Zero-Field Slow Magnetic Relaxation in Binuclear Dy Acetylacetonate Complex with Pyridine-N-Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. IR Spectroscopy
2.3. Elemental Analysis
2.4. X-ray Analysis
2.5. Magnetic Properties
2.6. Computational Methodology
3. Results and Discussion
3.1. Synthesis
3.2. X-ray Analysis
3.3. Magnetic Properties
3.3.1. Direct Current (dc) Magnetic Susceptibility of 1
3.3.2. Dynamic (ac) Magnetic Properties of 1
3.3.3. Theoretical Insight of the Dy Ions Magnetic Anisotropy in 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vincent, R.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W.; Balestro, F. Electronic read-out of a single nuclear spin using a molecular spin transistor. Nature 2012, 488, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.; Balestro, F.; Ballou, R.; Kliatskaya, S.; Ruben, M.; Wernsdorrer, W. Electrically driven nuclear spin resonance in single-molecule magnets. Science 2014, 344, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Shiddiq, M.; Komijani, D.; Duan, Y.; Gaita-Ariño, A.; Coronado, E.; Hill, S. Enhancing coherence in molecular spin qubits via atomic clock transitions. Nature 2016, 531, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Donati, F.; Rusponi, S.; Stepanow, S.; Wackerlin, C.; Singha, A.; Persichetti, L.; Baltic, R.; Diller, K.F.; Patthey, F.; Fernandes, E.; et al. Magnetic remanence in single atoms. Science 2016, 352, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Natterer, F.D.; Yang, K.; Paul, W.; Willke, P.; Choi, T.; Greber, T.; Heinrich, A.J.; Lutz, C.P. Reading and writing single-atom magnets. Nature 2017, 543, 226–228. [Google Scholar] [CrossRef] [Green Version]
- Benelli, C.; Gatteschi, D. Introduction to Molecular Magnetism: From Transition Metals to Lanthanides; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Tang, J.; Zhang, P. Lanthanide Single Molecule Magnets; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Verdaguer, M.; Launay, J.-P. Electrons in Molecules; From Basic Principles to Molecular Electronics; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Kronmuller, H.; Parkin, S. Handbook of Magnetism and Advanced Magnetic Materials; Wiley-VCH: Wehenheim, Germany, 2007; Volume 5. [Google Scholar]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular magnetic hysteresis at 60 kelvin in dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Zhan Shi, Z.; Li, D.; Li, Y.; An, N.; Shang, Y.; Sakiyama, H.; Muddassir, M.; Si, C. The regulation research of topology and magnetic exchange models of CPs through Co(II) concentration adjustment. J. Solid State Chem. 2023, 318, 123713. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. Strong exchange and magnetic blocking in N23−-radical-bridged lanthanide complexes. Nat. Chem. 2011, 3, 538–542. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Xu, G.-F.; Wernsdorfer, W.; Ungur, L.; Guo, Y.; Tang, J.; Zhang, H.-J.; Chibotaru, L.F.; Powell, A.K. Strong axiality and Ising exchange interaction suppress zero-field tunneling of magnetization of an asymmetric Dy2 single-molecule magnet. J. Am. Chem. Soc. 2011, 133, 11948–11951. [Google Scholar] [CrossRef]
- Blagg, R.J.; Ungur, L.; Tuna, F.; Speak, J.; Comar, P.; Collison, D.; Wernsdorfer, W.; McInnes, E.J.L.; Chibotaru, L.F.; Winpenny, R.E.P. Magnetic relaxation pathways in lanthanide single molecule magnets. Nat. Chem. 2013, 5, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Blagg, R.J.; Muryn, C.A.; McInnes, E.J.L.; Tuna, F.; Winpenny, R.E.P. Single pyramid magnets: Dy5 pyramids with slow magnetic relaxation to 40 K. Angew. Chem. Int. Ed. 2011, 29, 6530–6533. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Gamez, P.; Zhao, L.; Lin, S.-Y.; Deng, R.; Tang, J.; Zhang, H.-J. Two-step relaxation in a linear tetranuclear dysprosium(III) aggregate showing single-molecule magnet behavior. J. Am. Chem. Soc. 2010, 132, 8538–8539. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hewitt, I.; Madhu, N.T.; Chastanet, G.; Wernsdorfer, W.; Anson, C.A.; Benelli, C.; Sessoli, R.; Powell, A.K. Dysprosium triangles showing single-molecule magnet behavior of thermally excited spin states. Angew. Chem. Int. Ed. 2006, 45, 1729–1733. [Google Scholar] [CrossRef]
- Hewitt, I.J.; Tang, J.; Madhu, N.T.; Anson, C.E.; Lan, Y.; Luzon, J.; Etienne, M.; Sessoli, R.; Powell, A.K. Coupling Dy3 triangles enhances their slow magnetic relaxation. Angew. Chem. Int. Ed. 2010, 49, 6352–6356. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.-L.; Wang, T.-W.; Song, Y.; You, X.-Z. Slow relaxation processes and single-ion magnetic behaviors in dysprosium-containing complexes. Inorg. Chem. 2010, 49, 969–976. [Google Scholar] [CrossRef]
- Gorshkov, E.V.; Korchagin, D.V.; Yureva, E.A.; Shilov, G.V.; Zhidkov, M.V.; Dmitriev, A.I.; Efimov, N.N.; Palii, A.V.; Aldoshin, S.M. Effect of Ligand Substitution on Zero-Field Slow Magnetic Relaxation in Mononuclear Dy(III) β-Diketonate Complexes with Phenanthroline-Based Ligands. Magnetochemistry 2022, 8, 151. [Google Scholar] [CrossRef]
- Long, D.; Basalov, I.V.; Forosenko, N.V.; Lyssenko, K.A.; Mamontova, E.; Cherkasov, A.V.; Damjanović, M.; Chibotaru, L.F.; Guari, Y.; Larionova, J.; et al. Dysprosium Single-Molecule Magnets with Bulky Schiff Base Ligands: Modification of the Slow Relaxation of the Magnetization by Substituent Change. Chem. A Eur. J. 2019, 25, 474–478. [Google Scholar] [CrossRef]
- Canaj, A.B.; Dey, S.; Martí, E.R.; Wilson, C.; Rajaraman, G.; Murrie, M. Insight into D6h Symmetry: Targeting Strong Axiality in Stable Dysprosium(III) Hexagonal Bipyramidal Single-Ion Magnets. Angew. Chem. Int. Ed. 2019, 58, 14146–14151. [Google Scholar] [CrossRef] [Green Version]
- Mattei, C.A.; Montigaud, V.; Dorcet, V.; Riobé, F.; Argouarch, G.; Maury, O.; Le Guennic, B.; Cador, O.; Lalli, C.; Pointillart, F. Luminescent dysprosium single-molecule magnets made from designed chiral BINOL-derived bisphosphate ligands. Inorg. Chem. Front. 2021, 8, 963–976. [Google Scholar] [CrossRef]
- Parmar, V.S.; Mills, D.P.; Winpenny, R.E.P. Mononuclear Dysprosium Alkoxide and Aryloxide Single-Molecule Magnets. Chem.—A Eur. J. 2021, 27, 7625–7645. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Tolpygin, A.O.; Lyubov, D.M.; Rad’kova, N.Y.; Cherkasov, A.V.; Nelyubina, Y.V.; Guari, Y.; Larionova, J.; Trifonov, A.A. High magnetization reversal barriers in luminescent dysprosium octahedral and pentagonal bipyramidal single-molecule magnets based on fluorinated alkoxide ligands. Dalton Trans. 2021, 50, 8487–8496. [Google Scholar] [CrossRef]
- Ren, M.; Bao, S.-S.; Hoshino, N.; Akutagawa, T.; Wang, B.; Ding, Y.-C.; Wei, S.; Zheng, L.M. Solvent Responsive Magnetic Dynamics of a Dinuclear Dysprosium Single-Molecule Magnet. Chem. A Eur. J. 2013, 19, 9619–9628. [Google Scholar] [CrossRef] [PubMed]
- Layfield, R.A.; McDoual, J.J.W.; Sulway, S.A.; Tuna, F.; Collison, D.; Winpenny, R.E.P. Influence of the N-Bridging Ligand on Magnetic Relaxation in an Organometallic Dysprosium Single-Molecule Magnet. Chem.—A Eur. J. 2010, 16, 4442–4446. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Lan, Y.; Anson, C.A.; Powell, A.K. Anion-perturbed magnetic slow relaxation in planar {Dy4} clusters. Inorg. Chem. 2008, 47, 10813–10815. [Google Scholar] [CrossRef] [PubMed]
- Gamer, M.T.; Lan, Y.; Roesky, P.W.; Powell, A.K.; Clérac, R. Pentanuclear dysprosium hydroxy cluster showing single-molecule-magnet behavior. Inorg. Chem. 2008, 47, 6581–6583. [Google Scholar] [CrossRef]
- Wei, J.-Y.; Hsu, T.-J.; Wang, C.-W.; Kuo, C.-J.; Lin, P.-H. Alternate Synthetic Pathway Leading to Isolation of a Dinuclear Single-Molecule Magnet. Eur. J. Inorg. Chem. 2018, 29, 3397–3401. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, P.; Li, H.-F.; Tian, Y.-M.; Yan, P.-F.; Sun, W.-B. Structure and Single-Molecule Magnetic Property of a Dinuclear Dy2 Complex Bridged by the 4-Methylpyridine N-Oxide Ligand. Chem. A Eur. J. 2018, 32, 3668–3674. [Google Scholar]
- Han, T.; Giansiracusa, M.J.; Li, Z.-H.; Ding, Y.-S.; Chilton, N.F.; Winpenny, R.E.P.; Zheng, Y.-Z. Exchange-Biasing in a Dinuclear Dysprosium(III) Single-Molecule Magnet with a Large Energy Barrier for MagnetisationReversal. Chem. A Eur. J. 2020, 26, 6773–6777. [Google Scholar] [CrossRef]
- Ding, Y.S.; Chilton, N.F.; Winpenny, R.E.P.; Zheng, Y.-Z. On approaching the limit of molecular magnetic anisotropy: A near-perfect pentagonal bipyramidal dysprosium (III) single-molecule magnet. Angew. Chem. Int. Ed. 2016, 55, 16071–16074. [Google Scholar] [CrossRef]
- Zadrozny, J.M.; Xiao, D.J.; Atanasov, M.; Long, G.J.; Grandjean, F.; Neese, F.; Long, J.R. Magnetic blocking in a linear iron (I) complex. Nat. Chem. 2013, 5, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Petit, S.; Baril-Robert, F.; Pilet, G.; Reber, C.; Luneau, D. Luminescence spectroscopy of europium (III) and terbium (III) penta-, octa-and nonanuclear clusters with β-diketonate ligands. Dalton Trans. 2009, 34, 6809–6815. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, K.; Sun, Y.-C.; Brandão, P.; Jana, N.C.; Wang, X.-Y.; Panja, A. Macrocycle supported dinuclear lanthanide complexes with different β-diketonate co-ligands displaying zero field single-molecule magnetic behavior. New J. Chem. 2022, 46, 11722–11733. [Google Scholar] [CrossRef]
- Zhang, L.; Xiong, J.; Meng, Y.; Liu, T. Structures and magnetic relaxation properties of cyclopentadienyl/β-diketonate/trispyrazolylborate hybridized dysprosium single-molecule magnets. Chin. Chem. Lett. 2022, 108055. [Google Scholar] [CrossRef]
- Li, Y.; You, Y.; Zhao, P.; Liu, Z.-Y.; Zhang, Y.-Q.; Yang, E.-C.; Zhao, X.-J. Enhancing the Magnetic Anisotropy in Low-Symmetry Dy-Based Complexes by Tuning the Bond Length. Inorg. Chem. 2021, 60, 11419–11428. [Google Scholar] [CrossRef]
- Jiang, S.D.; Wang, B.W.; Gang Su, G.; Wang, Z.-M.; Gao, S. A Mononuclear Dysprosium Complex Featuring Single-Molecule-Magnet Behavior. Angew. Chem. 2010, 49, 7448–7451. [Google Scholar] [CrossRef]
- Guettas, D.; Gendron, F.; Garcia, G.F.; Riobé, F.; Roisnel, T.; Maury, O.; Pilet, G.; Cador, O.; Guennic, B.L. Luminescence-driven electronic structure determination in a textbook dimeric Dy(III)-based single molecule magnet. Chem. A Eur. J. 2020, 26, 4389–4395. [Google Scholar] [CrossRef]
- Yi, X.; Bernot, K.; Pointillart, F.; Poneti, G.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Sessoli, R. A Luminescent and Sublimable DyIII-Based Single-Molecule Magnet. Chem. A Eur. J. 2012, 18, 11379–11387. [Google Scholar] [CrossRef]
- Díaz-Ortega, I.F.; Herrera, J.M.; Aravena, D.; Ruiz, E.; Gupta, T.; Rajaraman, G.; Nojiri, H.; Colacio, E. Designing a Dy2 Single-Molecule Magnet with Two Well-Differentiated Relaxation Processes by Using a Nonsymmetric Bis-bidentate Bipyrimidine-N-Oxide Ligand: A Comparison with Mononuclear Counterparts. Inorg. Chem. 2018, 57, 6362–6375. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Pointillart, F.; Guennic, B.; Jung, J.; Daiguebonne, C.; Calvez, G.; Guillou, O.; Bernot, K. Rational engineering of dimeric Dy-based Single-Molecule Magnets for surface grafting. Polyhedron 2019, 164, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Kiefl, E.; Mannini, M.; Bernot, K.; Yi, X.; Amato, A.; Leviant, T.; Magnani, A.; Prokscha, T.; Suter, A.; Sessoli, R.; et al. Robust Magnetic Properties of a Sublimable Single-Molecule Magnet. ACS Nano 2016, 10, 5663–5669. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Calvez, G.; Daiguebonne, C.; Guillou, O.; Bernot, K. Rational organization of lanthanide-based SMM dimers into three-dimensional networks. Inorg. Chem. 2015, 54, 5213–5219. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Yi, X.; Jung, J.; Guillou, O.; Cador, O.; Pointillart, F.; Le Guennic, B.; Bernot, K. Optimization of Magnetic Relaxation and Isotopic Enrichment in Dimeric DyIII Single-Molecule Magnets. Eur. J. Inorg. Chem. 2018, 2018, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Cimatti, I.; Yi, X.; Sessoli, R.; Puget, M.; Guennic, B.L.; Jung, J.; Guizouarn, G.; Magnani, A.; Bernot, K.; Mannini, M. Chemical tailoring of single molecule magnet behavior in films of Dy(III) dimers. Appl. Surf. Sci. 2018, 432, 7–14. [Google Scholar] [CrossRef]
- Weissberger, A. Technique of Organic Chemistry; Interscience Pub: New York, NY, USA, 1955; Volume 7, p. 552. [Google Scholar]
- CrysAlis, P.R.O. Agilent Technologies; Agilent Technologies UK Ltd.: Oxfordshire, UK, 2011. [Google Scholar]
- Galván, I.F.; Vacher, M.; Alavi, A.; Angeli, C.; Aquilante, F.; Autschbach, J.; Bao, J.J.; Bokarev, S.I.; Bogdanov, N.A.; Carlson, R.K.; et al. OpenMolcas: From source code to insight. Chem. Theor. Comp. 2019, 15, 5925–5964. [Google Scholar] [CrossRef]
- Aquilante, F.; Autschbach, J.; Baiardi, A.; Battaglia, S.; Borin, V.A.; Chibotaru, L.F.; Conti, I.; De Vico, L.; Delcey, M.; Fdez, I.; et al. Modern quantum chemistry with [Open] Molcas. J. Chem. Phys. 2020, 152, 214117. [Google Scholar] [CrossRef]
- Howard, D.L.; Kjaergaard, H.G.; Huang, J.; Meuwly, M. Infrared and near-infrared spectroscopy of acetylacetone and hexafluoroacetylacetone. J. Phys. Chem. A 2015, 119, 7980–7990. [Google Scholar] [CrossRef]
- Brand, J.C.D.; Eglinton, G. Applications of Spectroscopy to Organic Chemistry; Daniel Davey and Co., Inc.: New York, NY, USA, 1965. [Google Scholar]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Manshi Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, Version 2.1; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]




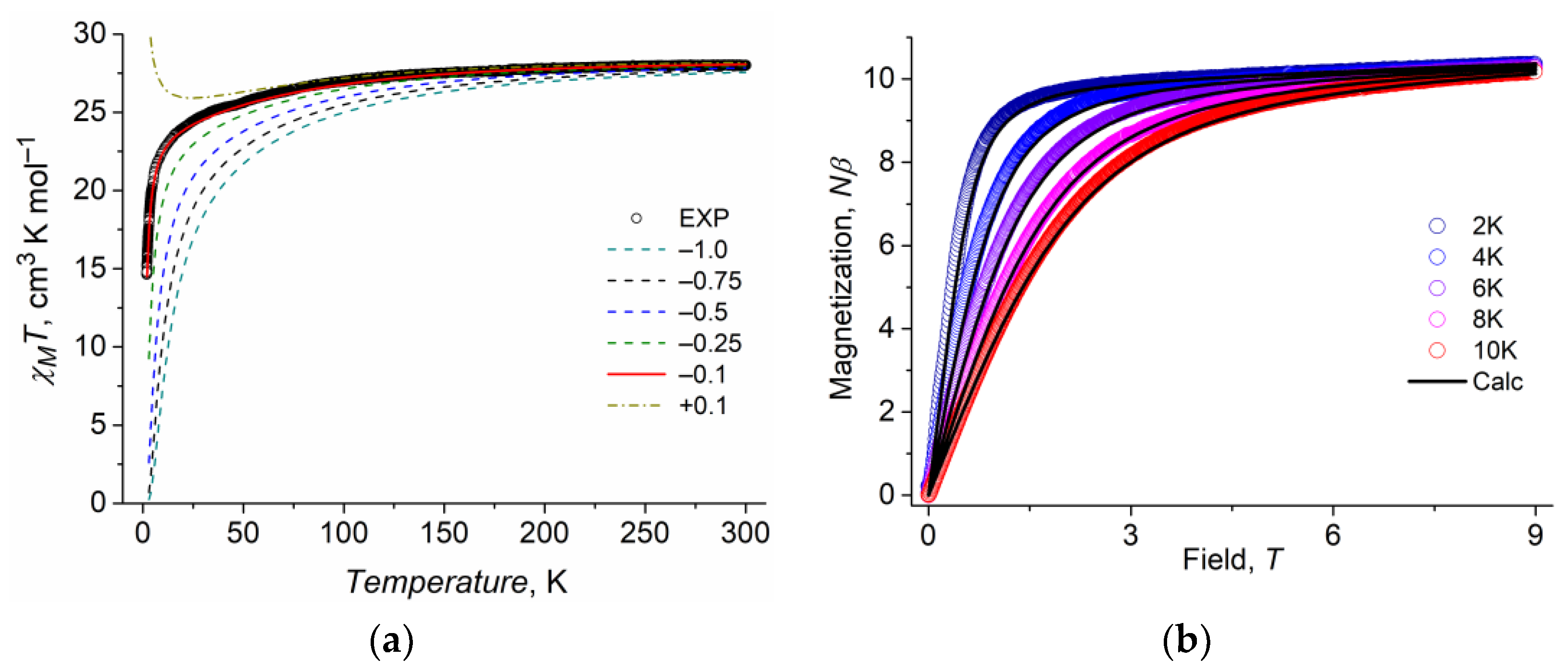
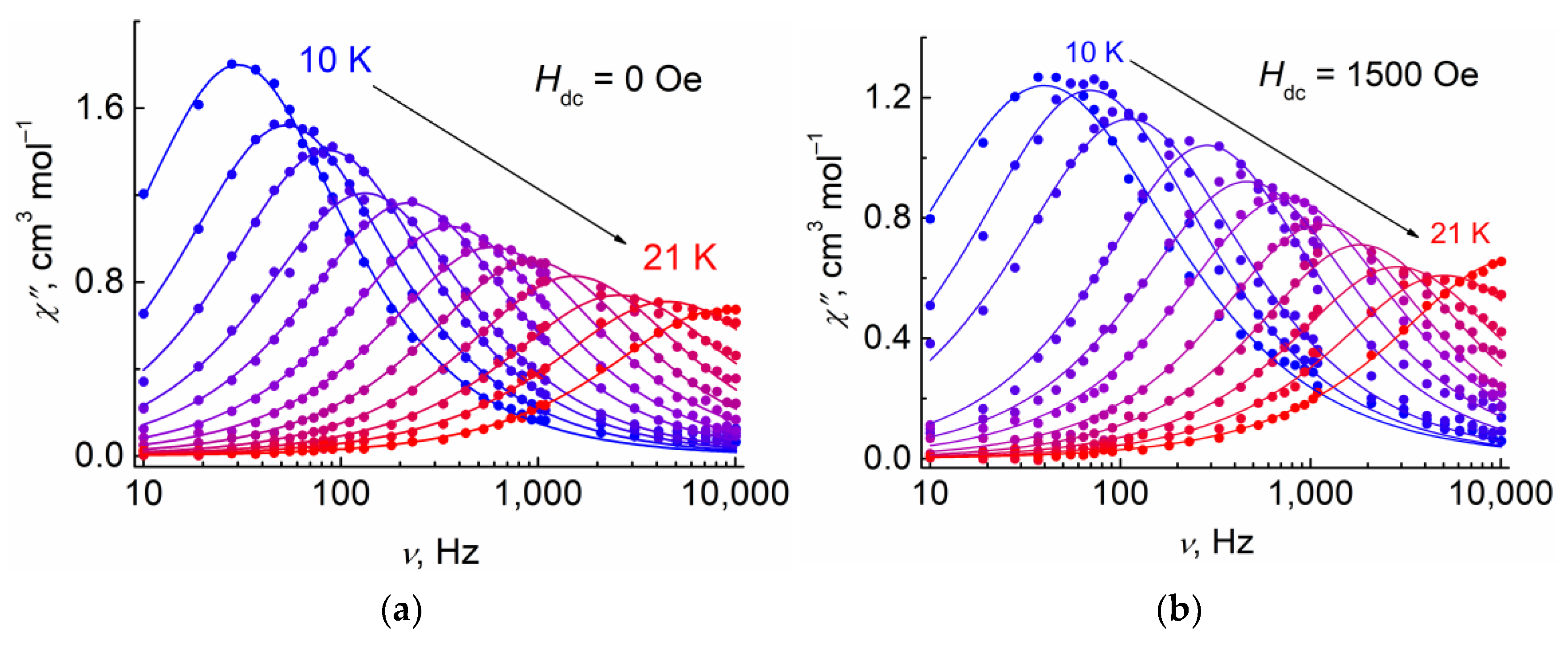
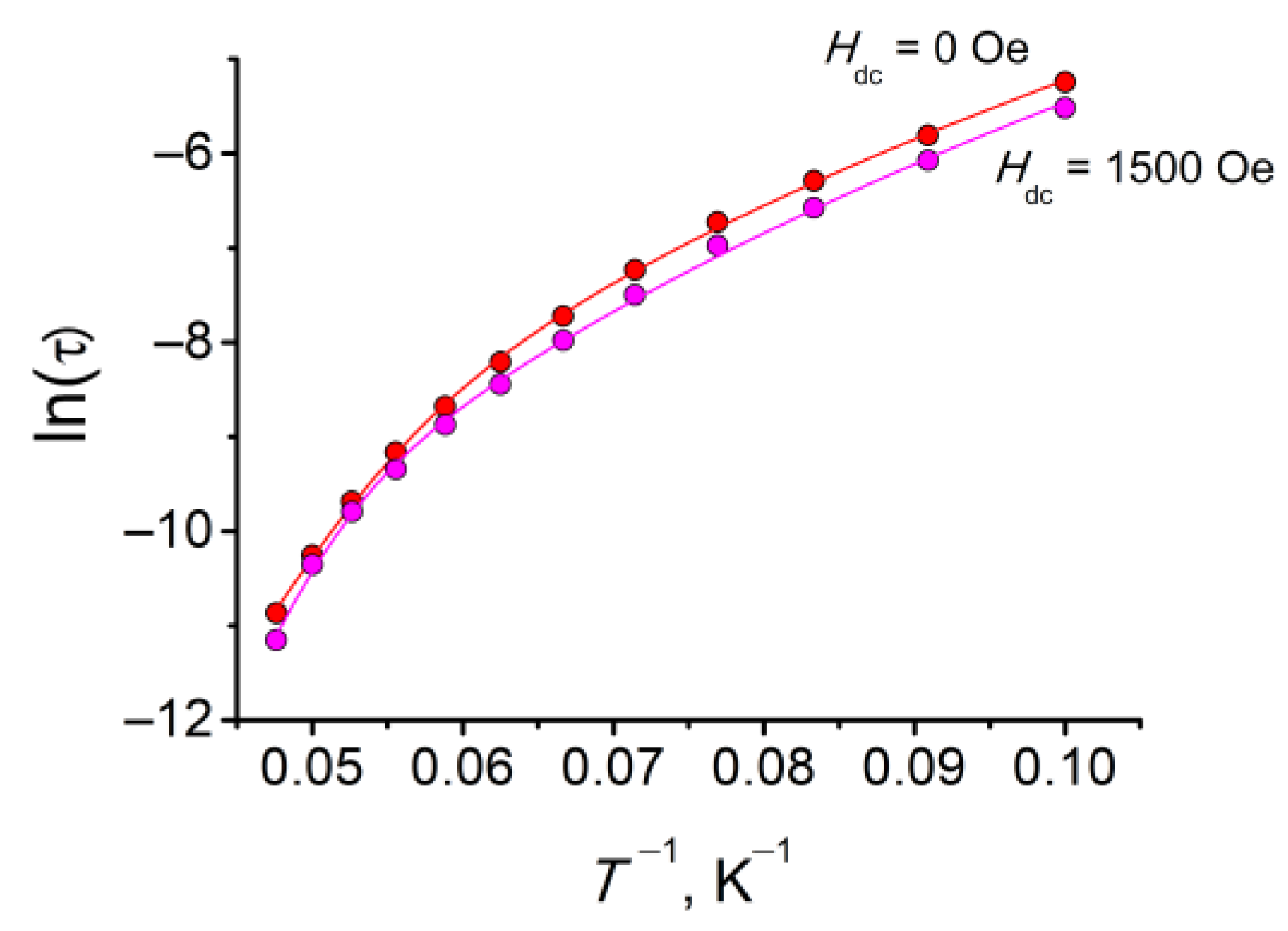
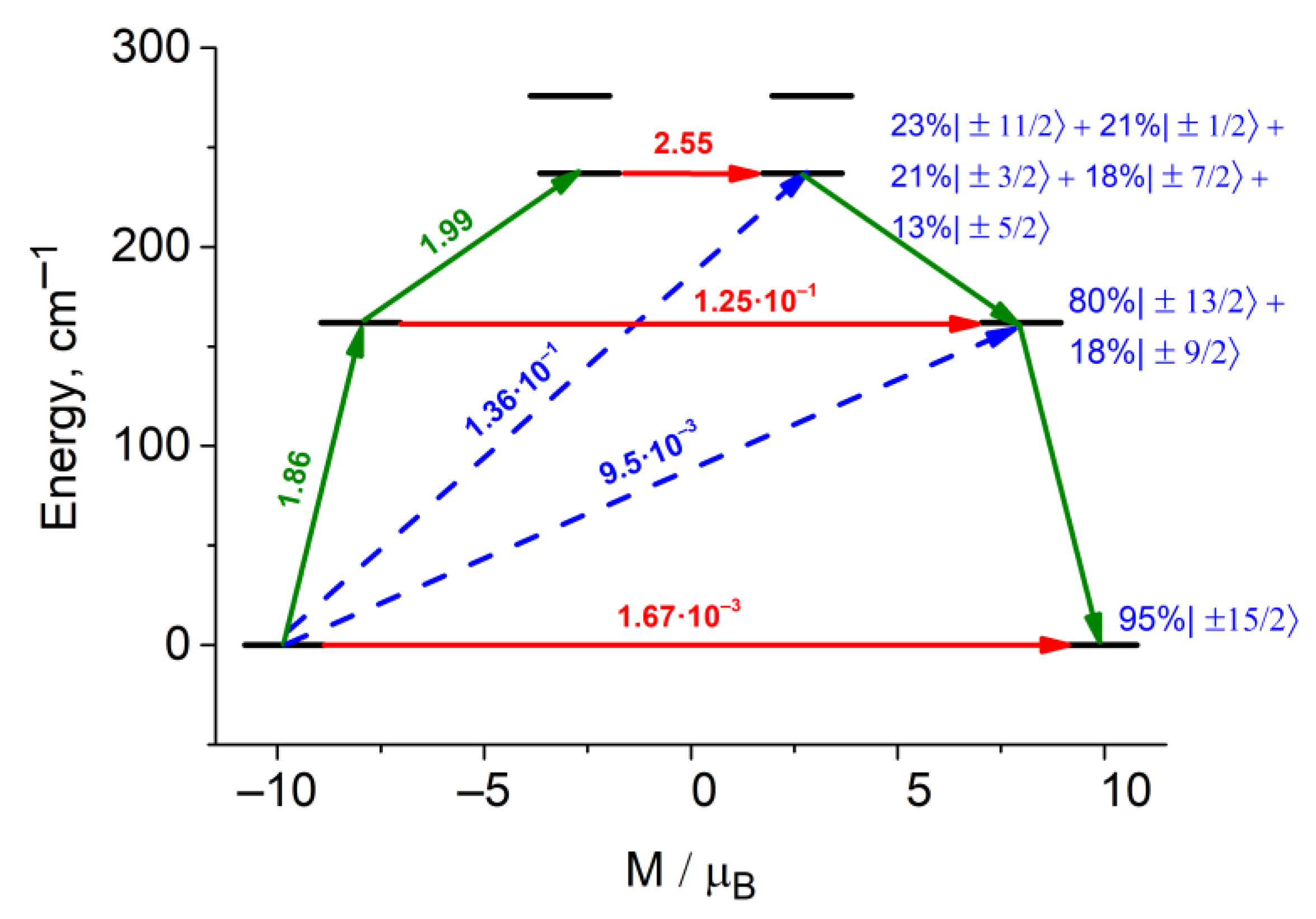
| Hdc, Oe | 0 | 1500 |
|---|---|---|
| τ0, s | 3.15 × 10–11 | 1.24 × 10–12 |
| Ueff, K | 287 | 338 |
| CRaman, s−1·K−n | 2.2 × 10–4 | 1.5 × 10–4 |
| nRaman | 5.93 | 6.20 |
| KD | Energy | gx | gy | gz |
|---|---|---|---|---|
| 1 | 0.0 | 0.003 | 0.007 | 19.666 |
| 2 | 162.0 | 0.340 | 0.407 | 16.020 |
| 3 | 236.9 | 3.871 | 4.262 | 13.147 |
| 4 | 275.8 | 6.851 | 5.849 | 3.310 |
| 5 | 308.9 | 3.271 | 5.709 | 12.247 |
| 6 | 372.6 | 0.032 | 0.080 | 18.336 |
| 7 | 433.8 | 0.006 | 0.055 | 16.535 |
| 8 | 493.7 | 0.023 | 0.066 | 18.388 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shtefanets, V.P.; Shilov, G.V.; Korchagin, D.V.; Yureva, E.A.; Dmitriev, A.I.; Zhidkov, M.V.; Morgunov, R.B.; Sanina, N.A.; Aldoshin, S.M. Zero-Field Slow Magnetic Relaxation in Binuclear Dy Acetylacetonate Complex with Pyridine-N-Oxide. Magnetochemistry 2023, 9, 105. https://doi.org/10.3390/magnetochemistry9040105
Shtefanets VP, Shilov GV, Korchagin DV, Yureva EA, Dmitriev AI, Zhidkov MV, Morgunov RB, Sanina NA, Aldoshin SM. Zero-Field Slow Magnetic Relaxation in Binuclear Dy Acetylacetonate Complex with Pyridine-N-Oxide. Magnetochemistry. 2023; 9(4):105. https://doi.org/10.3390/magnetochemistry9040105
Chicago/Turabian StyleShtefanets, Valeriya P., Gennady V. Shilov, Denis V. Korchagin, Elena A. Yureva, Alexei I. Dmitriev, Mikhail V. Zhidkov, Roman B. Morgunov, Nataliya A. Sanina, and Sergey M. Aldoshin. 2023. "Zero-Field Slow Magnetic Relaxation in Binuclear Dy Acetylacetonate Complex with Pyridine-N-Oxide" Magnetochemistry 9, no. 4: 105. https://doi.org/10.3390/magnetochemistry9040105
APA StyleShtefanets, V. P., Shilov, G. V., Korchagin, D. V., Yureva, E. A., Dmitriev, A. I., Zhidkov, M. V., Morgunov, R. B., Sanina, N. A., & Aldoshin, S. M. (2023). Zero-Field Slow Magnetic Relaxation in Binuclear Dy Acetylacetonate Complex with Pyridine-N-Oxide. Magnetochemistry, 9(4), 105. https://doi.org/10.3390/magnetochemistry9040105









