Vibronic Relaxation Pathways in Molecular Spin Qubit Na9[Ho(W5O18)2]·35H2O under Pressure
Abstract
:1. Introduction
2. Methods
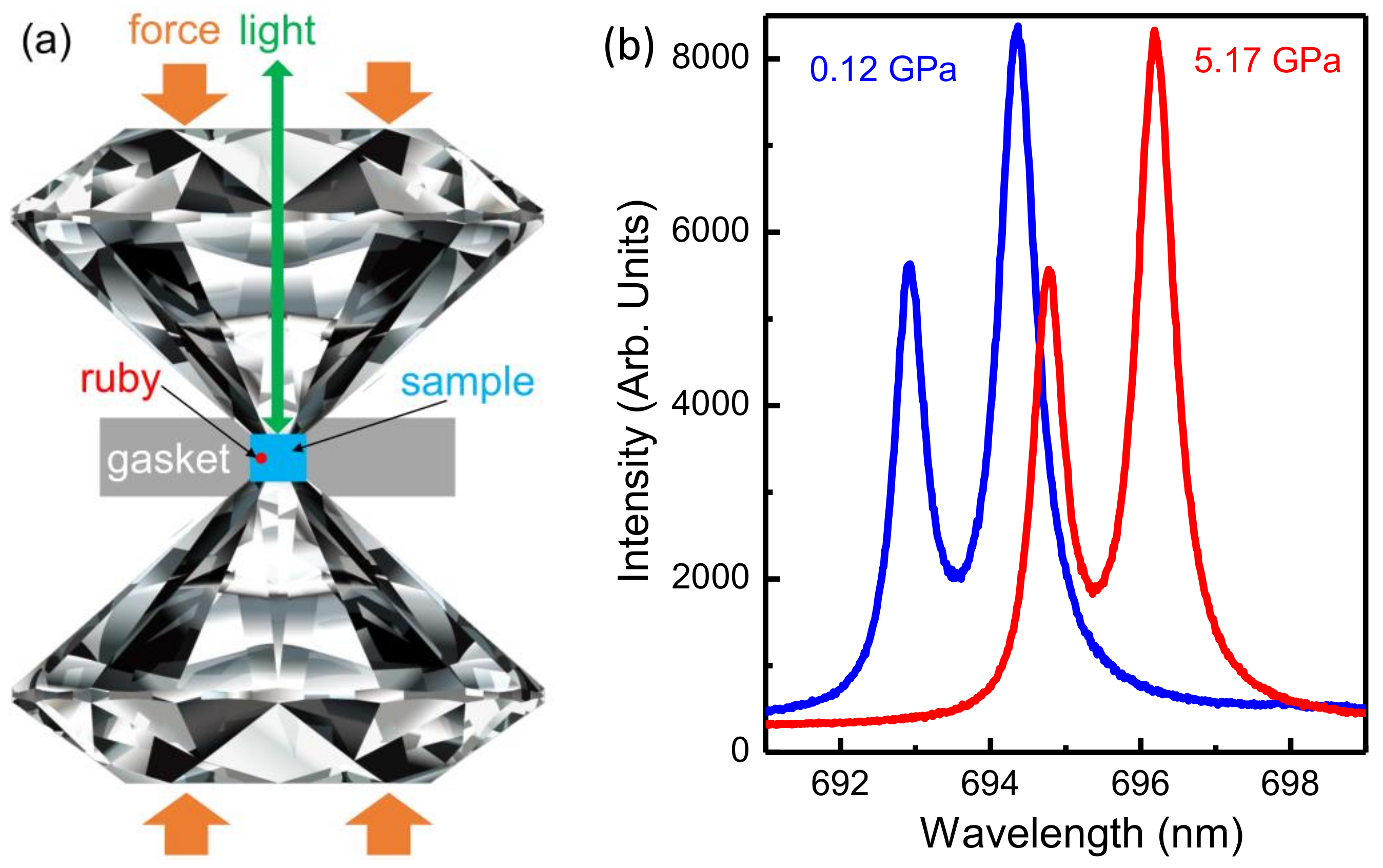
2.1. Experimental Setup
2.2. DFT Calculations
2.3. Semi-Empirical Crystal-Field Calculations
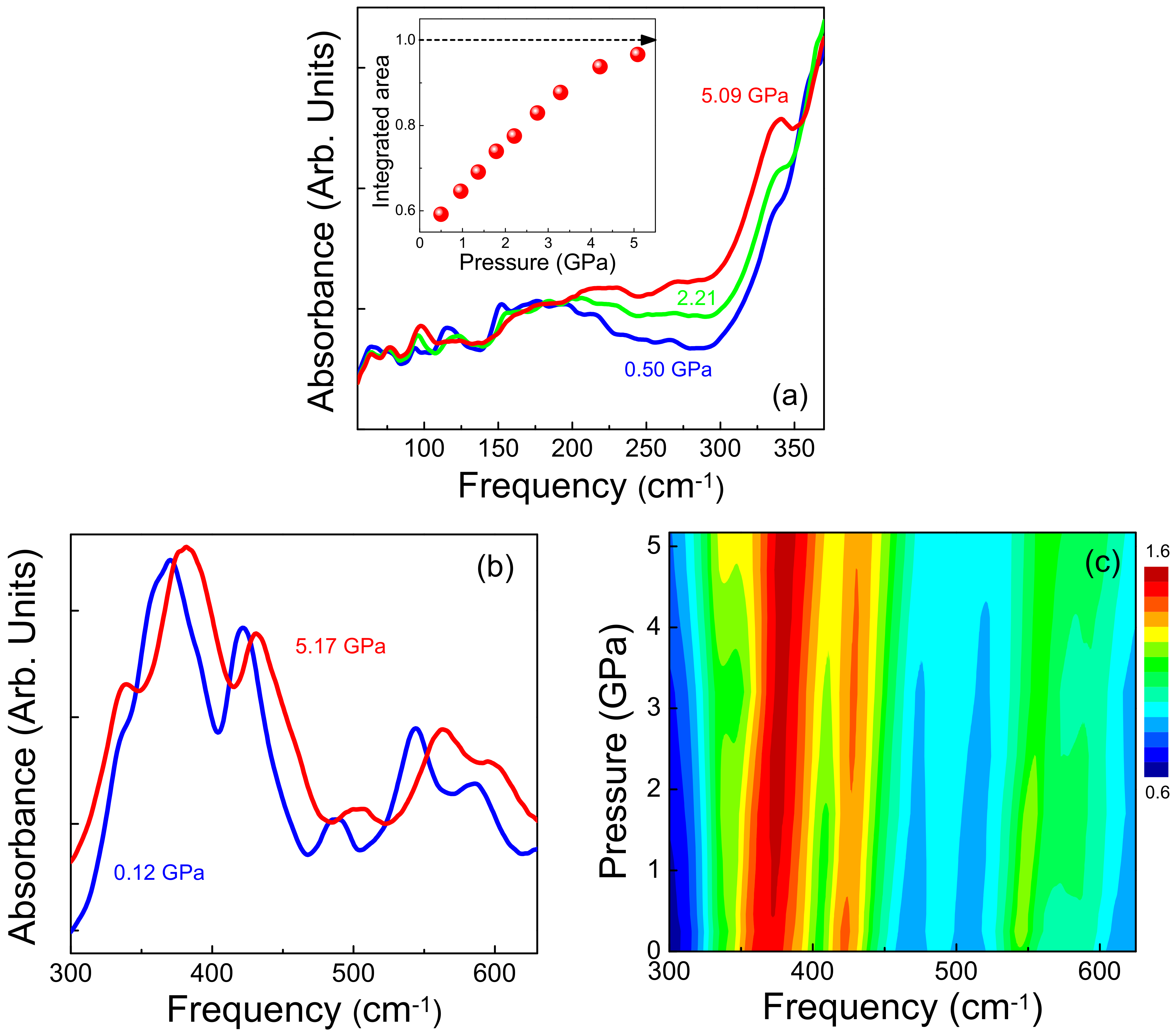
3. Results and Discussion
4. Summary and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woodruff, D.N.; Winpenny, R.E.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- McAdams, S.G.; Ariciu, A.-M.; Kostopoulos, A.K.; Walsh, J.P.; Tuna, F. Molecular single-ion magnets based on lanthanides and actinides: Design considerations and new advances in the context of quantum technologies. Coord. Chem. Rev. 2017, 346, 216–239. [Google Scholar] [CrossRef]
- Atzori, M.; Sessoli, R. The second quantum revolution: Role and challenges of molecular chemistry. J. Am. Chem. Soc. 2019, 141, 11339–11352. [Google Scholar] [CrossRef] [PubMed]
- Escalera-Moreno, L.; Baldoví, J.J.; Gaita-Ariño, A.; Coronado, E. Design of high-temperature f-block molecular nanomagnets through the control of vibration-induced spin relaxation. Chem. Sci. 2020, 11, 1593–1598. [Google Scholar] [CrossRef]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Baldoví, J.J.; Clemente-Juan, J.M.; Coronado, E.; Duan, Y.; Gaita-Ariño, A.; Gimenez-Saiz, C. Construction of a general library for the rational design of nanomagnets and spin qubits based on mononuclear f-block complexes: The polyoxometalate case. Inorg. Chem. 2014, 53, 9976–9980. [Google Scholar] [CrossRef]
- Chakarawet, K.; Atanasov, M.; Ellis, J.E.; Lukens, W.W., Jr.; Young, V.G., Jr.; Chatterjee, R.; Neese, F.; Long, J.R. Effect of spin–orbit coupling on phonon-mediated magnetic relaxation in a series of zero-valent vanadium, niobium, and tantalum isocyanide complexes. Inorg. Chem. 2021, 60, 18553–18560. [Google Scholar] [CrossRef]
- Gould, C.A.; McClain, K.R.; Reta, D.; Kragskow, J.G.C.; Marchiori, D.A.; Lachman, E.; Choi, E.-S.; Analytis, J.G.; Britt, R.D.; Chilton, N.F.; et al. Ultrahard magnetism from mixed-valence dilanthanide complexes with metal-metal bonding. Science 2022, 375, 198–202. [Google Scholar] [CrossRef]
- Duan, Y.; Rosaleny, L.E.; Coutinho, J.T.; Giménez-Santamarina, S.; Scheie, A.; Baldoví, J.J.; Cardona-Serra, S.; Gaita-Ariño, A. Data-driven design of molecular nanomagnets. Nat Commun 2022, 13, 7626. [Google Scholar] [CrossRef]
- Bader, K.; Dengler, D.; Lenz, S.; Endeward, B.; Jiang, S.-D.; Neugebauer, P.; Van Slageren, J. Room temperature quantum coherence in a potential molecular qubit. Nat. Commun. 2014, 5, 5304. [Google Scholar] [CrossRef] [Green Version]
- Zadrozny, J.M.; Niklas, J.; Poluektov, O.G.; Freedman, D.E. Millisecond coherence time in a tunable molecular electronic spin qubit. ACS Central Sci. 2015, 1, 488–492. [Google Scholar] [CrossRef]
- Atzori, M.; Tesi, L.; Morra, E.; Chiesa, M.; Sorace, L.; Sessoli, R. Room-temperature quantum coherence and rabi oscillations in vanadyl phthalocyanine: Toward multifunctional molecular spin qubits. J. Am. Chem. Soc. 2016, 138, 2154–2157. [Google Scholar] [CrossRef]
- Godfrin, C.; Ferhat, A.; Ballou, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W.; Balestro, F. Operating Quantum States in Single Magnetic Molecules: Implementation of Grover’s Quantum Algorithm. Phys. Rev. Lett. 2017, 119, 187702. [Google Scholar] [CrossRef]
- Gaita-Ariño, A.; Luis, F.; Hill, S.; Coronado, E. Molecular spins for quantum computation. Nat. Chem. 2019, 11, 301–309. [Google Scholar] [CrossRef]
- AlDamen, M.A.; Cardona-Serra, S.; Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A.; Marti-Gastaldo, C.; Luis, F.; Montero, O. Mononuclear lanthanide single molecule magnets based on the polyoxometalates [Ln(W5O18)2]9- and [Ln(β2-SiW11O39)2]13-, (LnIII = Tb, Dy, Ho, Er, Tm, and Yb). Inorg. Chem. 2009, 48, 3467–3479. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Goodwin, C.A.; Reta, D.; Ortu, F.; Chilton, N.F.; Mills, D.P. Synthesis and electronic structures of heavy lanthanide metallocenium cations. J. Am. Chem. Soc. 2017, 139, 18714–18724. [Google Scholar] [CrossRef]
- Long, J.; Ivanov, M.S.; Khomchenko, V.A.; Mamontova, E.; Thibaud, J.-M.; Rouquette, J.; Beaudhuin, M.; Granier, D.; Ferreira, R.A.S.; Carlos, L.D.; et al. Room temperature magnetoelectric coupling in a molecular ferroelectric ytterbium(III) complex. Science 2020, 367, 671–676. [Google Scholar] [CrossRef]
- Ghosh, S.; Datta, S.; Friend, L.; Cardona-Serra, S.; Gaita-Ariño, A.; Coronado, E.; Hill, S. Multi-frequency EPR studies of a mononuclear holmium single-molecule magnet based on the polyoxometalate [HoIII(W5O18)2]9-. Dalton Trans. 2012, 41, 13697–13704. [Google Scholar] [CrossRef]
- Shiddiq, M.; Komijani, D.; Duan, Y.; Gaita-Ariño, A.; Coronado, E.; Hill, S. Enhancing coherence in molecular spin qubits via atomic clock transitions. Nature 2016, 531, 348–351. [Google Scholar] [CrossRef]
- Vonci, M.; Giansiracusa, M.J.; Van den Heuvel, W.; Gable, R.W.; Moubaraki, B.; Murray, K.S.; Yu, D.; Mole, R.A.; Soncini, A.; Boskovic, C. Magnetic excitations in polyoxotungstate-supported lanthanoid single-molecule magnets: An inelastic neutron scattering and ab initio study. Inorg. Chem. 2017, 56, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Santamarina, S.; Cardona-Serra, S.; Clemente-Juan, J.M.; Gaita-Ariño, A.; Coronado, E. Exploiting clock transitions for the chemical design of resilient molecular spin qubits. Chem. Sci. 2020, 11, 10718–10728. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mrozek, J.; Duan, Y.; Ullah, A.; Baldoví, J.J.; Coronado, E.; Gaita-Ariño, A.; Ardavan, A. Quantum coherent spin-electric control in molecular nanomagnets. Nat. Phys. 2021, 17, 1205–1209. [Google Scholar] [CrossRef]
- Härtle, R.; Butzin, M.; Rubio-Pons, O.; Thoss, M. Quantum interference and decoherence in single-molecule junctions: How vibrations induce electrical current. Phys. Rev. Lett. 2011, 107, 046802. [Google Scholar] [CrossRef] [PubMed]
- Ballmann, S.; Härtle, R.; Coto, P.B.; Elbing, M.; Mayor, M.; Bryce, M.R.; Thoss, M.; Weber, H.B. Experimental evidence for quantum interference and vibrationally induced decoherence in single-molecule junctions. Phys. Rev. Lett. 2012, 109, 056801. [Google Scholar] [CrossRef]
- Hackenmuller, L.; Hornberger, K.; Brezger, B.; Zeilinger, A.; Arndt, M. Decoherence of matter waves by thermal emission of radiation. Nature 2004, 427, 711–714. [Google Scholar] [CrossRef]
- Chirolli, L.; Burkard, G. Decoherence in solid-state qubits. Adv. Phys. 2008, 57, 225–285. [Google Scholar] [CrossRef]
- Graham, M.J.; Zadrozny, J.M.; Shiddiq, M.; Anderson, J.S.; Fataftah, M.S.; Hill, S.; Freedman, D.E. Influence of electronic spin and spin–orbit coupling on decoherence in mononuclear transition metal complexes. J. Am. Chem. Soc. 2014, 136, 7623–7626. [Google Scholar] [CrossRef]
- Chen, J.; Hu, C.; Stanton, J.F.; Hill, S.; Cheng, H.-P.; Zhang, X.-G. Decoherence in molecular electron-spin qubits: Insights from quantum many-body simulations. J. Phys. Chem. Lett. 2020, 11, 2074–2078. [Google Scholar] [CrossRef]
- Ullah, A.; Hu, Z.; Cerda, J.; Aragó, J.; Gaita-Ariño, A. Electrical two-qubit gates within a pair of clock-qubit magnetic molecules. NPJ Quantum Inf. 2022, 8, 133. [Google Scholar] [CrossRef]
- Giansiracusa, M.J.; Kostopoulos, A.K.; Collison, D.; Winpenny, R.E.P.; Chilton, N.F. Correlating blocking temperatures with relaxation mechanisms in monometallic single-molecule magnets with high energy barriers (Ueff > 600 K). Chem. Commun. 2019, 55, 7025–7028. [Google Scholar] [CrossRef]
- Gu, L.; Wu, R. Origin of the anomalously low Raman exponents in single molecule magnets. Phys. Rev. B 2021, 103, 014401. [Google Scholar] [CrossRef]
- Kragskow, J.G.C.; Marbey, J.; Buch, C.D.; Nehrkorn, J.; Ozerov, M.; Piligkos, S.; Hill, S.; Chilton, N.F. Analysis of vibronic coupling in a 4f molecular magnet with FIRMS. Nat. Commun. 2022, 13, 825. [Google Scholar] [CrossRef]
- Lunghi, A.; Totti, F.; Sessoli, R.; Sanvito, S. The role of anharmonic phonons in under-barrier spin relaxation of single molecule magnets. Nat. Commun. 2017, 8, 14620. [Google Scholar] [CrossRef]
- Lunghi, A. Toward exact predictions of spin-phonon relaxation times: An ab initio implementation of open quantum systems theory. Sci. Adv. 2022, 8, eabn7880. [Google Scholar] [CrossRef]
- Mondal, S.; Lunghi, A. Spin-phonon decoherence in solid-state paramagnetic defects from first principles. arXiv 2022, arXiv:2212.11705. [Google Scholar]
- Santanni, F.; Albino, A.; Atzori, M.; Ranieri, D.; Salvadori, E.; Chiesa, M.; Lunghi, A.; Bencini, A.; Sorace, L.; Totti, F.; et al. Probing vibrational symmetry effects and nuclear spin economy principles in molecular spin qubits. Inorg. Chem. 2021, 60, 140–151. [Google Scholar]
- Blockmon, A.L.; Ullah, A.; Hughey, K.D.; Duan, Y.; O’Neal, K.R.; Baldovi, J.J.; Aragó, J.; Gaita-Ariño, A.; Coronado, E.; Musfeldt, J.L. Spectroscopic analysis of vibronic relaxation pathways in molecular spin qubit [Ho(W5O18)2]9-: Sparse spectra are key. Inorg. Chem. 2021, 60, 14096–14104. [Google Scholar] [CrossRef]
- Brinzari, T.V.; Haraldsen, J.T.; Chen, P.; Sun, Q.-C.; Kim, Y.; Tung, L.-C.; Litvinchuk, A.P.; Schlueter, J.A.; Smirnov, D.; Manson, J.L.; et al. Electron-phonon and magnetoelastic interactions in ferromagnetic Co[N(CN)2]2. Phys. Rev. Lett. 2013, 111, 047202. [Google Scholar] [CrossRef]
- Ullah, A.; Cerdá, J.; Baldoví, J.J.; Varganov, S.A.; Aragó, J.; Gaita-Ariño, A. In silico molecular engineering of dysprosocenium-based complexes to decouple spin energy levels from molecular vibrations. J. Phys. Chem. Lett. 2019, 10, 7678–7683. [Google Scholar] [CrossRef]
- Garlatti, E.; Tesi, L.; Lunghi, A.; Atzori, M.; Voneshen, D.; Santini, P.; Sanvito, S.; Guidi, T.; Sessoli, R.; Carretta, S. Unveiling phonons in a molecular qubit with four-dimensional inelastic neutron scattering and density functional theory. Nat. Comm. 2020, 11, 1751. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-J.; Von Kugelgen, S.; Krzyaniak, M.D.; Ji, W.; Dichtel, W.R.; Wasielewski, M.R.; Freedman, D.E. Spin and phonon design in modular arrays of molecular qubits. Chem. Mater. 2020, 32, 10200–10206. [Google Scholar] [CrossRef]
- Bayliss, S.L.; Deb, P.; Laorenza, D.W.; Onizhuk, M.; Galli, G.; Freedman, D.E.; Awschalom, D.D. Enhancing spin coherence in optically addressable molecular qubits through host-matrix control. Phys. Rev. X. 2022, 12, 031028. [Google Scholar] [CrossRef]
- Zvyagin, S.A.; Graf, D.; Sakurai, T.; Kimura, S.; Nojiri, H.; Wosnitza, J.; Ohta, H.; Ono, T.; Tanaka, H. Pressure-tuning the quantum spin Hamiltonian of the triangular lattice antiferromagnet Cs2CuCl4. Nat. Commun. 2019, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Escalera-Moreno, L.; Baldovi, J.J.; Gaita-Ariño, A.; Coronado, E. Spin states, vibrations and spin relaxation in molecular nanomagnets and spin qubits: A critical perspective. Chem. Sci. 2018, 9, 3265–3275. [Google Scholar] [CrossRef]
- Mao, H.K.; Bell, P.M.; Shaner, J.W.; Steinberg, D.J. Specific volume measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R1 fluorescence pressure gauge from 0.06 to 1 Mbar. J. Appl. Phys. 1976, 49, 3276–3283. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Cao, X.; Dolg, M. Valence basis sets for relativistic energy-consistent small-core lanthanide pseudopotentials. J. Chem. Phys. 2001, 115, 7348. [Google Scholar] [CrossRef]
- Cao, X.; Dolg, M. Segmented contraction scheme for small-core lanthanide pseudopotential basis sets. J. Mol. Struc.-THEOCHEM 2002, 581, 139–147. [Google Scholar] [CrossRef]
- Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted ab initio pseudopotentials for the rare earth elements. J. Chem. Phys. 1989, 90, 1730–1734. [Google Scholar] [CrossRef]
- Ross, R.; Powers, J.; Atashroo, T.; Ermler, W.; LaJohn, L.; Christiansen, P. Ab initio relativistic effective potentials with spin–orbit operators. IV. Cs through Rn. J. Chem. Phys. 1990, 93, 6654–6670. [Google Scholar] [CrossRef]
- Hariharan, P.; Pople, J. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Hehre, W.; Ditchfield, R.; Pople, J. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Baldoví, J.; Borrás-Almenar, J.; Clemente-Juan, J.; Coronado, E.; Gaita-Ariño, A. Modeling the properties of lanthanoid single-ion magnets using an effective point-charge approach. Dalton Trans. 2012, 41, 13705–13710. [Google Scholar] [CrossRef]
- Baldoví, J.J.; Cardona-Serra, S.; Clemente-Juan, J.M.; Coronado, E.; Gaita-Ariño, A.; Palii, A. SIMPRE: A software package to calculate crystal field parameters, energy levels, and magnetic properties on mononuclear lanthanoid complexes based on charge distributions. J. Comput. Chem. 2013, 34, 1961–1967. [Google Scholar] [CrossRef]
- Musfeldt, J.L.; Brinzari, T.V.; O’Neal, K.R.; Chen, P.; Schlueter, J.A.; Manson, J.L.; Litvinchuk, A.P.; Liu, Z. Pressure-temperature phase diagram reveals spin-lattice interactions in Co[N(CN)2]2. Inorg. Chem. 2017, 56, 4950–4955. [Google Scholar] [CrossRef]
- Hughey, K.D.; Harms, N.C.; O’Neal, K.R.; Clune, A.J.; Monroe, J.C.; Blockmon, A.L.; Landee, C.P.; Liu, Z.; Ozerov, M.; Musfeldt, J.L. Spin-lattice coupling across the magnetic quantum phase transition in Cu-containing coordination polymers. Inorg. Chem. 2020, 59, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Clune, A.; Harms, N.C.; O’Neal, K.R.; Hughey, K.D.; Smith, K.; Obeysekera, D.; Haddock, J.; Dalal, N.; Yang, J.; Liu, Z.; et al. Developing the pressure—Temperature—Magnetic field phase diagram of multiferroic [(CH3)2NH2]Mn(HCOO)3. Inorg. Chem. 2020, 59, 10083–10090. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musfeldt, J.L.; Liu, Z.; López-Alcalá, D.; Duan, Y.; Gaita-Ariño, A.; Baldoví, J.J.; Coronado, E. Vibronic Relaxation Pathways in Molecular Spin Qubit Na9[Ho(W5O18)2]·35H2O under Pressure. Magnetochemistry 2023, 9, 53. https://doi.org/10.3390/magnetochemistry9020053
Musfeldt JL, Liu Z, López-Alcalá D, Duan Y, Gaita-Ariño A, Baldoví JJ, Coronado E. Vibronic Relaxation Pathways in Molecular Spin Qubit Na9[Ho(W5O18)2]·35H2O under Pressure. Magnetochemistry. 2023; 9(2):53. https://doi.org/10.3390/magnetochemistry9020053
Chicago/Turabian StyleMusfeldt, Janice L., Zhenxian Liu, Diego López-Alcalá, Yan Duan, Alejandro Gaita-Ariño, José J. Baldoví, and Eugenio Coronado. 2023. "Vibronic Relaxation Pathways in Molecular Spin Qubit Na9[Ho(W5O18)2]·35H2O under Pressure" Magnetochemistry 9, no. 2: 53. https://doi.org/10.3390/magnetochemistry9020053
APA StyleMusfeldt, J. L., Liu, Z., López-Alcalá, D., Duan, Y., Gaita-Ariño, A., Baldoví, J. J., & Coronado, E. (2023). Vibronic Relaxation Pathways in Molecular Spin Qubit Na9[Ho(W5O18)2]·35H2O under Pressure. Magnetochemistry, 9(2), 53. https://doi.org/10.3390/magnetochemistry9020053







