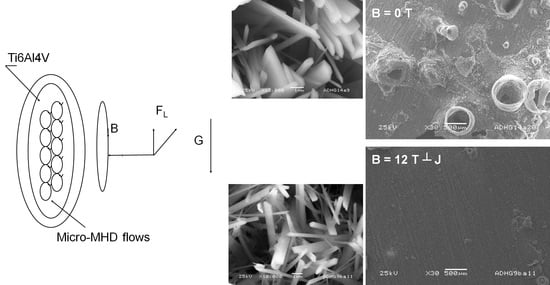
Influence of a Constant Perpendicular High Magnetic Field on the Electrodeposition of Calcium Phosphate Coating
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
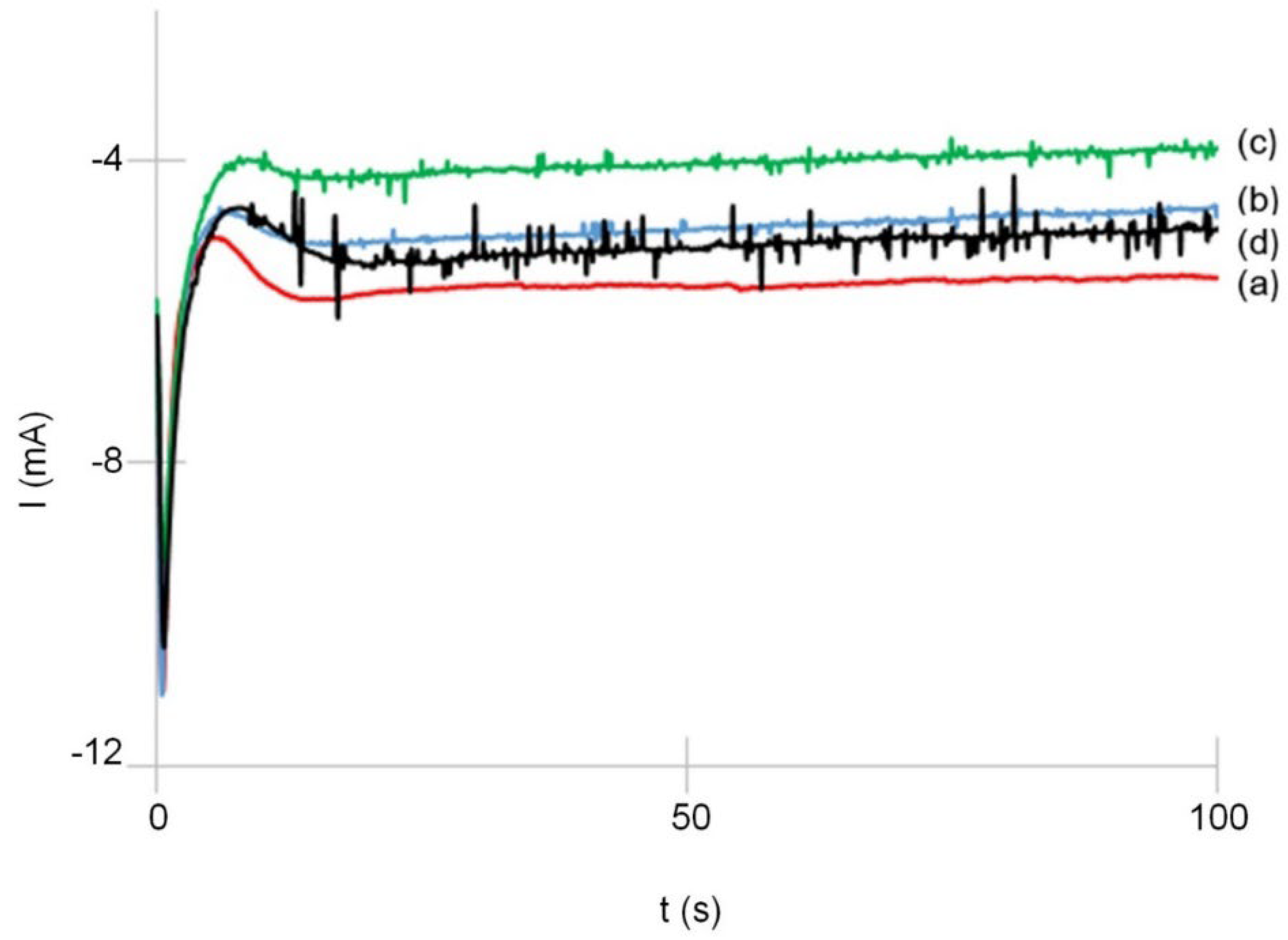
3.1. Analysis of Transient Curves
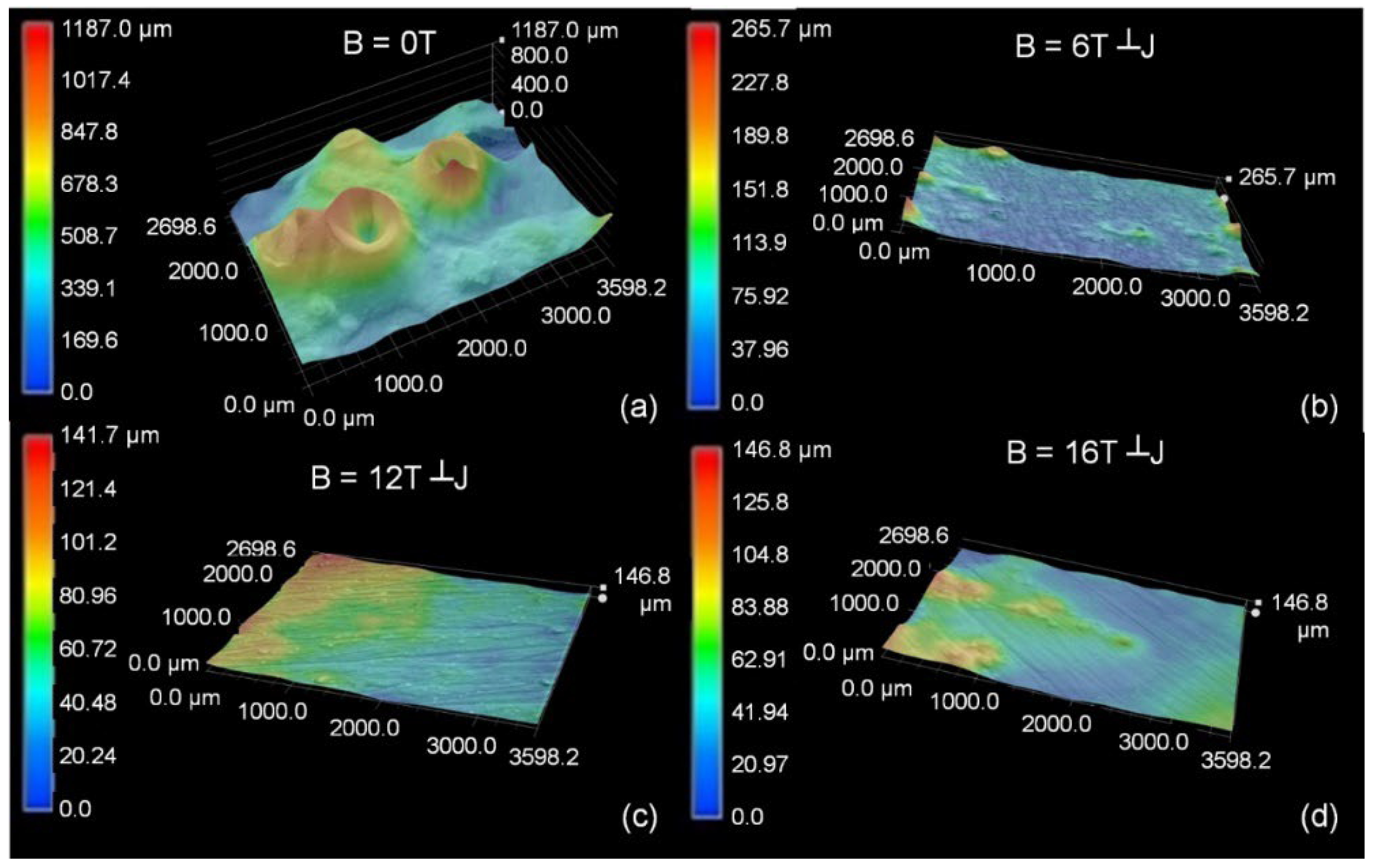
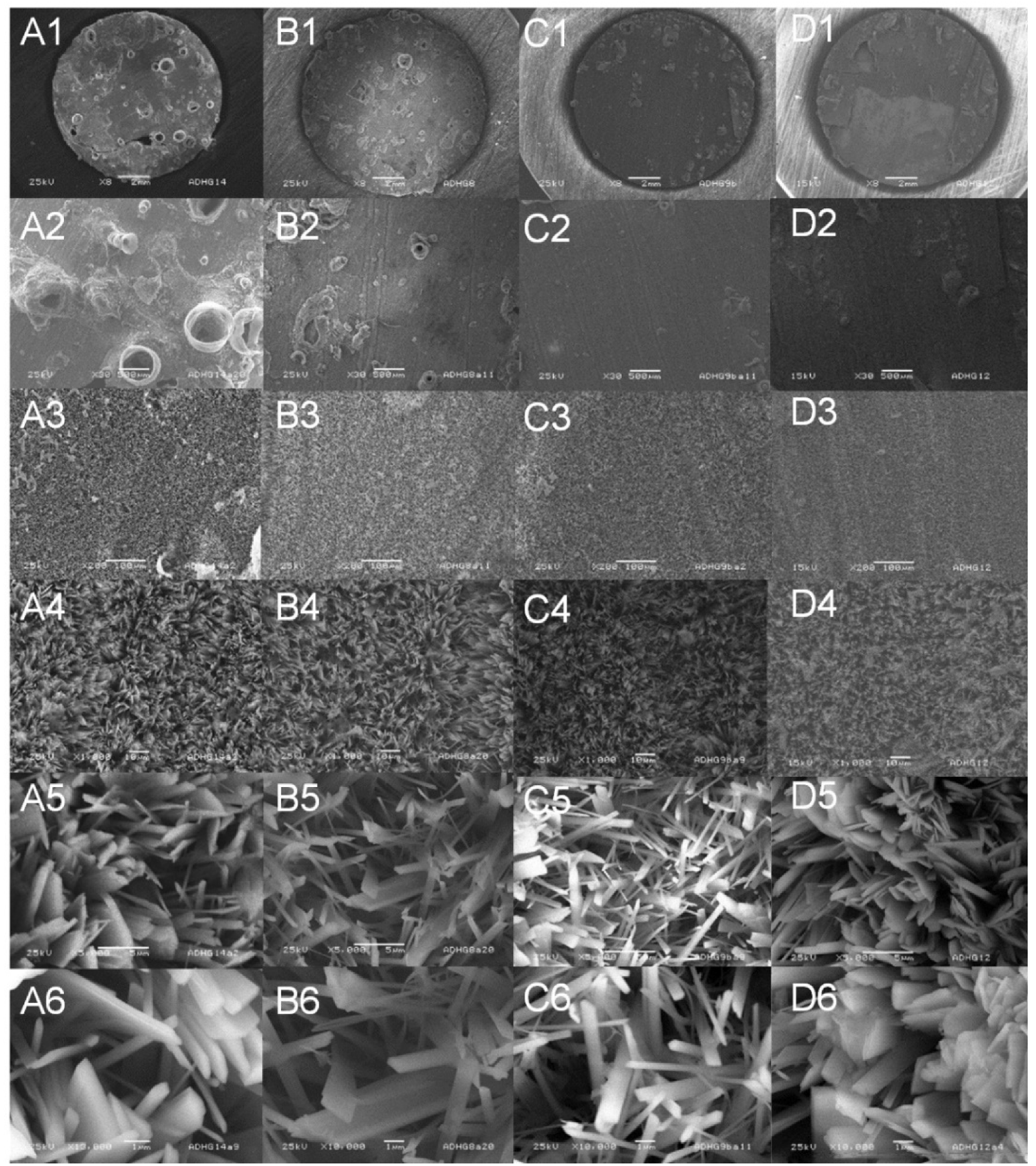
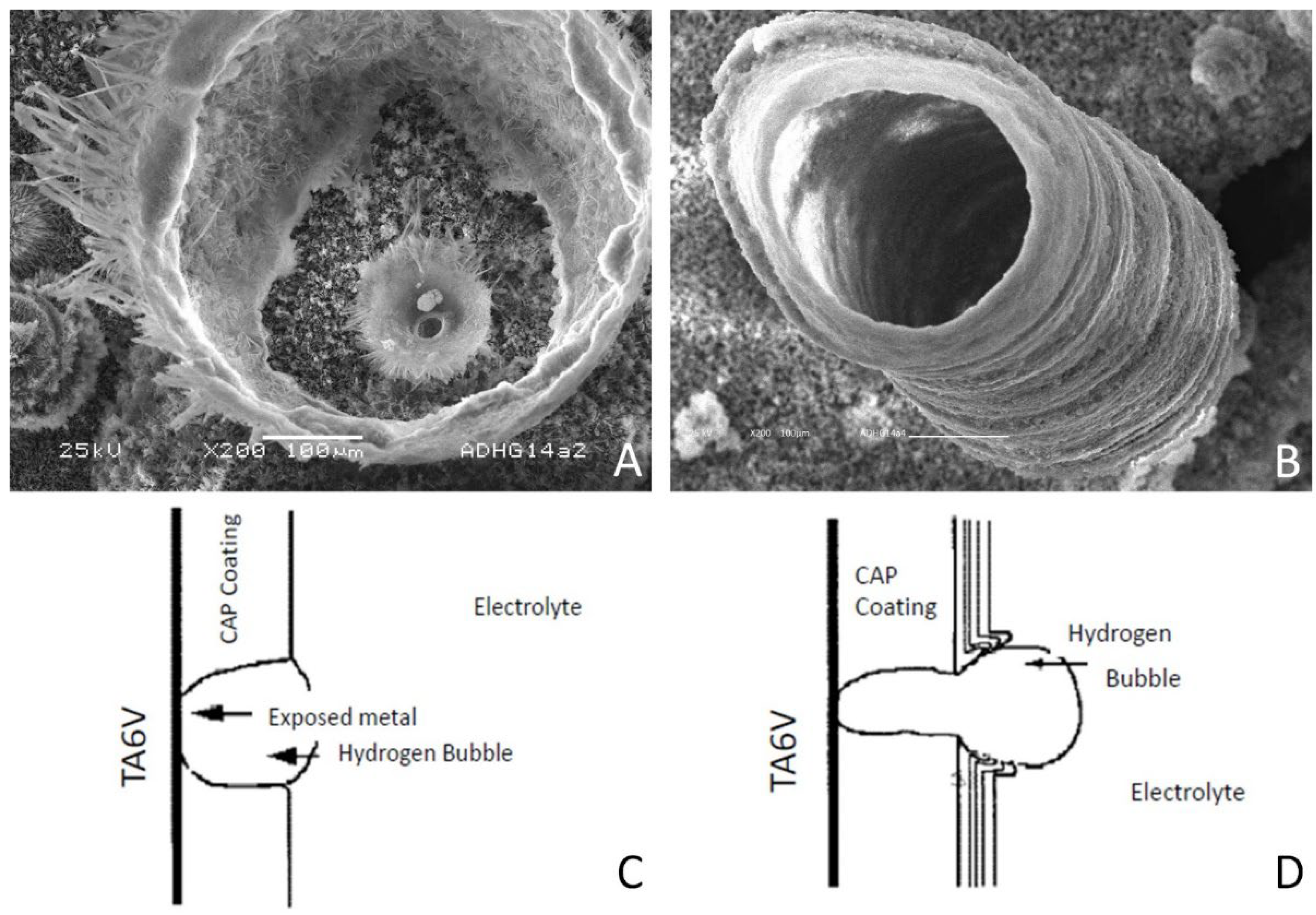
3.2. Surface Topography and Morphology
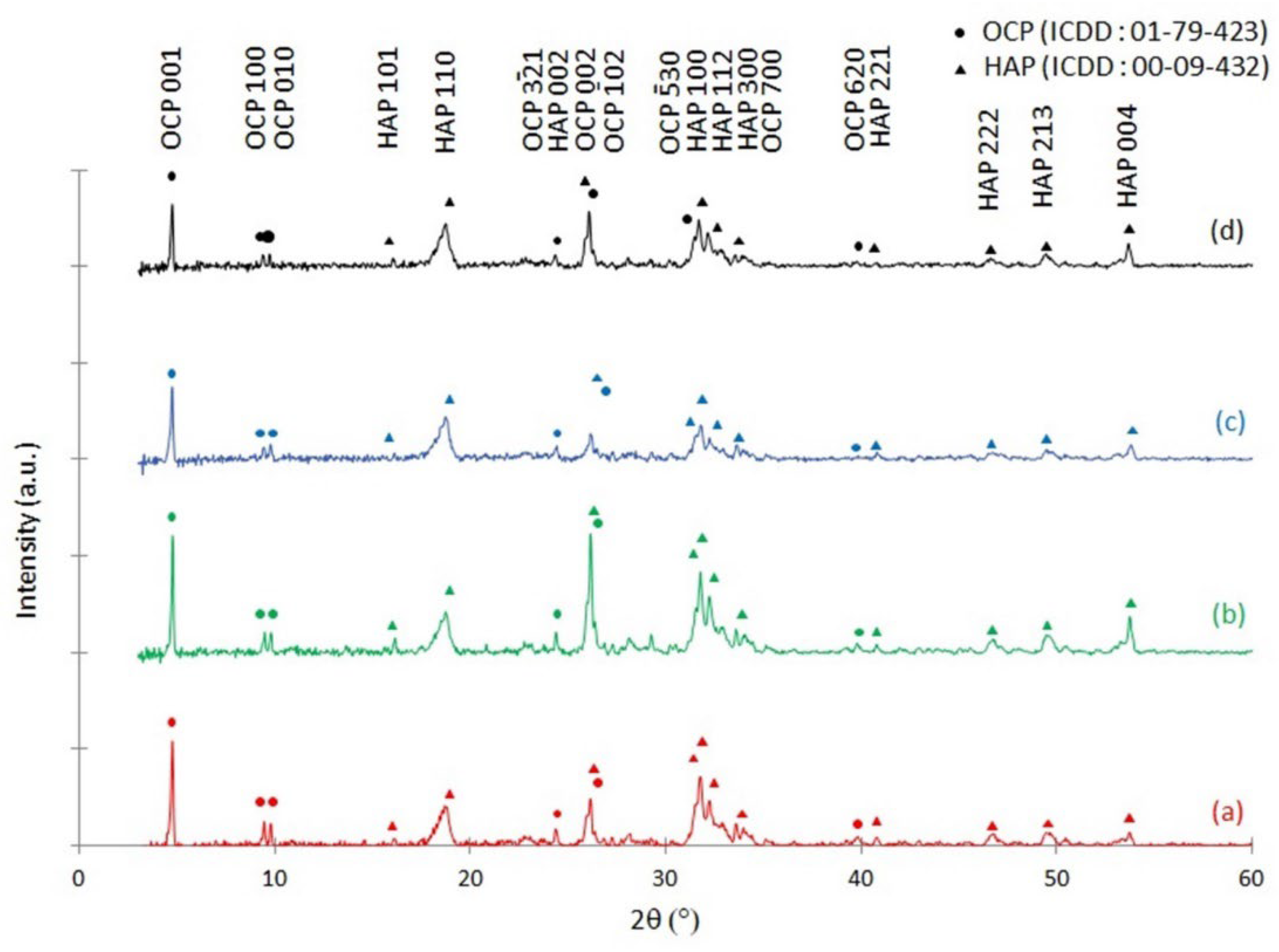
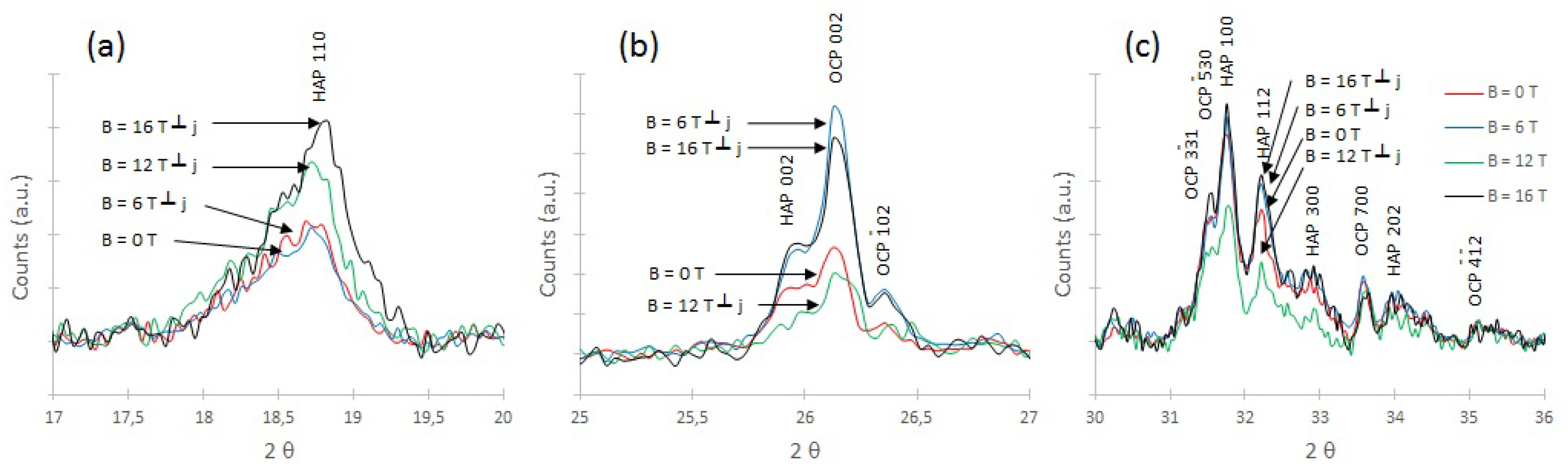
3.3. X-ray Diffraction
3.4. Chemical Composition
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorozhkin, S.V. Calcium Orthophosphates as Bioceramics: State of the Art. J. Funct. Biomater. 2010, 1, 22–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, O.; Kamakura, S.; Katagiri, T.; Nakamura, M.; Zhao, B.; Honda, Y.; Kamijo, R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca—Deficient hydroxyapatite. Biomaterials 2006, 27, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; Kwon, T.Y.; Kim, K.H. Direct nanocrystalline hydroxyapatite formation on titanium from ultrasonated electrochemical bath at physiological pH. Mater. Sci. Eng. C 2008, 28, 1265–1270. [Google Scholar] [CrossRef]
- Li, T.T.; Ling, L.; Lin, M.C.; Peng, H.K.; Ren, H.T.; Lou, C.W.; Lin, J.H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Uribe, R.; Uvillus, A.; Fernandez, L.; Bonilla, O.; Jara, A.; Gonzales, G. Electrochemical Deposition of Hydroxyapatite on Stainless Steel Coated with Tantalum/Tantalum Nitride Using Simulated Body Fluid as an Electrolytic Medium. Coatings 2022, 12, 440. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A.; Dietrich, D.; Steinhaeuser, S.; Wielage, B. Electrocrystallization of nanocrystallite calcium phosphate coatings on titanium substrate at different current densities. Surf. Coat. Technol. 2008, 202, 5895–5900. [Google Scholar] [CrossRef]
- Blackwood, D.J.; Seah, K.H.W. Electrochemical cathodic deposition of hydroxyapatite: Improvements in adhesion and crystallinity. Mater. Sci. Eng. C 2009, 29, 1233–1238. [Google Scholar] [CrossRef]
- Popa, M.V.; Moreno, J.M.C.; Popa, M.; Vasilescu, E.; Drob, P.; Vasilescu, C.; Drob, S.I.J.S.; Technology, C. Electrochemical deposition of bioactive coatings on Ti and Ti–6Al–4V surfaces. Surf. Coat. Technol. 2011, 205, 4776–4783. [Google Scholar] [CrossRef]
- Wang, S.H.; Shih, W.J.; Li, W.L.; Hon, M.H.; Wang, M.C. Morphology of calcium phosphate coatings deposited on a Ti–6Al–4V substrate by an electrolytic method under 80 Torr. J. Europ. Ceram. Soc. 2005, 25, 3287–3292. [Google Scholar] [CrossRef]
- Mokabber, T.; Lu, L.Q.; Van Rijn, P.; Vakis, A.I.; Pei, Y.T. Crystal growth mechanism of calcium phosphate coatings on titanium by electrochemical deposition. Surf. Coat. Technol. 2018, 334, 526–535. [Google Scholar] [CrossRef]
- Drevet, R.; Benhayoune, H.; Wortham, L.; Potiron, S.; Douglade, J.; Laurent-Maquin, D. Effects of pulsed current and H2O2 amount on the composition of electrodeposited calcium phosphate coatings. Mater. Charact. 2010, 61, 786–795. [Google Scholar] [CrossRef]
- Chopart, J.P.; Aaboubi, O.; Msellak, K. Electrochemical characterization of Ni-Fe alloy codeposition under MHD control. J. Solid State Electrochem. 2007, 11, 703–710. [Google Scholar] [CrossRef]
- Aogaki, R.; Fueki, K.; Mukaibo, T. Application of Magnetohydrodynamic Effect to the Analysis of Electrochemical Reactions -1. MHD Flow of an Electrolyte Solution in an Electrode-Cell with a Short Rectangular Channel. Denki Kagaku (Presently Electrochem.) 1975, 43, 504–508. [Google Scholar] [CrossRef]
- Aogaki, R.; Morimoto, R.; Asanuma, M. Nonequilibrium fluctuations in micro-MHD effects on electrodeposition. J. Magn. Magn. Mater. 2010, 322, 1664–1668. [Google Scholar] [CrossRef] [Green Version]
- Daltin, A.L.; Baudart, P.; Debray, F.P.; Chopart, J.P. Morphology change of electrodeposited calcium phosphate coating on Ti6Al4V substrate under high magnetic field. J. Iron Steel Res. Int. 2012, 19, 1162–1165. [Google Scholar]
- Tohidi, P.M.; Safavi, M.S.; Etminanfar, M.; Khalil-Allafi, J. Pulsed electrodeposition of compact, corrosion resistant, and bioactive HAp coatings by application of optimized magnetic field. Mater. Chem. Phys. 2020, 254, 123511. [Google Scholar] [CrossRef]
- Liu, C.; Tian, A.; Yang, H.; Xu, Q.; Xue, X. Electrodeposited hydroxyapatite coatings on the TiO2 nanotube in static magnetic field. Appl. Surf. Sci. 2013, 287, 218–222. [Google Scholar] [CrossRef]
- Zhao, X.N.; Li, H.J.; Li, K.Z.; Lu, J.H.; Zhang, L.L.; Cao, S. Electrodeposition of nanostructured calcium phosphate coating under magnetic field. Mater. Technol. 2012, 27, 371–374. [Google Scholar] [CrossRef]
- Tian, A.; Xue, X.; Liu, C.; He, J.; Yang, Z. Electrodeposited hydroxyapatite coatings in staticmagnetic field. Mater. Lett. 2010, 64, 1197–1199. [Google Scholar] [CrossRef]
- Daltin, A.L.; Ahmed Chekkat, A.; Millet, P.; Chopart, J.P. Electrocrystallization kinetics of hydroxyapatite and calcium phosphate coatings under magnetic field. Magnetohydrodynamics 2012, 48, 261–270. [Google Scholar]
- Seyedraoufi, Z.; Mirdamadi, S. Effects of pulse electrodeposition parameters and alkali treatment on the properties of nanohydroxyapatite coating on porous Mg–Zn scaffold for bone tissue engineering application. Mater. Chem. Phys. 2014, 148, 519–527. [Google Scholar] [CrossRef]
- Daltin, A.L.; Chopart, J.P. Morphology of magneto-electrodeposited Cu2O microcrystals. CrystEngComm 2011, 13, 3373–3377. [Google Scholar] [CrossRef]
- Habibovic, P.; van der Valk, C.M.; van Blitterswijk, C.A.; De Groot, K.; Meijer, G. Influence of octacalcium phosphate coating on osteoinductive properties of biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 373–380. [Google Scholar] [CrossRef]
- Li, D.; Gao, Y.; Wang, Q.; Li, G.; Wu, C.; Daltin, A.L.; Chopart, J.P. Tailoring the morphology, structure and magnetic properties of electrodeposited CoFe films onto Si(100) by in-situ uniform and gradient high magnetic fields. J. Electrochem. Soc. 2016, 163, D836–D841. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daltin, A.-L.; Chopart, J.-P. Influence of a Constant Perpendicular High Magnetic Field on the Electrodeposition of Calcium Phosphate Coating. Magnetochemistry 2022, 8, 62. https://doi.org/10.3390/magnetochemistry8060062
Daltin A-L, Chopart J-P. Influence of a Constant Perpendicular High Magnetic Field on the Electrodeposition of Calcium Phosphate Coating. Magnetochemistry. 2022; 8(6):62. https://doi.org/10.3390/magnetochemistry8060062
Chicago/Turabian StyleDaltin, Anne-Lise, and Jean-Paul Chopart. 2022. "Influence of a Constant Perpendicular High Magnetic Field on the Electrodeposition of Calcium Phosphate Coating" Magnetochemistry 8, no. 6: 62. https://doi.org/10.3390/magnetochemistry8060062
APA StyleDaltin, A.-L., & Chopart, J.-P. (2022). Influence of a Constant Perpendicular High Magnetic Field on the Electrodeposition of Calcium Phosphate Coating. Magnetochemistry, 8(6), 62. https://doi.org/10.3390/magnetochemistry8060062