Magnetic Switchability via Thermal-Induced Structural Phase Transitions in Molecular Solids
Abstract
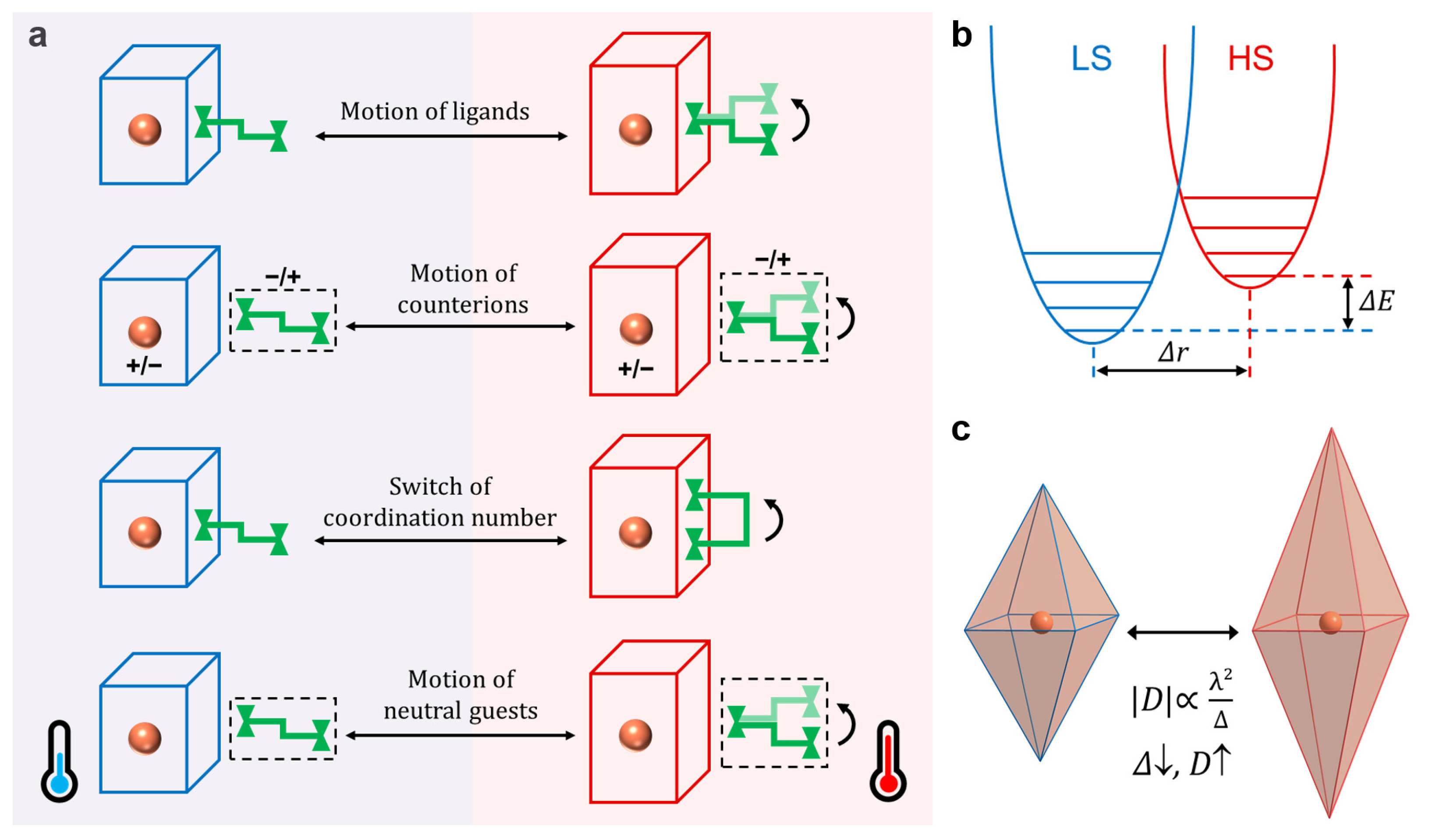
:1. Introduction
2. Thermal-Induced Dynamics of Ligands
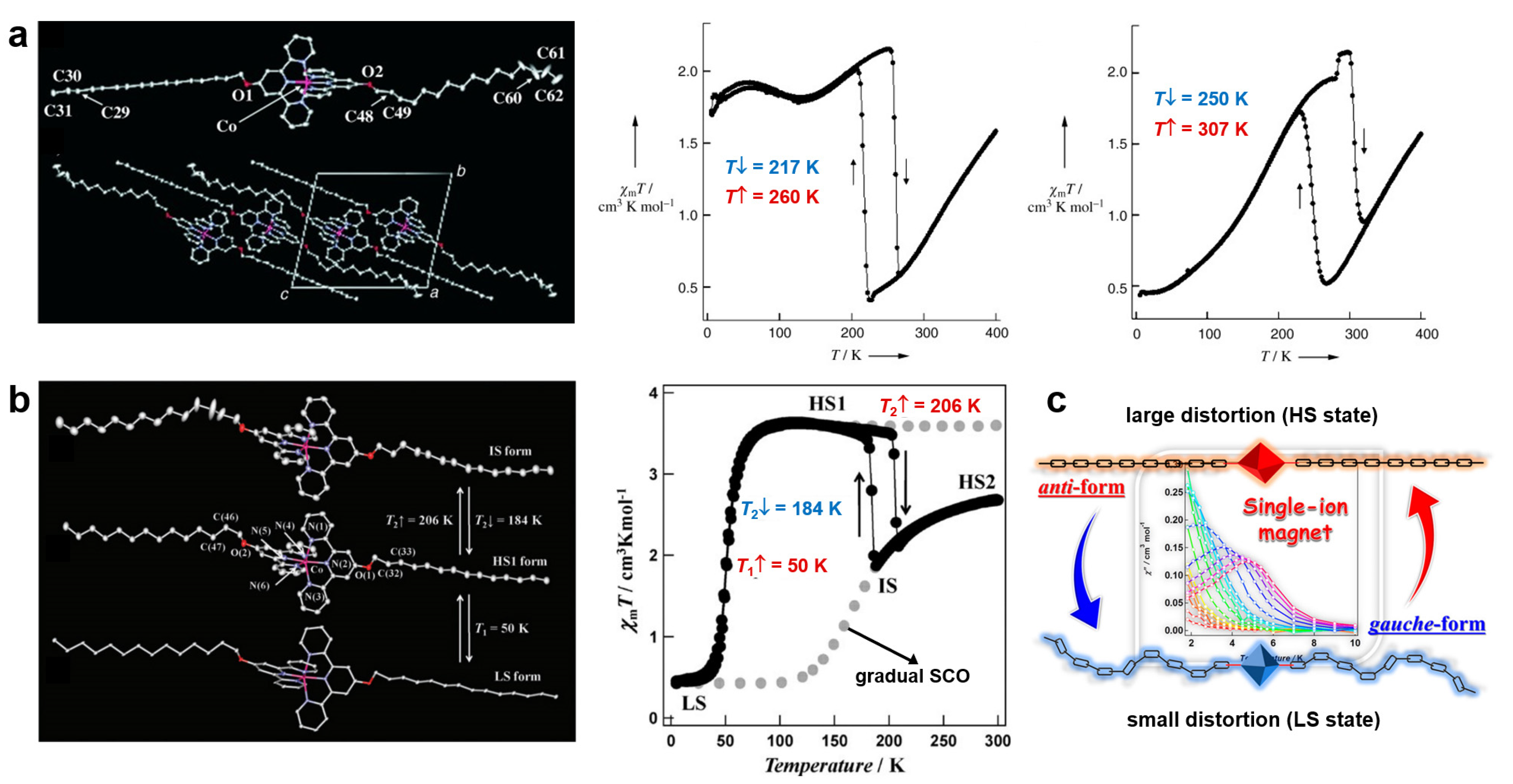
2.1. Alkyl Chains

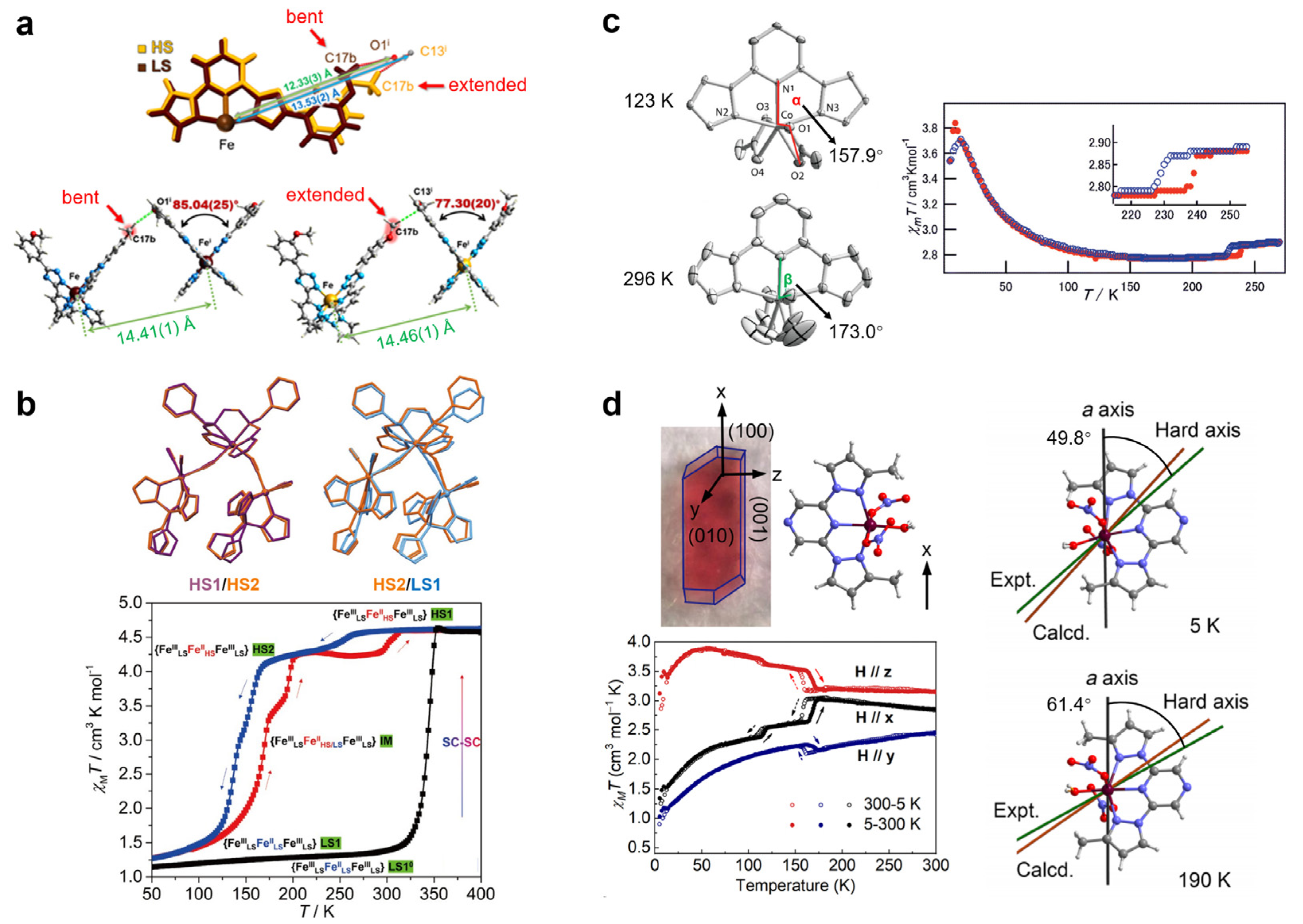
2.2. Non-Alkyl Groups
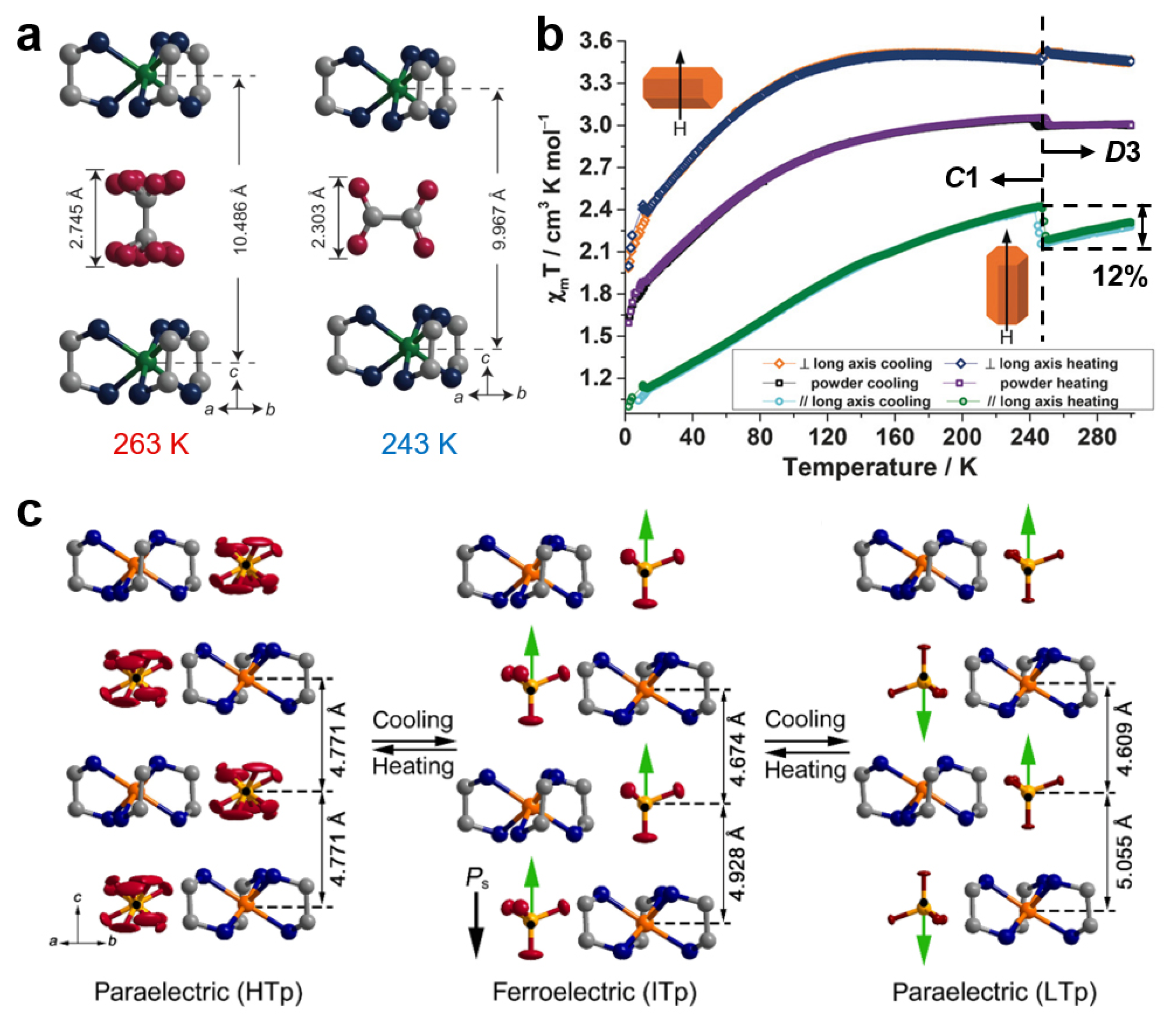
3. Thermal-Induced Dynamics of Counterions
3.1. Countercations
3.2. Counteranions
4. Thermal-Induced Dynamics of Coordination Number
5. Thermal-Induced Dynamics of Neutral Guests
6. Summary and Perspective
- (1)
- One of the most important goals of magnetic switches is to achieve wide thermal hysteresis centered at room temperature. This means that both states can be accessible at the same temperature for such materials. The thermal hysteresis width varies greatly among the complexes with thermal-induced phase transitions summarized herein (Table 1). Apparently, the modulation of the orbital angular momentum is usually accompanied by a narrower thermal hysteresis compared to those of the spin transition. The thermal hysteresis of [FeII(2-(5-(3-methoxy-4H-1,2,4-triazol-3-yl)-6-(1H-pyrazol-1-yl))pyridine)] can even reach 105 K, which is not inferior to the conventional SCO complexes with large hysteresis [99,100,101,102]. This demonstrates the potential of flexible complexes that can undergo structural phase transitions. The supramolecular interactions (hydrogen bonding, π···π stacking, etc.) may have an important influence on the intermolecular cooperative effect, which in turn can regulate the phase transition temperature as well as the thermal hysteresis width. As a result, when designing structural phase transition complexes, supramolecular cooperativity should be appropriately introduced and explored. However, the detailed mechanism has not yet been elucidated at present. A lot of exploration is still needed to achieve a deeper understanding by further realizing targeted design and synthesis.
- (2)
- The reversible switching of coordination number is more pronounced to modulate the orbital contribution, and it has the potential to facilely construct the supramolecular structures by using neutral guests, since the neutral guests do not require compatible counterion fragments to compensate for the charge imbalance. However, there are few studies of these two types. To disrupt this scarcity, the tactics for designing these types need to be further explored.
- (3)
- Likewise, the stimuli of light, electric or magnetic field may cause reversible changes in the crystal structure to regulate magnetic properties, thus providing research interest and promising applications in optical switches and magnetoelectric devices. Furthermore, apart from the sole control of heat, light and electric or magnetic field, it is wonderful to realize the response of a single moiety to multiple stimuli, as well as the cooperative response of multiple moieties to multiple stimuli.
- (4)
- Finally, the precise design of materials with switchable magnetic characteristics is still challenging. To guide the development of high-performance functional materials, it is necessary to have a deeper understanding of the roles between crystal structures and structural phase transition, as well as magneto-structural correlation. More importantly, designing and realizing multifunctional materials with strong synergistic effects between magnetic, electric, fluorescent, and other physical properties is very helpful.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gütlich, P.; Hauser, A.; Spiering, H. Thermal and Optical Switching of Iron(II) Complexes. Angew. Chem. Int. Ed. Engl. 1994, 33, 2024–2054. [Google Scholar] [CrossRef]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nat. Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Ni, Z.P.; Liu, J.L.; Hoque, M.N.; Liu, W.; Li, J.Y.; Chen, Y.C.; Tong, M.L. Recent advances in guest effects on spin-crossover behavior in Hofmann-type metal-organic frameworks. Coord. Chem. Rev. 2017, 335, 28–43. [Google Scholar] [CrossRef]
- Zhao, L.; Meng, Y.S.; Liu, Q.; Sato, O.; Shi, Q.; Oshio, H.; Liu, T. Switching the magnetic hysteresis of an [Feii–NC–Wv]-based coordination polymer by photoinduced reversible spin crossover. Nat. Chem. 2021, 13, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Ohkoshi, S.i.; Imoto, K.; Tsunobuchi, Y.; Takano, S.; Tokoro, H. Light-induced spin-crossover magnet. Nat. Chem. 2011, 3, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.C.; Real, J.A. Thermo-, piezo-, photo- and chemo-switchable spin crossover iron(II)-metallocyanate based coordination polymers. Coord. Chem. Rev. 2011, 255, 2068–2093. [Google Scholar] [CrossRef]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Brooker, S. Spin crossover with thermal hysteresis: Practicalities and lessons learnt. Chem. Soc. Rev. 2015, 44, 2880–2892. [Google Scholar] [CrossRef] [Green Version]
- Halcrow, M.A. (Ed.) Spin-Crossover Materials: Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Heersche, H.B.; de Groot, Z.; Folk, J.A.; van der Zant, H.S.J.; Romeike, C.; Wegewijs, M.R.; Zobbi, L.; Barreca, D.; Tondello, E.; Cornia, A. Electron Transport through Single Mn12 Molecular Magnets. Phys. Rev. Lett. 2006, 96, 206801. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, P.; Riso, C. Magnetic force microscopy revealing long range molecule impact on magnetic tunnel junction based molecular spintronics devices. Org. Electron. 2019, 75, 105421. [Google Scholar] [CrossRef] [Green Version]
- Dei, A.; Gatteschi, D.; Sangregorio, C.; Sorace, L. Quinonoid Metal Complexes: Toward Molecular Switches. Acc. Chem. Res. 2004, 37, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Tezgerevska, T.; Alley, K.G.; Boskovic, C. Valence tautomerism in metal complexes: Stimulated and reversible intramolecular electron transfer between metal centers and organic ligands. Coord. Chem. Rev. 2014, 268, 23–40. [Google Scholar] [CrossRef]
- Sato, O.; Tao, J.; Zhang, Y.Z. Control of Magnetic Properties through External Stimuli. Angew. Chem. Int. Ed. 2007, 46, 2152–2187. [Google Scholar] [CrossRef]
- Sato, O. Dynamic molecular crystals with switchable physical properties. Nat. Chem. 2016, 8, 644–656. [Google Scholar] [CrossRef]
- Yao, Z.S.; Tang, Z.; Tao, J. Bistable molecular materials with dynamic structures. Chem. Commun. 2020, 56, 2071–2086. [Google Scholar] [CrossRef] [PubMed]
- Binder, K. Theory of first-order phase transitions. Rep. Prog. Phys. 1987, 50, 783. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, R.G. Ferroelectric Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1163–1195. [Google Scholar] [CrossRef]
- Cowley, R.A. Structural phase transitions I. Landau theory. Adv. Phys. 1980, 29, 13–59. [Google Scholar] [CrossRef]
- Müller, U. Symmetry Relations at Phase Transitions; Oxford University Press: New York, NY, USA, 2013; pp. 196–215. [Google Scholar]
- Clarke, J.B.; Hastie, J.W.; Kihlborg, L.H.E.; Metselaar, R.; Thackeray, M.M. Definitions of terms relating to phase transitions of the solid state (IUPAC Recommendations 1994). Pure Appl. Chem. 1994, 66, 577–594. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Han, X.B.; Zhang, W. Structural phase transition-associated dielectric transition and ferroelectricity in coordination compounds. Coord. Chem. Rev. 2019, 378, 561–576. [Google Scholar] [CrossRef]
- Benelli, C.; Gatteschi, D. Introduction to Molecular Magnetism: From Transition Metals to Lanthanides; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Gao, S. (Ed.) Molecular Nanomagnets and Related Phenomena; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Boča, R. Zero-field splitting in metal complexes. Coord. Chem. Rev. 2004, 248, 757–815. [Google Scholar] [CrossRef]
- Gomez-Coca, S.; Cremades, E.; Aliaga-Alcalde, N.; Ruiz, E. Mononuclear Single-Molecule Magnets: Tailoring the Magnetic Anisotropy of First-Row Transition-Metal Complexes. J. Am. Chem. Soc. 2013, 135, 7010–7018. [Google Scholar] [CrossRef] [PubMed]
- Novikov, V.V.; Pavlov, A.A.; Nelyubina, Y.V.; Boulon, M.-E.; Varzatskii, O.A.; Voloshin, Y.Z.; Winpenny, R.E.P. A Trigonal Prismatic Mononuclear Cobalt(II) Complex Showing Single-Molecule Magnet Behavior. J. Am. Chem. Soc. 2015, 137, 9792–9795. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, X.H.; Deng, Y.F.; Zhang, X.Y.; Chang, X.Y.; Zheng, Z.; Zhang, Y.Z. Azido-Cyanide Mixed-Bridged FeIII–NiII Complexes. Inorg. Chem. 2020, 59, 16215–16224. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Xiao, D.J.; Atanasov, M.; Long, G.J.; Grandjean, F.; Neese, F.; Long, J.R. Magnetic blocking in a linear iron(I) complex. Nat. Chem. 2013, 5, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.N.; Du, J.Z.; Zhang, Y.Q.; Leng, X.B.; Yang, M.W.; Jiang, S.D.; Wang, Z.X.; Ouyang, Z.W.; Deng, L.; Wang, B.W.; et al. Two-Coordinate Co(II) Imido Complexes as Outstanding Single-Molecule Magnets. J. Am. Chem. Soc. 2017, 139, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Kassem, S.; van Leeuwen, T.; Lubbe, A.S.; Wilson, M.R.; Feringa, B.L.; Leigh, D.A. Artificial molecular motors. Chem. Soc. Rev. 2017, 46, 2592–2621. [Google Scholar] [CrossRef] [Green Version]
- Coskun, A.; Banaszak, M.; Astumian, R.D.; Stoddart, J.F.; Grzybowski, B.A. Great expectations: Can artificial molecular machines deliver on their promise? Chem. Soc. Rev. 2012, 41, 19–30. [Google Scholar] [CrossRef]
- Browne, W.R.; Feringa, B.L. Making molecular machines work. Nat. Nanotechnol. 2006, 1, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef] [Green Version]
- Moulin, E.; Faour, L.; Carmona-Vargas, C.C.; Giuseppone, N. From Molecular Machines to Stimuli-Responsive Materials. Adv. Mater. 2020, 32, 1906036. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, L.; Tilby, M.J.; Szpera, R.; Smith, O.A.; Bunce, H.A.P.; Fletcher, S.P. Molecular machines for catalysis. Nat. Rev. Chem. 2018, 2, 0117. [Google Scholar] [CrossRef]
- Aprahamian, I. The Future of Molecular Machines. ACS Cent. Sci. 2020, 6, 347–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.L.; Southerland, H.; Li, J.R.; Prosvirin, A.V.; Zhao, H.; Dunbar, K.R. Crystal-to-Crystal Transformation of Magnets Based on Heptacyanomolybdate(III) Involving Dramatic Changes in Coordination Mode and Ordering Temperature. Angew. Chem. Int. Ed. 2012, 51, 9321–9324. [Google Scholar] [CrossRef]
- Zheng, T.; Clemente-Juan, J.M.; Ma, J.; Dong, L.; Bao, S.S.; Huang, J.; Coronado, E.; Zheng, L.M. Breathing Effect in a Cobalt Phosphonate upon Dehydration/Rehydration: A Single-Crystal-to-Single-Crystal Study. Chem. Eur. J. 2013, 19, 16394–16402. [Google Scholar] [CrossRef]
- Fan, K.; Xu, F.; Kurmoo, M.; Huang, X.D.; Liao, C.H.; Bao, S.S.; Xue, F.; Zheng, L.M. Metal–Metalloligand Coordination Polymer Embedding Triangular Cobalt–Oxo Clusters: Solvent- and Temperature-Induced Crystal to Crystal Transformations and Associated Magnetism. Inorg. Chem. 2020, 59, 8935–8945. [Google Scholar] [CrossRef] [PubMed]
- Galyametdinov, Y.; Ksenofontov, V.; Prosvirin, A.; Ovchinnikov, I.; Ivanova, G.; Gütlich, P.; Haase, W. First Example of Coexistence of Thermal Spin Transition and Liquid-Crystal Properties. Angew. Chem. Int. Ed. 2001, 40, 4269–4271. [Google Scholar] [CrossRef]
- Fujigaya, T.; Jiang, D.L.; Aida, T. Switching of Spin States Triggered by a Phase Transition: Spin-Crossover Properties of Self-Assembled Iron(II) Complexes with Alkyl-Tethered Triazole Ligands. J. Am. Chem. Soc. 2003, 125, 14690–14691. [Google Scholar] [CrossRef]
- Hayami, S.; Shigeyoshi, Y.; Akita, M.; Inoue, K.; Kato, K.; Osaka, K.; Takata, M.; Kawajiri, R.; Mitani, T.; Maeda, Y. Reverse Spin Transition Triggered by a Structural Phase Transition. Angew. Chem. Int. Ed. 2005, 44, 4899–4903. [Google Scholar] [CrossRef]
- Zheng, J.; Kwak, K.; Xie, J.; Fayer, M.D. Ultrafast Carbon-Carbon Single-Bond Rotational Isomerization in Room-Temperature Solution. Science 2006, 313, 1951–1955. [Google Scholar] [CrossRef] [Green Version]
- Sorai, M.; Saito, K. Alkyl chains acting as entropy reservoir in liquid crystalline materials. Chem. Rec. 2003, 3, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Hayami, S.; Murata, K.; Urakami, D.; Kojima, Y.; Akita, M.; Inoue, K. Dynamic structural conversion in a spin-crossover cobalt(ii) compound with long alkyl chains. Chem. Commun. 2008, 6510–6512. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, F.; Ohtani, R.; Nakamura, M.; Lindoy, L.F.; Hayami, S. Slow Magnetic Relaxation Triggered by a Structural Phase Transition in Long-Chain-Alkylated Cobalt(II) Single-Ion Magnets. Inorg. Chem. 2019, 58, 7409–7415. [Google Scholar] [CrossRef] [PubMed]
- Seredyuk, M.; Muñoz, M.C.; Castro, M.; Romero-Morcillo, T.; Gaspar, A.B.; Real, J.A. Unprecedented Multi-Stable Spin Crossover Molecular Material with Two Thermal Memory Channels. Chem. Eur. J. 2013, 19, 6591–6596. [Google Scholar] [CrossRef] [PubMed]
- Rosario-Amorin, D.; Dechambenoit, P.; Bentaleb, A.; Rouzières, M.; Mathonière, C.; Clérac, R. Multistability at Room Temperature in a Bent-Shaped Spin-Crossover Complex Decorated with Long Alkyl Chains. J. Am. Chem. Soc. 2018, 140, 98–101. [Google Scholar] [CrossRef]
- Zhao, Q.; Xue, J.P.; Liu, Z.K.; Yao, Z.S.; Tao, J. Spin-crossover iron(ii) long-chain complex with slow spin equilibrium at low temperatures. Dalton Trans. 2021, 50, 11106–11112. [Google Scholar] [CrossRef] [PubMed]
- Su, S.Q.; Kamachi, T.; Yao, Z.S.; Huang, Y.G.; Shiota, Y.; Yoshizawa, K.; Azuma, N.; Miyazaki, Y.; Nakano, M.; Maruta, G.; et al. Assembling an alkyl rotor to access abrupt and reversible crystalline deformation of a cobalt(II) complex. Nat. Commun. 2015, 6, 8810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seredyuk, M.; Znovjyak, K.; Valverde-Muñoz, F.J.; da Silva, I.; Muñoz, M.C.; Moroz, Y.S.; Real, J.A. 105 K Wide Room Temperature Spin Transition Memory Due to a Supramolecular Latch Mechanism. J. Am. Chem. Soc. 2022, 144, 14297–14309. [Google Scholar] [CrossRef]
- Zhao, X.H.; Shao, D.; Chen, J.T.; Gan, D.X.; Yang, J.; Zhang, Y.Z. A trinuclear {FeIII2FeII} complex involving both spin and non-spin transitions exhibits three-step and wide thermal hysteresis. Sci. China Chem. 2022, 65, 532–538. [Google Scholar] [CrossRef]
- Juhász, G.; Matsuda, R.; Kanegawa, S.; Inoue, K.; Sato, O.; Yoshizawa, K. Bistability of Magnetization without Spin-Transition in a High-Spin Cobalt(II) Complex due to Angular Momentum Quenching. J. Am. Chem. Soc. 2009, 131, 4560–4561. [Google Scholar] [CrossRef]
- Su, S.Q.; Wu, S.Q.; Hagihala, M.; Miao, P.; Tan, Z.; Torii, S.; Kamiyama, T.; Xiao, T.; Wang, Z.; Ouyang, Z.; et al. Water-oriented magnetic anisotropy transition. Nat. Commun. 2021, 12, 2738. [Google Scholar] [CrossRef] [PubMed]
- Akutagawa, T.; Shitagami, K.; Nishihara, S.; Takeda, S.; Hasegawa, T.; Nakamura, T.; Hosokoshi, Y.; Inoue, K.; Ikeuchi, S.; Miyazaki, Y.; et al. Molecular Rotor of Cs2([18]crown-6)3 in the Solid State Coupled with the Magnetism of [Ni(dmit)2]. J. Am. Chem. Soc. 2005, 127, 4397–4402. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, Y.; Xiong, R.G.; Yoshikawa, H.; Awaga, K. Exceptional Dielectric Phase Transitions in a Perovskite-Type Cage Compound. Angew. Chem. Int. Ed. 2010, 49, 6608–6610. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Gan, L.; Zhang, S.W.; Gao, S. Perovskite-like Metal Formates with Weak Ferromagnetism and as Precursors to Amorphous Materials. Inorg. Chem. 2004, 43, 4615–4625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, B.; Otsuka, T.; Inoue, K.; Kobayashi, H.; Kurmoo, M. Anionic NaCl-type frameworks of [MnII(HCOO)3−], templated by alkylammonium, exhibit weak ferromagnetism. Dalton. Trans. 2004, 4, 2209–2216. [Google Scholar] [CrossRef]
- Jain, P.; Dalal, N.S.; Toby, B.H.; Kroto, H.W.; Cheetham, A.K. Order−Disorder Antiferroelectric Phase Transition in a Hybrid Inorganic−Organic Framework with the Perovskite Architecture. J. Am. Chem. Soc. 2008, 130, 10450–10451. [Google Scholar] [CrossRef]
- Jain, P.; Ramachandran, V.; Clark, R.J.; Zhou, H.D.; Toby, B.H.; Dalal, N.S.; Kroto, H.W.; Cheetham, A.K. Multiferroic Behavior Associated with an Order−Disorder Hydrogen Bonding Transition in Metal−Organic Frameworks (MOFs) with the Perovskite ABX3 Architecture. J. Am. Chem. Soc. 2009, 131, 13625–13627. [Google Scholar] [CrossRef]
- Besara, T.; Jain, P.; Dalal, N.S.; Kuhns, P.L.; Reyes, A.P.; Kroto, H.W.; Cheetham, A.K. Mechanism of the order–disorder phase transition, and glassy behavior in the metal-organic framework [(CH3)2NH2]Zn(HCOO)3. Proc. Natl. Acad. Sci. USA 2011, 108, 6828–6832. [Google Scholar] [CrossRef] [Green Version]
- Thomson, R.I.; Jain, P.; Cheetham, A.K.; Carpenter, M.A. Elastic relaxation behavior, magnetoelastic coupling, and order-disorder processes in multiferroic metal-organic frameworks. Phys. Rev. B 2012, 86, 214304. [Google Scholar] [CrossRef] [Green Version]
- Fu, D.W.; Zhang, W.; Cai, H.L.; Zhang, Y.; Ge, J.Z.; Xiong, R.G.; Huang, S.D.; Nakamura, T. A Multiferroic Perdeutero Metal–Organic Framework. Angew. Chem. Int. Ed. 2011, 50, 11947–11951. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, H.Y.; Graf, R.; Spiess, H.W.; Yao, Y.F.; Zhu, R.Q.; Xiong, R.G. Tunable and Switchable Dielectric Constant in an Amphidynamic Crystal. J. Am. Chem. Soc. 2013, 135, 5230–5233. [Google Scholar] [CrossRef]
- Zhao, X.H.; Huang, X.C.; Zhang, S.L.; Shao, D.; Wei, H.Y.; Wang, X.Y. Cation-Dependent Magnetic Ordering and Room-Temperature Bistability in Azido-Bridged Perovskite-Type Compounds. J. Am. Chem. Soc. 2013, 135, 16006–16009. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.L.; Zhang, Y.; Fu, D.W.; Zhang, W.; Liu, T.; Yoshikawa, H.; Awaga, K.; Xiong, R.G. Above-Room-Temperature Magnetodielectric Coupling in a Possible Molecule-Based Multiferroic: Triethylmethylammonium Tetrabromoferrate(III). J. Am. Chem. Soc. 2012, 134, 18487–18490. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.P.; Ye, Q.; Li, Q.; Wang, H.T.; Fu, D.W.; Zhang, Y.; Xiong, R.G. Novel Phase-Transition Materials Coupled with Switchable Dielectric, Magnetic, and Optical Properties: [(CH3)4P][FeCl4] and [(CH3)4P][FeBr4]. Chem. Mater. 2014, 26, 6042–6049. [Google Scholar] [CrossRef]
- Han, S.; Zhang, J.; Teng, B.; Ji, C.; Zhang, W.; Sun, Z.; Luo, J. Inorganic–organic hybrid switchable dielectric materials with the coexistence of magnetic anomalies induced by reversible high-temperature phase transition. J. Mater. Chem. C 2017, 5, 8509–8515. [Google Scholar] [CrossRef]
- Yao, Z.S.; Mito, M.; Kamachi, T.; Shiota, Y.; Yoshizawa, K.; Azuma, N.; Miyazaki, Y.; Takahashi, K.; Zhang, K.; Nakanishi, T.; et al. Molecular motor-driven abrupt anisotropic shape change in a single crystal of a Ni complex. Nat. Chem. 2014, 6, 1079–1083. [Google Scholar] [CrossRef]
- Yao, Z.S.; Wu, S.Q.; Kitagawa, Y.; Su, S.Q.; Huang, Y.G.; Li, G.L.; Ni, Z.H.; Nojiri, H.; Shiota, Y.; Yoshizawa, K.; et al. Anisotropic Change in the Magnetic Susceptibility of a Dynamic Single Crystal of a Cobalt(II) Complex. Angew. Chem. Int. Ed. 2017, 56, 717–721. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.Q.; Xue, J.P.; Wang, X.L.; Sato, O.; Yao, Z.S.; Tao, J. A Molecular Crystal Shows Multiple Correlated Magnetic and Ferroelectric Switchings. CCS Chem. 2020, 3, 2464–2472. [Google Scholar] [CrossRef]
- Venkataramani, S.; Jana, U.; Dommaschk, M.; Sönnichsen, F.D.; Tuczek, F.; Herges, R. Magnetic Bistability of Molecules in Homogeneous Solution at Room Temperature. Science 2011, 331, 445–448. [Google Scholar] [CrossRef]
- Thies, S.; Sell, H.; Schütt, C.; Bornholdt, C.; Näther, C.; Tuczek, F.; Herges, R. Light-Induced Spin Change by Photodissociable External Ligands: A New Principle for Magnetic Switching of Molecules. J. Am. Chem. Soc. 2011, 133, 16243–16250. [Google Scholar] [CrossRef]
- Su, S.Q.; Wu, S.Q.; Baker, M.L.; Bencok, P.; Azuma, N.; Miyazaki, Y.; Nakano, M.; Kang, S.; Shiota, Y.; Yoshizawa, K.; et al. Quenching and Restoration of Orbital Angular Momentum through a Dynamic Bond in a Cobalt(II) Complex. J. Am. Chem. Soc. 2020, 142, 11434–11441. [Google Scholar] [CrossRef] [PubMed]
- Guionneau, P.; Le Gac, F.; Kaiba, A.; Costa, J.S.; Chasseau, D.; Létard, J.-F. A reversible metal–ligand bond break associated to a spin-crossover. Chem. Commun. 2007, 36, 3723–3725. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.A.; McMonagle, C.J.; Wilson, C.; Probert, M.R.; Murrie, M. Trigonal to Pentagonal Bipyramidal Coordination Switching in a Co(II) Single-Ion Magnet. Inorg. Chem. 2019, 58, 9691–9697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, S.N.; Su, D.; Ruan, Z.Y.; Zhou, Y.Q.; Deng, W.; Zhang, W.X.; Sun, Y.; Liu, J.L.; Tong, M.L. Reversible Switchability of Magnetic Anisotropy and Magnetodielectric Effect Induced by Intermolecular Motion. Angew. Chem. Int. Ed. 2022, 61, e202204700. [Google Scholar] [CrossRef] [PubMed]
- Vogelsberg, C.S.; Garcia-Garibay, M.A. Crystalline molecular machines: Function, phase order, dimensionality, and composition. Chem. Soc. Rev. 2012, 41, 1892–1910. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, C.T.; Zeng, Y.; Ji, C.M.; Du, Z.Y.; Zhang, W.X.; Chen, X.M. Crystalline Supramolecular Gyroscope with a Water Molecule as an Ultrasmall Polar Rotator Modulated by Charge-Assisted Hydrogen Bonds. J. Am. Chem. Soc. 2017, 139, 8086–8089. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Zhang, T.; Gu, Z.X.; Zhang, Z.X.; Fu, D.W.; Chen, X.G.; Zhang, H.Y.; Xiong, R.G. Record Enhancement of Curie Temperature in Host–Guest Inclusion Ferroelectrics. J. Am. Chem. Soc. 2021, 143, 5091–5098. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.W.; Zhang, W.; Cai, H.L.; Zhang, Y.; Ge, J.Z.; Xiong, R.G.; Huang, S.D. Supramolecular Bola-Like Ferroelectric: 4-Methoxyanilinium Tetrafluoroborate-18-crown-6. J. Am. Chem. Soc. 2011, 133, 12780–12786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Lu, S.Q.; Chen, X.; Xiong, R.G.; Tang, Y.Y. The first high-temperature multiaxial ferroelectric host–guest inclusion compound. Chem. Commun. 2019, 55, 11571–11574. [Google Scholar] [CrossRef] [PubMed]
- Siegler, M.A.; Hao, X.; Parkin, S.; Brock, C.P. [Ni(H2O)6](NO3)2·(15-crown-5)·2H2O: Three phase transitions and an intermediate modulated phase stable over a range of ca 40 K. Acta Cryst. B 2011, 67, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.B.; Tian, Z.F.; Duan, H.B.; Zhu, Z.P.; He, W.; Hong, T.Y.; Yu, G.; He, Y.J.; Yang, J.K. [(18-Crown-6)K][Fe(1)Cl(1)4]0.5[Fe(2)Cl(2)4]0.5: A Multifunctional Molecular Switch of Dielectric, Conductivity and Magnetic Properties. Chem. Asian J. 2018, 13, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Gu, Z.F.; Xiong, J.-B.; Gao, J.X.; Liu, Y.; Wang, B.; Tan, Y.H.; Xu, Q. Unusual Sequential Reversible Phase Transitions Containing Switchable Dielectric Behaviors in Cyclopentyl Ammonium 18-Crown-6 Perchlorate. Chem. Mater. 2016, 28, 4476–4482. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Di, F.F.; Li, P.F.; Xiong, R.G. Crown Ether Host-Guest Molecular Ferroelectrics. Chem. Eur. J. 2022, 28, e202102990. [Google Scholar] [CrossRef]
- Rado, G.T.; Folen, V.J. Observation of the Magnetically Induced Magnetoelectric Effect and Evidence for Antiferromagnetic Domains. Phys. Rev. Lett. 1961, 7, 310–311. [Google Scholar] [CrossRef]
- Kimura, T.; Goto, T.; Shintani, H.; Ishizaka, K.; Arima, T.; Tokura, Y. Magnetic control of ferroelectric polarization. Nature 2003, 426, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Hur, N.; Park, S.; Sharma, P.A.; Ahn, J.S.; Guha, S.; Cheong, S.W. Electric polarization reversal and memory in a multiferroic material induced by magnetic fields. Nature 2004, 429, 392–395. [Google Scholar] [CrossRef]
- Dos santos-García, A.J.; Solana-Madruga, E.; Ritter, C.; Andrada-Chacón, A.; Sánchez-Benítez, J.; Mompean, F.J.; Garcia-Hernandez, M.; Sáez-Puche, R.; Schmidt, R. Large Magnetoelectric Coupling Near Room Temperature in Synthetic Melanostibite Mn2FeSbO6. Angew. Chem. Int. Ed. 2017, 56, 4438–4442. [Google Scholar] [CrossRef]
- Wang, Y.X.; Ma, Y.; Chai, Y.; Shi, W.; Sun, Y.; Cheng, P. Observation of Magnetodielectric Effect in a Dysprosium-Based Single-Molecule Magnet. J. Am. Chem. Soc. 2018, 140, 7795–7798. [Google Scholar] [CrossRef]
- Wang, Y.X.; Shi, W.; Li, H.; Song, Y.; Fang, L.; Lan, Y.; Powell, A.K.; Wernsdorfer, W.; Ungur, L.; Chibotaru, L.F.; et al. A single-molecule magnet assembly exhibiting a dielectric transition at 470 K. Chem. Sci. 2012, 3, 3366–3370. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, J.Y.; Huang, R.K.; Chen, M.K.; Xue, W.; Zhang, W.X.; Zeng, M.H.; Chen, X.M. Spin-reorientation-induced magnetodielectric coupling effects in two layered perovskite magnets. Chem. Sci. 2018, 9, 7413–7418. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Shen, S.; Cong, J.; Yan, L.; Wang, S.; Sun, Y. Observation of Resonant Quantum Magnetoelectric Effect in a Multiferroic Metal–Organic Framework. J. Am. Chem. Soc. 2016, 138, 782–785. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, F.; Horiuchi, S.; Tokunaga, M.; Fujioka, J.; Tokura, Y. Ferroelectricity in a one-dimensional organic quantum magnet. Nat. Phys. 2010, 6, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, X.; Zhao, H.X.; Ren, Y.P.; Zhuang, G.L.; Long, L.S.; Zheng, L.S. The Mechanism of the Magnetodielectric Response in a Molecule-Based Trinuclear Iron Cluster Material. Angew. Chem. Int. Ed. 2020, 59, 14409–14413. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, R.; Zenno, H.; Sekine, Y.; Nakaya, M.; Akita, M.; Kosumi, D.; Lindoy, L.F.; Hayami, S. A Ferroelectric Metallomesogen Exhibiting Field-Induced Slow Magnetic Relaxation. Chem. Eur. J. 2022, 28, e202103367. [Google Scholar] [CrossRef]
- Harding, D.J.; Harding, P.; Phonsri, W. Spin crossover in iron(III) complexes. Coord. Chem. Rev. 2016, 313, 38–61. [Google Scholar] [CrossRef]
- Kumar, K.S.; Heinrich, B.; Vela, S.; Moreno-Pineda, E.; Bailly, C.; Ruben, M. Bi-stable spin-crossover characteristics of a highly distorted [Fe(1-BPP-COOC2H5)2](ClO4)2·CH3CN complex. Dalton. Trans. 2019, 48, 3825–3830. [Google Scholar] [CrossRef]
- Halcrow, M.A. Spin-crossover Compounds with Wide Thermal Hysteresis. Chem. Lett. 2014, 43, 1178–1188. [Google Scholar] [CrossRef]
- Grzywa, M.; Röß-Ohlenroth, R.; Muschielok, C.; Oberhofer, H.; Błachowski, A.; Żukrowski, J.; Vieweg, D.; von Nidda, H.-A.K.; Volkmer, D. Cooperative Large-Hysteresis Spin-Crossover Transition in the Iron(II) Triazolate [Fe(ta)2] Metal–Organic Framework. Inorg. Chem. 2020, 59, 10501–10511. [Google Scholar] [CrossRef]

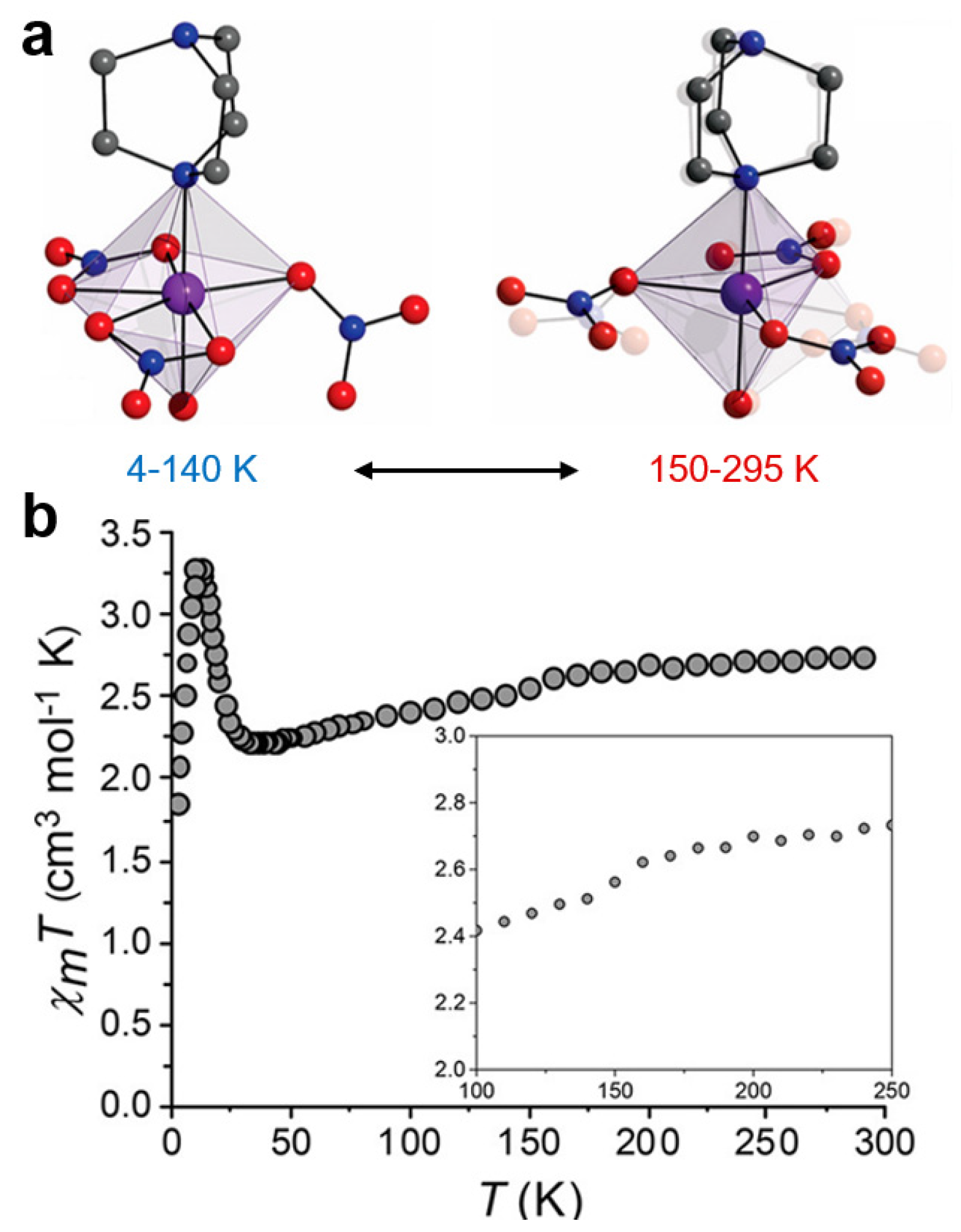

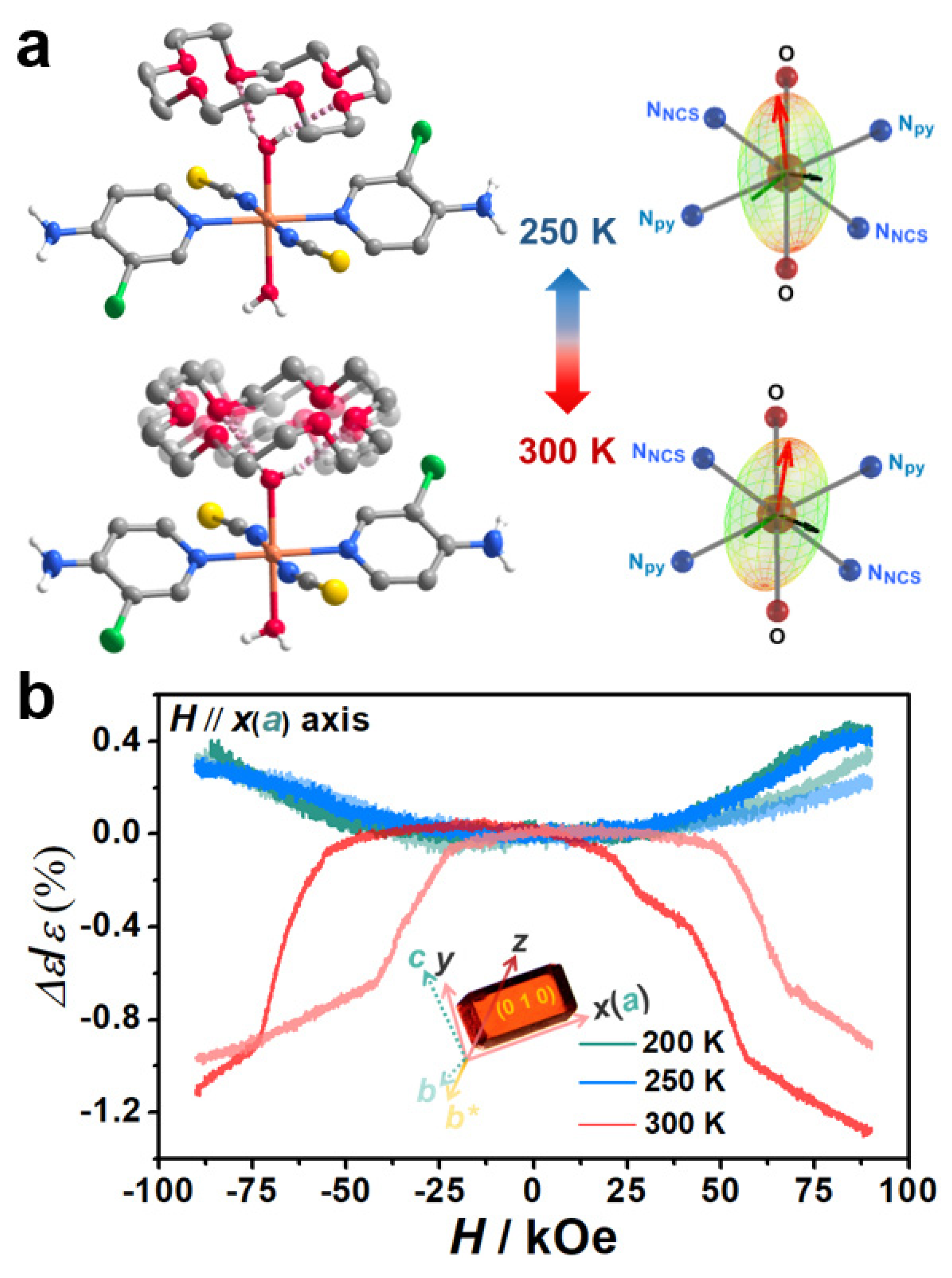
| Complex | T↓/K | T↑/K | ∆T/K | Comment | Ref. |
|---|---|---|---|---|---|
| [CoII(C14-terpy)2](BF4)2 | 250 | 307 | 57 | [43] | |
| [CoII(C16-terpy)2](BF4)2 | 217 | 260 | 43 | [43] | |
| [CoII(C14-terpy)2](BF4)2∙MeOH | 184 | 206 | 22 | [46] | |
| [FeII(nBu-im)3(tren)](PF6)2 | 115 | 129 | 14 | Scan rate: 4 K min–1 | [48] |
| 135 | 176 | 41 | Scan rate: 0.1 K min–1 | ||
| [FeII(C10-pbh)2] | – | ca. 298 | 1.2 | [49] | |
| [FeII(2-(5-(3-methoxy-4H-1,2,4-triazol-3-yl)-6-(1H-pyrazol-1-yl))pyridine)] | 255 | 360 | 105 | [52] | |
| {[(pzTp)FeIII(CN)3]2[FeII(L)]} | 256 | 300 | 44 | 0.5 or 1 K min–1 Scan-rate dependence | [53] |
| [CoII(NO3)2(2,6-di(pyrazol-1-yl)pyrazine)] | 228 | 240 | 12 | [54] | |
| [CoII(ONO2)2(H2O)(mprpz)] | – | ca. 110 | 7 | Low-temperature phase ↔ intermediate phase | [55] |
| 155 | 165 | 10 | Intermediate phase ↔ high-temperature phase | ||
| [CH3NH3][MnII(N3)3] | 264 | 277 | 13 | [66] | |
| [(CH3)2NH2][MnII(N3)3] | 286 | 298 | 12 | ||
| [(CH3)3NH][MnII(N3)3] | 363 | 356 | 7 | ||
| [(CH3)4N][MnII(N3)3] | 305 | 309 | 4 | ||
| [(CH3CH2)3(CH3)N][FeIIIBr4] | 361 | 366 | 5 | [67] | |
| [(CH3)4P][FeIIIBr4] | 368 | 374 | 6 | [68] | |
| [CoII(en)3](ox) | – | ca. 250 | 4 | [71] | |
| [CoII(en)3](SO4) | – | ca. 177 | 4 | [72] | |
| [CoII(NO3)2(ethyl-2,6-di(1H-pyrazol-1-yl)isonicotinate)] | – | ca. 128.5 | 14 | [75] | |
| [CoII(NCS)2(H2O)2(4-amino-3-chloropyridine)2]∙(18-crown-6) | 277.6 | 281.2 | 3.6 | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.-N.; Yao, C.-Y.; Liu, J.-L.; Tong, M.-L. Magnetic Switchability via Thermal-Induced Structural Phase Transitions in Molecular Solids. Magnetochemistry 2023, 9, 80. https://doi.org/10.3390/magnetochemistry9030080
Du S-N, Yao C-Y, Liu J-L, Tong M-L. Magnetic Switchability via Thermal-Induced Structural Phase Transitions in Molecular Solids. Magnetochemistry. 2023; 9(3):80. https://doi.org/10.3390/magnetochemistry9030080
Chicago/Turabian StyleDu, Shan-Nan, Chan-Ying Yao, Jun-Liang Liu, and Ming-Liang Tong. 2023. "Magnetic Switchability via Thermal-Induced Structural Phase Transitions in Molecular Solids" Magnetochemistry 9, no. 3: 80. https://doi.org/10.3390/magnetochemistry9030080
APA StyleDu, S. -N., Yao, C. -Y., Liu, J. -L., & Tong, M. -L. (2023). Magnetic Switchability via Thermal-Induced Structural Phase Transitions in Molecular Solids. Magnetochemistry, 9(3), 80. https://doi.org/10.3390/magnetochemistry9030080








