Fluorescent Single-Core and Multi-Core Nanoprobes as Cell Trackers and Magnetic Nanoheaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
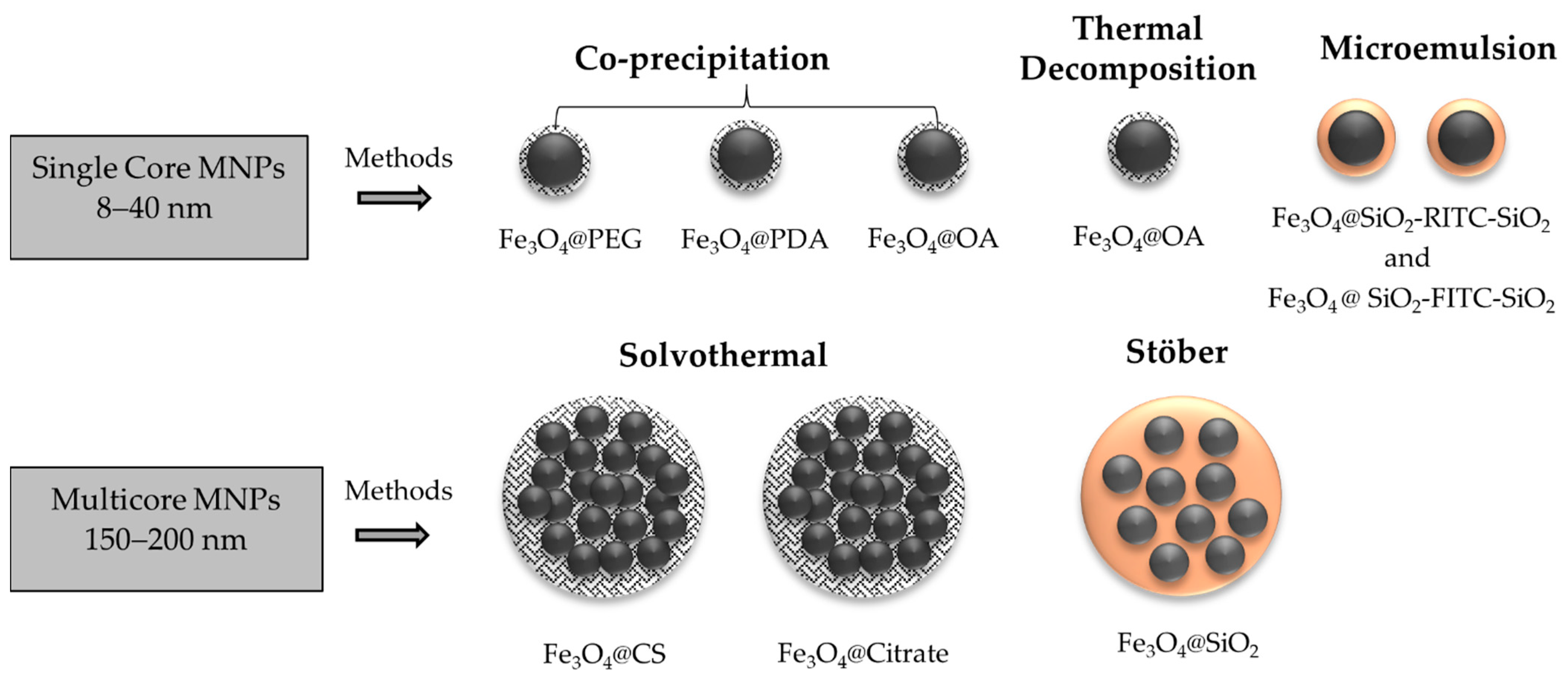
2.2. Synthesis of MNPs
2.2.1. MNPs Prepared by Co-Precipitation Method
2.2.2. MNPs Prepared by Thermal Decomposition Method
2.2.3. MNPs Prepared by Microemulsion Method
2.2.4. MNPs Prepared by Solvothermal Method
2.2.5. MNPs Prepared by Stöber Method
2.3. Physicochemical Characterization
2.3.1. XRD Structural Characterization
2.3.2. Microscopy Morphological Characterization
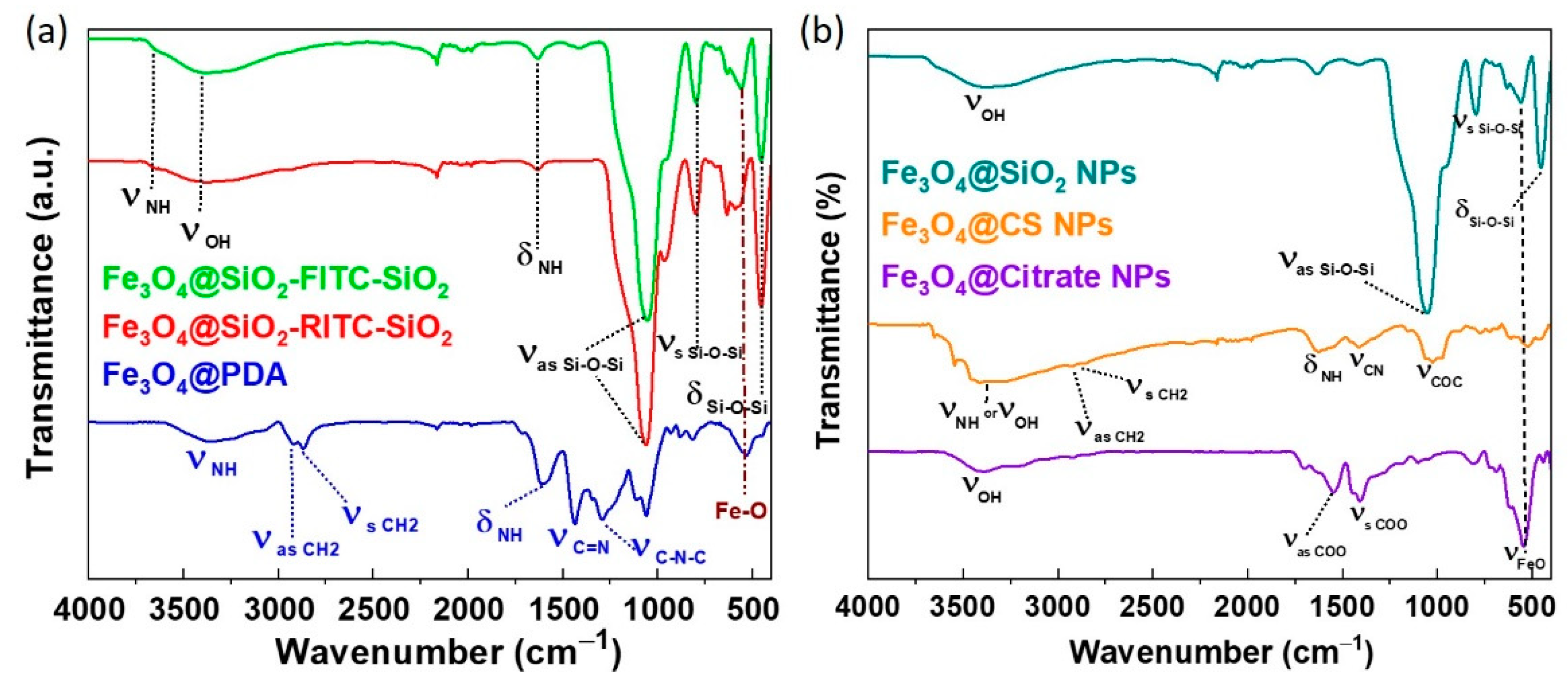
2.3.3. Surface Chemistry Characterization
2.3.4. Hydrodynamic Particle Size and Zeta Potential Measurements
2.3.5. Compositional Characterization
2.3.6. Optical Properties
2.3.7. DC Magnetic Characterization
2.3.8. AC Magnetic Characterization
2.3.9. Magnetic Hyperthermia Characterization
3. Results and Discussion
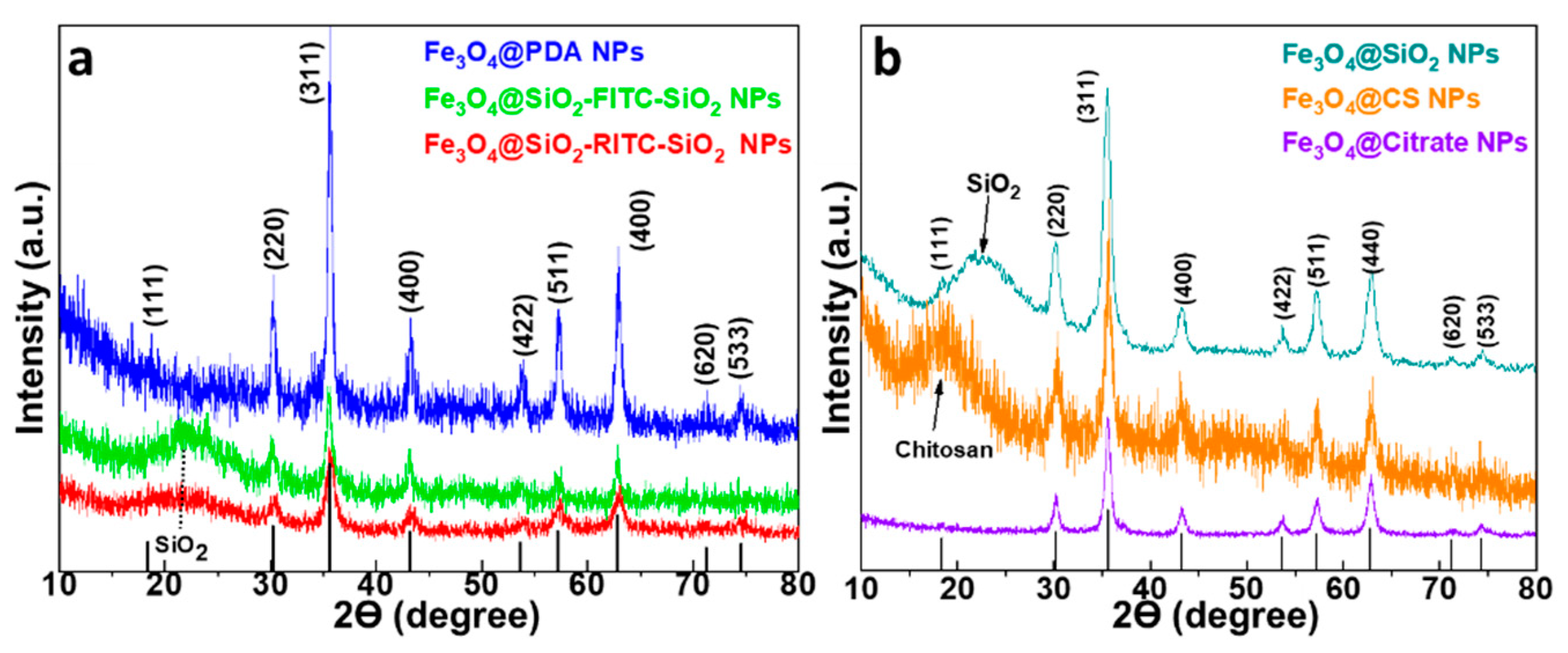
3.1. X-ray Diffraction (XRD)
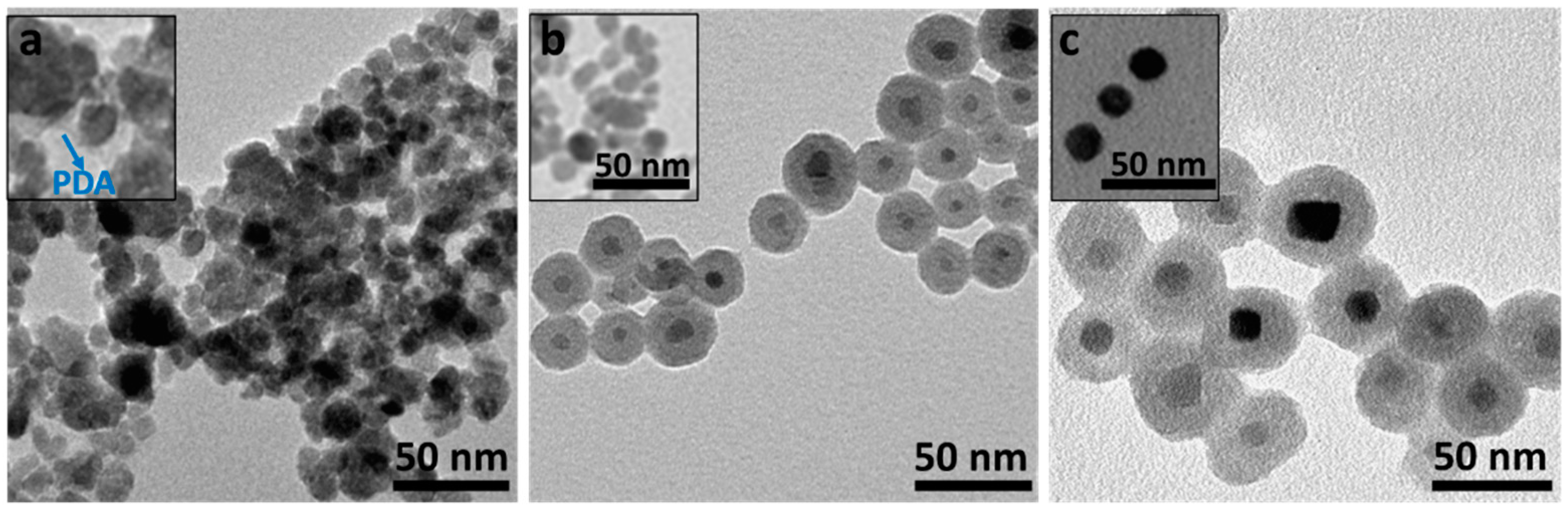
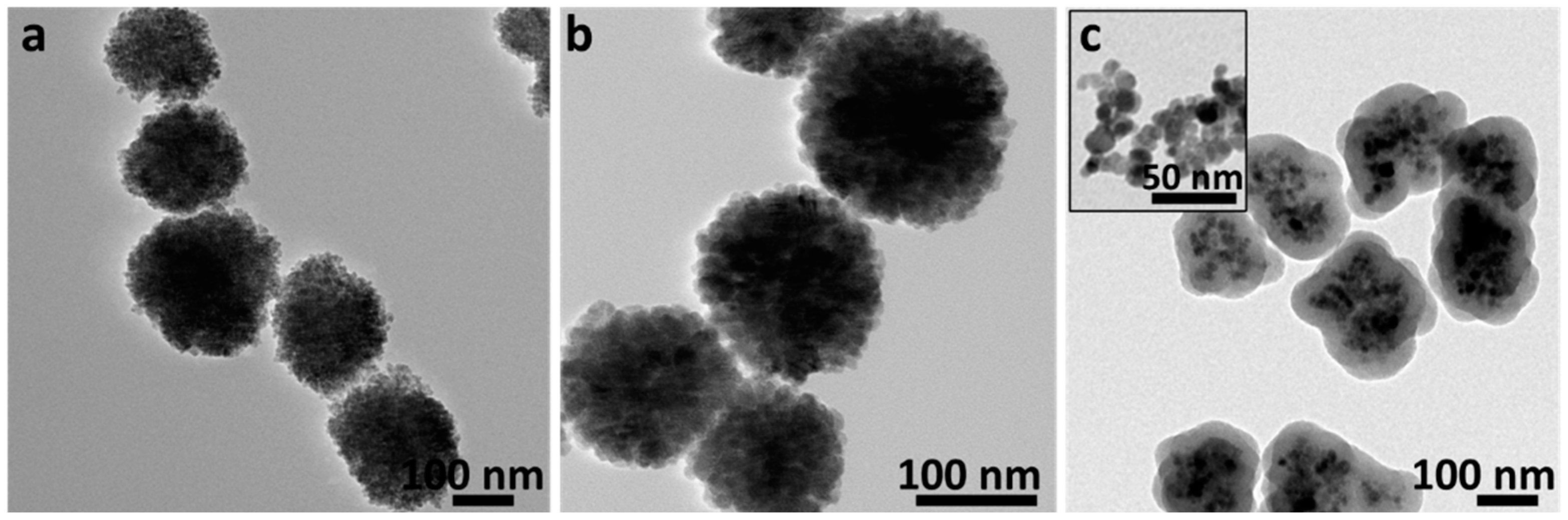
3.2. Transmission Electron Microscopy (TEM)
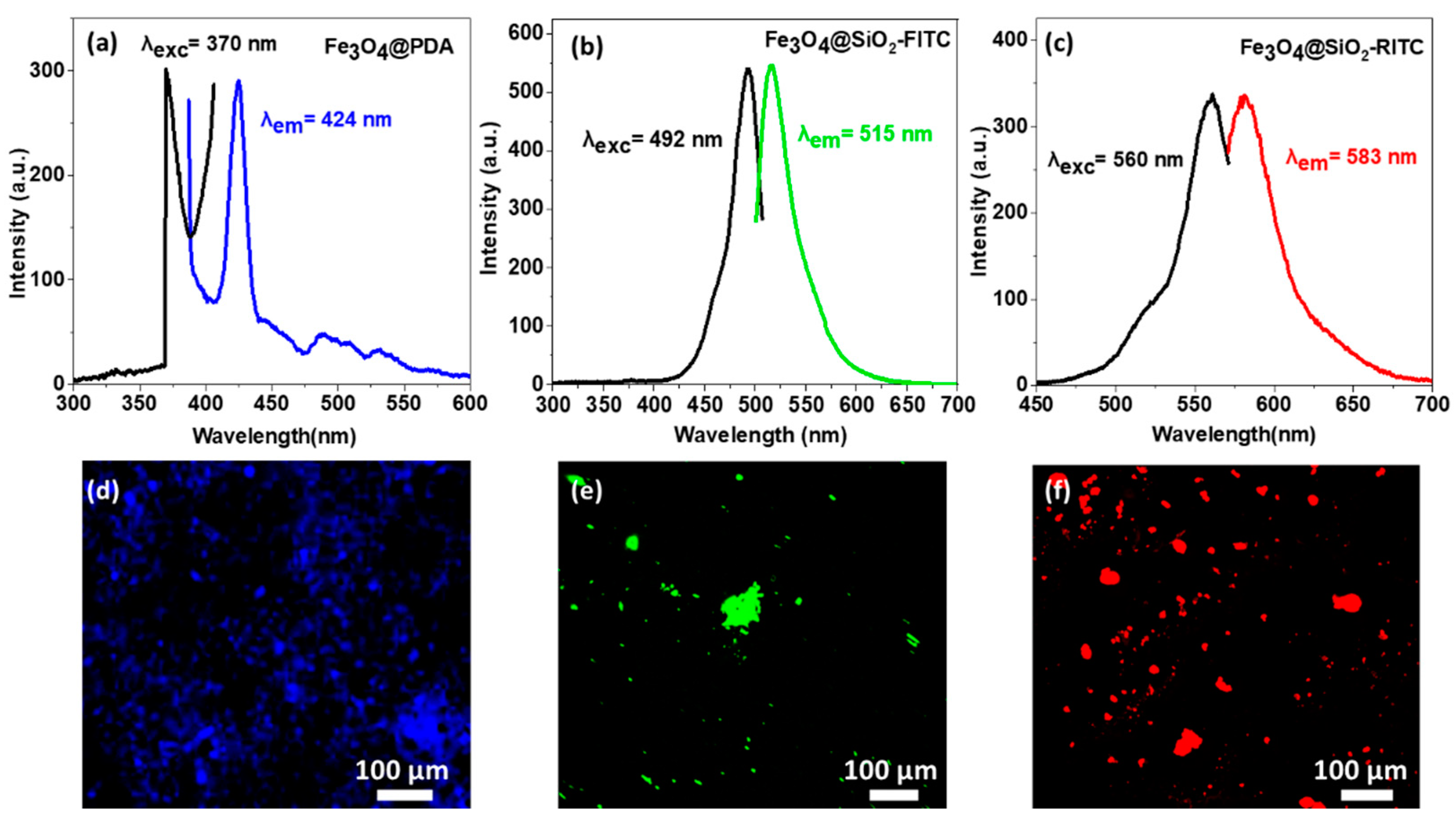
3.3. Optical Characterization of Fluorescent Single-Core MNPs
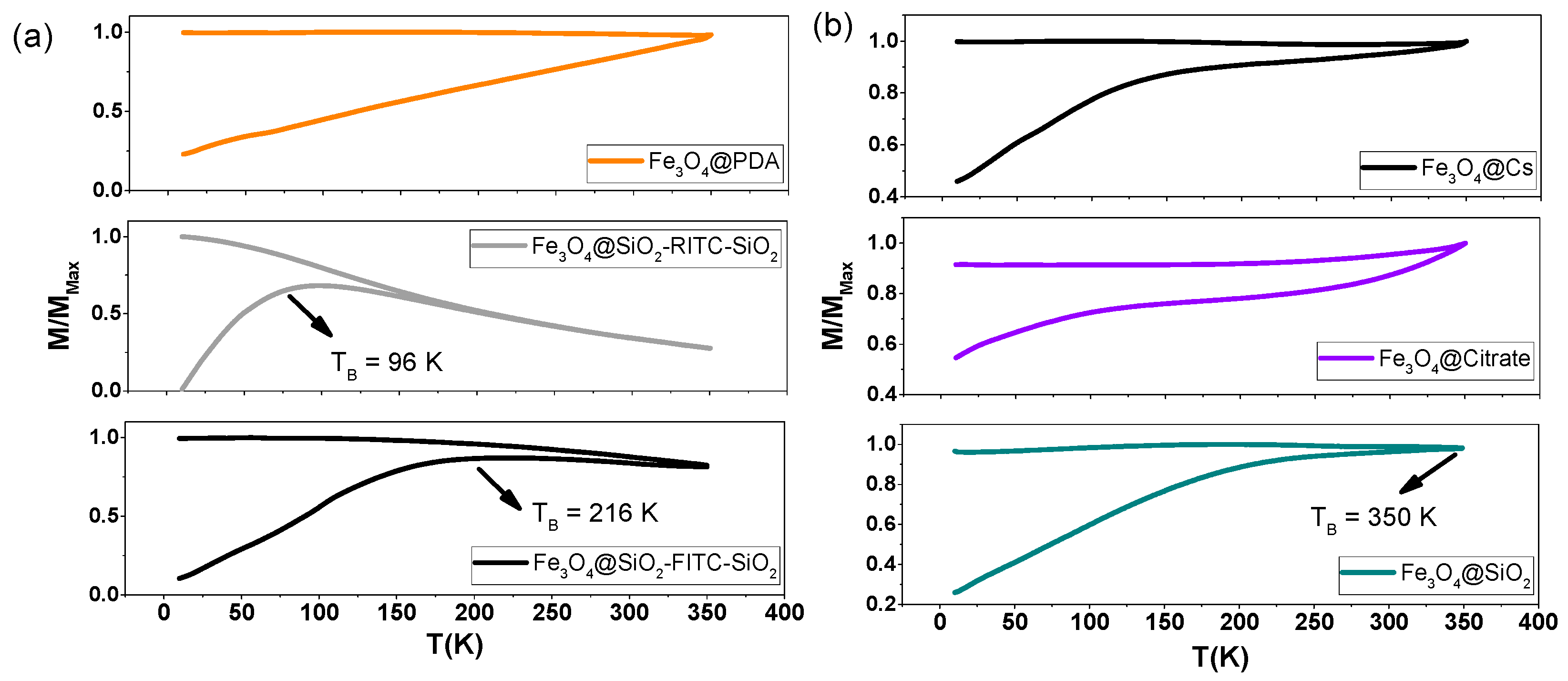
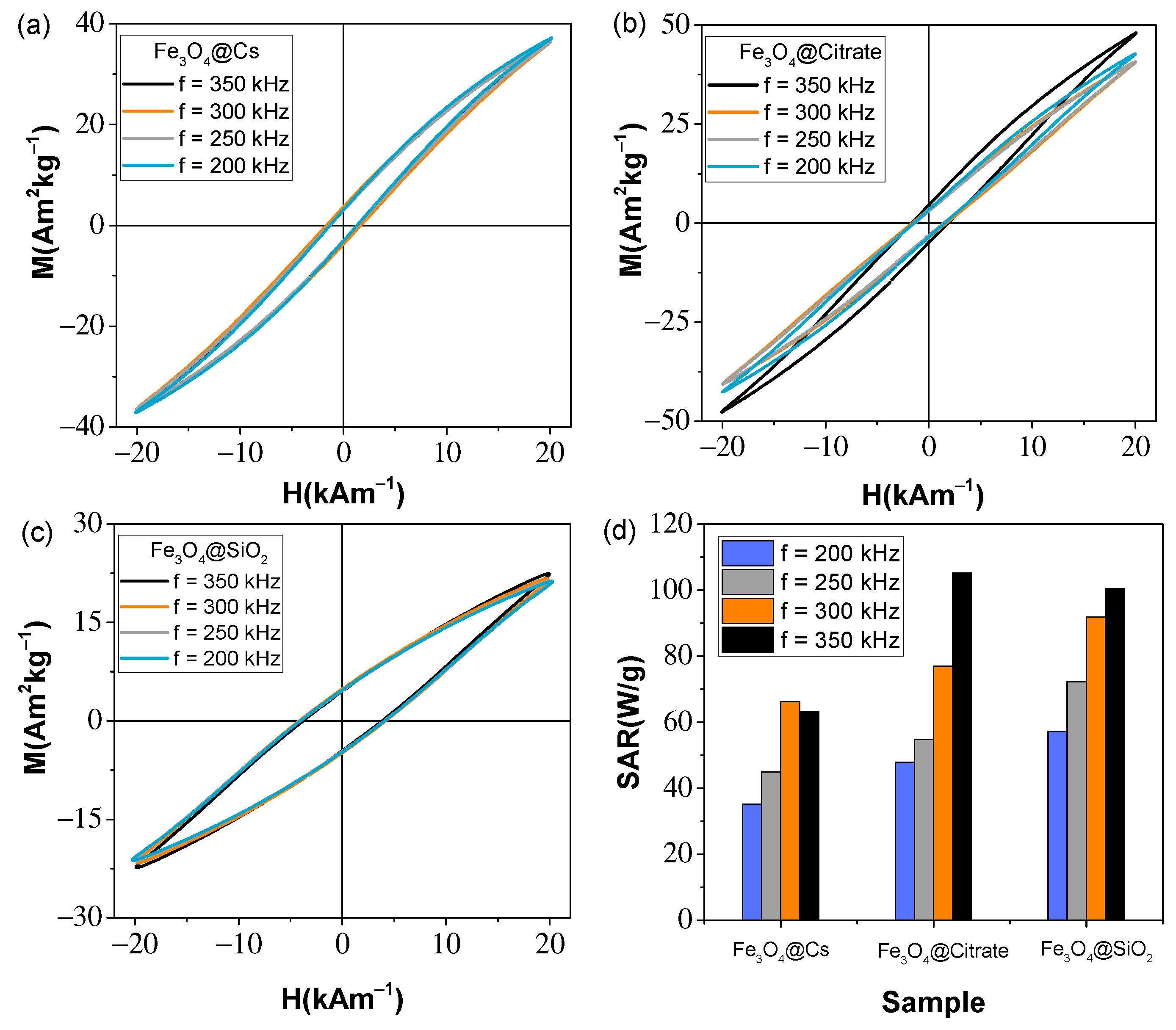
3.4. Magnetic Characterization
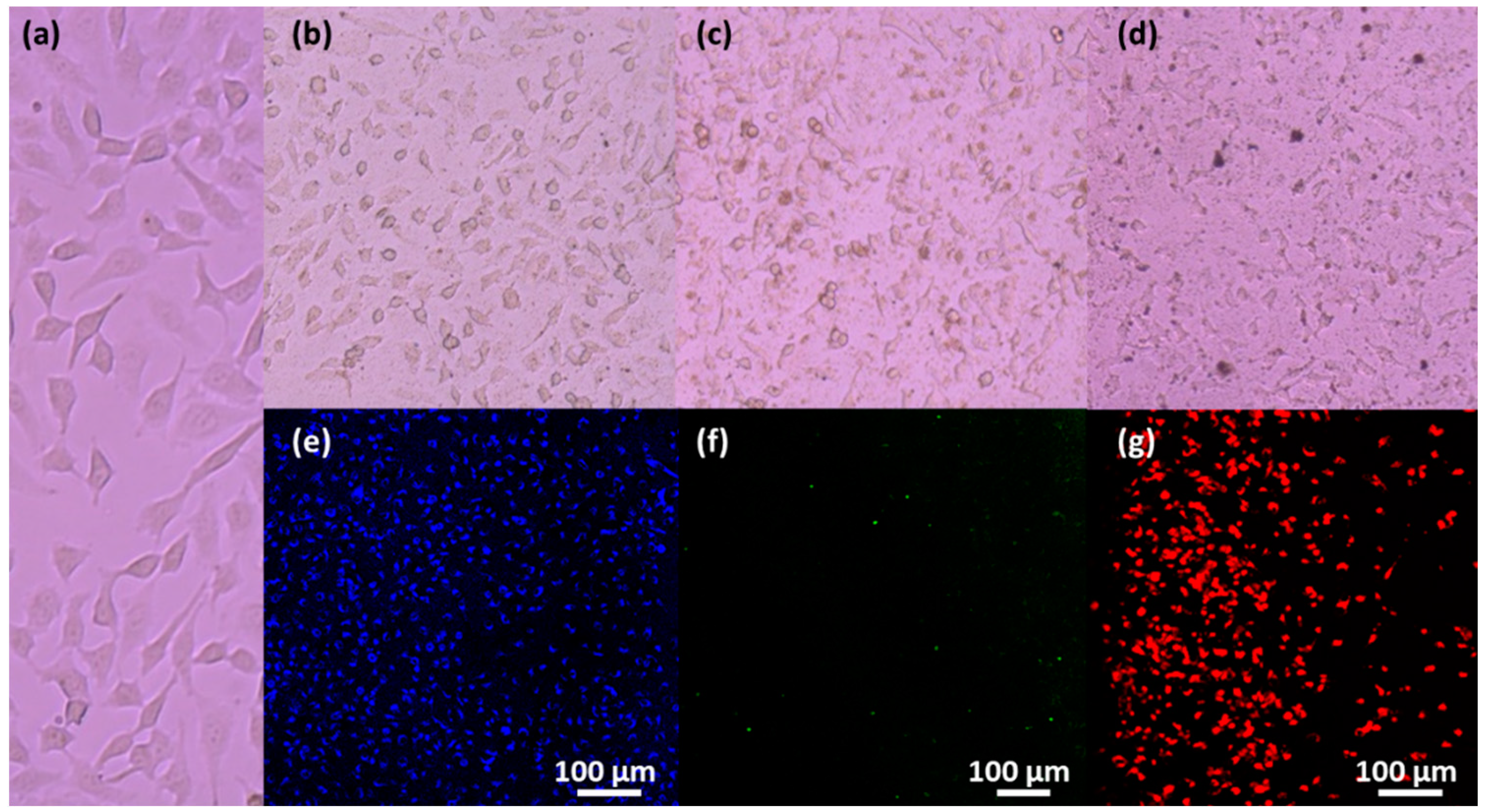
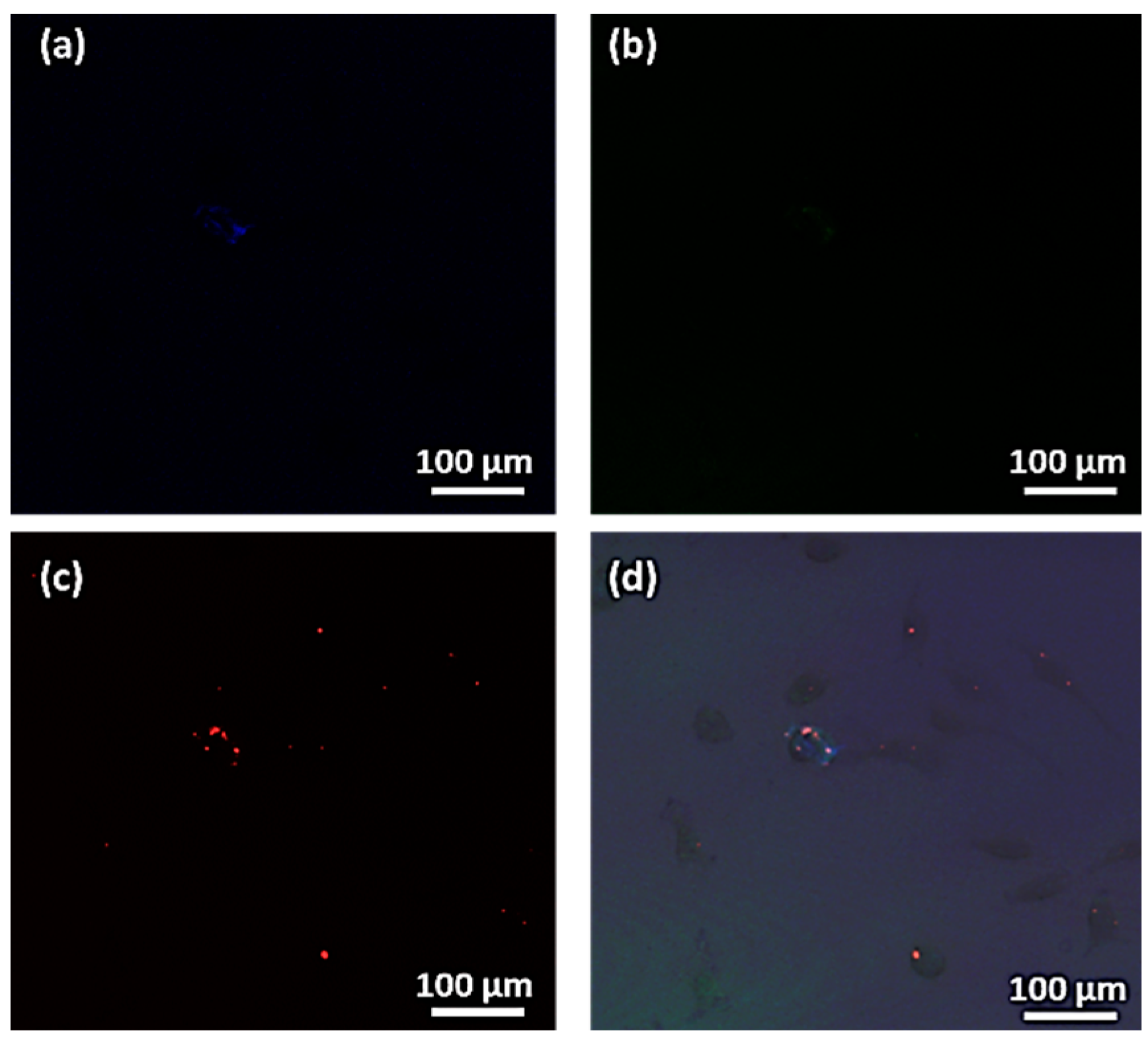
3.5. Magnetic Uptake and Cell Isolation with Fluorescence Single-Core MNPs
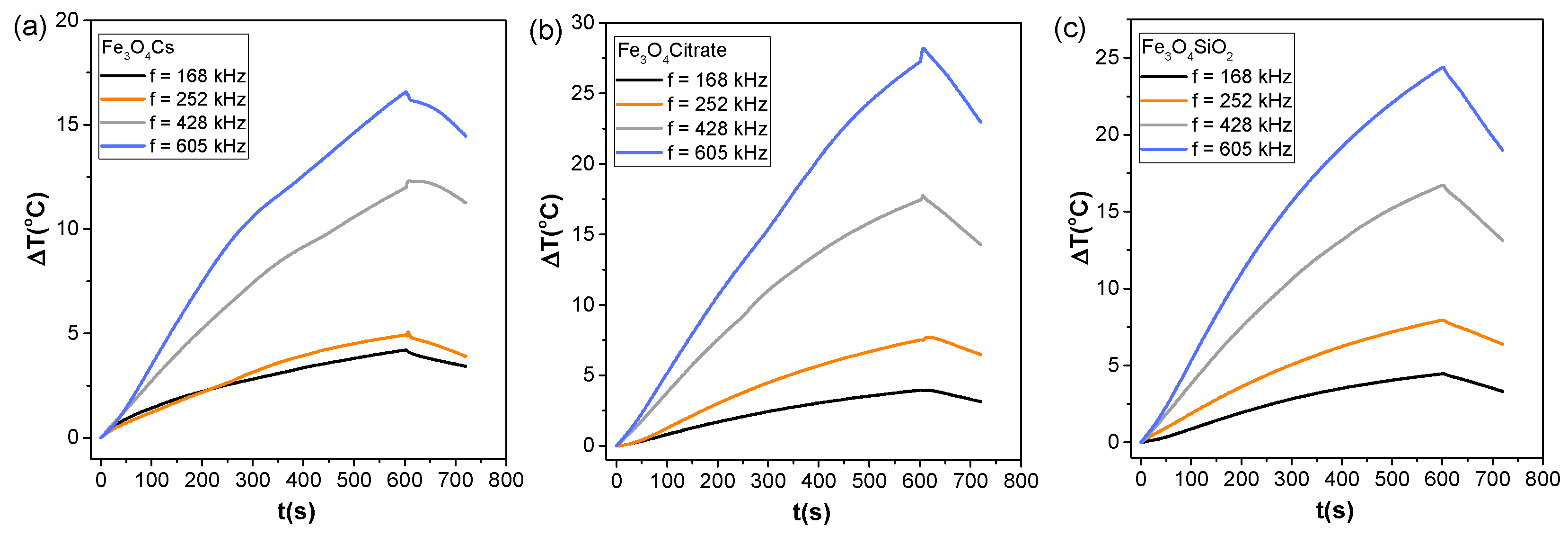
3.6. Heat Capacity of Multi-Core MNPs for Magnetic Hyperthermia and Nanowarming
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Arias, L.S.; Pessan, J.P.; Vieira, A.P.M.; de Lima, T.M.T.; Delbem, A.C.B.; Monteiro, D.R. Iron Oxide Nanoparticles for Biomedical Applications: A Perspective on Synthesis, Drugs, Antimicrobial Activity, and Toxicity. Antibiotics 2018, 7, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yáñez-Vilar, S.; Sánchez-Andújar, M.; Gómez-Aguirre, C.; Mira, J.; Señarís-Rodríguez, M.A.; Castro-García, S. A Simple Solvothermal Synthesis of MFe2O4 (M = Mn, Co and Ni) Nanoparticles. J. Solid State Chem. 2009, 182, 2685–2690. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, J.; Wang, Z.; Zhou, Z. Solvothermal Synthesis of Size-Controlled Monodispersed Superparamagnetic Iron Oxide Nanoparticles. Appl. Sci. 2019, 9, 5157. [Google Scholar] [CrossRef] [Green Version]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (Ionp) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef]
- de Montferrand, C.; Hu, L.; Milosevic, I.; Russier, V.; Bonnin, D.; Motte, L.; Brioude, A.; Lalatonne, Y. Iron Oxide Nanoparticles with Sizes, Shapes and Compositions Resulting in Different Magnetization Signatures as Potential Labels for Multiparametric Detection. Acta Biomater. 2013, 9, 6150–6157. [Google Scholar] [CrossRef]
- Song, C.; Sun, W.; Xiao, Y.; Shi, X. Ultrasmall Iron Oxide Nanoparticles: Synthesis, Surface Modification, Assembly, and Biomedical Applications. Drug Discov. Today 2019, 24, 835–844. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Tang, R.; Qiu, H.; Wang, C.; Zhou, Z. Solvothermal Synthesis and Characterization of Monodisperse Superparamagnetic Iron Oxide Nanoparticles. J. Magn. Magn. Mater. 2015, 379, 226–231. [Google Scholar] [CrossRef]
- Gutiérrez, L.; Costo, R.; Grüttner, C.; Westphal, F.; Gehrke, N.; Heinke, D.; Fornara, A.; Pankhurst, Q.A.; Johansson, C.; Veintemillas-Verdaguer, S.; et al. Synthesis Methods to Prepare Single- and Multi-Core Iron Oxide Nanoparticles for Biomedical Applications. Dalton Trans. 2015, 44, 2943–2952. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, Surface Functionalization and Application of Fe3O4 Magnetic Nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Bean, C.P.; Livingston, J.D. Superparamagnetism. J. Appl. Phys. 1959, 30, S120–S129. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological Applications of Magnetic Nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic Iron Oxide Nanoparticles for Imaging, Targeting and Treatment of Primary and Metastatic Tumors of the Brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Zelepukin, I.V.; Yaremenko, A.V.; Ivanov, I.N.; Yuryev, M.V.; Cherkasov, V.R.; Deyev, S.M.; Nikitin, P.I.; Nikitin, M.P. Long-Term Fate of Magnetic Particles in Mice: A Comprehensive Study. ACS Nano 2021, 15, 11341–11357. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Saha, R.; Wong, D.; Wang, J.P. Magnetic Particle Spectroscopy-Based Bioassays: Methods, Applications, Advances, and Future Opportunities. J. Phys. D Appl. Phys. 2019, 52, 173001. [Google Scholar] [CrossRef]
- Ramalho, J.; Ramalho, M.; Jay, M.; Burke, L.M.; Semelka, R.C. Gadolinium Toxicity and Treatment. Magn. Reson. Imaging 2016, 34, 1394–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gradinaru, L.M.; Barbalata Mandru, M.; Drobota, M.; Aflori, M.; Butnaru, M.; Spiridon, M.; Doroftei, F.; Aradoaei, M.; Ciobanu, R.C.; Vlad, S. Composite Materials Based on Iron Oxide Nanoparticles and Polyurethane for Improving the Quality of Mri. Polymers 2021, 13, 4316. [Google Scholar] [CrossRef]
- Kader, A.; Kaufmann, J.O.; Mangarova, D.B.; Moeckel, J.; Brangsch, J.; Adams, L.C.; Zhao, J.; Reimann, C.; Saatz, J.; Traub, H.; et al. Iron Oxide Nanoparticles for Visualization of Prostate Cancer in MRI. Cancers 2022, 14, 2909. [Google Scholar] [CrossRef]
- Wu, M.; Li, X.; Guo, Q.; Li, J.; Xu, G.; Li, G.; Wang, J.; Zhang, X. Magnetic Mesoporous Silica Nanoparticles-Aided Dual MR/NIRF Imaging to Identify Macrophage Enrichment in Atherosclerotic Plaques. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102330. [Google Scholar] [CrossRef]
- Ledda, M.; Fioretti, D.; Lolli, M.G.; Papi, M.; di Gioia, C.; Carletti, R.; Ciasca, G.; Foglia, S.; Palmieri, V.; Marchese, R.; et al. Biocompatibility Assessment of Sub-5 Nm Silica-Coated Superparamagnetic Iron Oxide Nanoparticles in Human Stem Cells and in Mice for Potential Application in Nanomedicine. Nanoscale 2020, 12, 1759–1778. [Google Scholar] [CrossRef]
- García, R.S.; Stafford, S.; Gun’ko, Y.K. Recent Progress in Synthesis and Functionalization of Multimodal Fluorescent-Magnetic Nanoparticles for Biological Applications. Appl. Sci. 2018, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Molaei, M.J.; Salimi, E. Magneto-Fluorescent Superparamagnetic Fe3O4@SiO2@alginate/Carbon Quantum Dots Nanohybrid for Drug Delivery. Mater. Chem. Phys. 2022, 288, 126361. [Google Scholar] [CrossRef]
- Li, X.; Lin, H.; Li, Q.; Xue, J.; Xu, Y.; Zhuang, L. Recyclable Magnetic Fluorescent Fe3O4@SiO2Core-Shell Nanoparticles Decorated with Carbon Dots for Fluoride Ion Removal. ACS Appl. Nano Mater. 2021, 4, 3062–3074. [Google Scholar] [CrossRef]
- Siciliano, G.; Monteduro, A.G.; Turco, A.; Primiceri, E.; Rizzato, S.; Depalo, N.; Curri, M.L.; Maruccio, G. Polydopamine-Coated Magnetic Iron Oxide Nanoparticles: From Design to Applications. Nanomaterials 2022, 12, 1145. [Google Scholar] [CrossRef]
- Lin, L.S.; Cong, Z.X.; Cao, J.B.; Ke, K.M.; Peng, Q.L.; Gao, J.; Yang, H.H.; Liu, G.; Chen, X. Multifunctional Fe3O4@polydopamine Core-Shell Nanocomposites for Intracellular MRNA Detection and Imaging-Guided Photothermal Therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Sallem, F.; Mirjolet, C.; Nury, T.; Sahoo, S.K.; Millot, N.; Kumar, R. Polydopamine Modified Superparamagnetic Iron Oxide Nanoparticles as Multifunctional Nanocarrier for Targeted Prostate Cancer Treatment. Nanomaterials 2019, 9, 138. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Millot, N.; Maurizi, L.; Lizard, G.; Kumar, R. Taurine-Conjugated Mussel-Inspired Iron Oxide Nanoparticles with an Elongated Shape for Effective Delivery of Doxorubicin into the Tumor Cells. ACS Omega 2020, 5, 16165–16175. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, J.; Li, J.; Fan, Y.; Wang, J.; Sun, W.; Yang, H.; Peng, C.; Shen, M.; Shi, X. Polydopamine-Coated Magnetic Mesoporous Silica Nanoparticles for Multimodal Cancer Theranostics. J. Mater. Chem. B 2019, 7, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Morato, Y.L.; Paredes, K.O.; Chamizo, L.L.; Marciello, M.; Filice, M. Recent Advances in Multimodal Molecular Imaging of Cancer Mediated by Hybrid Magnetic Nanoparticles. Polymers 2021, 13, 2989. [Google Scholar] [CrossRef]
- Chekina, N.; Horák, D.; Jendelová, P.; Trchová, M.; Bene, M.J.; Hrubý, M.; Herynek, V.; Turnovcová, K.; Syková, E. Fluorescent Magnetic Nanoparticles for Biomedical Applications. J. Mater. Chem. 2011, 21, 7630–7639. [Google Scholar] [CrossRef]
- Lartigue, L.; Coupeau, M.; Lesault, M. Luminophore and Magnetic Multicore Nanoassemblies for Dual-Mode MRI and Fluorescence Imaging. Nanomaterials 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piñeiro, Y.; Vargas, Z.; Rivas, J.; Lõpez-Quintela, M.A. Iron Oxide Based Nanoparticles for Magnetic Hyperthermia Strategies in Biological Applications. Eur. J. Inorg. Chem. 2015, 2015, 4495–4509. [Google Scholar] [CrossRef]
- Chiu-Lam, A.; Staples, E.; Pepine, C.J.; Rinaldi, C. Perfusion, Cryopreservation, and Nanowarming of Whole Hearts Using Colloidally Stable Magnetic Cryopreservation Agent Solutions. Sci. Adv. 2021, 7, eabe3005. [Google Scholar]
- Gao, Z.; Ring, H.L.; Sharma, A.; Namsrai, B.; Tran, N.; Finger, E.B.; Garwood, M.; Haynes, C.L.; Bischof, J.C. Preparation of Scalable Silica-Coated Iron Oxide Nanoparticles for Nanowarming. Adv. Sci. 2020, 7, 1901624. [Google Scholar] [CrossRef] [Green Version]
- Ovejero, J.G.; Spizzo, F.; Morales, M.P.; del Bianco, L. Nanoparticles for Magnetic Heating: When Two (or More) Is Better than One. Materials 2021, 14, 6416. [Google Scholar] [CrossRef]
- Qiu, P.; Jensen, C.; Charity, N.; Towner, R.; Mao, C. Oil Phase Evaporation-Induced Self-Assembly of Hydrophobic Nanoparticles into Spherical Clusters with Controlled Surface Chemistry in an Oil-in-Water Dispersion and Comparison of Behaviors of Individual and Clustered Iron Oxide Nanoparticles. J. Am. Chem. Soc. 2010, 132, 17724–17732. [Google Scholar] [CrossRef] [Green Version]
- Chen, O.; Riedemann, L.; Etoc, F.; Herrmann, H.; Coppey, M.; Barch, M.; Farrar, C.T.; Zhao, J.; Bruns, O.T.; Wei, H.; et al. Magneto-Fluorescent Core-Shell Supernanoparticles. Nat. Commun. 2014, 5, 5093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, S.D.; Choi, C.; Khamwannah, J.; Jin, S. Magnetically Vectored Delivery of Cancer Drug Using Remotely On-off Switchable Nano Capsules. IEEE Trans. Magn. 2013, 49, 349–352. [Google Scholar] [CrossRef]
- Elsaidy, A.; Vallejo, J.P.; Salgueiriño, V.; Lugo, L. Tuning the Thermal Properties of Aqueous Nanofluids by Taking Advantage of Size-Customized Clusters of Iron Oxide Nanoparticles. J. Mol. Liq. 2021, 344, 117727. [Google Scholar] [CrossRef]
- Ranoo, S.; Lahiri, B.B.; Damodaran, S.P.; Philip, J. Tuning Magnetic Heating Efficiency of Colloidal Dispersions of Iron Oxide Nano-Clusters by Varying the Surfactant Concentration during Solvothermal Synthesis. J. Mol. Liq. 2022, 360, 119444. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Yan, Y.; Roberts, C.; Liu, Z.; Chen, Y. The Role of Dipole Interactions in Hyperthermia Heating Colloidal Clusters of Densely-Packed Superparamagnetic Nanoparticles. Sci. Rep. 2018, 8, 4704. [Google Scholar] [CrossRef] [PubMed]
- Skumiel, A.; Kertmen, A.; Nowaczyk, G. Investigation of the Magnetic Hyperthermia Effect in an Aqueous Dispersion of Colloidosomal Nanoparticle Clusters. J. Mol. Liq. 2019, 283, 91–95. [Google Scholar] [CrossRef]
- Dutz, S.; Clement, J.H.; Eberbeck, D.; Gelbrich, T.; Hergt, R.; Müller, R.; Wotschadlo, J.; Zeisberger, M. Ferrofluids of Magnetic Multicore Nanoparticles for Biomedical Applications. J. Magn. Magn. Mater. 2009, 321, 1501–1504. [Google Scholar] [CrossRef]
- Casula, M.F.; Conca, E.; Bakaimi, I.; Sathya, A.; Materia, M.E.; Casu, A.; Falqui, A.; Sogne, E.; Pellegrino, T.; Kanaras, A.G. Manganese doped-iron oxide nanoparticle clusters and their potential as agents for magnetic resonance imaging and hyperthermia. Phys. Chem. Chem. Phys. 2016, 18, 16848. [Google Scholar] [CrossRef]
- Vamvakidis, K.; Mourdikoudis, S.; Makridis, A.; Paulidou, E.; Angelakeris, M.; Dendrinou-Samara, C. Magnetic Hyperthermia Efficiency and MRI Contrast Sensitivity of Colloidal Soft/Hard Ferrite Nanoclusters. J. Colloid Interface Sci. 2018, 511, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Choo, E.S.G.; Ahmed, A.S.; Zhao, L.Y.; Yang, Y.; Ramanujan, R.V.; Xue, J.M.; di Fan, D.; Fan, H.M.; Ding, J. Magnetic Nanoparticle-Loaded Polymer Nanospheres as Magnetic Hyperthermia Agents. J. Mater. Chem. B 2014, 2, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Piñeiro, Y.; Gómez, M.G.; Alves, L.d.C.; Prieto, A.A.; Acevedo, P.G.; Gudiña, R.S.; Puig, J.; Teijeiro, C.; Vilar, S.Y.; Rivas, J. Hybrid Nanostructured Magnetite Nanoparticles: From Bio-Detection and Theragnostics to Regenerative Medicine. Magnetochemistry 2020, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Massart, R. Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, X.; Wu, D.; Chen, Q.; Huang, D.; Sun, C.; Xin, J.; Ni, K.; Gao, J. Anisotropic Shaped Iron Oxide Nanostructures: Controlled Synthesis and Proton Relaxation Shortening Effects. Chem. Mater. 2015, 27, 3505–3515. [Google Scholar] [CrossRef]
- Fang, Y.; Loc, W.S.; Lu, W.; Fang, J. Synthesis of In2O3@SiO2 Core-Shell Nanoparticles with Enhanced Deeper Energy Level Emissions of In 2O3. Langmuir 2011, 27, 14091–14095. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, J.; Lee, S.S.; Ying, J.Y. Reverse Microemulsion-Mediated Synthesis of Silica-Coated Gold and Silver Nanoparticles. Langmuir 2008, 24, 5842–5848. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jia, W.; Lin, C.; Fan, G.; Jin, Y.; Chen, X.; Chen, G. Facile Synthesis of Folate-Conjugated Magnetic/Fluorescent Bifunctional Microspheres. Nanoscale Res. Lett. 2014, 9, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Wang, F.; Cai, W.; Zhang, X. Trisodium Citrate-Assisted Synthesis of Highly Water-Dispersible and Superparamagnetic Mesoporous Fe3O4 Hollow Microspheres via Solvothermal Process. J. Alloys Compd. 2015, 636, 34–39. [Google Scholar] [CrossRef]
- Alavi Nikje, M.M.; Farahmand Nejad, M.A.; Shabani, K.; Haghshenas, M. Preparation of Magnetic Polyurethane Rigid Foam Nanocomposites. Colloid Polym. Sci. 2013, 291, 903–909. [Google Scholar] [CrossRef]
- Saleh, T.A. Structural Characterization of Hybrid Materials. In Polymer Hybrid Materials and Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 213–240. [Google Scholar]
- Coker, V.S.; Bell, A.M.T.; Pearce, C.I.; Patrick, R.A.D.; van der Laan, G.; Lloyd, J.R. Time-Resolved Synchrotron Powder X-Ray Diffraction Study of Magnetite Formation by the Fe(III)-Reducing Bacterium Geobacter Sulfurreducens. Am. Mineral. 2008, 93, 540–547. [Google Scholar] [CrossRef]
- Chitra, K.; Annadurai, G. Fluorescent Silica Nanoparticles in the Detection and Control of the Growth of Pathogen. J. Nanotechnol. 2013, 2013, 509628. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Dutta, P.K.; Koh, J. A Physico-Chemical and Biological Study of Novel Chitosan-Chloroquinoline Derivative for Biomedical Applications. Int. J. Biol. Macromol. 2011, 49, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Church, J.S.; Woodhead, A.L. Infrared and Raman Spectroscopic Studies on Iron Oxide Magnetic Nano-Particles and Their Surface Modifications. J. Magn. Magn. Mater. 2012, 324, 1543–1550. [Google Scholar] [CrossRef]
- Rajan, A.; Sharma, M.; Sahu, N.K. Assessing Magnetic and Inductive Thermal Properties of Various Surfactants Functionalised Fe3O4 Nanoparticles for Hyperthermia. Sci. Rep. 2020, 10, 15045. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Fan, F.; Zhang, Y.; Pei, Z.; Wang, W.; Pei, Y. A Facile Approach for Fabrication of Core-Shell Magnetic Molecularly Imprinted Nanospheres towards Hypericin. Polymers 2017, 9, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haddad, P.S.; Duarte, E.L.; Baptista, M.S.; Goya, G.F.; Leite, C.A.P.; Itri, R. Synthesis and Characterization of Silica-Coated Magnetic Nanoparticles. In Surface and Colloid Science; Progress in Colloid and Polymer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 128, pp. 232–238. [Google Scholar]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Mazario, E.; Sánchez-Marcos, J.; Menéndez, N.; Herrasti, P.; García-Hernández, M.; Muñoz-Bonilla, A. One-Pot Electrochemical Synthesis of Polydopamine Coated Magnetite Nanoparticles. RSC Adv. 2014, 4, 48353–48361. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Wang, C.; Hu, J.; Fu, S. Chitosan Nanoparticles as a Novel Delivery System for Ammonium Glycyrrhizinate. Int. J. Pharm. 2005, 295, 235–245. [Google Scholar] [CrossRef]
- Sikora, A.; Shard, A.G.; Minelli, C. Size and ζ-Potential Measurement of Silica Nanoparticles in Serum Using Tunable Resistive Pulse Sensing. Langmuir 2016, 32, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Franco-Ulloa, S.; Tatulli, G.; Bore, S.L.; Moglianetti, M.; Pompa, P.P.; Cascella, M.; de Vivo, M. Dispersion State Phase Diagram of Citrate-Coated Metallic Nanoparticles in Saline Solutions. Nat. Commun. 2020, 11, 5422. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Luo, S.; Xu, D.; Liu, S.; Wu, N.; Yao, W.; Zhang, X.; Zheng, L.; Lin, X. Silica-Polydopamine Hybrids as Light-Induced Oxidase Mimics for Colorimetric Detection of Pyrophosphate. Analyst 2020, 145, 424–433. [Google Scholar] [CrossRef]
- Wu, S.; Li, Z.; Han, J.; Han, S. Dual Colored Mesoporous Silica Nanoparticles with PH Activable Rhodamine-Lactam for Ratiometric Sensing of Lysosomal Acidity. Chem. Commun. 2011, 47, 11276–11278. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Osorio, Z.; da Silva-Candal, A.; Piñeiro, Y.; Iglesias-Rey, R.; Sobrino, T.; Campos, F.; Castillo, J.; Rivas, J. Multifunctional Superparamagnetic Stiff Nanoreservoirs for Blood Brain Barrier Applications. Nanomaterials 2019, 9, 449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucheryavy, P.; He, J.; John, V.T.; Maharjan, P.; Spinu, L.; Goloverda, G.Z.; Kolesnichenko, V.L. Superparamagnetic Iron Oxide Nanoparticles with Variable Size and an Iron Oxidation State as Prospective Imaging Agents. Langmuir 2013, 29, 710–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Docampo, M.A.; Testa-Anta, M.; Rivas-Murias, B.; Salgueiriño, V. Clusters of Magnetite-Maghemite Nanocrystals with a Chemically-Tailored Average Diameter. J. Nanosci. Nanotechnol. 2019, 19, 4930–4937. [Google Scholar] [CrossRef] [PubMed]
- Smolensky, E.D.; Park, H.Y.E.; Zhou, Y.; Rolla, G.A.; Marjańska, M.; Botta, M.; Pierre, V.C. Scaling Laws at the Nanosize: The Effect of Particle Size and Shape on the Magnetism and Relaxivity of Iron Oxide Nanoparticle Contrast Agents. J. Mater. Chem. B 2013, 1, 2818–2828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas Rojas, P.C.; Tancredi, P.; Moscoso Londoño, O.; Knobel, M.; Socolovsky, L.M. Tuning Dipolar Magnetic Interactions by Controlling Individual Silica Coating of Iron Oxide Nanoparticles. J. Magn. Magn. Mater. 2018, 451, 688–696. [Google Scholar] [CrossRef]
- Bi, Q.; Song, X.; Hu, A.; Luo, T.; Jin, R.; Ai, H.; Nie, Y. Magnetofection: Magic Magnetic Nanoparticles for Efficient Gene Delivery. Chin. Chem. Lett. 2020, 31, 3041–3046. [Google Scholar] [CrossRef]
- Ni, J.S.; Li, Y.; Yue, W.; Liu, B.; Li, K. Nanoparticle-Based Cell Trackers for Biomedical Applications. Theranostics 2020, 10, 1923–1947. [Google Scholar] [CrossRef] [PubMed]
- Sajib, M.; Albers, E.; Langeland, M.; Undeland, I. Understanding the Effect of Temperature and Time on Protein Degree of Hydrolysis and Lipid Oxidation during Ensilaging of Herring (Clupea harengus) Filleting Co-Products. Sci. Rep. 2020, 10, 9590. [Google Scholar] [CrossRef]
- Graf, C.; Gao, Q.; Schütz, I.; Noufele, C.N.; Ruan, W.; Posselt, U.; Korotianskiy, E.; Nordmeyer, D.; Rancan, F.; Hadam, S.; et al. Surface Functionalization of Silica Nanoparticles Supports Colloidal Stability in Physiological Media and Facilitates Internalization in Cells. Langmuir 2012, 28, 7598–7613. [Google Scholar] [CrossRef]
- Cabrera, D.; Coene, A.; Leliaert, J.; Artés-Ibáñez, E.J.; Dupré, L.; Telling, N.D.; Teran, F.J. Dynamical Magnetic Response of Iron Oxide Nanoparticles Inside Live Cells. ACS Nano 2018, 12, 2741–2752. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, J.G.; Cabrera, D.; Carrey, J.; Valdivielso, T.; Salas, G.; Teran, F.J. Effects of Inter- and Intra-Aggregate Magnetic Dipolar Interactions on the Magnetic Heating Efficiency of Iron Oxide Nanoparticles. Phys. Chem. Chem. Phys. 2016, 18, 10954–10963. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Reeves, D.B.; Ferguson, R.M.; Weaver, J.B.; Krishnan, K.M. Mixed Brownian Alignment and Néel Rotations in Superparamagnetic Iron Oxide Nanoparticle Suspensions Driven by an Ac Field. Phys. Rev. B Condens. Matter Mater. Phys. 2015, 92, 094438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutz, S.; Hergt, R. Magnetic Nanoparticle Heating and Heat Transfer on a Microscale: Basic Principles, Realities and Physical Limitations of Hyperthermia for Tumour Therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 Nanoparticles and Their Magnetic Properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Peng, H.; Wen, Y.; Li, N. Re-Examination of Characteristic FTIR Spectrum of Secondary Layer in Bilayer Oleic Acid-Coated Fe3O4 Nanoparticles. Appl. Surf. Sci. 2010, 256, 3093–3097. [Google Scholar] [CrossRef]
- Gopal, S.V.; Chitrambalam, S.; Joe, I.H. Coherent Source Interaction, Third-Order Nonlinear Response of Synthesized PEG Coated Magnetite Nanoparticles in Polyethylene Glycol and Its Application. Opt. Laser Technol. 2018, 98, 84–91. [Google Scholar] [CrossRef]

| Core | Coating | Layer Content (wt %) | DH (nm) | DXRD (nm) | DTEM (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|---|---|---|
| Single-Core | PDA | 41.70 | 163.00 ± 131.50 | 12.27 | 14.90 | 0.445 | (+) 28.70 |
| SiO2-RITC | 75.10 | 44.14 ± 19.73 | 9.28 | 22.50 | 0.195 | (−) 38.00 | |
| SiO2-FITC | 82.60 | 142.20 ± 50.47 | 8.59 | 40.09 | 0.265 | (−) 45.50 | |
| Multi-Core | CS | 34.10 | 201.20 ± 52.56 | 13.04 | 178.44 | 0.259 | (+) 12.00 |
| Citrate | 7.10 | 339.80 ± 218.00 | 13.25 | 145.96 | 0.233 | (−) 50.50 | |
| SiO2 | 78.1 | 215.50 ± 61.91 | 9.82 | 197.27 | 0.146 | (−) 45.93 |
| Coating | MS (emug−1) | MR (emug−1) | HC (Oe) | TB (K) | |
|---|---|---|---|---|---|
| Single-Core | PDA | 65.38 | 5.74 | 51.7 | >350 |
| SiO2-RITC | 71.44 | 0.65 | 3.53 | 96 | |
| SiO2-FITC | 49.89 | 3.35 | 13.45 | 216 | |
| Multi-Core | CS | 67.35 | 0.70 | 3.85 | >350 |
| Citrate | 67.45 | 2.33 | 17.19 | >350 | |
| SiO2 | 67.05 | 1.44 | 10.18 | ~350 |
| Sample | HC-AC (kAm−1) | MR-AC (Am2kg−1) | MMax-AC (Am2kg−1) | Area (mJkg−1) | SARAC (Wg−1) | SARMH (Wg−1) | ΔT (°C) |
|---|---|---|---|---|---|---|---|
| Fe3O4@Cs | 1.4 | 3.1 | 36.9 | 181.4 | 63.2 | 19.56 | 16.54 |
| Fe3O4@Citrate | 1.8 | 4.7 | 47.8 | 302.2 | 105.2 | 34.13 | 27.24 |
| Fe3O4@SiO2 | 3.8 | 4.5 | 22.4 | 288.5 | 100.5 | 34.55 | 24.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García Acevedo, P.; González Gómez, M.A.; Arnosa Prieto, Á.; De Castro Alves, L.; Seco Gudiña, R.; Piñeiro, Y.; Rivas, J. Fluorescent Single-Core and Multi-Core Nanoprobes as Cell Trackers and Magnetic Nanoheaters. Magnetochemistry 2022, 8, 83. https://doi.org/10.3390/magnetochemistry8080083
García Acevedo P, González Gómez MA, Arnosa Prieto Á, De Castro Alves L, Seco Gudiña R, Piñeiro Y, Rivas J. Fluorescent Single-Core and Multi-Core Nanoprobes as Cell Trackers and Magnetic Nanoheaters. Magnetochemistry. 2022; 8(8):83. https://doi.org/10.3390/magnetochemistry8080083
Chicago/Turabian StyleGarcía Acevedo, Pelayo, Manuel A. González Gómez, Ángela Arnosa Prieto, Lisandra De Castro Alves, Román Seco Gudiña, Yolanda Piñeiro, and José Rivas. 2022. "Fluorescent Single-Core and Multi-Core Nanoprobes as Cell Trackers and Magnetic Nanoheaters" Magnetochemistry 8, no. 8: 83. https://doi.org/10.3390/magnetochemistry8080083
APA StyleGarcía Acevedo, P., González Gómez, M. A., Arnosa Prieto, Á., De Castro Alves, L., Seco Gudiña, R., Piñeiro, Y., & Rivas, J. (2022). Fluorescent Single-Core and Multi-Core Nanoprobes as Cell Trackers and Magnetic Nanoheaters. Magnetochemistry, 8(8), 83. https://doi.org/10.3390/magnetochemistry8080083







