Something You Need Might Be under Your Feet: Molecular Magnetism of Heavy Kramers Lanthanide Hydrated Chlorides and Their Complexes with Polydentate Terpy Ligand
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure Features
2.2. Magnetic Properties of the Complexes
3. Materials and Methods
3.1. Synthesis of [Ln(H2O)4(terpy)Cl]Cl2·3H2O (2Ln) Compounds
3.2. Computations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaz, R.C.; Esteves, I.O.; Oliveira, W.X.; Honorato, J.; Martins, F.T.; Marques, L.F.; dos Santos, G.L.; Ricardo, O.; Freire, R.O.; Jesus, L.T.; et al. Mononuclear lanthanide(III)-oxamate complexes as new photoluminescent field-induced single-molecule magnets: Solid-state photophysical and magnetic properties. Dalton Trans. 2020, 49, 16106–16124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Guo, M.; Li, X.L.; Tang, J. Molecular magnetism of lanthanide: Advances and perspectives. Coord. Chem. Rev. 2019, 378, 350–364. [Google Scholar] [CrossRef]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-ac to dysprosium metallocenes, a travel in time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Parmar, V.S.; Mills, D.P.; Winpenny, R.E.P. Mononuclear Dysprosium Alkoxide and Aryloxide Single-Molecule Magnets. Chem. Eur. J. 2021, 27, 7625–7645. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guo, M.; Tang, J. Recent Developments in Lanthanide Single-Molecule Magnets. Chem. Asian J. 2017, 12, 2772–2779. [Google Scholar] [CrossRef]
- Cador, O.; Le Guennic, B.; Pointillart, F. Electro-activity and magnetic switching in lanthanide-based single-molecule magnets. Inorg. Chem. Front. 2019, 6, 3398–3417. [Google Scholar] [CrossRef] [Green Version]
- Casanovas, B.; Porcar, O.; Speed, S.; Vicente, R.; Font-Bardía, M.; El Fallah, M.S. Field-Induced SMM and Vis/NIR Luminescence on Mononuclear Lanthanide Complexes with 9-Anthracenecarboxylate and 2,2′:6,2″-Terpyridine. Magnetochemistry 2021, 7, 124. [Google Scholar] [CrossRef]
- Canaj, A.B.; Sing, M.K.; Wilson, C.; Rajaraman, G.; Murrie, M. Chemical and in silico tuning of the magnetisation reversal barrier in pentagonal bipyramidal Dy(III) single-ion magnets. Chem. Comm. 2018, 54, 8273–8276. [Google Scholar] [CrossRef] [Green Version]
- Li, L.L.; Su, H.D.; Liu, S.; Xu, Y.C.; Wang, W.Z. A new air- and moisture-stable pentagonal-bipyramidal Dy III single-ion magnet based on the HMPA ligand. Dalton Trans. 2019, 48, 2213–2219. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Efimov, N.N.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Vasilyev, P.N.; Novotortsev, V.M. Novel mononuclear Ln complexes with pyrazine-2-carboxylate and acetylacetonate co-ligands: Remarkable single molecule magnet behavior of a Yb derivative. Dalton Trans. 2017, 46, 11806–11816. [Google Scholar] [CrossRef]
- Jiménez, J.R.; Diaz-Ortega, I.F.; Ruiz, E.; Aravena, D.; Pope, S.J.A.; Colacio, E.; Herrera, J.M. Lanthanide Tetrazolate Complexes Combining Single-Molecule Magnet and Luminescence Properties: The Effect of the Replacement of Tetrazolate N3 by β-Diketonate Ligands on the Anisotropy Energy Barrier. Chem. Eur. J. 2016, 22, 14548–14559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, M.; Ishikawa, N.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Static Magnetic-Field-Induced Phase Lag in the Magnetization Response of Tris(dipicolinato)lanthanides. Inorg. Chem. 2006, 45, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Boulon, M.E.; Cucinotta, G.; Luzon, J.; Degl’Innocenti, C.; Perfetti, M.; Bernot, K.; Calvez, G.; Caneschi, A.; Sessoli, R. Magnetic Anisotropy and Spin-Parity Effect Along the Series of Lanthanide Complexes with DOTA. Angew. Chem. Int. Ed. 2013, 52, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Mylonas-Margaritis, I.; Maniaki, D.; Mayans, J.; Ciammaruchi, L.; Bekiari, V.; Raptopoulou, C.P.; Psycharis, V.; Christodoulou, S.; Escuer, A.; Perlepes, S.P. Mononuclear Lanthanide(III)-Salicylideneaniline Complexes: Synthetic, Structural, Spectroscopic, and Magnetic Studies. Magnetochemistry 2018, 4, 45. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.L.; Yuan, K.; Leng, J.D.; Ungur, L.; Wernsdorfer, W.; Guo, F.S.; Chibotaru, L.F.; Tong, M.L. A Six-Coordinate Ytterbium Complex Exhibiting Easy-Plane Anisotropy and Field-Induced Single-Ion Magnet Behavior. Inorg. Chem. 2012, 51, 8538–8544. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.Q.; Zhang, P.; Zhao, L.; Guo, M.; Tang, J. Single-Molecule Magnet Behavior Enhanced by Synergic Effect of Single-Ion Anisotropy and Magnetic Interactions. Inorg. Chem. 2017, 56, 7882–7889. [Google Scholar] [CrossRef]
- Bar, A.K.; Kalita, P.; Sutter, J.P.; Chandrasekhar, V. Pentagonal-Bipyramid Ln(III) Complexes Exhibiting Single-Ion-Magnet Behavior: A Rational Synthetic Approach for a Rigid Equatorial Plane. Inorg. Chem. 2018, 57, 2398–2401. [Google Scholar] [CrossRef]
- Saha, R.; Goswami, S.; Biswas, S.; Steele, I.M.; Dey, K.; Jana, A.D.; Kumar, S. A dynamic metal–organic supramolecular host based on weak π-stacking interactions incorporating 2D water-chloride-methanolic supramolecular sheet. Inorg. Chim. Acta 2014, 423, 123–132. [Google Scholar] [CrossRef]
- Puntus, L.N.; Lyssenko, K.A.; Antipin, M.Y.; Bünzli, J.C.G. Role of Inner- and Outer-Sphere Bonding in the Sensitization of Eu III-Luminescence Deciphered by Combined Analysis of Experimental Electron Density Distribution Function and Photophysical Data. Inorg. Chem. 2008, 47, 2008–11095. [Google Scholar] [CrossRef]
- Puntus, L.N.; Lyssenko, K.A.; Pekareva, I.S.; Bünzli, J.C.G. Intermolecular Interactions as Actors in Energy-Transfer Processes in Lanthanide Complexes with 2,2′-Bipyridine. J. Phys. Chem. B 2009, 113, 9265–9277. [Google Scholar] [CrossRef]
- Lhoste, J.; Henry, N.; Loiseau, T.; Abraham, F. Molecular assemblies of trichloride neodymium and europium complexes chelated by 1,10-phenanthroline. Polyhedron 2011, 30, 1289–1294. [Google Scholar] [CrossRef]
- Alfi, N.; Khorasani-Motlagh, M.; Rezvani, A.R.; Noroozifar, M.; Molčanov, K. Synthesis, characterization, crystal structure, DNA/BSA binding ability and antibacterial activity of asymmetric europium complex based on 1,10-phenanthroline. J. Mol. Struct. 2017, 1137, 771–783. [Google Scholar] [CrossRef]
- Petrosyants, S.P.; Ilyukhin, A.B.; Gavrikov, A.V.; Mikhlina, Y.A.; Puntus, L.N.; Varaksina, E.A.; Efimov, N.N.; Novotortsev, V.M. Luminescent and magnetic properties of mononuclear lanthanide thiocyanates with terpyridine as auxiliary ligand. Inorg. Chim. Acta 2019, 486, 499–505. [Google Scholar] [CrossRef]
- Petrosyants, S.P.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Gavrikov, A.V.; Vasilyev, P.N.; Novotortsev, V.M. Mononuclear Dysprosium Thiocyanate Complexes with 2,2′-Bipyridine and 1,10-Phenanthroline: Synthesis, Crystal Structures, SIM Behavior, and Solid-Phase Transformations. Eur. J. Inorg. Chem. 2017, 2017, 3561–3569. [Google Scholar] [CrossRef]
- Petrosyants, S.P.; Ilyukhin, A.B.; Efimov, N.N.; Gavrikov, A.V.; Novotortsev, V.M. Self-assembly and SMM properties of lanthanide cyanocobaltate chain complexes with terpyridine as blocking ligand. Inorg. Chim. Acta 2018, 482, 813–820. [Google Scholar] [CrossRef]
- Giansiracus, M.J.; Al-Badran, S.; Kostopoulos, A.K.; Whitehead, G.F.S.; Collison, D.; Tuna, F.; Winpenny, R.E.P.; Chilton, N.F. A Large Barrier Single-Molecule Magnet without Magnetic Memory. Dalton Trans. 2019, 48, 10795–107981. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The terpyridine isomer game: From chelate to coordination network building block. Chem. Commun. 2020, 56, 10786–10794. [Google Scholar] [CrossRef]
- Bell, M.T.; Smith, A.J. Structure of hexaaquadichloroyttrium(III) chloride. Acta Crystallogr. Sect. C 1990, 46, 960–962. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Kepert, C.; Lu, W.; Skelton, B.; White, A. Structural Systematics of Rare Earth Complexes. V. The Hydrated 1: 1 Adducts of 2,2′:6′,2″-Terpyridine With the Lanthanoid(III) Chlorides. Aust. J. Chem. 1994, 47, 365–384. [Google Scholar] [CrossRef]
- Glick, M.D.; Radonovich, L.J. Stereochemistry of the chloropentaaquoterpyridylpraseodymium(III) ion. Inorg. Chem. 1971, 10, 1463–1468. [Google Scholar] [CrossRef]
- Lhoste, J.; Pérez-Campos, A.; Henry, N.; Loiseau, T.; Rabu, P.; Abraham, F. Chain-like and dinuclear coordination polymers in lanthanide (Nd, Eu) oxochloride complexes with 2,2′:6′,2″-terpyridine: Synthesis, XRD structure and magnetic properties. Dalton Trans. 2011, 40, 9136–9144. [Google Scholar] [CrossRef] [PubMed]
- Curnock, E.; Levason, W.; Light, M.E.; Luthra, S.K.; McRobbie, G.; Monzittu, F.M.; Reid, G.; Williams, R.N. Group 3 metal trihalide complexes with neutral N-donor ligands-exploring their affinity towards fluoride. Dalton Trans. 2018, 7, 6059–6068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kepert, C.J.; Skeleton, B.W.; White, A.H. Structural Systematics of Rare Earth Complexes. VII. Crystal Structure of Bis(2,2′/6′,2ESC-Terpyridinium) Octaaquaterbium(III) Heptachloride Hydrate. Aust. J. Chem. 1994, 47, 391–396. [Google Scholar] [CrossRef]
- Babeshkin, K.A.; Gavrikov, A.V.; Petrosyants, S.P.; Ilyukhin, A.B.; Belova, E.V.; Efimov, N.N. Unexpected Supremacy of Non-Dysprosium Single-Ion Magnets within a Series of Isomorphic Lanthanide Cyanocobaltate(III) Complexes. Eur. J. Inorg. Chem. 2000, 46, 4380–4390. [Google Scholar] [CrossRef]
- Feng, M.; Tong, M.L. Single Ion Magnets from 3d to 5f: Developments and Strategies. Chem. Eur. J. 2018, 24, 7574–7594. [Google Scholar] [CrossRef]
- Mamontova, E.; Long, J.; Ferreira, R.; Botas, A.; Luneau, D.; Guari, Y.; Carlos, L.; Larionova, J. Magneto-Luminescence Correlation in the Textbook Dysprosium(III) Nitrate Single-Ion Magnet. Magnetochemistry 2016, 2, 41. [Google Scholar] [CrossRef] [Green Version]
- Habib, F.; Brunet, G.; Vieru, V.; Korobkov, I.; Chibotaru, L.F.; Murugesu, M. Significant Enhancement of Energy Barriers in Dinuclear Dysprosium Single-Molecule Magnets through Electron-Withdrawing Effects. J. Am. Chem. Soc. 2013, 135, 13242–13245. [Google Scholar] [CrossRef]
- Petrosyants, S.P.; Babeshkin, K.A.; Gavrikov, A.V.; Ilyukhin, A.B.; Belova, E.V.; Efimov, N.N. Towards comparative investigation of Er- and Yb-based SMMs: The effect of the coordination environment configuration on the magnetic relaxation in the series of heteroleptic thiocyanate complexes. Dalton Trans. 2019, 48, 12644–12655, and references therein. [Google Scholar] [CrossRef]
- Pointillart, F.; Cador, O.; Le Guennic, B.; Ouahab, L. Uncommon Lanthanide Ions in Purely 4f Single Molecule Magnets. Coord. Chem. Rev. 2017, 346, 150–175. [Google Scholar] [CrossRef]
- Borah, A.; Murugavel, R. Magnetic relaxation in single-ion magnets formed by less-studied lanthanide ions Ce(III), Nd(III), Gd(III), Ho(III), Tm(II/III) and Yb(III). Coord. Chem. Rev. 2022, 453, 214288, and references therein. [Google Scholar] [CrossRef]
- Mondal, A.; Konar, S. Strong Equatorial Crystal Field Enhances the Axial Anisotropy and Energy Barrier for Spin Reversal Process in Yb2 Single Molecule Magnets. Chem. Eur. J. 2021, 27, 3449–3456. [Google Scholar] [CrossRef]
- Leng, J.D.; Hua, Q.Y.; Liu, W.T.; Tao, Z.X.; Tan, N.W.; Wang, Y.F.; Lin, W.Q. Slow magnetic relaxation of mononuclear complexes based on uncommon Kramers lanthanide ions Ce III, Sm III and Yb III. Dalton Trans. 2022, 51, 12661–12669. [Google Scholar] [CrossRef]
- Handzlik, G.; Magott, M.; Arczyński, M.; Sheveleva, A.M.; Tuna, F.; Baran, S.; Pinkowicz, D. Identical anomalous Raman relaxation exponent in a family of Single Ion Magnets: Towards reliable Raman relaxation determination. Dalton Trans. 2020, 49, 11942–11949. [Google Scholar] [CrossRef]
- Fondo, M.; Corredoira-Vázquez, J.; García Deibe, A.M.; Matalobos, J.S.; Amoza, M.; Botas, A.M.P.; Ferreira, R.A.S.; Carlos, L.D.; Colacio, E. Field-Induced slow magnetic relaxation and luminescence thermometry in a mononuclear ytterbium complex. Inorg. Chem. Front. 2020, 7, 3019–3029. [Google Scholar] [CrossRef]
- Han, L.Z.; Jiao, C.Q.; Chen, W.C.; Shao, K.Z.; Jin, L.Y.; Su, Z.M. Assembly of tetra-nuclear Yb III-containing selenotungstate clusters: Synthesis, structures, and magnetic properties. Dalton Trans. 2021, 50, 11535–11541. [Google Scholar] [CrossRef]
- Shmelev, M.A.; Polunin, R.A.; Gogoleva, N.V.; Evstifeev, I.S.; Vasilyev, P.N.; Dmitriev, A.A.; Varaksina, E.A.; Efimov, N.N.; Taydakov, I.V.; Sidorov, A.A.; et al. Cadmium-Inspired Self-Polymerization of {LnIIICd2} Units: Structure, Magnetic and Photoluminescent Properties of Novel Trimethylacetate 1D-Polymers (Ln = Sm, Eu, Tb, Dy, Ho, Er, Yb). Molecules 2021, 26, 4296. [Google Scholar] [CrossRef]
- Mondal, A.; Konar, S. A remarkable energy barrier for spin reversal in a field induced dinuclear ytterbium single molecule magnet. Dalton Trans. 2021, 50, 13666–13670. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Efimov, N.N.; Ilyukhin, A.B.; Dobrokhotova, Z.V.; Novotortsev, V.M. Yb3+ can be much better than Dy3+: SMM properties and controllable self-assembly of novel lanthanide 3,5-dinitrobenzoate-acetylacetonate complexes. Dalton Trans. 2018, 47, 6199–6209. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, v2.1; University of Barcelona: Barcelona, Spain; The Hebrew University of Jerusalem: Jerusalem, Israel, 2013. [Google Scholar]
- Aravena, D.; Ruiz, E. Shedding Light on the Single-Molecule Magnet Behavior of Mononuclear Dy III Complexes. Inorg. Chem. 2013, 52, 13770–13778. [Google Scholar] [CrossRef] [PubMed]
- Petrosyants, S.P.; Babeshkin, K.A.; Ilyukhin, A.B.; Belova, E.V.; Efimov, N.N. Complexes of Lanthanide (Dy, Er, Yb) Thiocyanates with Tetramethylphenanthroline. Synthesis, Thermolysis, and SMM Properties. Russ. J. Coord. Chem. 2021, 47, 244–252. [Google Scholar] [CrossRef]
- Petrosyants, S.P.; Babeshkin, K.A.; Ilyukhin, A.B.; Efimov, N.N. Molecular Magnets Based on Mononuclear Aqua and Aqua-Chloro Lanthanide (Tb, Dy, Er, Yb) Complexes with Bipyridine. Russ. J. Coord. Chem. 2021, 47, 165–173. [Google Scholar] [CrossRef]
- Galván, I.F.; Vacher, M.; Alavi, A.; Angeli, C.; Aquilante, F.; Autschbach, J.; Bao, J.J.; Bokarev, S.I.; Bogdanov, N.A.; Carlson, R.K.; et al. OpenMolcas: From Source Code to Insight. J. Chem. Theory Comput. 2019, 15, 5925–5964. [Google Scholar] [CrossRef]
- Aquilante, F.; Autschbach, J.; Baiardi, A.; Battaglia, S.; Borin, V.A.; Chibotaru, L.F.; Conti, I.; De Vico, L.; Delcey, M.; Galván, I.F.; et al. Modern quantum chemistry with [Open] Molcas. J. Chem. Phys. 2020, 152, 214117. [Google Scholar] [CrossRef]
- Reiher, M. Relativistic Douglas-Kroll-Hess theory. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 139–149. [Google Scholar] [CrossRef]
- Wolf, A.; Reiher, M.; Hess, B.A. The generalized Douglas–Kroll transformation. J. Chem. Phys. 2002, 117, 9215–9226. [Google Scholar] [CrossRef] [Green Version]
- Chibotaru, L.F.; Ungur, L.; Soncini, A. The Origin of Nonmagnetic Kramers Doublets in the Ground State of Dysprosium Triangles: Evidence for a Toroidal Magnetic Moment. Angew. Chem. Int. Ed. 2008, 47, 4126–4129. [Google Scholar] [CrossRef] [Green Version]
- Chibotaru, L.F.; Ungur, L. Ab initio calculation of anisotropic magnetic properties of complexes. I. Unique definition of pseudospin Hamiltonians and their derivation. J. Chem. Phys. 2012, 137, 064112. [Google Scholar] [CrossRef]

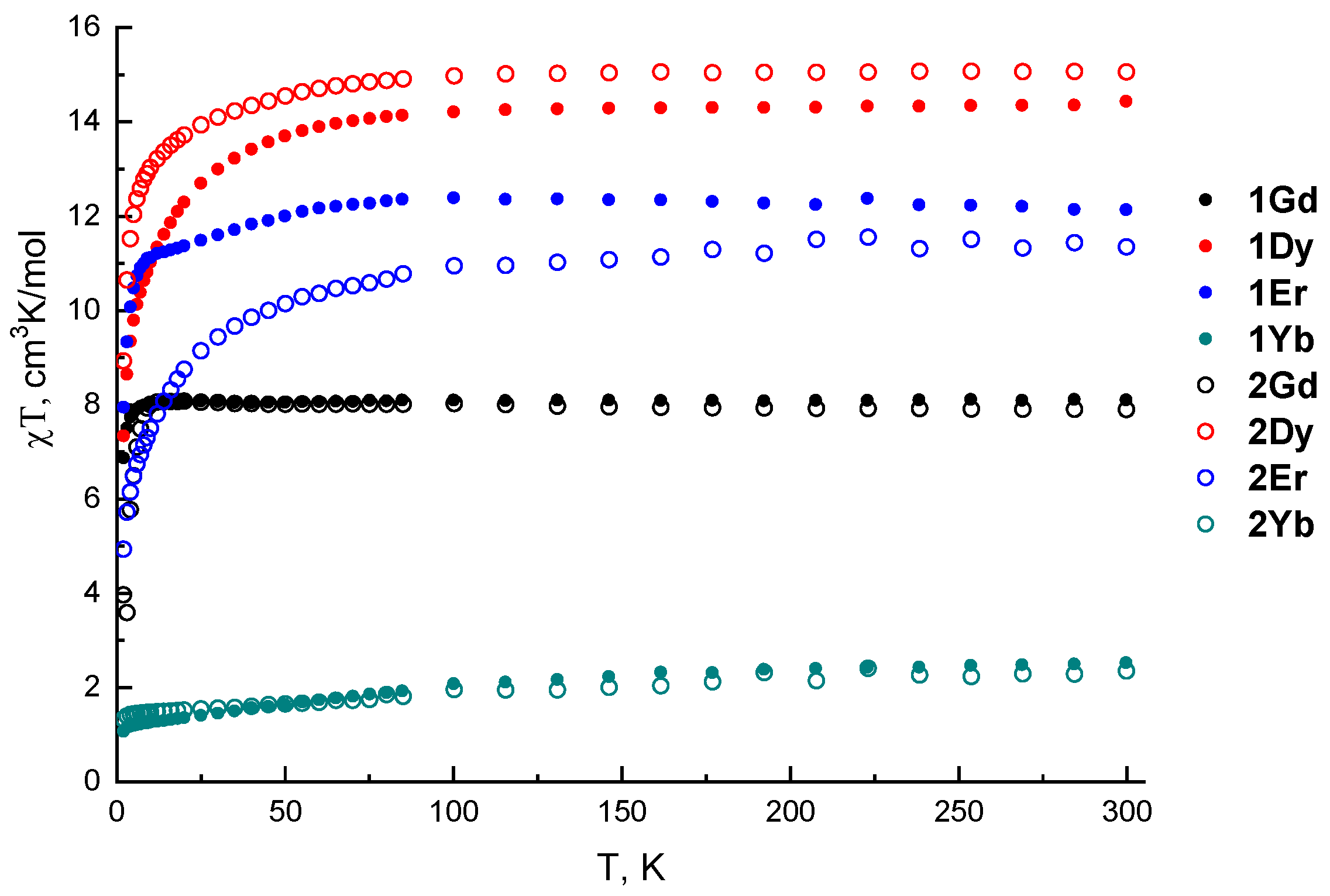
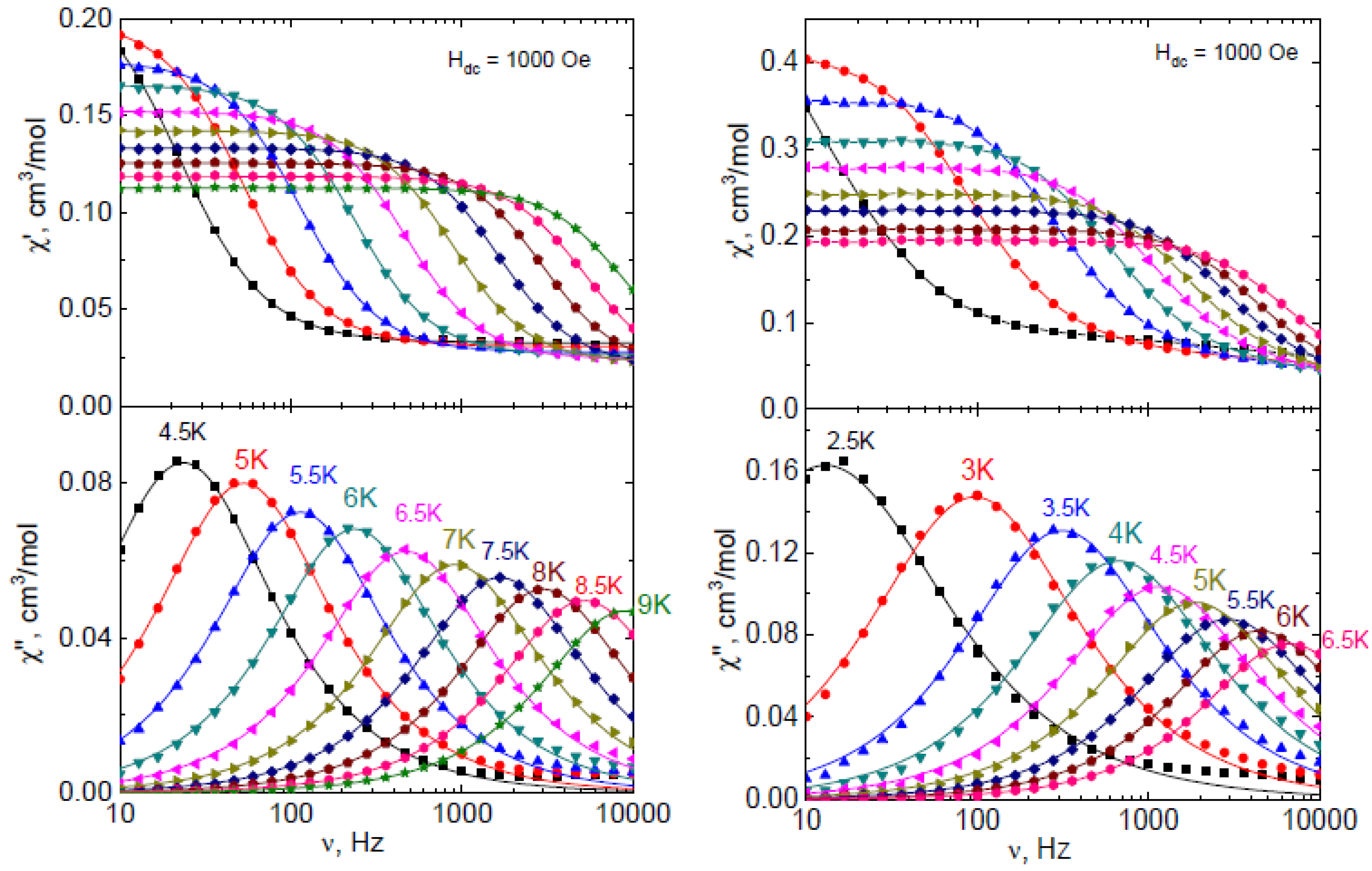
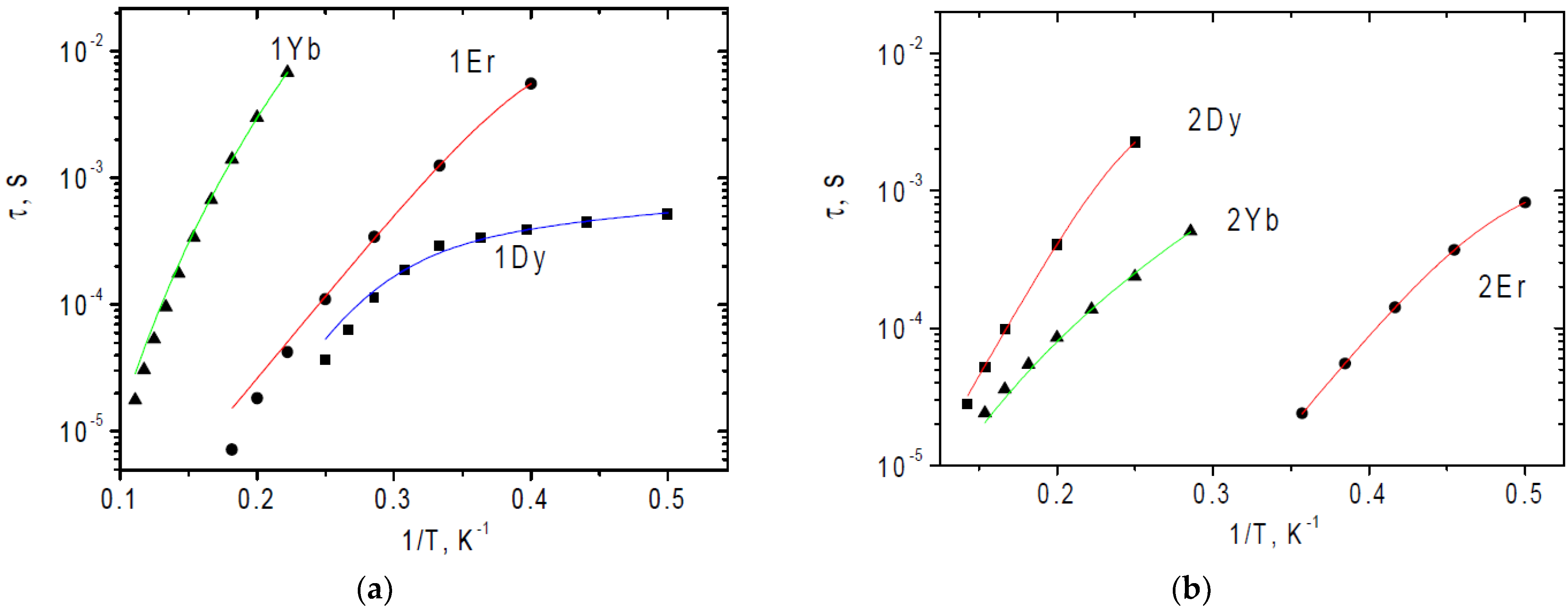
| Complex | χT (300 K) | χT Theor. | χT (2 K) |
|---|---|---|---|
| 1Gd | 8.10 | 7.88 | 6.87 |
| 1Dy | 14.44 | 14.17 | 7.34 |
| 1Er | 12.14 | 11.48 | 7.95 |
| 1Yb | 2.53 | 2.57 | 1.07 |
| 2Gd | 7.90 | 7.88 | 3.96 |
| 2Dy | 15.06 | 14.17 | 8.93 |
| 2Er | 11.35 | 11.48 | 4.94 |
| 2Yb | 2.35 | 2.57 | 1.33 |
| Complex | 1Dy | 1Er | 1Yb | 2Dy | 2Er | 2Yb | |
|---|---|---|---|---|---|---|---|
| Field, Oe | 1000 | 1000 | 1000 | 1500 | 1000 | 1000 | |
| T range, K | 2–4 | 2.5–5.5 | 4.5–9 | 4–7 | 2–4 | 3.5–6.5 | |
| Raman | C, K−n_Raman·s−1 | 0.057 * | - | 0.001 | - | - | 3.0 |
| nRaman | 9 ** | - | 7.9 | - | - | 5.2 | |
| Direct | Adirect, K−1 Oe−n_direct s−1 | 9.3 × 10−10 * | 3.4 × 10−11 * | - | 1.1 × 10−11 * | 3.9 × 10−10 * | - |
| ndirect | 4 ** | 4 ** | - | 4 ** | 4 ** | - | |
| Orbach | Ueff/kB, K (Ueff, cm−1) | - | 30 * (21) | - | 46 * (32) | 32 * (22) | - |
| τ0, s | - | 6.5 × 10−8 * | - | 4.3 × 10−8 * | 2.8 × 10−10 * | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrosyants, S.P.; Babeshkin, K.A.; Ilyukhin, A.B.; Koroteev, P.S.; Efimov, N.N. Something You Need Might Be under Your Feet: Molecular Magnetism of Heavy Kramers Lanthanide Hydrated Chlorides and Their Complexes with Polydentate Terpy Ligand. Magnetochemistry 2023, 9, 31. https://doi.org/10.3390/magnetochemistry9010031
Petrosyants SP, Babeshkin KA, Ilyukhin AB, Koroteev PS, Efimov NN. Something You Need Might Be under Your Feet: Molecular Magnetism of Heavy Kramers Lanthanide Hydrated Chlorides and Their Complexes with Polydentate Terpy Ligand. Magnetochemistry. 2023; 9(1):31. https://doi.org/10.3390/magnetochemistry9010031
Chicago/Turabian StylePetrosyants, Svetlana P., Konstantin A. Babeshkin, Andrey B. Ilyukhin, Pavel S. Koroteev, and Nikolay N. Efimov. 2023. "Something You Need Might Be under Your Feet: Molecular Magnetism of Heavy Kramers Lanthanide Hydrated Chlorides and Their Complexes with Polydentate Terpy Ligand" Magnetochemistry 9, no. 1: 31. https://doi.org/10.3390/magnetochemistry9010031
APA StylePetrosyants, S. P., Babeshkin, K. A., Ilyukhin, A. B., Koroteev, P. S., & Efimov, N. N. (2023). Something You Need Might Be under Your Feet: Molecular Magnetism of Heavy Kramers Lanthanide Hydrated Chlorides and Their Complexes with Polydentate Terpy Ligand. Magnetochemistry, 9(1), 31. https://doi.org/10.3390/magnetochemistry9010031






