Study of Defect-Induced Chemical Modifications in Spinel Zinc-Ferrites Nanostructures by In-Depth XPS Investigation
Abstract
1. Introduction
2. Experimental Method
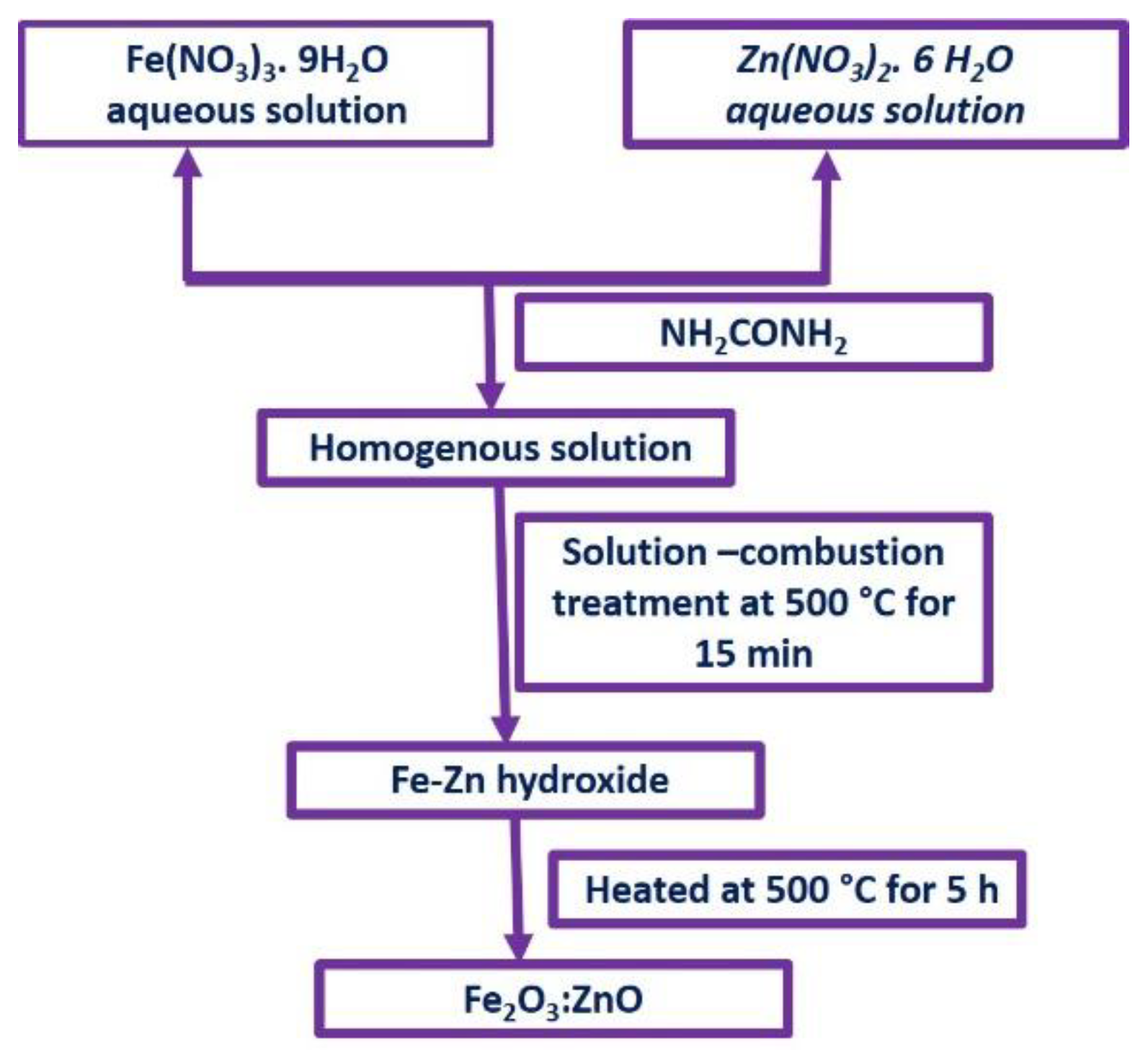
2.1. Synthesis of Fe2O3 and Fe2O3:Zn Nanostructures
Reagents
2.2. Synthesis of Pure Fe2O3 NPs
2.3. Synthesis of Fe2O3:Zn NPs
2.4. Material Testing Procedure
3. Results and Discussion
3.1. Crystallographic Structure & Phase Identification: X-ray Diffraction (XRD)
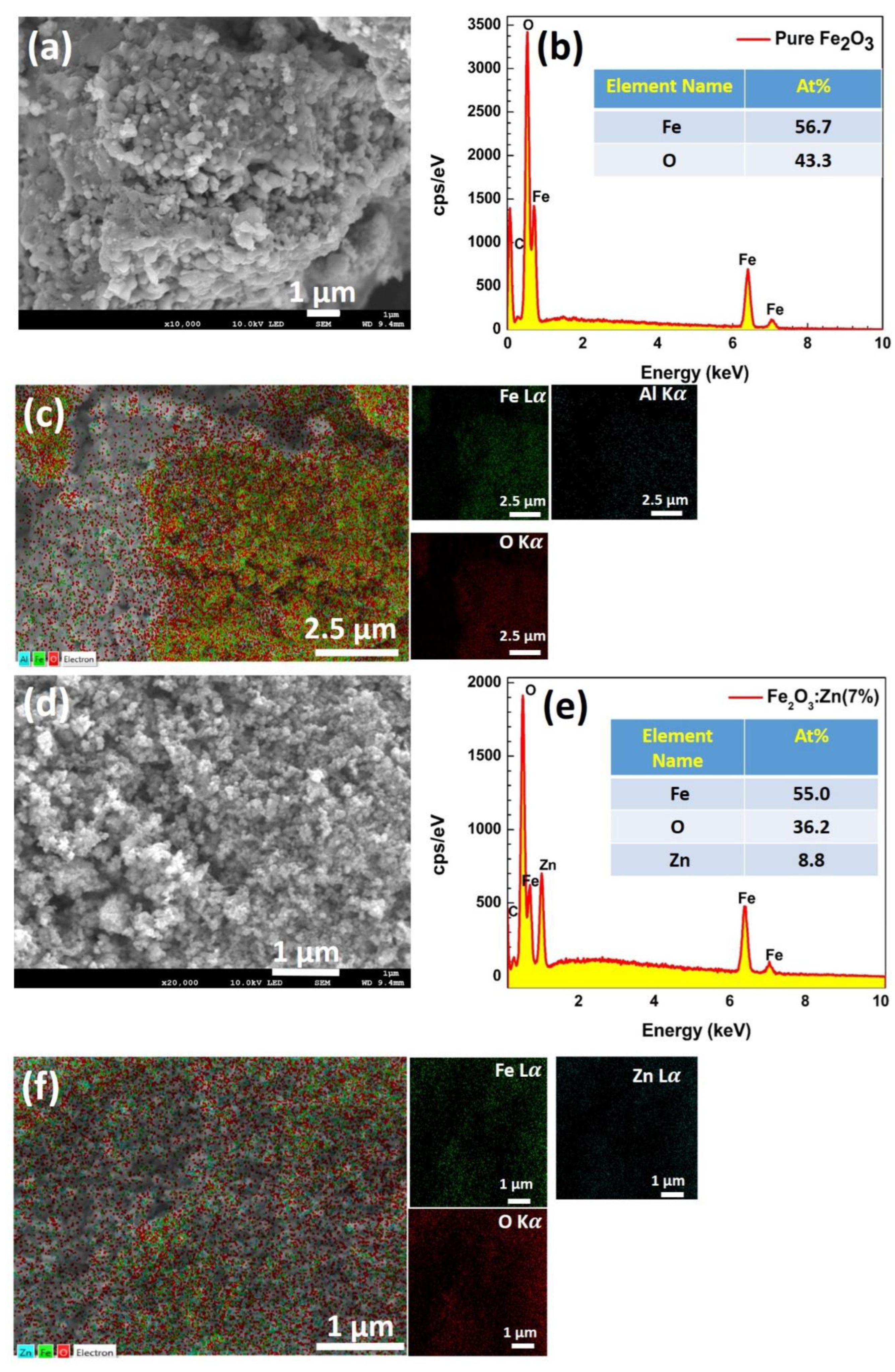
3.2. Surface Morphology & Elemental Analysis
3.3. Morphological Studies of Fe2O3:Zn: TEM
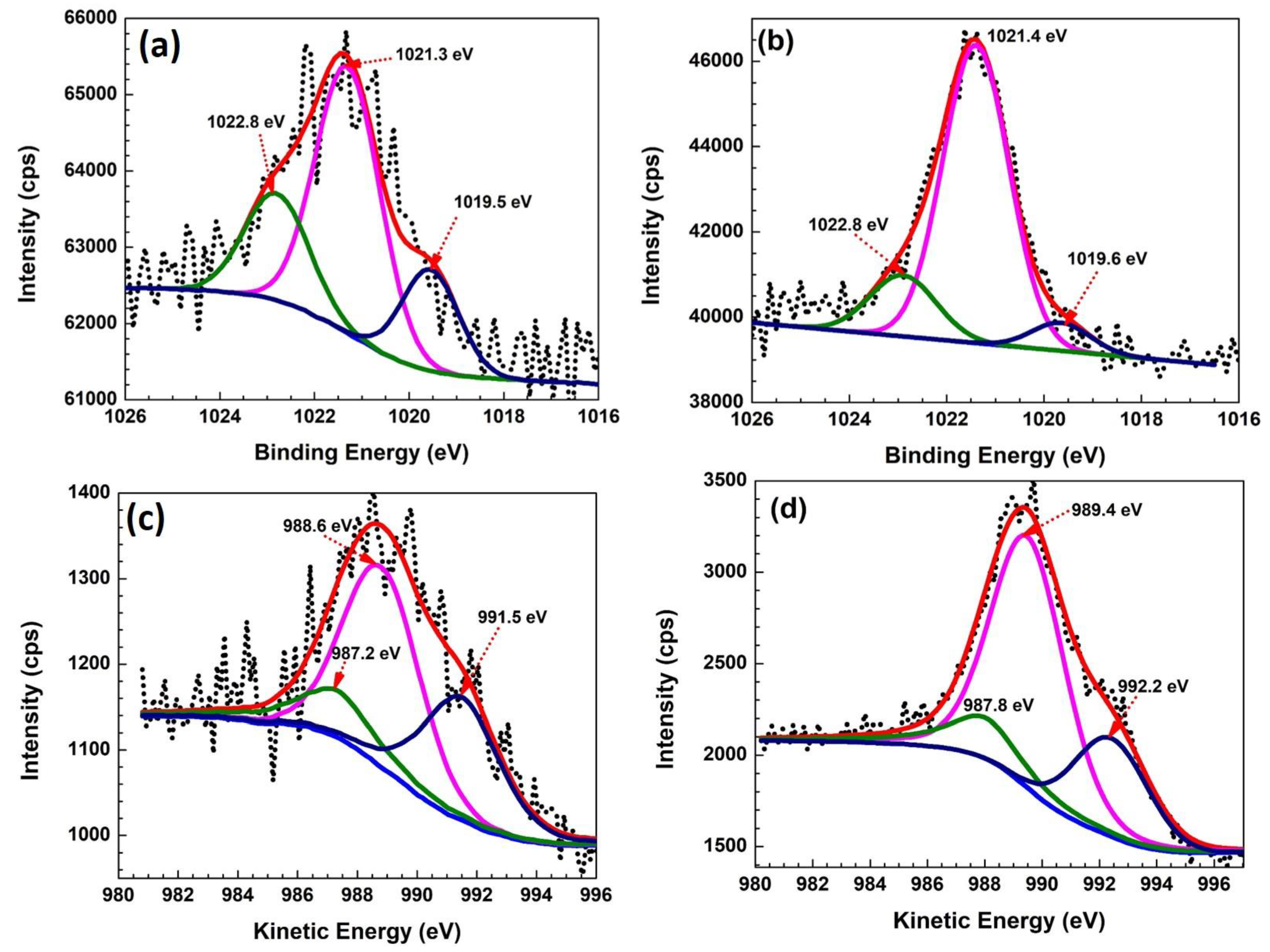
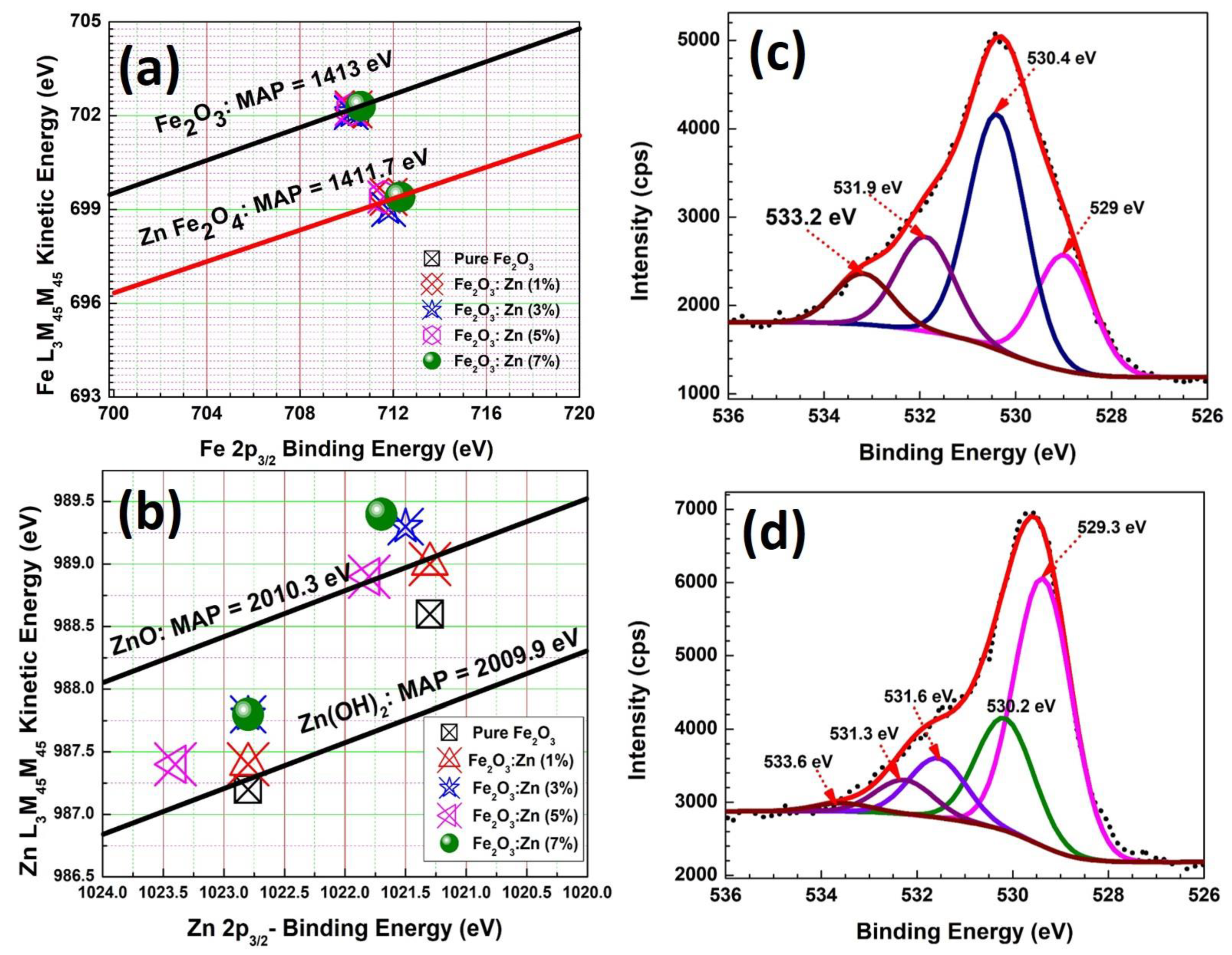
3.4. Surface Analysis Studies of Fe2O3:Zn: XPS
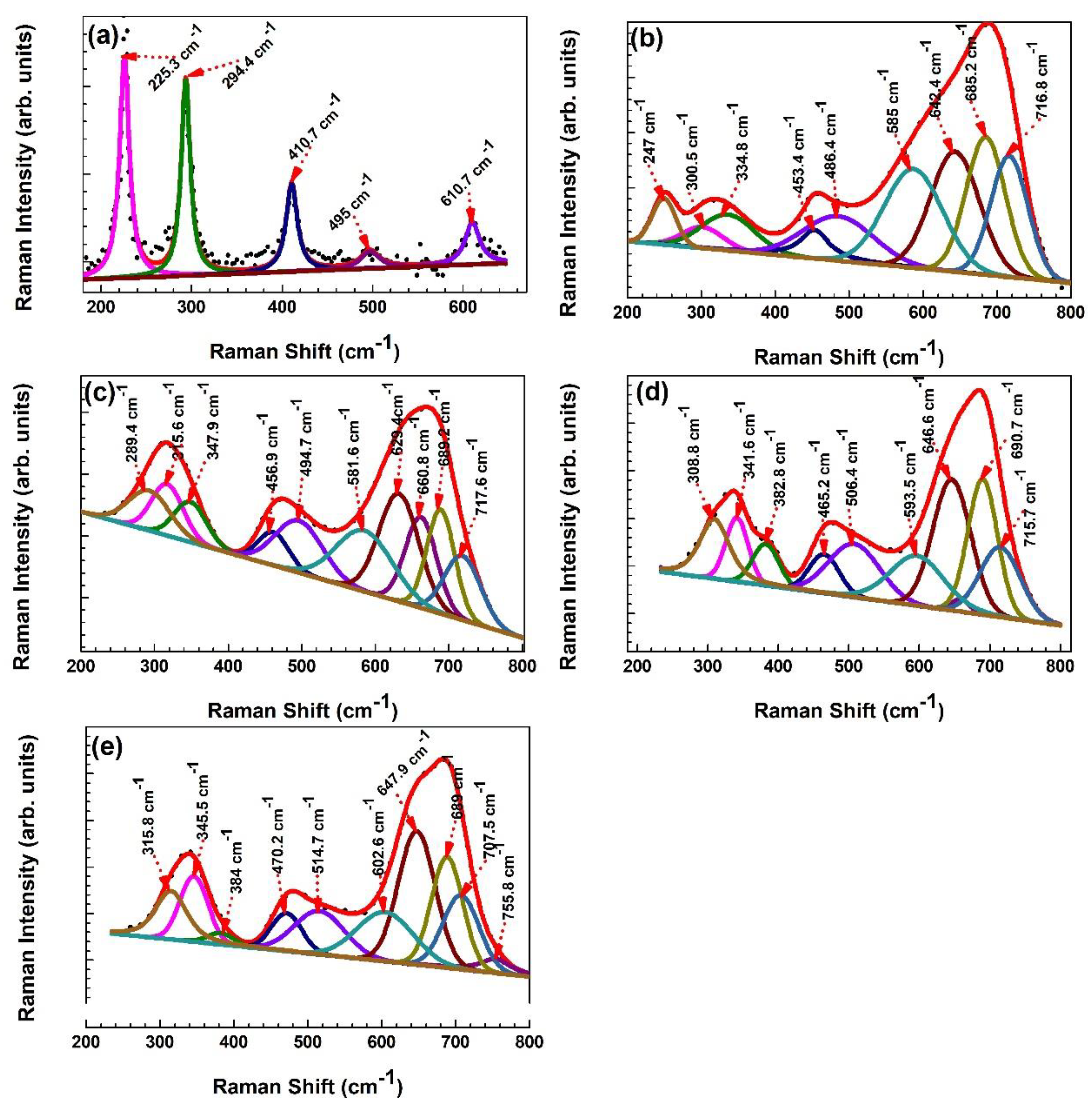
3.5. Bonding of Fe2O3:Zn Nanostructures: Raman Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.; Zafar, H.; Zia, M.; ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Rakshit, R.; Alam, M.; Mandal, K. Magnetic and Electronic Properties of Zn -Doped Fe3O4 Hollow Nanospheres. Phys. Rev. Appl. 2019, 11, 024059. [Google Scholar] [CrossRef]
- Mondal, D.; Phukan, G.; Paul, N.; Borah, J. Improved self heating and optical properties of bifunctional Fe3O4/ZnS nanocomposites for magnetic hyperthermia application. J. Magn. Magn. Mater. 2021, 528, 167809. [Google Scholar] [CrossRef]
- Ge, S.; Shi, X.; Sun, K.; Li, C.; Uher, C.; Baker, J.R., Jr.; Banaszak Holl, M.M.; Orr, B.G. Facile Hydrothermal Synthesis of Iron Oxide Nanoparticles with Tunable Magnetic Properties. J. Phys. Chem. C 2009, 113, 13593–13599. [Google Scholar] [CrossRef]
- Pu, Y.; Tao, X.; Zeng, X.; Le, Y.; Chen, J.-F. Synthesis of Co–Cu–Zn doped Fe3O4 nanoparticles with tunable morphology and magnetic properties. J. Magn. Magn. Mater. 2010, 322, 1985–1990. [Google Scholar] [CrossRef]
- Bram, S.; Gordon, M.N.; Carbonell, M.A.; Pink, M.; Stein, B.D.; Morgan, D.G.; Aguilà, D.; Aromí, G.; Skrabalak, S.E.; Losovyj, Y.; et al. Zn2+ Ion Surface Enrichment in Doped Iron Oxide Nanoparticles Leads to Charge Carrier Density Enhancement. ACS Omega 2018, 3, 16328–16337. [Google Scholar] [CrossRef] [PubMed]
- Priya, R.S.; Kumar, E.R.; Balamurugan, A.; Srinivas, C. Green synthesized MgFe2O4 ferrites nanoparticles for biomedical applications. Appl. Phys. A 2021, 127, 538. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef]
- Agrawal, S.; Parveen, A.; Azam, A. Structural, electrical, and optomagnetic tweaking of Zn doped CoFe2−ZnO4− nanoparticles. J. Magn. Magn. Mater. 2016, 414, 144–152. [Google Scholar] [CrossRef]
- Dantas, J.; Leal, E.; Mapossa, A.; Cornejo, D.; Costa, A. Magnetic nanocatalysts of Ni0.5Zn0.5Fe2O4 doped with Cu and performance evaluation in transesterification reaction for biodiesel production. Fuel 2017, 191, 463–471. [Google Scholar] [CrossRef]
- Besenhard, M.O.; LaGrow, A.P.; Hodzic, A.; Kriechbaum, M.; Panariello, L.; Bais, G.; Loizou, K.; Damilos, S.; Cruz, M.M.; Thanh, N.T.K.; et al. Co-precipitation synthesis of stable iron oxide nanoparticles with NaOH: New insights and continuous production via flow chemistry. Chem. Eng. J. 2020, 399, 125740. [Google Scholar] [CrossRef]
- Su, X.; Chen, S.; Zhou, Z. Synthesis and characterization of monodisperse porous α-Al2O3 nanoparticles. Appl. Surf. Sci. 2012, 258, 5712–5715. [Google Scholar] [CrossRef]
- Joseph, J.A.; Nair, S.B.; John, S.S.; Remillard, S.K.; Shaji, S.; Philip, R.R. Zinc-doped iron oxide nanostructures for enhanced photocatalytic and antimicrobial applications. J. Appl. Electrochem. 2021, 51, 521–538. [Google Scholar] [CrossRef]
- Choodamani, C.; Nagabhushana, G.; Ashoka, S.; Prasad, B.D.; Rudraswamy, B.; Chandrappa, G. Structural and magnetic studies of Mg(1−x)ZnxFe2O4 nanoparticles prepared by a solution combustion method. J. Alloys Compd. 2013, 578, 103–109. [Google Scholar] [CrossRef]
- Pradhan, G.K.; Martha, S.; Parida, K.M. Synthesis of Multifunctional Nanostructured Zinc–Iron Mixed Oxide Photocatalyst by a Simple Solution-Combustion Technique. ACS Appl. Mater. Interfaces 2011, 4, 707–713. [Google Scholar] [CrossRef]
- Kumar, P.; Inwati, G.K.; Mathpal, M.C.; Ghosh, S.; Roos, W.; Swart, H. Defects induced enhancement of antifungal activities of Zn doped CuO nanostructures. Appl. Surf. Sci. 2021, 560, 150026. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Ghosh, S.; Inwati, G.K.; Maze, J.R.; Duvenhage, M.-M.; Roos, W.; Swart, H. Plasmonic Au nanoparticles embedded in glass: Study of TOF-SIMS, XPS and its enhanced antimicrobial activities. J. Alloys Compd. 2022, 909, 164789. [Google Scholar] [CrossRef]
- Leveneur, J.; Waterhouse, G.I.N.; Kennedy, J.; Metson, J.B.; Mitchell, D.R.G. Nucleation and Growth of Fe Nanoparticles in SiO2: A TEM, XPS, and Fe L-Edge XANES Investigation. J. Phys. Chem. C 2011, 115, 20978–20985. [Google Scholar] [CrossRef]
- McLoughlin, T.; Babbitt, W.R.; Himmer, P.A.; Nakagawa, W. Auger electron spectroscopy for surface ferroelectric domain differentiation in selectively poled MgO:LiNbO3. Opt. Mater. Express 2020, 10, 2379–2393. [Google Scholar] [CrossRef]
- Mouder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Coporation: Waltham, MA, USA, 1992. [Google Scholar]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef]
- Pramila, S.; Ranganatha, V.L.; Nagaraju, G.; Mallikarjunaswamy, C. Microwave and combustion methods: A comparative study of synthesis, characterization, and applications of NiO nanoparticles. Inorg. Nano-Metal Chem. 2022, 1–12. [Google Scholar] [CrossRef]
- Maslen, E.N.; Streltsov, V.A.; Streltsova, N.R.; Ishizawa, N. Synchrotron X-ray study of the electron density in α-Fe2O3. Acta Crystallogr. Sect. B Struct. Sci. 1994, 50, 435–441. [Google Scholar] [CrossRef]
- Schäfer, W.; Kockelmann, W.A.; Kirfel, A.; Potzel, W.; Burghart, F.; Kalvius, G.; Martin, A.; Kaczmarek, W.; Campbell, S. Structural and Magnetic Variations of ZnFe2O4 Spinels-Neutron Powder Diffraction Studies. Mater. Sci. Forum 2000, 321–324, 802–807. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, V.; Singh, J.P.; Kumar, A.; Chahal, S.; Sachdev, K.; Chae, K.; Kumar, A.; Asokan, K.; Kanjilal, D. Investigations on magnetic and electrical properties of Zn doped Fe2O3 nanoparticles and their correlation with local electronic structures. J. Magn. Magn. Mater. 2019, 489, 165398. [Google Scholar] [CrossRef]
- Chahal, S.; Kumar, A.; Kumar, P. Zn Doped α-Fe2O3: An Efficient Material for UV Driven Photocatalysis and Electrical Conductivity Suman. Crystals 2020, 10, 273. [Google Scholar]
- Kumar, P.; Kumar, A.; Rizvi, M.A.; Moosvi, S.K.; Krishnan, V.; Duvenhage, M.; Roos, W.; Swart, H. Surface, optical and photocatalytic properties of Rb doped ZnO nanoparticles. Appl. Surf. Sci. 2020, 514, 145930. [Google Scholar] [CrossRef]
- Kumar, P.; Mathpal, M.C.; Jagannath, G.; Prakash, J.; Maze, J.; Roos, W.D.; Swart, H.C. Optical limiting applications of resonating plasmonic Au nanoparticles in a dielectric glass medium. Nanotechnology 2021, 32, 345709. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Wang, S.; Meng, C.; Bai, Y.; Wang, Y.; Liu, P.; Pan, L.; Zhang, L.; Yin, Z.; Tang, N. Synergy Promotion of Elemental Doping and Oxygen Vacancies in Fe2O3 Nanorods for Photoelectrochemical Water Splitting. ACS Appl. Nano Mater. 2022, 5, 6781–6791. [Google Scholar] [CrossRef]
- Al Khabouri, S.; Al Harthi, S.; Maekawa, T.; Nagaoka, Y.; Elzain, M.E.; Al Hinai, A.; Al-Rawas, A.D.; Gismelseed, A.M.; Yousif, A.A. Composition, Electronic and Magnetic Investigation of the Encapsulated ZnFe2O4 Nanoparticles in Multiwall Carbon Nanotubes Containing Ni Residuals. Nanoscale Res. Lett. 2015, 10, 262. [Google Scholar] [CrossRef]
- Fu, B.; Hower, J.C.; Dai, S.; Mardon, S.M.; Liu, G. Determination of Chemical Speciation of Arsenic and Selenium in High-As Coal Combustion Ash by X-ray Photoelectron Spectroscopy: Examples from a Kentucky Stoker Ash. ACS Omega 2018, 3, 17637–17645. [Google Scholar] [CrossRef] [PubMed]
- Beisinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. The role of the Auger parameter in XPS studies of nickel metal, halides and oxides. Phys. Chem. Chem. Phys. 2012, 14, 2434–2442. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, B.; Rangam, N.; Jiricek, P.; Gordeev, I.; Tóth, J.; Kövér, L.; Mohai, M.; Borowicz, P. Surface Study of Fe3O4 Nanoparticles Functionalized With Biocompatible Adsorbed Molecules. Front. Chem. 2019, 7, 642. [Google Scholar] [CrossRef] [PubMed]
- Rivera, L.M.R.; Machado, J.G.; Mathpal, M.C.; Chaves, N.L.; Gregurec, D.; Báo, S.N.; Paterno, L.G.; Moya, S.E.; Azevedo, R.B.; Soler, M.A.G. Functional glucosamine-iron oxide nanocarriers. J. Mater. Res. 2020, 35, 1726–1737. [Google Scholar] [CrossRef]
- Mathpal, M.C.; Tripathi, A.K.; Singh, M.K.; Gairola, S.P.; Pandey, S.N.; Agarwal, A. Effect of annealing temperature on Raman spectra of TiO2 nanoparticles. Chem. Phys. Lett. 2013, 555, 182–186. [Google Scholar] [CrossRef]
- Gaur, L.K.; Mathpal, M.C.; Kumar, P.; Gairola, S.; Agrahari, V.; Martinez, M.; Aragon, F.; Soler, M.A.; Swart, H.; Agarwal, A. Observations of phonon anharmonicity and microstructure changes by the laser power dependent Raman spectra in Co doped SnO2 nanoparticles. J. Alloys Compd. 2020, 831, 154836. [Google Scholar] [CrossRef]
- Aquino, C.L.E.; Balela, M.D.L. Thermally grown Zn-doped hematite (α-Fe2O3) nanostructures for efficient adsorption of Cr(VI) and Fenton-assisted degradation of methyl orange. SN Appl. Sci. 2020, 2, 2099. [Google Scholar] [CrossRef]
- Jubb, A.; Allen, H.C. Vibrational Spectroscopic Characterization of Hematite, Maghemite, and Magnetite Thin Films Produced by Vapor Deposition. ACS Appl. Mater. Interfaces 2010, 2, 2804–2812. [Google Scholar] [CrossRef]
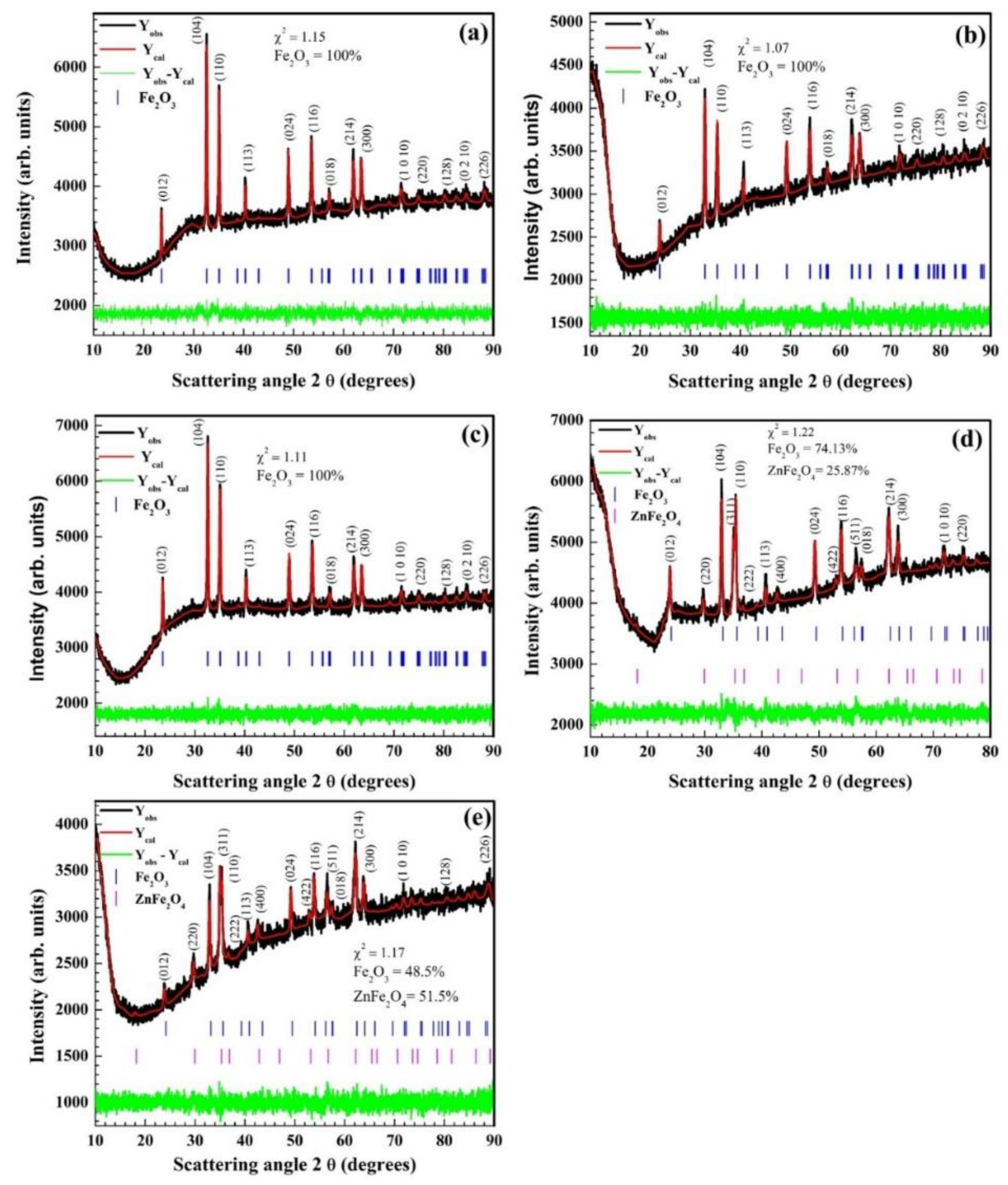


| Zn (%) | α–Fe2O3 Phase | ZnFe2O4 Phase | ||||||
|---|---|---|---|---|---|---|---|---|
| a (Å) | c (Å) | Phase Fraction (%) | Density (g/cm3) | Crystallite Size (nm) | a (Å) | Phase Fraction (%) | Density (g/cm3) | |
| 0 | 5.026 | 13.722 | 100 | 4.94 | 44.5 | -- | 0 | -- |
| 1 | 5.031 | 13.726 | 100 | 4.99 | 26 | -- | 0 | -- |
| 3 | 5.024 | 13.717 | 100 | 5.09 | 33 | -- | 0 | -- |
| 5 | 5.032 | 13.731 | 74.13 | 5.07 | 27 | 8.429 | 25.87 | 5.02 |
| 7 | 5.032 | 13.738 | 48.50 | 5.08 | 24 | 8.458 | 51.50 | 5.17 |
| Name of Phase of the Nanomaterials | Raman Vibration Mode (in cm−1), [Reference in Bracket] | Pure Fe2O3 | 1% Zn-doped | 3% Zn-doped | 5% Zn-doped | 7% Zn-doped |
|---|---|---|---|---|---|---|
| Hematite (α−Fe2O3) | A1g modes [25,26,39] | 225.3 495 | 486.4 | 494.7 | 506.4 | 514.7 |
| Hematite (α−Fe2O3) | Eg modes [25,26,38] | 294.4 410.7 610.7 | 247 300.5 585 | 289.4 315.6, 581.6, 629.4 | 308.8 593.5 | 315.8 602.6 |
| Maghemite (γ−Fe2O3) | Eg or T2g modes [34,38,39] | 334.8 | 347.9 | 341.6 | 345.5 | |
| Hematite (α−Fe2O3) | A2u modes [39] | 382.8 | 384 | |||
| Hematite (α−Fe2O3) | Eu −one phonon mode [39] | 453.4 | 456.9 | 465.2 | 470.2 | |
| Hematite (α−Fe2O3) | Eu −LO (longitudinal optical mode due to the defects) [38,39] | 642.4 | 660.8 | 646.6 | 647.9 | |
| Zinc Ferrite (ZnFe2O4) | A1g modes [25,38,39] | 685.2 | 689.2 | 690.7 | 689 | |
| Maghemite (γ−Fe2O3) | A1g modes [35,39] | 716.8 | 717.6 | 715.7 | 707.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, P.; Mathpal, M.C.; Inwati, G.K.; Kumar, S.; Duvenhage, M.-M.; Roos, W.D.; Swart, H.C. Study of Defect-Induced Chemical Modifications in Spinel Zinc-Ferrites Nanostructures by In-Depth XPS Investigation. Magnetochemistry 2023, 9, 20. https://doi.org/10.3390/magnetochemistry9010020
Kumar P, Mathpal MC, Inwati GK, Kumar S, Duvenhage M-M, Roos WD, Swart HC. Study of Defect-Induced Chemical Modifications in Spinel Zinc-Ferrites Nanostructures by In-Depth XPS Investigation. Magnetochemistry. 2023; 9(1):20. https://doi.org/10.3390/magnetochemistry9010020
Chicago/Turabian StyleKumar, Promod, Mohan Chandra Mathpal, Gajendra Kumar Inwati, Sanjay Kumar, Mart-Mari Duvenhage, Wiets Daniel Roos, and Hendrik C. Swart. 2023. "Study of Defect-Induced Chemical Modifications in Spinel Zinc-Ferrites Nanostructures by In-Depth XPS Investigation" Magnetochemistry 9, no. 1: 20. https://doi.org/10.3390/magnetochemistry9010020
APA StyleKumar, P., Mathpal, M. C., Inwati, G. K., Kumar, S., Duvenhage, M.-M., Roos, W. D., & Swart, H. C. (2023). Study of Defect-Induced Chemical Modifications in Spinel Zinc-Ferrites Nanostructures by In-Depth XPS Investigation. Magnetochemistry, 9(1), 20. https://doi.org/10.3390/magnetochemistry9010020









