Magnetism and Electronic State of Iron Ions on the Surface and in the Core of TiO2 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results
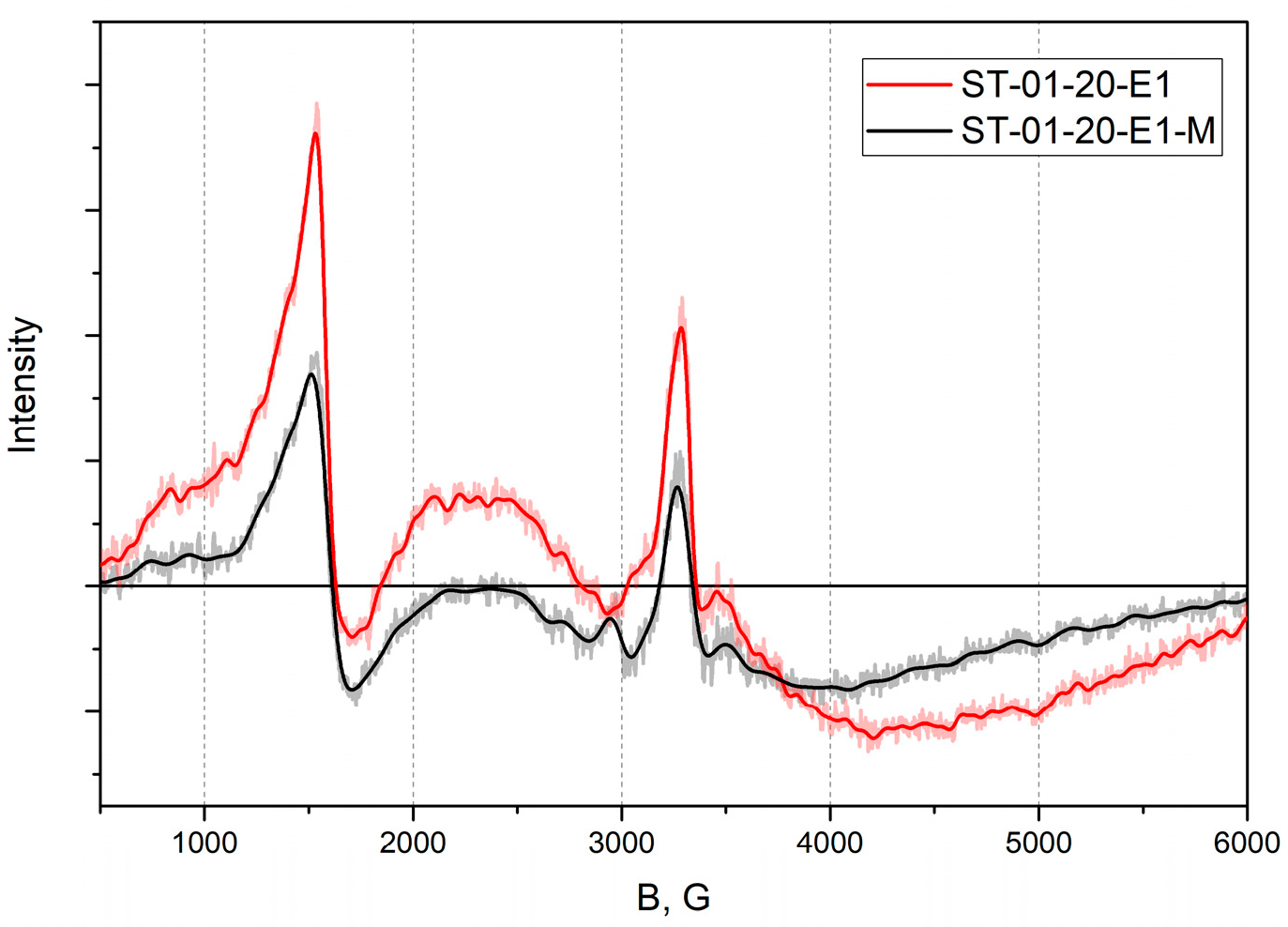
3.1. EPR Investigations of TiO2 Nanopowders with Fe Deposited on the Surface and in the Core of Nanoparticles of Different Size
3.2. Results of Theoretical Study and Experimental Investigation of the Magnetic Properties of the Surface and the Cores of TiO2: Fe Nanoparticles and Discussion
3.2.1. Theoretical Study of Magnetism of the Surface and the Core of TiO2-Based Nanocrystalline Systems Doped with Iron
3.2.2. Mössbauer Spectroscopy of Nanocrystalline TiO2 Samples with Localization of Fe Ions on the Surface and in the Core
3.3. Magnetic Properties of ST-01, ST-21 Nanopowders Doped with Iron
3.3.1. Analysis of Magnetization Curves for Samples ST-01, ST-21 Doped Iron
3.3.2. Estimation of the Effect of the Low-Spin State of Fe3+ on the Magnetization of TiO2 Samples
3.3.3. Analysis of Effective Magnetic Moments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Groß, A. Theoretical Surface Science: A Microscopic Perspective. In Theoretical Surface Science: A Microscopic Perspective; Springer: Berlin/Heidelberg, Germany, 2009; pp. 165–184. ISBN 978-3-540-68966-9. [Google Scholar]
- Mohamed, A.E.-M.A.; Mohamed, M.A. Nanoparticles: Magnetism and Applications. In Magnetic Nanostructures; Abd-Elsalam, K.A., Mohamed, M.A., Prasad, R., Eds.; Nanotechnology in the Life Sciences; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–12. ISBN 978-3-030-16438-6. [Google Scholar]
- Pryazhnikov, D.V.; Kubrakova, I.V. Surface-Modified Magnetic Nanoscale Materials: Preparation and Study of Their Structure, Composition, and Properties. J. Anal. Chem. 2021, 76, 685–706. [Google Scholar] [CrossRef]
- Li, N.; Zhang, W.; Wang, D.; Li, G.; Zhao, Y. Synthesis and Applications of TiO2-based Nanostructures as Photocatalytic Materials. Chem. Asian J. 2022, 17, e202200822. [Google Scholar] [CrossRef] [PubMed]
- Fàbrega, C.; Andreu, T.; Cabot, A.; Morante, J.R. Location and Catalytic Role of Iron Species in TiO2:Fe Photocatalysts: An EPR Study. J. Photochem. Photobiol. A Chem. 2010, 211, 170–175. [Google Scholar] [CrossRef]
- Areerachakul, N.; Sakulkhaemaruethai, S.; Johir, M.A.H.; Kandasamy, J.; Vigneswaran, S. Photocatalytic Degradation of Organic Pollutants from Wastewater Using Aluminium Doped Titanium Dioxide. J. Water Process Eng. 2019, 27, 177–184. [Google Scholar] [CrossRef]
- Chernyshev, A.P. Mechanism of the Order-Disorder Transition in Ultra-Small Metal Nanoparticles. Phys. E Low-Dimens. Syst. Nanostructures 2018, 104, 111–115. [Google Scholar] [CrossRef]
- Howard-Fabretto, L.; Andersson, G.G. Metal Clusters on Semiconductor Surfaces and Application in Catalysis with a Focus on Au and Ru. Adv. Mater. 2020, 32, 1904122. [Google Scholar] [CrossRef] [PubMed]
- Doud, E.A.; Voevodin, A.; Hochuli, T.J.; Champsaur, A.M.; Nuckolls, C.; Roy, X. Superatoms in Materials Science. Nat. Rev. Mater. 2020, 5, 371–387. [Google Scholar] [CrossRef]
- Banach, E.; Bürgi, T. Metal Nanoclusters as Versatile Building Blocks for Hierarchical Structures. Helv. Chim. Acta 2022, 105, e202100186. [Google Scholar] [CrossRef]
- Mason, J.L.; Folluo, C.N.; Jarrold, C.C. More than Little Fragments of Matter: Electronic and Molecular Structures of Clusters. J. Chem. Phys. 2021, 154, 200901. [Google Scholar] [CrossRef]
- Wang, H.; Lu, J. A Review on Particle Size Effect in Metal-Catalyzed Heterogeneous Reactions. Chin. J. Chem. 2020, 38, 1422–1444. [Google Scholar] [CrossRef]
- Baletto, F. Structural Properties of Sub-Nanometer Metallic Clusters. J. Phys. Condens. Matter 2019, 31, 113001. [Google Scholar] [CrossRef] [PubMed]
- Reimann, S.M.; Koskinen, M.; Häkkinen, H.; Lindelof, P.E.; Manninen, M. Magic Triangular and Tetrahedral Clusters. Phys. Rev. B 1997, 56, 12147–12150. [Google Scholar] [CrossRef]
- Illy, S.; Tillement, O.; Machizaud, F.; Dubois, J.M.; Massicot, F.; Fort, Y.; Ghanbaja, J. First Direct Evidence of Size-Dependent Structural Transition in Nanosized Nickel Particles. Philos. Mag. A 1999, 79, 1021–1031. [Google Scholar] [CrossRef]
- Jones, R.O. Cluster Geometries from Density Functional Calculations—Prospects and Limitations. In The European Physical Journal D; Châtelain, A., Bonard, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 81–84. ISBN 978-3-642-88190-9. [Google Scholar]
- Yasuda, H.; Mori, H. Effect of Cluster Size on the Chemical Ordering in Nanometer-Sized Au-75at%Cu Alloy Clusters. Z. Für Phys. D At. Mol. Clust. 1996, 37, 181–186. [Google Scholar] [CrossRef]
- Doye, J.P.K. Physical Perspectives on the Global Optimization of Atomic Clusters. In Global Optimization; Pintér, J.D., Ed.; Nonconvex Optimization and Its Applications; Springer: Berlin/Heidelberg, Germany, 2006; Volume 85, pp. 103–139. ISBN 978-0-387-30408-3. [Google Scholar]
- Baletto, F.; Ferrando, R. Structural Properties of Nanoclusters: Energetic, Thermodynamic, and Kinetic Effects. Rev. Mod. Phys. 2005, 77, 371–423. [Google Scholar] [CrossRef]
- Ohtani, B.; Mahaney, O.O.P.; Amano, F.; Murakami, N.; Abe, R. What Are Titania Photocatalysts?―An Exploratory Correlation of Photocatalytic Activity with Structural and Physical Properties. J. Adv. Oxid. Technol. 2010, 13, 247–261. [Google Scholar] [CrossRef]
- Gupta, A.; Zhang, R.; Kumar, P.; Kumar, V.; Kumar, A. Nano-Structured Dilute Magnetic Semiconductors for Efficient Spintronics at Room Temperature. Magnetochemistry 2020, 6, 15. [Google Scholar] [CrossRef]
- Chouhan, L.; Bouzerar, G.; Srivastava, S.K. Effect of Mg-Doping in Tailoring D0 Ferromagnetism of Rutile TiO2 Compounds for Spintronics Application. J. Mater. Sci Mater. Electron. 2021, 32, 11193–11201. [Google Scholar] [CrossRef]
- Wertheim, G.K.; Remeika, J.P. Mössbauer Effect Hyperfine Structure of Trivalent Fe57 in Corundum. Phys. Lett. 1964, 10, 14–15. [Google Scholar] [CrossRef]
- Katsuhisa, T. ESR and Mössbauer Studies on Structure and Magnetic Properties of Iron-Containing Amorphous Oxides. Ph.D. Thesis, Kyoto University, Kyoto, Japan, 1991. [Google Scholar]
- Yermakov, A.Y.; Boukhvalov, D.W.; Volegov, A.S.; Uimin, M.A.; Zakharova, G.S.; Korolev, A.V.; Rosenfeld, E.V.; Mesilov, V.V.; Minin, A.S.; Galakhov, V.R.; et al. Unconventional Magnetism of Non-Uniform Distribution of Co in TiO2 Nanoparticles. J. Alloys Compd. 2020, 826, 154194. [Google Scholar] [CrossRef]
- Molochnikov, L.S.; Borodin, K.I.; Yermakov, A.E.; Uimin, M.A.; Minin, A.S.; Vostroknutova, A.V.; Eremina, R.M.; Kurkin, M.I.; Konev, S.F.; Konev, A.S.; et al. Magnetism and Temperature Dependence of Nano-TiO2: Fe EPR Spectra. Mater. Chem. Phys. 2022, 276, 125327. [Google Scholar] [CrossRef]
- Bingham, P.A. Magnetism and Temperature Dependence of Nano-TiO2: Fe EPR Spectra, Chapter 5, Electron Spin Resonance. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2000. [Google Scholar]
- Ferretti, A.M.; Forni, L.; Oliva, C.; Ponti, A. EPR Study of Iron-Doped MFI Zeolite and Silicalite Catalysts: Effect of Treatments after Synthesis. Res. Chem. Intermed. 2002, 28, 101–116. [Google Scholar] [CrossRef]
- Matsnev, M.E.; Rusakov, V.S. SpectrRelax: An Application for Mössbauer Spectra Modeling and Fitting. In Proceedings of the International Conference MSMS-12, Olomouc, Czech Republic, 11–15 June 2012; pp. 178–185. [Google Scholar]
- Yu, S.; Yun, H.J.; Lee, D.M.; Yi, J. Preparation and Characterization of Fe-Doped TiO2 Nanoparticles as a Support for a High Performance CO Oxidation Catalyst. J. Mater. Chem. 2012, 22, 12629. [Google Scholar] [CrossRef]
- Adán, C.; Bahamonde, A.; Fernández-García, M.; Martínez-Arias, A. Structure and Activity of Nanosized Iron-Doped Anatase TiO2 Catalysts for Phenol Photocatalytic Degradation. Appl. Catal. B Environ. 2007, 72, 11–17. [Google Scholar] [CrossRef]
- Yermakov, A.Y.; Gubkin, A.F.; Korolev, A.V.; Molochnikov, L.S.; Uimin, M.A.; Rosenfeld, E.V.; Kurkin, M.I.; Minin, A.S.; Volegov, A.S.; Boukhvalov, D.W.; et al. Formation of Fe–Fe Antiferromagnetic Dimers in Doped TiO2:Fe Nanoparticles. J. Phys. Chem. C 2019, 123, 1494–1505. [Google Scholar] [CrossRef]
- Vasil’yev, A.N.; Zvereva, Y.A.; Volkova, O.S.; Markina, M.M. Nizkorazmernyy magnetizm. In Nizkorazmernyy Magnetizm; Izdatel’stvo Fizmatlit: Moscow, Russia, 2017; p. 336. [Google Scholar]
- Dong, F.; Wang, H.; Wu, Z.; Qiu, J. Marked Enhancement of Photocatalytic Activity and Photochemical Stability of N–Doped TiO2 Nanocrystals by Fe3+/Fe2+ Surface Modification. J. Colloid Interface Sci. 2010, 343, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Thorp, J.S.; Eggleston, H.S. Rhombic Symmetry Sites in Fe/TiO2 Powders. J. Mater. Sci. Lett. 1985, 4, 1140–1142. [Google Scholar] [CrossRef]
- Janes, R. Structural and Spectroscopic Studies of Iron (III) Doped Titania Powders Prepared by Sol-Gel Synthesis and Hydrothermal Processing. Dye. Pigment. 2004, 62, 199–212. [Google Scholar] [CrossRef]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA Method for Ab Initio Order- N Materials Simulation. J. Phys. Condens. Matter 2002, 14, 2745–2779. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient Pseudopotentials for Plane-Wave Calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]









| No. | Sample | Calcination T, °C | Fe Conc., wt.% |
|---|---|---|---|
| Location of iron atoms on the surface of TiO2 nanoparticles | |||
| 1 | ST-01-20 | 20 | 4.17 |
| 2 | ST-21-20 | 20 | 2.09 |
| 3 | ST-41-20 | 20 | 0.52 |
| 4 | ST-01-20-E1-1 | 20 | 0.42 |
| 5 | ST-01-20-E1-2 | 20 | 0.33 |
| 6 | ST-01-20-E1-3 | 20 | 0.51 |
| 7 | ST-01-20-E1-4 | 20 | 1.44 |
| 8 | ST-21-20-E1 | 20 | 0.18 |
| Location of iron atoms in the core of TiO2 nanoparticles | |||
| 9 | ST-01-100-E1 | 100 | 1.09 |
| 10 | ST-01-300-E1-E2 | 300 | 1.62 |
| 11 | ST-21-100-E1 | 100 | 0.26 |
| 12 | ST-21-300-E1 | 300 | 0.57 |
| 13 | ST-21-400-E1 | 400 | 0.91 |
| Host | Configuration of Impurities | Formation Energy, eV/Fe | Magnetic Moments, μB | Occupancy | CN | d(Fe-Fe), Å | d(Fe-O), Å |
|---|---|---|---|---|---|---|---|
| Core | FeTi | −0.04 | 3.80 | 3d6 4s0 | 6 | --- | 1.91, 1.99 |
| FeTi + FeTi | −0.13 | 3.73 | 3d6 4s0 | 6 | 3.11 | 1.90, 1.98 | |
| Core + vO | FeTi | −1.68 | 4.27 | 3d5 4s0 | 5 | --- | 1.90, 1.94, 2.08 |
| FeTi + FeTi | −1.41 | 4.26, 4.31 | 3d5 4s0 | 5 | 3.01 | 1.94, 2.00, 2.06 | |
| (110) | Fei | −5.73 | 4.14 | 3d5 4s0 | 5 | --- | 1.93, 2.02 |
| FeTi + Fei | −3.83 | 4.20, 1.12 | 3d5 4s0 (HS, LS) | 5 | 2.72 | 1.90, 1.93, 2.02 | |
| (110) + vO | Fei | −2.98 | 3.69 | 3d6 4s0 | 3 | --- | 1.90 |
| FeTi + Fei | −3.14 | 3.63, 4.27 | 3d5 4s0, 3d6 4s0 | 3, 5 | 2.82 | 1.90 | |
| (101) | Fei | −4.53 | 3.83 | 3d6 4s0 | 3 | --- | 1.90, 2.01 |
| FeTi + Fei | −3.14 | 3.92, 4.24 | 3d6 4s0, 3d5 4s0 | 3, 4 | 4.52 | 1.82, 1.94, 2.08 | |
| (101) + vO | Fei | −5.18 | 3.88 | 3d6 4s0 | 3 | --- | 1.90, 2.07 |
| FeTi + Fei | −3.43 | 3.82, 4.19 | 3d6 4s0, 3d5 4s0 | 3, 3 | 4.75 | 1.89, 2.02 |
| Sample | Annealing | Etching | Mass. % Fe57 |
|---|---|---|---|
| ST-01-20-E1-M | no | HNO3 | 4.00 |
| ST-01-300-E1-E2-M | 300 °C | HCl, Na2S2O6 | 2.72 |
| ST-01-20-E1-M | ST-01-300-E1-E2-M | |||||
|---|---|---|---|---|---|---|
| I, rel.un. | IS, mm/s | QS, mm/s | I, rel.un. | IS, mm/s | QS, mm/s | |
| 1 | 0.72 ± 0.01 | 0.343 ± 0.007 | 0.60 ± 0.01 | 0.318 ± 0.002 | 0.35 ± 0.002 | 0.89 ± 0.01 |
| 2 | 0.28 ± 0.02 | 0.337 ± 0.005 | 1.04 ± 0.01 | 0.515 ± 0.002 | 0.367 ± 0.001 | 0.56 ± 0.01 |
| 3 | - | - | - | 0.167 ± 0.001 | 0.318 ± 0.003 | 1.32 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yermakov, A.Y.; Uimin, M.A.; Boukhvalov, D.W.; Minin, A.S.; Kleinerman, N.M.; Naumov, S.P.; Volegov, A.S.; Starichenko, D.V.; Borodin, K.I.; Gaviko, V.S.; et al. Magnetism and Electronic State of Iron Ions on the Surface and in the Core of TiO2 Nanoparticles. Magnetochemistry 2023, 9, 198. https://doi.org/10.3390/magnetochemistry9080198
Yermakov AY, Uimin MA, Boukhvalov DW, Minin AS, Kleinerman NM, Naumov SP, Volegov AS, Starichenko DV, Borodin KI, Gaviko VS, et al. Magnetism and Electronic State of Iron Ions on the Surface and in the Core of TiO2 Nanoparticles. Magnetochemistry. 2023; 9(8):198. https://doi.org/10.3390/magnetochemistry9080198
Chicago/Turabian StyleYermakov, Anatoly Ye., Mikhail A. Uimin, Danil W. Boukhvalov, Artem S. Minin, Nadezhda M. Kleinerman, Sergey P. Naumov, Aleksey S. Volegov, Denis V. Starichenko, Kirill I. Borodin, Vasily S. Gaviko, and et al. 2023. "Magnetism and Electronic State of Iron Ions on the Surface and in the Core of TiO2 Nanoparticles" Magnetochemistry 9, no. 8: 198. https://doi.org/10.3390/magnetochemistry9080198
APA StyleYermakov, A. Y., Uimin, M. A., Boukhvalov, D. W., Minin, A. S., Kleinerman, N. M., Naumov, S. P., Volegov, A. S., Starichenko, D. V., Borodin, K. I., Gaviko, V. S., Konev, S. F., & Cherepanov, N. A. (2023). Magnetism and Electronic State of Iron Ions on the Surface and in the Core of TiO2 Nanoparticles. Magnetochemistry, 9(8), 198. https://doi.org/10.3390/magnetochemistry9080198







