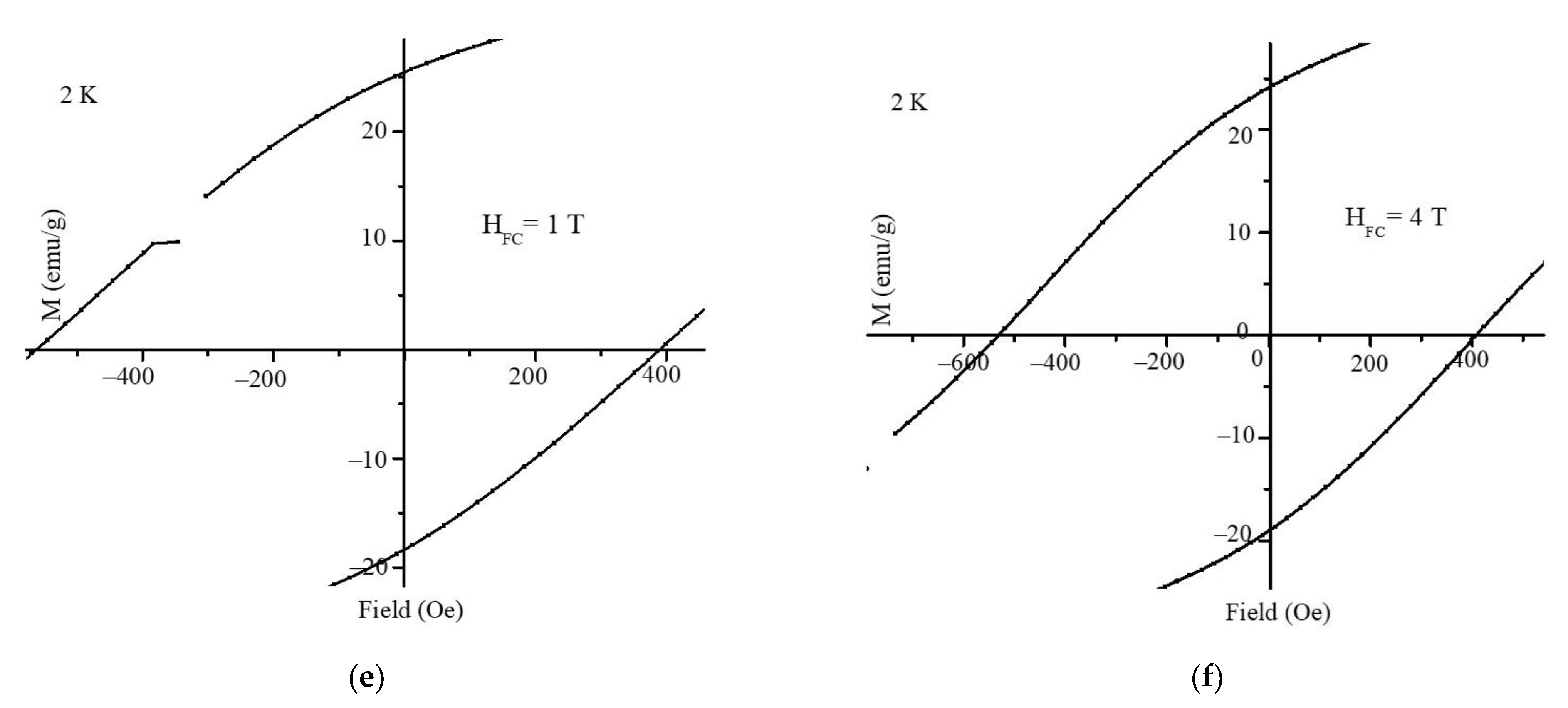
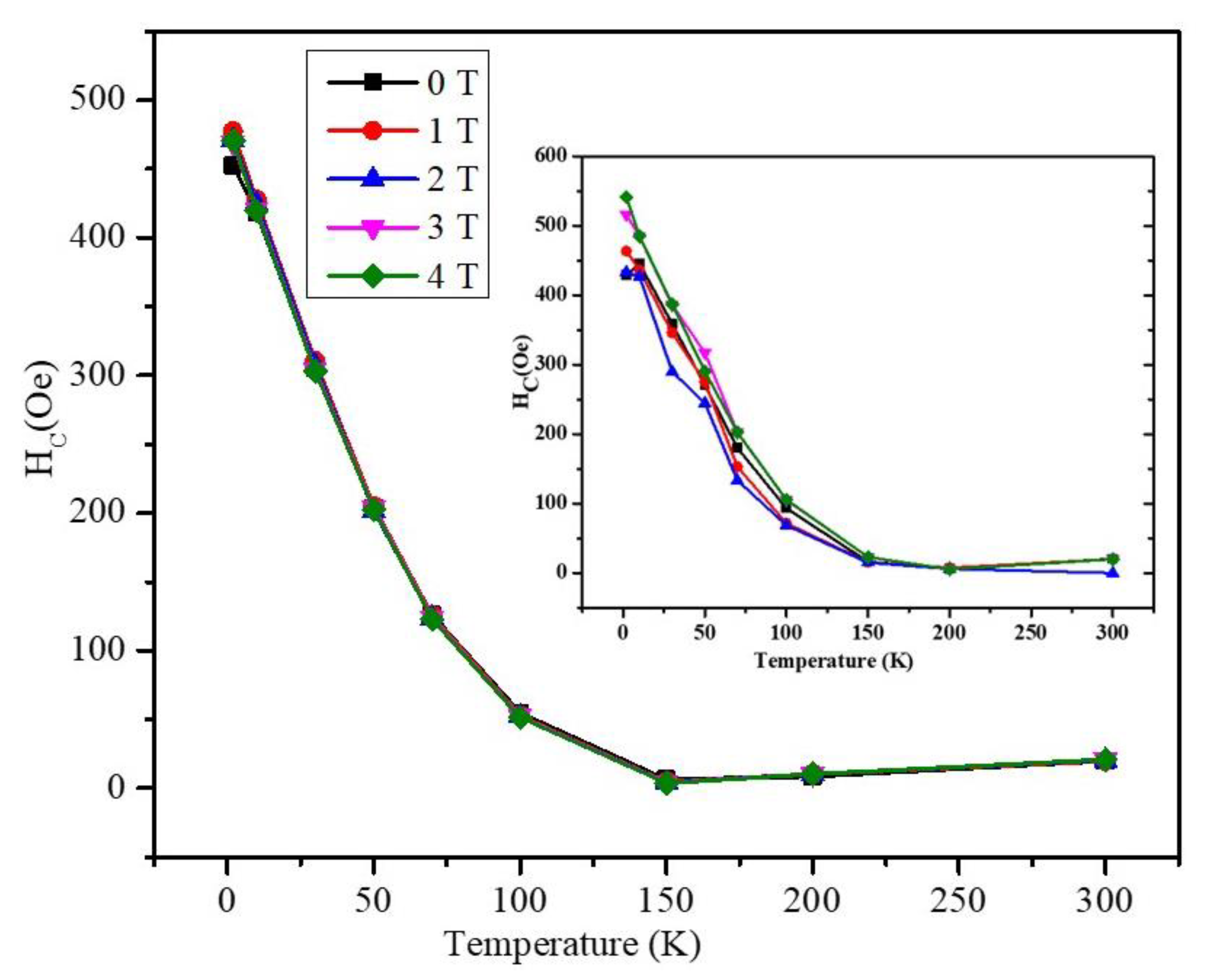
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Hasany, S.F.; Abdurahman, N.H.; Sunarti, A.R.; Jose, R. Magnetic Iron Oxide Nanoparticles: Chemical Synthesis and Applications Review. Curr. Nanosci. 2013, 9, 561–575. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Tarasevych, A.V.; Kukhar, V.P.; Boukherroub, R.; Szunerits, S. Recent Advances in Surface Chemistry Strategies for the Fabrication of Functional Iron Oxide Based Magnetic Nanoparticles. Nanoscale 2013, 5, 10729–10752. [Google Scholar] [CrossRef]
- Shokrollahi, H. A Review of the Magnetic Properties, Synthesis Methods and Applications of Maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Li, Z.; Chanéac, C.; Berger, G.; Delaunay, S.; Graff, A.; Lefèvre, G. Mechanism and Kinetics of Magnetite Oxidation under Hydrothermal Conditions. RSC Adv. 2019, 9, 33633–33642. [Google Scholar] [CrossRef] [Green Version]
- Coduri, M.; Masala, P.; Del Bianco, L.; Spizzo, F.; Ceresoli, D.; Castellano, C.; Cappelli, S.; Oliva, C.; Checchia, S.; Allieta, M.; et al. Local Structure and Magnetism of Fe2O3 Maghemite Nanocrystals: The Role of Crystal Dimension. Nanomaterials 2020, 10, 867. [Google Scholar] [CrossRef]
- Ponomar, V.P.; Antonenko, T.S.; Vyshnevskyi, O.A.; Brik, A.B. Thermally Induced Changes in the Magnetic Properties of Iron Oxide Nanoparticles under Reducing and Oxidizing Conditions. Adv. Powder Technol. 2020, 31, 2587–2596. [Google Scholar] [CrossRef]
- Yüksel, Y. Effects of the Particle Size and Shape of the Magnetic Nanoparticles on the Magnetic Hyperthermia and Exchange Bias Properties☆. Phys. B Condens. Matter 2019, 575, 411689. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Skumryev, V.; Stoyanov, S.; Zhang, Y.; Hadjipanayis, G.; Givord, D.; Nogués, J. Beating the Superparamagnetic Limit with Exchange Bias. Nature 2003, 423, 850–853. [Google Scholar] [CrossRef]
- Mercante, L.A.; Melo, W.W.M.; Granada, M.; Troiani, H.E.; Macedo, W.A.A.; Ardison, J.D.; Vaz, M.G.F.; Novak, M.A. Magnetic Properties of Nanoscale Crystalline Maghemite Obtained by a New Synthetic Route. J. Magn. Magn. Mater. 2012, 324, 3029–3033. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.S.; Mathieu, R.; Lee, S.S.; Normile, P.S.; Singh, G.; Nordblad, P.; Toro, J.A.D. Size-Dependent Surface Effects in Maghemite Nanoparticles and Its Impact on Interparticle Interactions in Dense Assemblies. Nanotechnology 2015, 26, 475703. [Google Scholar] [CrossRef]
- Di Corato, R.; Aloisi, A.; Rella, S.; Greneche, J.-M.; Pugliese, G.; Pellegrino, T.; Malitesta, C.; Rinaldi, R. Maghemite Nanoparticles with Enhanced Magnetic Properties: One-Pot Preparation and Ultrastable Dextran Shell. ACS Appl. Mater. Interfaces 2018, 10, 20271–20280. [Google Scholar] [CrossRef] [PubMed]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [Green Version]
- Fantechi, E.; Campo, G.; Carta, D.; Corrias, A.; de Julián Fernández, C.; Gatteschi, D.; Innocenti, C.; Pineider, F.; Rugi, F.; Sangregorio, C. Exploring the Effect of Co Doping in Fine Maghemite Nanoparticles. J. Phys. Chem. C 2012, 116, 8261–8270. [Google Scholar] [CrossRef]
- Can, M.M.; Bairam, C.; Aksoy, S.; Kuruca, D.S.; Kaneko, S.; Aktaş, Z.; Öncül, M.O. Effect of Ti Atoms on Néel Relaxation Mechanism at Magnetic Heating Performance of Iron Oxide Nanoparticles. Coatings 2022, 12, 481. [Google Scholar] [CrossRef]
- Liu, X.; Pichon, B.P.; Ulhaq, C.; Lefèvre, C.; Grenèche, J.-M.; Bégin, D.; Bégin-Colin, S. Systematic Study of Exchange Coupling in Core–Shell Fe3−δO4@CoO Nanoparticles. Chem. Mater. 2015, 27, 4073–4081. [Google Scholar] [CrossRef]
- Silva, F.G.; Depeyrot, J.; Raikher, Y.L.; Stepanov, V.I.; Poperechny, I.S.; Aquino, R.; Ballon, G.; Geshev, J.; Dubois, E.; Perzynski, R. Exchange-Bias and Magnetic Anisotropy Fields in Core–Shell Ferrite Nanoparticles. Sci. Rep. 2021, 11, 5474. [Google Scholar] [CrossRef]
- Muhammed Shameem, P.V.; Senthil Kumar, M. Training Effect of the Exchange Bias in Sputter Deposited Fe3O4 Thin Films with Varying Thickness. J. Magn. Magn. Mater. 2018, 458, 241–252. [Google Scholar] [CrossRef]
- Kim, W.; Suh, C.-Y.; Cho, S.-W.; Roh, K.-M.; Kwon, H.; Song, K.; Shon, I.-J. A New Method for the Identification and Quantification of Magnetite–Maghemite Mixture Using Conventional X-ray Diffraction Technique. Talanta 2012, 94, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Cervellino, A.; Frison, R.; Cernuto, G.; Guagliardi, A.; Masciocchi, N. Lattice Parameters and Site Occupancy Factors of Magnetite–Maghemite Core–Shell Nanoparticles. A Critical Study. J. Appl. Cryst. 2014, 47, 1755–1761. [Google Scholar] [CrossRef]
- Lee, K.; Jang, J.; Nakano, H.; Nakagawa, S.; Paek, S.H.; Bae, S. External Magnetic Field Dependent Shift of Superparamagnetic Blocking Temperature Due to Core/Surface Disordered Spin Interactions. Nanotechnology 2017, 28, 075710. [Google Scholar] [CrossRef] [PubMed]
- Nayek, C.; Manna, K.; Bhattacharjee, G.; Murugavel, P.; Obaidat, I. Investigating Size- and Temperature-Dependent Coercivity and Saturation Magnetization in PEG Coated Fe3O4 Nanoparticles. Magnetochemistry 2017, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, I.M.; Nayek, C.; Manna, K.; Bhattacharjee, G.; Al-Omari, I.A.; Gismelseed, A. Investigating Exchange Bias and Coercivity in Fe3O4–γ-Fe2O3 Core–Shell Nanoparticles of Fixed Core Diameter and Variable Shell Thicknesses. Nanomaterials 2017, 7, 415. [Google Scholar] [CrossRef] [Green Version]
- Alaabed, S.; Obaidat, I.M.; Khaleel, A.; AbuShareb, A.; Abdi, I.; Farouk, M.; Narayanaswamy, V.; Al-Omari, I.A. Positive and Negative Exchange Bias in Maghemite Nanoparticles. Mater. Today Proc. 2020, 28, 611–614. [Google Scholar] [CrossRef]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E.J.; Foner, S. Surface Spin Disorder in Ferrite Nanoparticles (Invited). J. Appl. Phys. 1997, 81, 5552–5557. [Google Scholar] [CrossRef]
- Mørup, S.; Brok, E.; Frandsen, C. Spin Structures in Magnetic Nanoparticles. J. Nanomater. 2013, 2013, e720629. [Google Scholar] [CrossRef] [Green Version]
- Obaidat, I.; Mohite, V.; Issa, B.; Tit, N.; Haik, Y. Predicting a Major Role of Surface Spins in the Magnetic Properties of Ferrite Nanoparticles. Cryst. Res. Technol. 2009, 44, 489–494. [Google Scholar] [CrossRef]
- Kodama, R.H. Magnetic Nanoparticles. J. Magn. Magn. Mater. 1999, 200, 359–372. [Google Scholar] [CrossRef]
- Luis, F.; Torres, J.M.; García, L.M.; Bartolomé, J.; Stankiewicz, J.; Petroff, F.; Fettar, F.; Maurice, J.-L.; Vaurès, A. Enhancement of the Magnetic Anisotropy of Nanometer-Sized Co Clusters: Influence of the Surface and of Interparticle Interactions. Phys. Rev. B 2002, 65, 094409. [Google Scholar] [CrossRef] [Green Version]
- Bødker, F.; Mørup, S.; Linderoth, S. Surface Effects in Metallic Iron Nanoparticles. Phys. Rev. Lett. 1994, 72, 282–285. [Google Scholar] [CrossRef] [PubMed]



| Refinement Parameters | |
|---|---|
| Lattice Constant (a = b = c) (Å) | 8.3344 |
| α = β= γ (º) | 90.000 |
| Density (g/cm3) | 4.774 |
| V (Å3) | 578.917 |
| Bragg R-factor | 18.6 |
| Rf-factor | 28.0 |
| Chi2 | 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narayanaswamy, V.; Al-Omari, I.A.; Kamzin, A.S.; Khurshid, H.; Khaleel, A.; Issa, B.; Obaidat, I.M. Coercivity and Exchange Bias in Ti-Doped Maghemite Nanoparticles. Magnetochemistry 2022, 8, 165. https://doi.org/10.3390/magnetochemistry8120165
Narayanaswamy V, Al-Omari IA, Kamzin AS, Khurshid H, Khaleel A, Issa B, Obaidat IM. Coercivity and Exchange Bias in Ti-Doped Maghemite Nanoparticles. Magnetochemistry. 2022; 8(12):165. https://doi.org/10.3390/magnetochemistry8120165
Chicago/Turabian StyleNarayanaswamy, Venkatesha, Imaddin A. Al-Omari, Aleksandr S. Kamzin, Hafsa Khurshid, Abbas Khaleel, Bashar Issa, and Ihab M. Obaidat. 2022. "Coercivity and Exchange Bias in Ti-Doped Maghemite Nanoparticles" Magnetochemistry 8, no. 12: 165. https://doi.org/10.3390/magnetochemistry8120165
APA StyleNarayanaswamy, V., Al-Omari, I. A., Kamzin, A. S., Khurshid, H., Khaleel, A., Issa, B., & Obaidat, I. M. (2022). Coercivity and Exchange Bias in Ti-Doped Maghemite Nanoparticles. Magnetochemistry, 8(12), 165. https://doi.org/10.3390/magnetochemistry8120165






