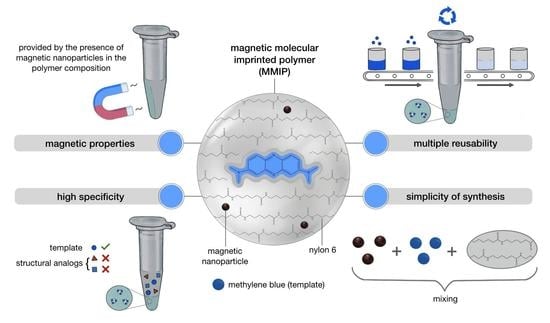
Preparation of Magnetic Molecularly Imprinted Polymer for Methylene Blue Capture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Magnetic Nanoparticles (MNPs)
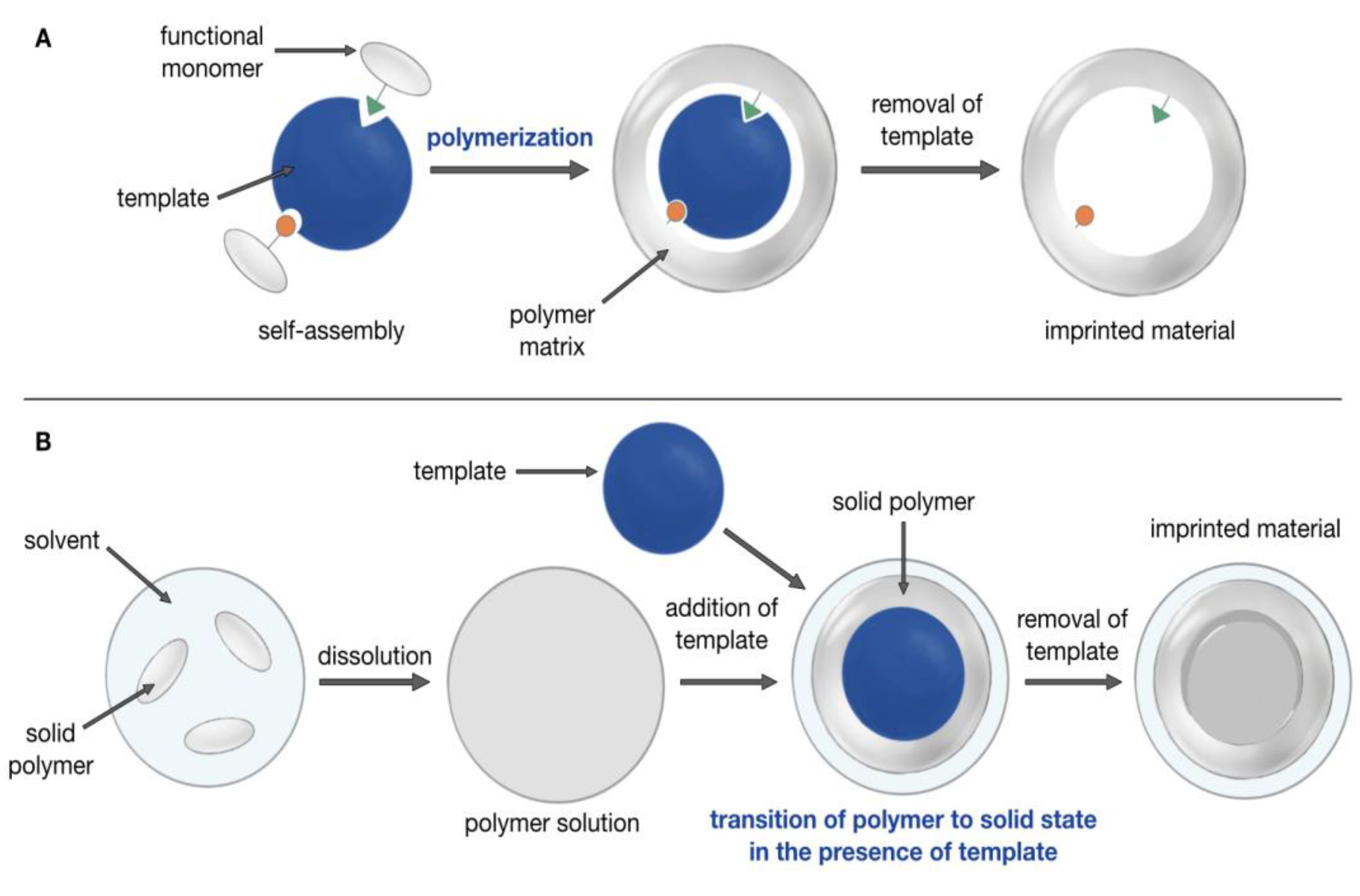
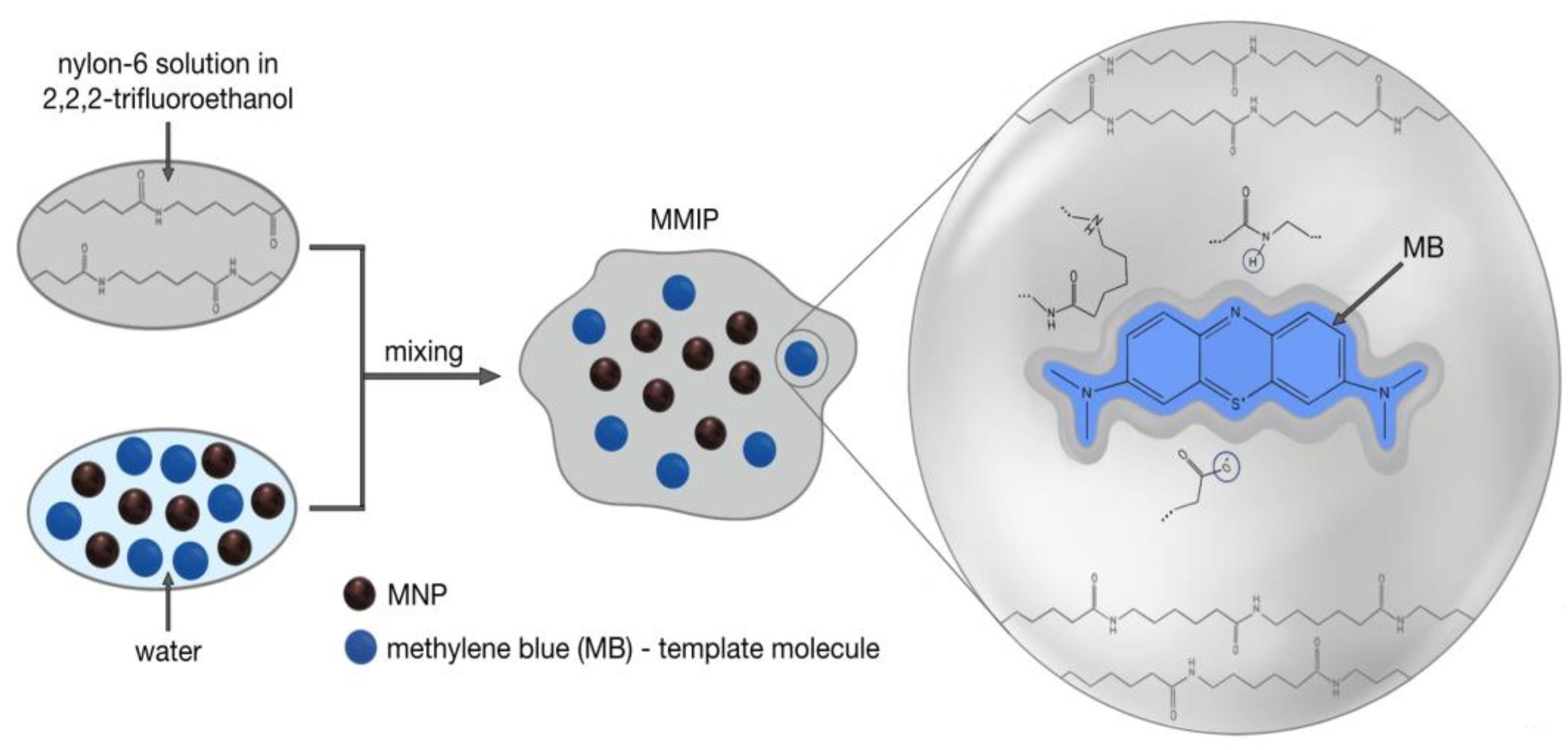
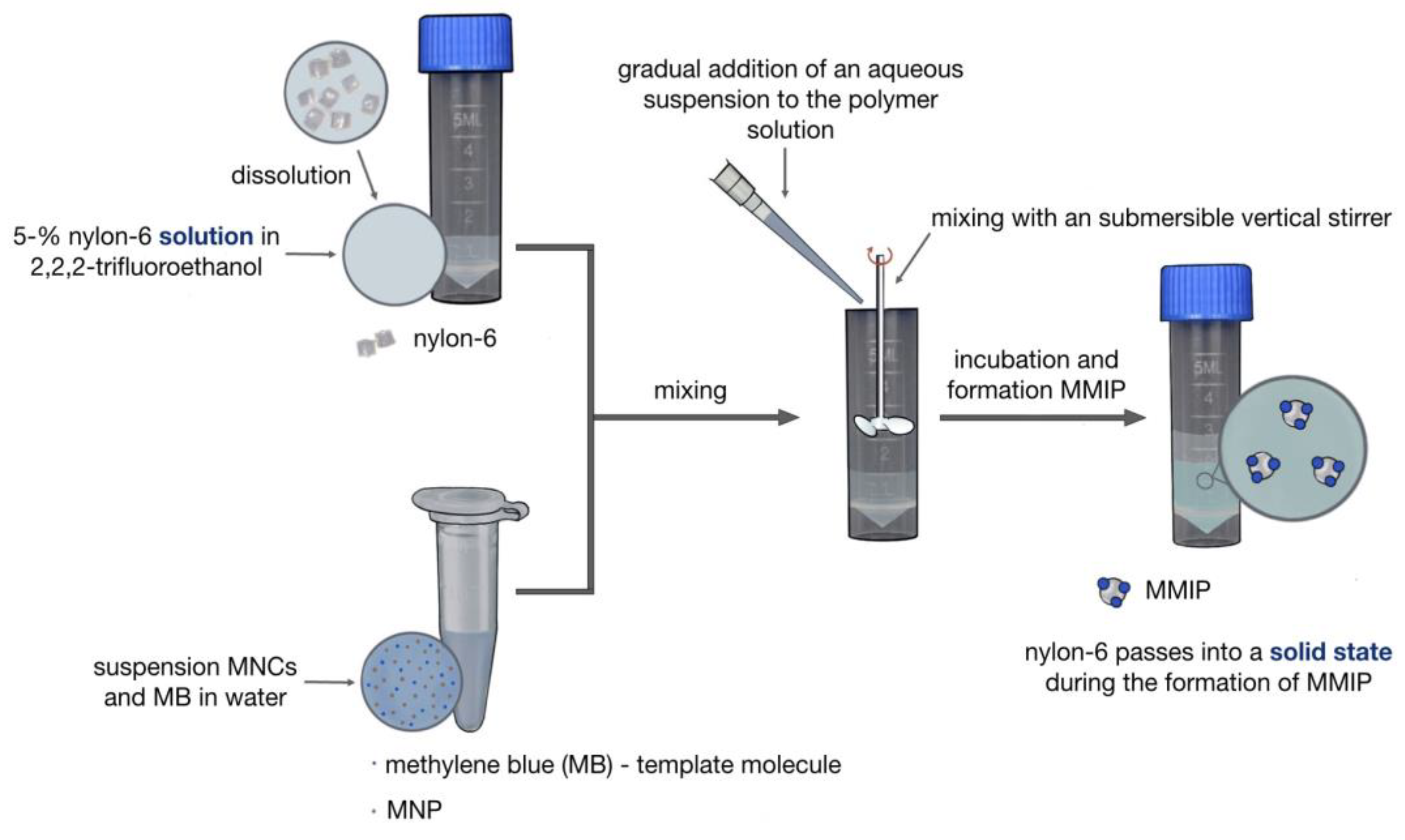
2.3. Synthesis of MMIPs
2.4. Synthesis of MNIPs
2.5. MMIPs Characterization
2.6. Methylene Blue (MB) Capture Analysis for MMIPs and MNIPs
2.7. Reusability Study of MMIPs
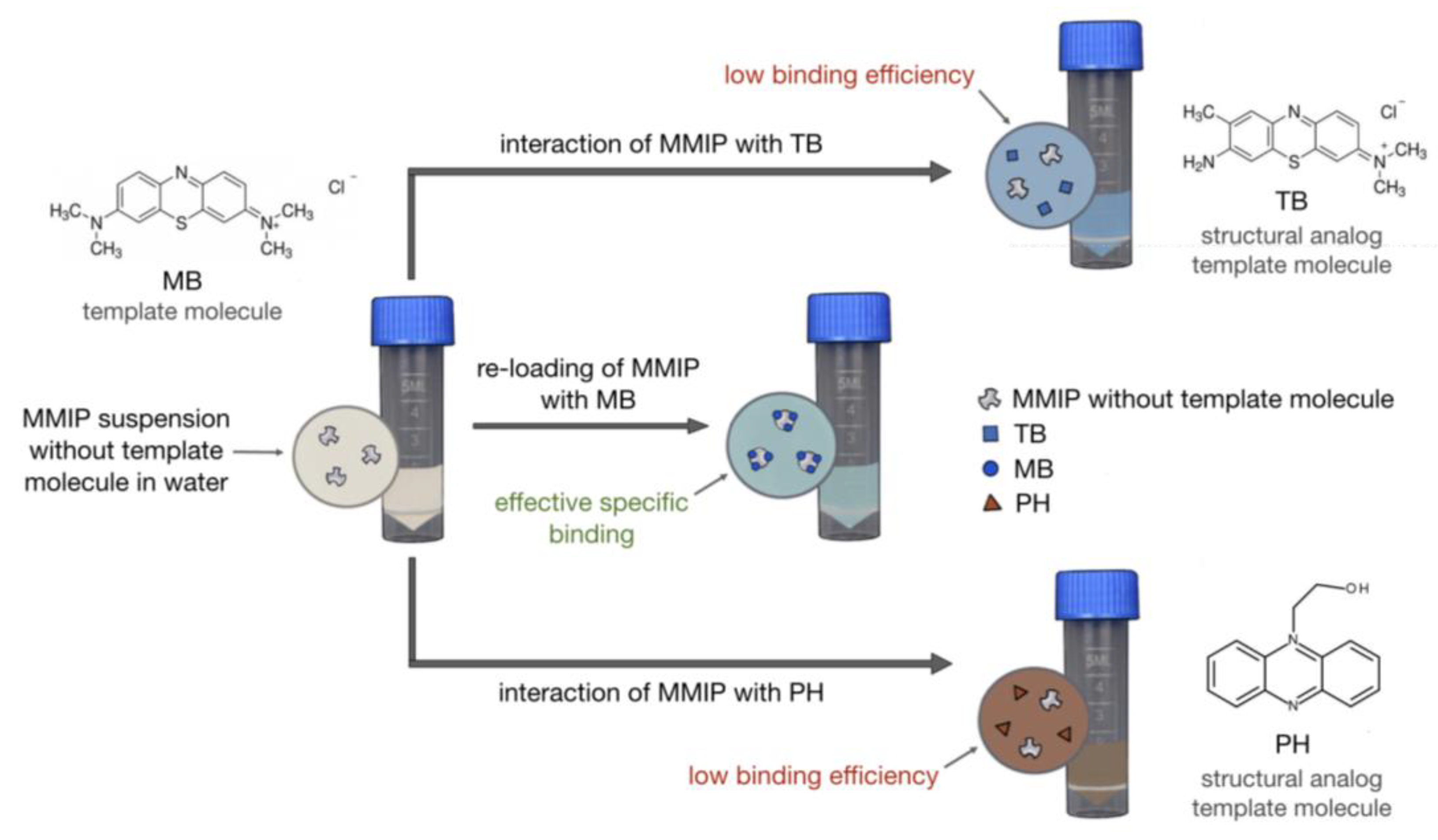
2.8. Selectivity Study of MMIPs with MB, Toluidine Blue (TB), and Hydroxyethylphenazine (PH)
2.9. MB Capture by MMIPs from the Lake Water MB Solution
3. Results and Discussion
3.1. Synthesis of Magnetic Imprinted Polymers (MMIPs) and Magnetic Nonimprinted Polymers (MNIPs)
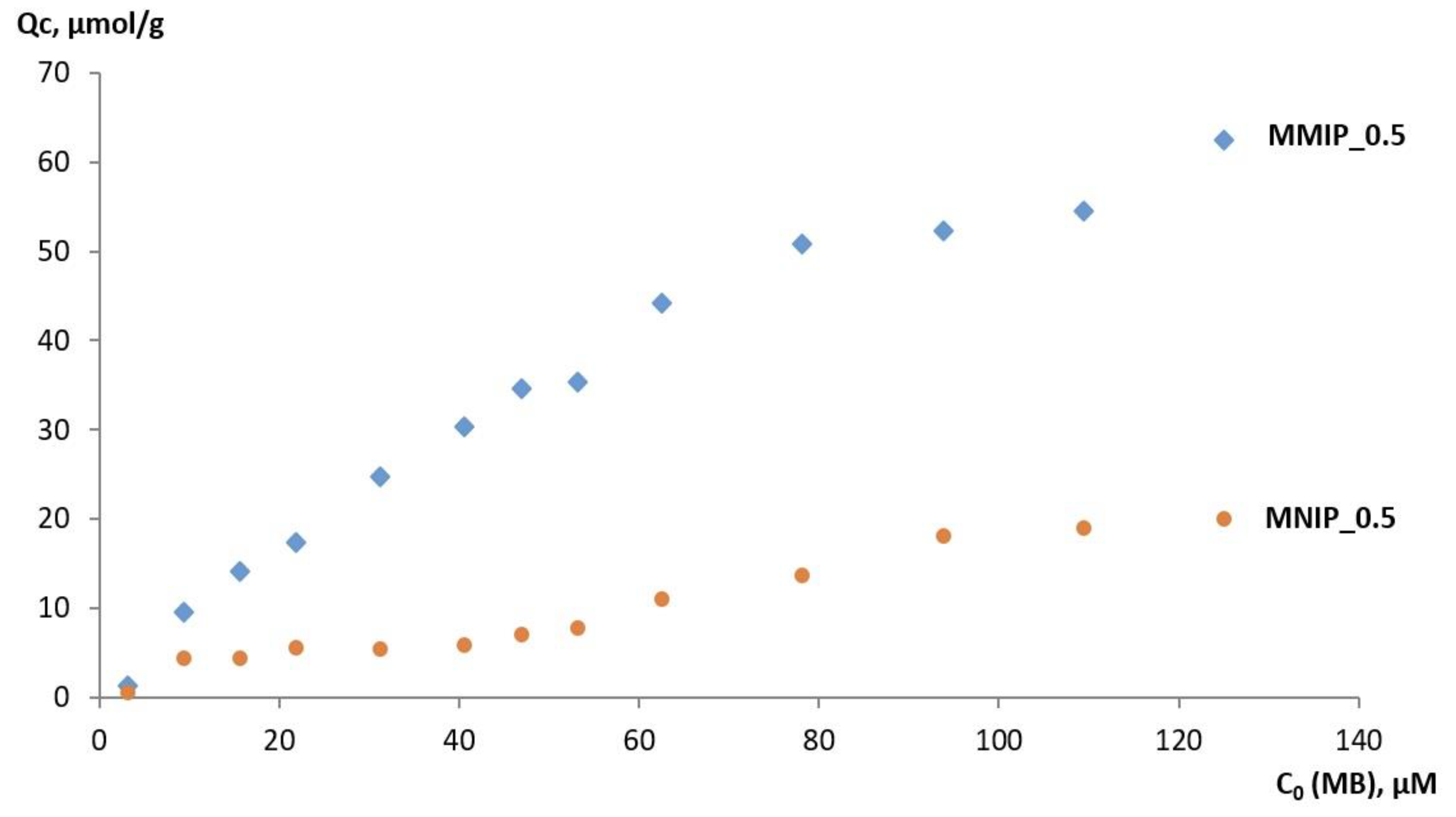
3.2. MB Capture by MMIPs and MNIPs
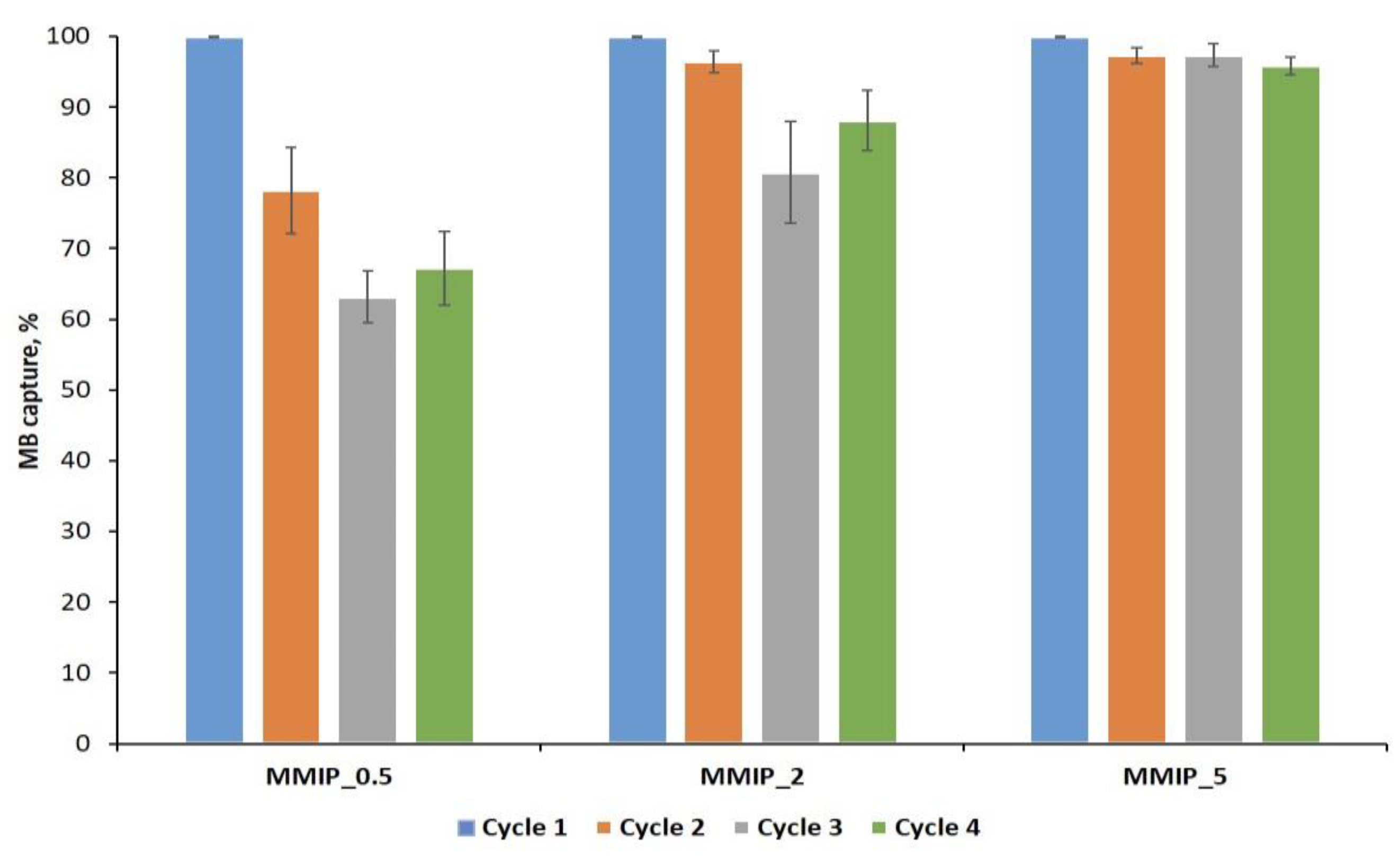
3.3. Reusability Study of MMIPs
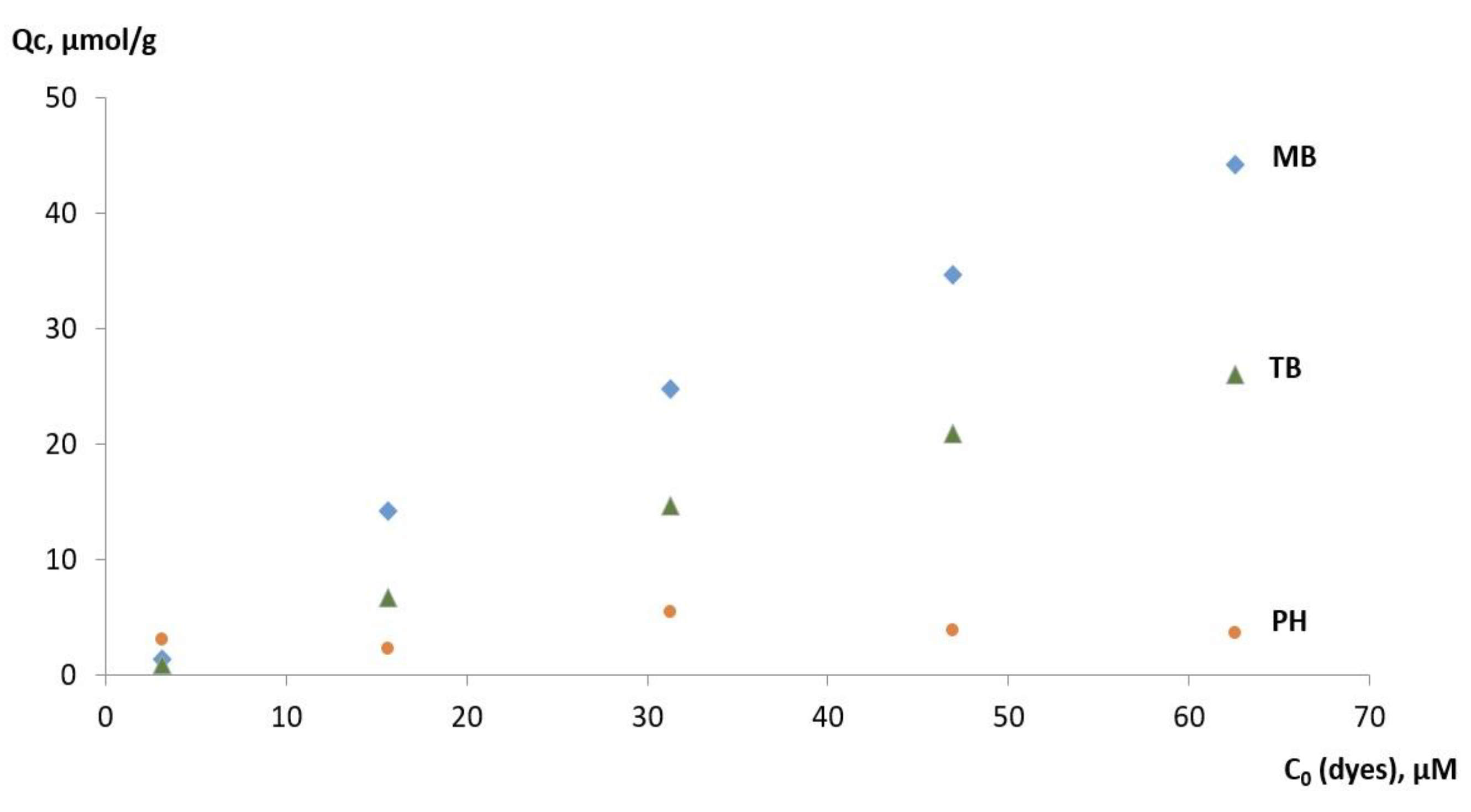
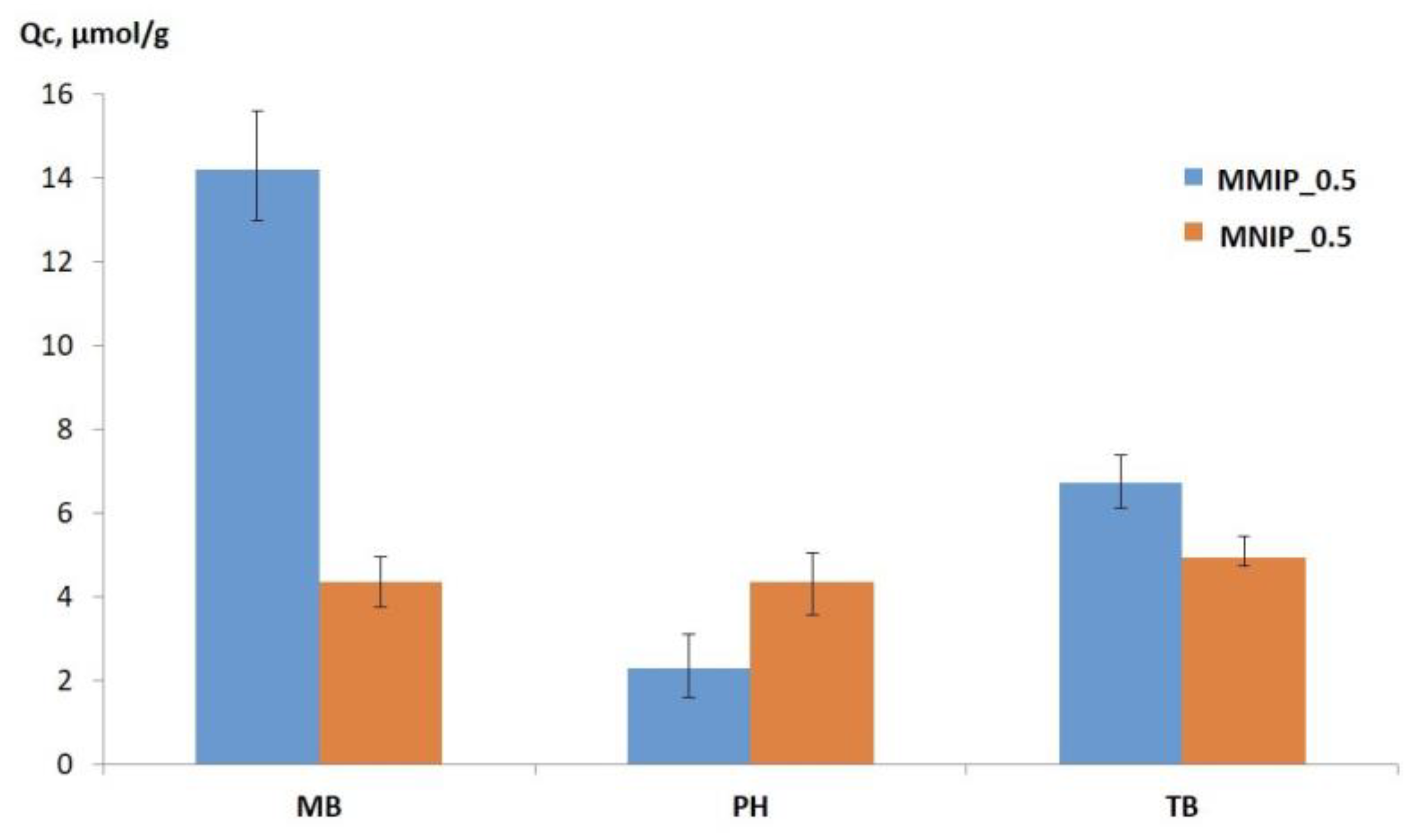
3.4. Selectivity of MMIP Binding of MB and Its Analogs
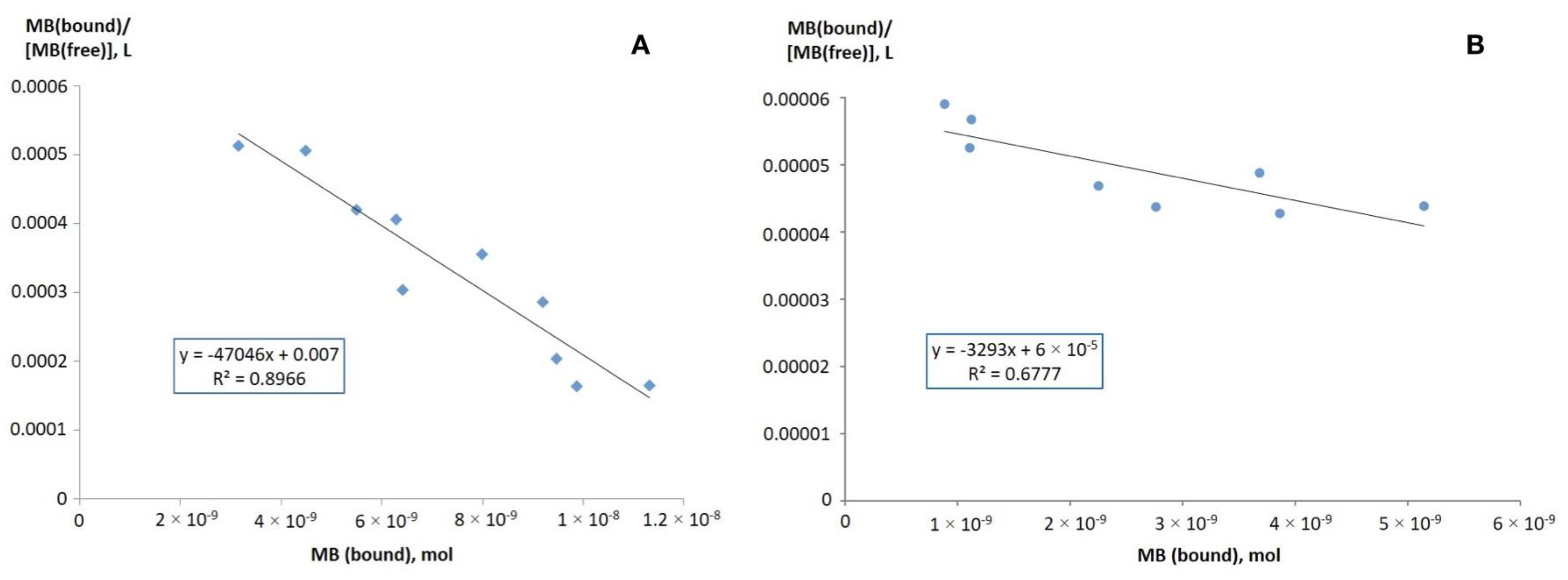
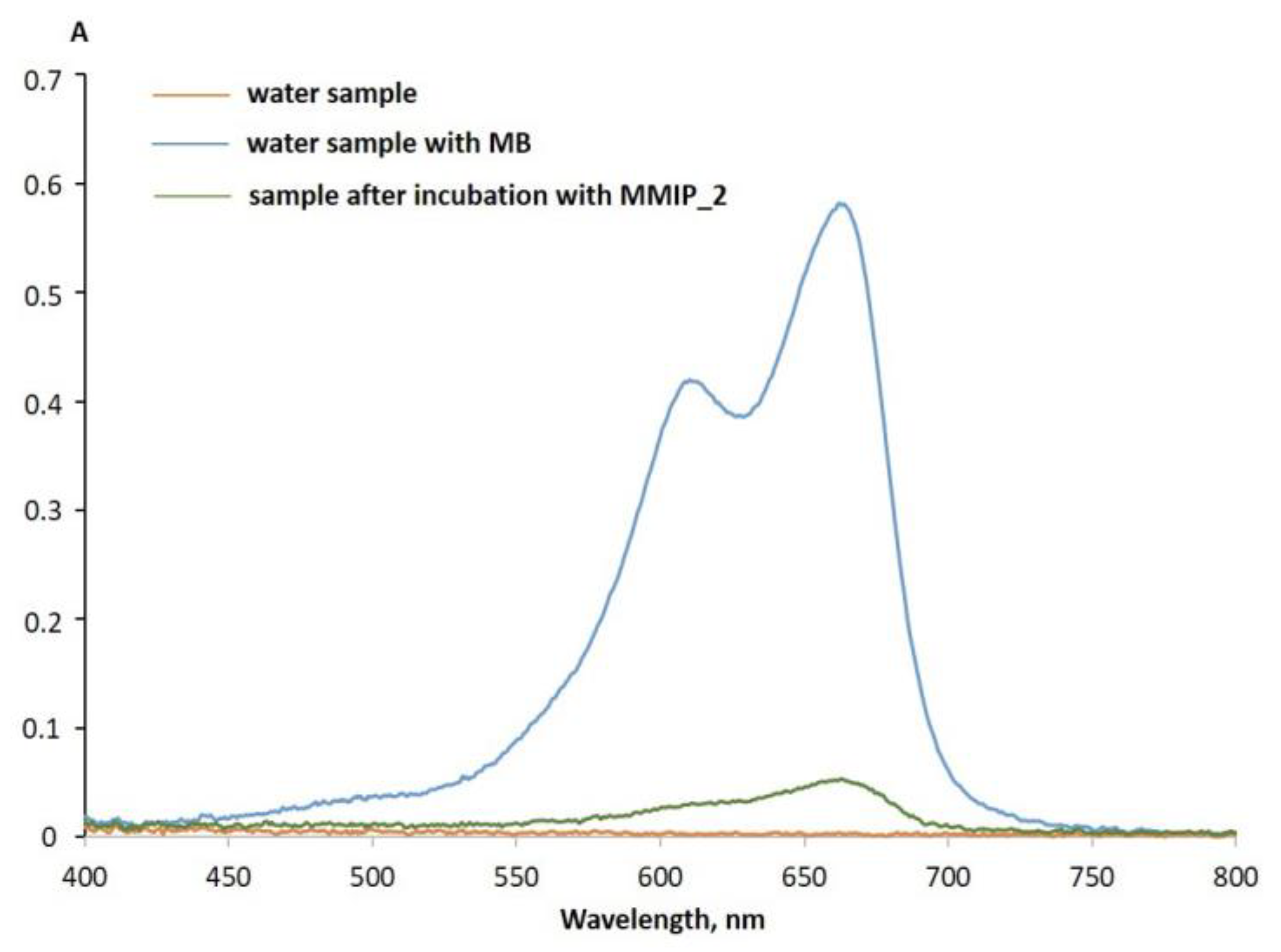
3.5. MMIP Interaction with a Model MB Solution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khitous, A.; Molinaro, C.; Gree, S.; Haupt, K.; Soppera, O. Plasmon-Induced Photopolymerization of Molecularly Imprinted Polymers for Nanosensor Applications. Adv. Mater. Interfaces 2023, 10, 2201651. [Google Scholar] [CrossRef]
- Meng, H.; Zhao, T.; Jing, T.; Zeng, Y.; Wang, N. Preparation and Properties of Novel Magnetic Methylene Blue Molecularly Imprinted Polymer. Polym. Sci. Ser. B 2021, 63, 245–256. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Tang, Z.; Niu, H.; Cai, Y.; Meng, W.; Wu, F.; Giesy, J.P. Synthesis of Magnetic Metal-Organic Framework (MOF) for Efficient Removal of Organic Dyes from Water. Sci. Rep. 2015, 5, 11849. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.G.; Carvalho, N.V.; Paterno, L.G.; Moura, L.D.; Filomeno, C.L.; de Paula, E.; Báo, S.N. Methylene Blue Associated with Maghemite Nanoparticles Has Antitumor Activity in Breast and Ovarian Carcinoma Cell Lines. Cancer Nanotechnol. 2021, 12, 11. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Popadić, D.; Gavrilov, N.; Ignjatović, L.; Krajišnik, D.; Mentus, S.; Milojević-Rakić, M.; Bajuk-Bogdanović, D. How to Obtain Maximum Environmental Applicability from Natural Silicates. Catalysts 2022, 12, 519. [Google Scholar] [CrossRef]
- Ramin, N.A.; Ramachandran, M.R.; Saleh, N.M.; Mat Ali, Z.M.; Asman, S. Magnetic Nanoparticles Molecularly Imprinted Polymers: A Review. Curr. Nanosci. 2022, 18, 372–400. [Google Scholar] [CrossRef]
- Meseguer-Lloret, S.; Torres-Cartas, S.; Gómez-Benito, C.; Herrero-Martínez, J.M. Magnetic Molecularly Imprinted Polymer for the Simultaneous Selective Extraction of Phenoxy Acid Herbicides from Environmental Water Samples. Talanta 2022, 239, 123082. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly Imprinted Polymer Micro- and Nano-Particles: A Review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef]
- Dmitrienko, E.V.; Pyshnaya, I.A.; Martyanov, O.N.; Pyshnyi, D. V Molecularly Imprinted Polymers for Biomedical and Biotechnological Applications. Russ. Chem. Rev. 2016, 85, 513–536. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Kılıç, S.; Esen, C.; Denizli, A. Molecularly Imprinted Polymer-Based Sensors for Protein Detection. Polymers 2023, 15, 629. [Google Scholar] [CrossRef] [PubMed]
- Ariani, M.D.; Zuhrotun, A.; Manesiotis, P.; Hasanah, A.N. Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products. Polymers 2022, 14, 1389. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Q.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. The Preparation of Layered Hierarchical and Cube-Shaped Magnetic Fe3O4/CaCO3 for Efficient Enrichment of Pb(II) from Aqueous Solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100600. [Google Scholar] [CrossRef]
- Murdaya, N.; Triadenda, A.L.; Rahayu, D.; Hasanah, A.N. A Review: Using Multiple Templates for Molecular Imprinted Polymer: Is It Good? Polymers 2022, 14, 4441. [Google Scholar] [CrossRef]
- Huang, X.C.; Ma, J.K.; Wei, S.L. Preparation and Application of a Novel Magnetic Molecularly Imprinted Polymer for Simultaneous and Rapid Determination of Three Trace Endocrine Disrupting Chemicals in Lake Water and Milk Samples. Anal. Bioanal. Chem. 2020, 412, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.L.; Liu, W.T.; Huang, X.C.; Ma, J.K. Preparation and Application of a Magnetic Plasticizer as a Molecularly Imprinted Polymer Adsorbing Material for the Determination of Phthalic Acid Esters in Aqueous Samples. J. Sep. Sci. 2018, 41, 3806–3814. [Google Scholar] [CrossRef]
- Ma, J.K.; Huang, X.C.; Wei, S.L. Rapid Determination of Antiviral Medication Ribavirin in Different Feedstuffs Using a Novel Magnetic Molecularly Imprinted Polymer Coupled with High-Performance Liquid Chromatography. J. Sep. Sci. 2019, 42, 3372–3381. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Yu, X. Magnetic Molecularly Imprinted Polymers: Synthesis and Applications in the Selective Extraction of Antibiotics. Front. Chem. 2021, 9, 706311. [Google Scholar] [CrossRef]
- Lazar, M.M.; Ghiorghita, C.A.; Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Ion-Imprinted Polymeric Materials for Selective Adsorption of Heavy Metal Ions from Aqueous Solution. Molecules 2023, 28, 2798. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Sun, D.; Yan, S.; Wen, Y.; Wang, Y.; Li, G.; Liu, H.; Li, J.; Song, Z. Recent Advances in Molecularly Imprinted Polymers for Antibiotic Analysis. Molecules 2023, 28, 335. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Yuan, Y.; Wu, Y. Iron-Based Magnetic Molecular Imprinted Polymers and Their Application in Removal and Determination of Di-n-Pentyl Phthalate in Aqueous Media. R. Soc. Open Sci. 2017, 4, 170672. [Google Scholar] [CrossRef] [PubMed]
- Urriza-Arsuaga, I.; Guadaño-Sánchez, M.; Urraca, J.L. Current Trends in Molecular Imprinting: Strategies, Applications and Determination of Target Molecules in Spain. Int. J. Mol. Sci. 2023, 24, 1915. [Google Scholar] [CrossRef] [PubMed]
- Poonia, K.; Raizada, P.; Singh, A.; Verma, N.; Ahamad, T.; Alshehri, S.M.; Khan, A.A.P.; Singh, P.; Hussain, C.M. Magnetic Molecularly Imprinted Polymer Photocatalysts: Synthesis, Applications and Future Perspective. J. Ind. Eng. Chem. 2022, 113, 1–14. [Google Scholar] [CrossRef]
- Wei, S.; Li, J.; Liu, Y.; Ma, J. Development of Magnetic Molecularly Imprinted Polymers with Double Templates for the Rapid and Selective Determination of Amphenicol Antibiotics in Water, Blood, and Egg Samples. J. Chromatogr. A 2016, 1473, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Goyal, G.; Bhakta, S.; Mishra, P. Surface Molecularly Imprinted Biomimetic Magnetic Nanoparticles for Enantioseparation. ACS Appl. Nano Mater. 2019, 2, 6747–6756. [Google Scholar] [CrossRef]
- Dinc, M.; Esen, C.; Mizaikoff, B. Recent Advances on Core–Shell Magnetic Molecularly Imprinted Polymers for Biomacromolecules. Trends Anal. Chem. 2019, 114, 202–217. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, Y.; Xu, W.; Liu, Y.; Zhou, Y. Adsorption and Specific Recognition of DNA by Using Imprinted Polymer Layers Grafted onto Ionic Liquid Functionalized Magnetic Microspheres. Microchim. Acta 2017, 184, 4433–4441. [Google Scholar] [CrossRef]
- Popova, V.; Dmitrienko, E.; Chubarov, A. Magnetic Nanocomposites and Imprinted Polymers for Biomedical Applications of Nucleic Acids. Magnetochemistry 2023, 9, 12. [Google Scholar] [CrossRef]
- Petrov, K.D.; Chubarov, A.S. Magnetite Nanoparticles for Biomedical Applications. Encyclopedia 2022, 2, 1811–1828. [Google Scholar] [CrossRef]
- Bobrikova, E.; Chubarov, A.; Dmitrienko, E. The Effect of PH and Buffer on Oligonucleotide Affinity for Iron Oxide Nanoparticles. Magnetochemistry 2021, 7, 128. [Google Scholar] [CrossRef]
- Pilvenyte, G.; Ratautaite, V.; Boguzaite, R.; Ramanavicius, A.; Viter, R.; Ramanavicius, S. Molecularly Imprinted Polymers for the Determination of Cancer Biomarkers. Int. J. Mol. Sci. 2023, 24, 4105. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhao, X.; Ma, Y.; Zhang, H.; Pan, G. Molecularly Imprinted Nanomaterials with Stimuli Responsiveness for Applications in Biomedicine. Molecules 2023, 28, 918. [Google Scholar] [CrossRef]
- Gagliardi, M. Design and Development of Molecularly Imprinted Biodegradable Polymers for Nanomedicine. Adv. Ind. Eng. Polym. Res. 2023. [Google Scholar] [CrossRef]
- Balcer, E.; Sobiech, M. Molecularly Imprinted Carriers for Diagnostics and Therapy—A Critical Appraisal. Pharmaceutics 2023, 15, 1647. [Google Scholar] [CrossRef]
- Garnier, M.; Sabbah, M.; Ménager, C.; Griffete, N. Hybrid Molecularly Imprinted Polymers: The Future of Nanomedicine? Nanomaterials 2021, 11, 3091. [Google Scholar] [CrossRef]
- Sanadgol, N.; Wackerlig, J. Developments of Smart Drug-Delivery Systems Based on Magnetic Molecularly Imprinted Polymers for Targeted Cancer Therapy: A Short Review. Pharmaceutics 2020, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Dmitrienko, E.V.; Bulushev, R.D.; Haupt, K.; Kosolobov, S.S.; Latyshev, A.V.; Pyshnaya, I.A.; Pyshnyi, D.V. A Simple Approach to Prepare Molecularly Imprinted Polymers from Nylon-6. J. Mol. Recognit. 2013, 26, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Shakiba, M.; Rezvani Ghomi, E.; Khosravi, F.; Jouybar, S.; Bigham, A.; Zare, M.; Abdouss, M.; Moaref, R.; Ramakrishna, S. Nylon—A Material Introduction and Overview for Biomedical Applications. Polym. Adv. Technol. 2021, 32, 3368–3383. [Google Scholar] [CrossRef]
- Bulgakova, A.; Chubarov, A.; Dmitrienko, E. Magnetic Nylon 6 Nanocomposites for the Microextraction of Nucleic Acids from Biological Samples. Magnetochemistry 2022, 8, 85. [Google Scholar] [CrossRef]
- Reyes-Gallardo, E.M.; Lucena, R.; Cárdenas, S. Silica Nanoparticles-Nylon 6 Composites: Synthesis, Characterization and Potential Use as Sorbent. RSC Adv. 2017, 7, 2308–2314. [Google Scholar] [CrossRef]
- Mahfuz, H.; Hasan, M.; Dhanak, V.; Beamson, G.; Stewart, J.; Rangari, V.; Wei, X.; Khabashesku, V.; Jeelani, S. Reinforcement of Nylon 6 with Functionalized Silica Nanoparticles for Enhanced Tensile Strength and Modulus. Nanotechnology 2008, 19, 445702. [Google Scholar] [CrossRef]
- Popova, V.; Poletaeva, Y.; Chubarov, A.; Pyshnyi, D.; Dmitrienko, E. Doxorubicin-Loaded Silica Nanocomposites for Cancer Treatment. Coatings 2023, 13, 324. [Google Scholar] [CrossRef]
- Kovrigina, E.; Poletaeva, Y.; Zheng, Y.; Chubarov, A. Nylon-6-Coated Doxorubicin-Loaded Magnetic Nanoparticles and Nanocapsules for Cancer Treatment. Magnetochemistry 2023, 9, 106. [Google Scholar] [CrossRef]
- Yang, F.; Fu, D.; Li, P.; Sui, X.; Xie, Y.; Chi, J.; Liu, J.; Huang, B. Magnetic Molecularly Imprinted Polymers for the Separation and Enrichment of Cannabidiol from Hemp Leaf Samples. ACS Omega 2022, 8, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic Nanoparticle Systems for Nanomedicine—A Materials Science Perspective. Magnetochemistry 2020, 6, 2. [Google Scholar] [CrossRef]
- Hepel, M. Magnetic Nanoparticles for Nanomedicine. Magnetochemistry 2020, 6, 3. [Google Scholar] [CrossRef]
- Niu, M.; Pham-Huy, C.; He, H. Core-Shell Nanoparticles Coated with Molecularly Imprinted Polymers: A Review. Microchim. Acta 2016, 183, 2677–2695. [Google Scholar] [CrossRef]
- Kirillov, V.L.; Balaev, D.A.; Semenov, S.V.; Shaikhutdinov, K.A.; Martyanov, O.N. Size Control in the Formation of Magnetite Nanoparticles in the Presence of Citrate Ions. Mater. Chem. Phys. 2014, 145, 75–81. [Google Scholar] [CrossRef]
- Kirillov, V.L.; Yakushkin, S.S.; Balaev, D.A.; Dubrovskiy, A.A.; Semenov, S.V.; Knyazev, Y.V.; Bayukov, O.A.; Velikanov, D.A.; Yatsenko, D.A.; Martyanov, O.N. Dimethylsulfoxide as a Media for One-Stage Synthesis of the Fe3O4 -Based Ferro Fl Uids with a Controllable Size Distribution. Mater. Chem. Phys. 2019, 225, 292–297. [Google Scholar] [CrossRef]
- Ibarra, J.; Melendres, J.; Almada, M.; Burboa, M.G.; Taboada, P.; Juárez, J.; Valdez, M.A. Synthesis and Characterization of Magnetite/PLGA/Chitosan Nanoparticles. Mater. Res. Express 2015, 2, 95010. [Google Scholar] [CrossRef]
- Popova, V.; Poletaeva, Y.; Chubarov, A.; Dmitrienko, E. PH-Responsible Doxorubicin-Loaded Fe3O4@CaCO3 Nanocomposites for Cancer Treatment. Pharmaceutics 2023, 15, 771. [Google Scholar] [CrossRef] [PubMed]
- Kowalik, P.; Elbaum, D.; Mikulski, J.; Fronc, K.; Kamińska, I.; Morais, P.C.; Eduardo De Souza, P.; Nunes, R.B.; Veiga-Souza, F.H.; Gruzeł, G.; et al. Upconversion Fluorescence Imaging of HeLa Cells Using ROS Generating SiO2-Coated Lanthanide-Doped NaYF4 Nanoconstructs. RSC Adv. 2017, 7, 30262–30273. [Google Scholar] [CrossRef]
- Stoia, M.; Istratie, R.; Păcurariu, C. Investigation of Magnetite Nanoparticles Stability in Air by Thermal Analysis and FTIR Spectroscopy. J. Therm. Anal. Calorim. 2016, 125, 1185–1198. [Google Scholar] [CrossRef]
- Vasanthan, N. Crystallinity Determination of Nylon 66 by Density Measurement and Fourier Transform Infrared (FTIR) Spectroscopy. J. Chem. Educ. 2012, 89, 387–390. [Google Scholar] [CrossRef]
- Mahdi, H.A. An FTIR Study of Characterization of Neat and UV Stabilized Nylon 6,6 Polymer Films. J. Pure Appl. Sci. 2011, 24, 86–90. [Google Scholar]
- Khori, N.K.E.M.; Salmiati; Hadibarata, T.; Yusop, Z. A Combination of Waste Biomass Activated Carbon and Nylon Nanofiber for Removal of Triclosan from Aqueous Solutions. J. Environ. Treat. Tech. 2020, 8, 1036–1045. [Google Scholar] [CrossRef]
- Gao, R.; Hao, Y.; Zhao, S.; Zhang, L.; Cui, X.; Liu, D.; Tang, Y.; Zheng, Y. Novel Magnetic Multi-Template Molecularly Imprinted Polymers for Specific Separation and Determination of Three Endocrine Disrupting Compounds Simultaneously in Environmental Water Samples. RSC Adv. 2014, 4, 56798–56808. [Google Scholar] [CrossRef]
- Ansari, S.; Karimi, M. Recent Configurations and Progressive Uses of Magnetic Molecularly Imprinted Polymers for Drug Analysis. Talanta 2017, 167, 470–485. [Google Scholar] [CrossRef] [PubMed]

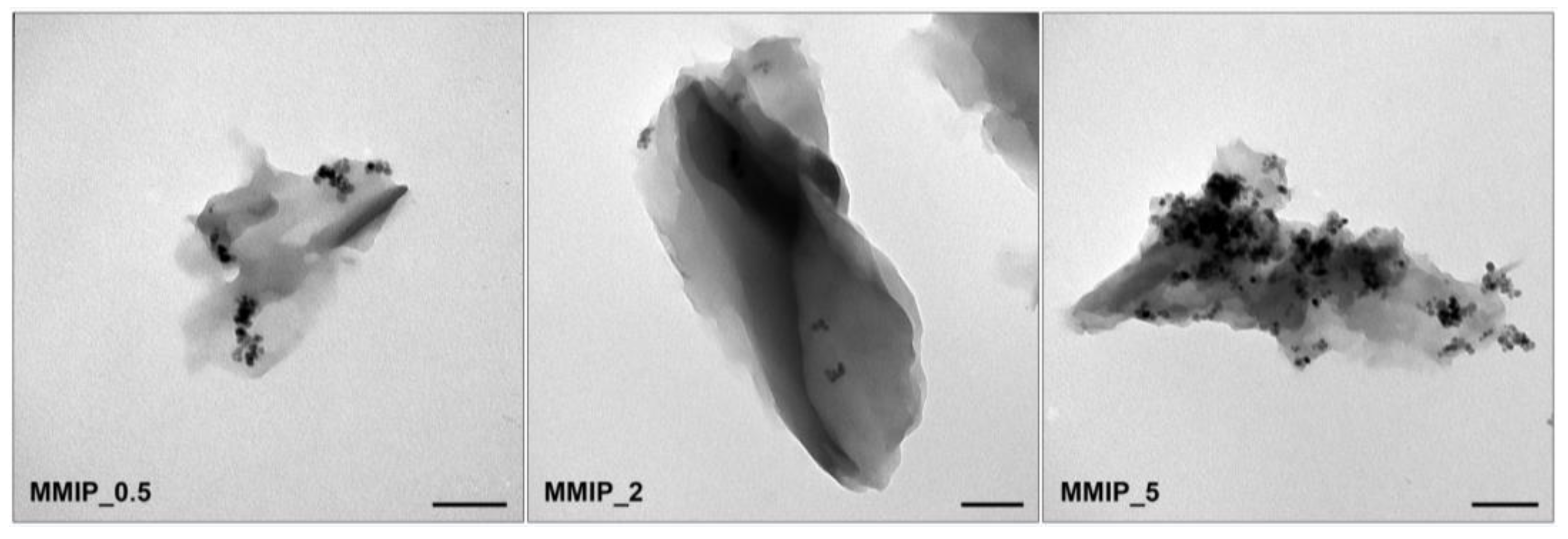
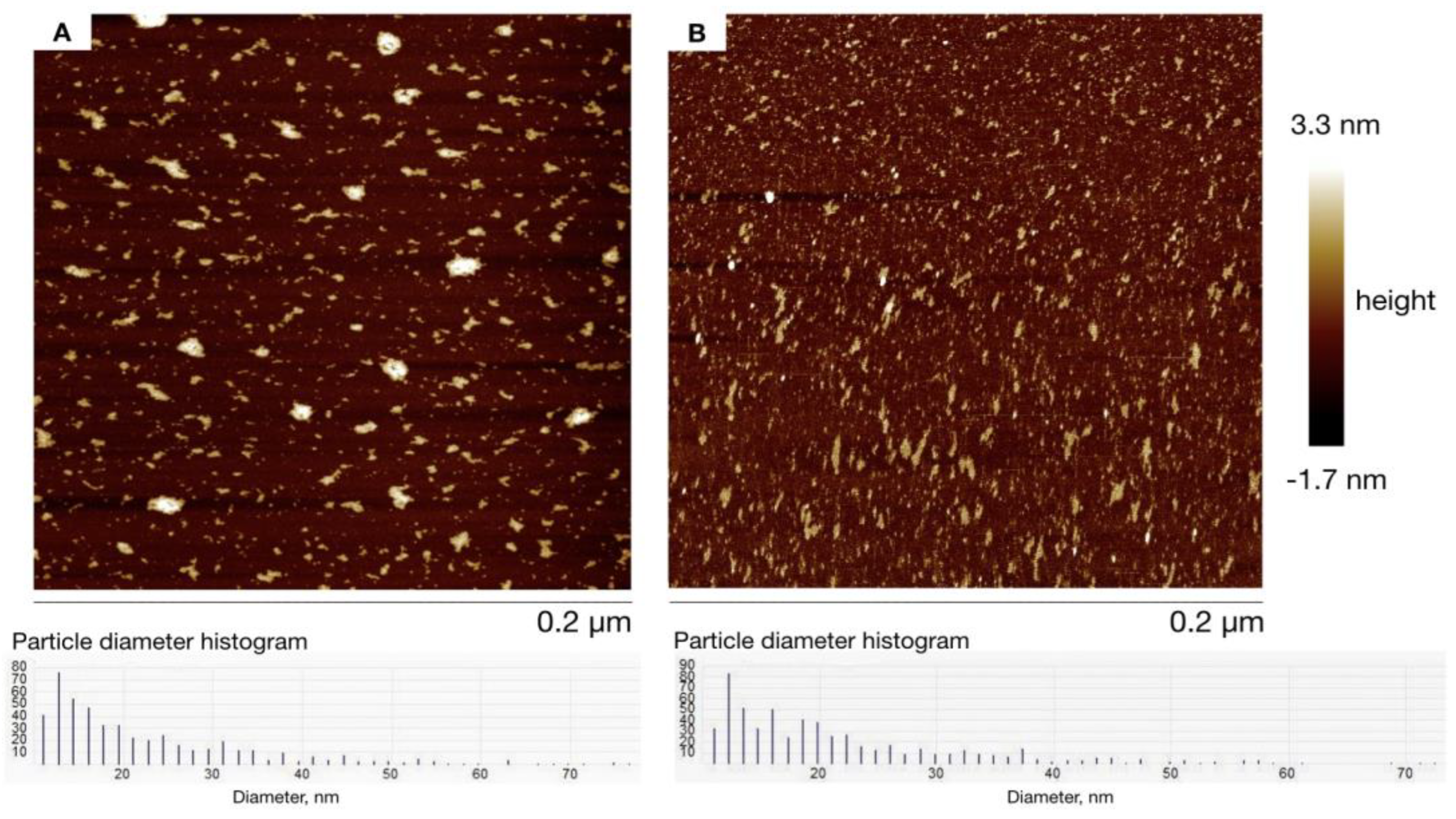
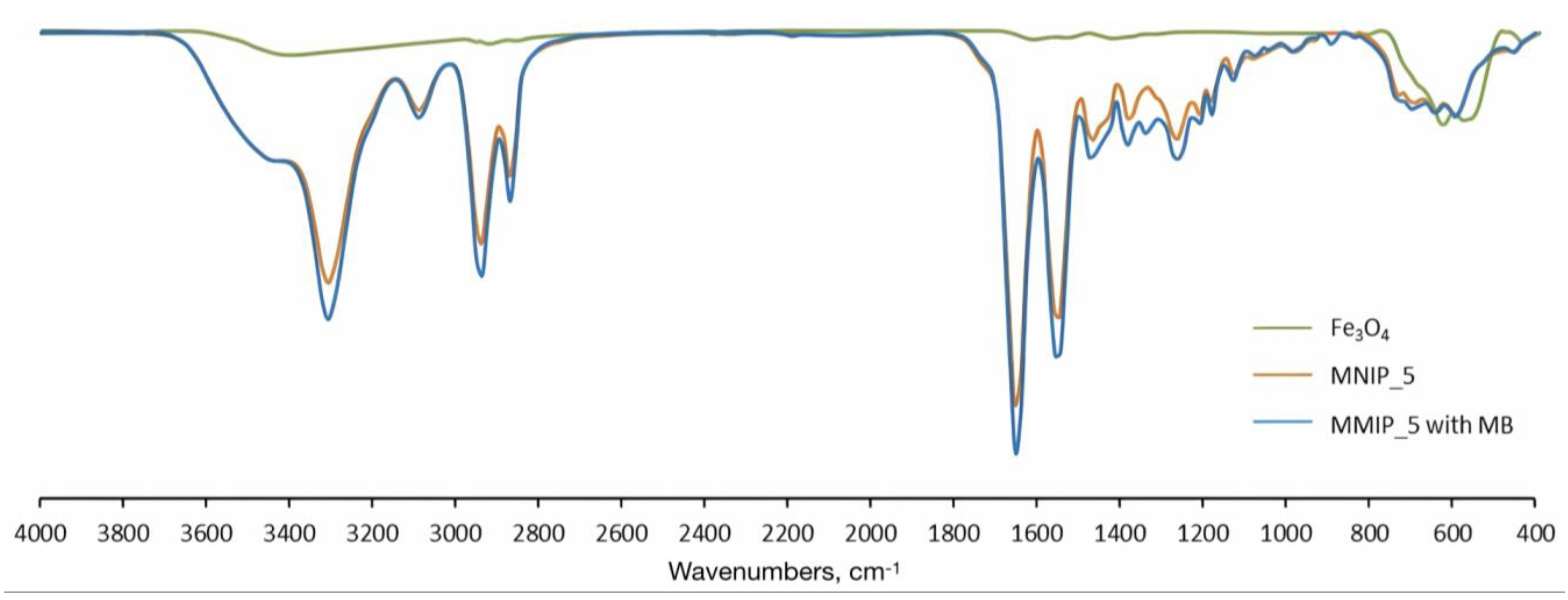
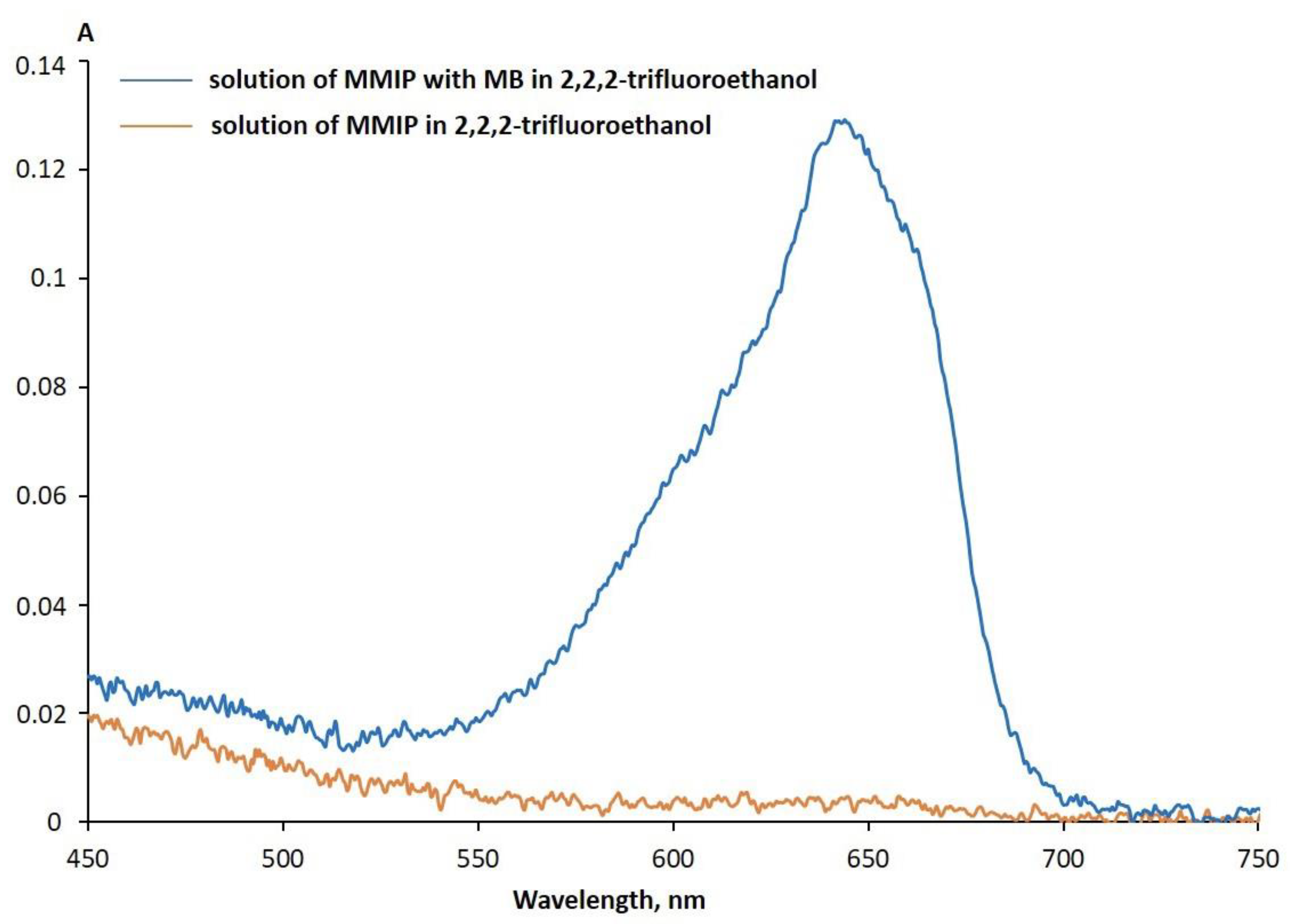

| Sample Name | MNPs Amount for Synthesis | Hydrodynamic Diameter, nm |
|---|---|---|
| MMIP_0.5 | 0.5 mg | 66.7 ± 10.2 |
| MMIP_2 | 2 mg | 103.1 ± 24.6 |
| MMIP_5 | 5 mg | 173.8 ± 24.5 |
| MNIP_0.5 | 0.5 mg | 86.9 ± 29.1 |
| MNIP_2 | 2 mg | 105.0 ± 17.2 |
| MNIP_5 | 5 mg | 144.1 ± 23.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedelnikova, A.; Poletaeva, Y.; Golyshev, V.; Chubarov, A.; Dmitrienko, E. Preparation of Magnetic Molecularly Imprinted Polymer for Methylene Blue Capture. Magnetochemistry 2023, 9, 196. https://doi.org/10.3390/magnetochemistry9080196
Sedelnikova A, Poletaeva Y, Golyshev V, Chubarov A, Dmitrienko E. Preparation of Magnetic Molecularly Imprinted Polymer for Methylene Blue Capture. Magnetochemistry. 2023; 9(8):196. https://doi.org/10.3390/magnetochemistry9080196
Chicago/Turabian StyleSedelnikova, Anastasia, Yuliya Poletaeva, Victor Golyshev, Alexey Chubarov, and Elena Dmitrienko. 2023. "Preparation of Magnetic Molecularly Imprinted Polymer for Methylene Blue Capture" Magnetochemistry 9, no. 8: 196. https://doi.org/10.3390/magnetochemistry9080196
APA StyleSedelnikova, A., Poletaeva, Y., Golyshev, V., Chubarov, A., & Dmitrienko, E. (2023). Preparation of Magnetic Molecularly Imprinted Polymer for Methylene Blue Capture. Magnetochemistry, 9(8), 196. https://doi.org/10.3390/magnetochemistry9080196