Investigation of Heat Transfer Enhancement Techniques on a Scalable Novel Hybrid Thermal Management Strategy for Lithium-Ion Battery Packs
Abstract
:1. Introduction
2. Description of the Proposed Hybrid Strategy
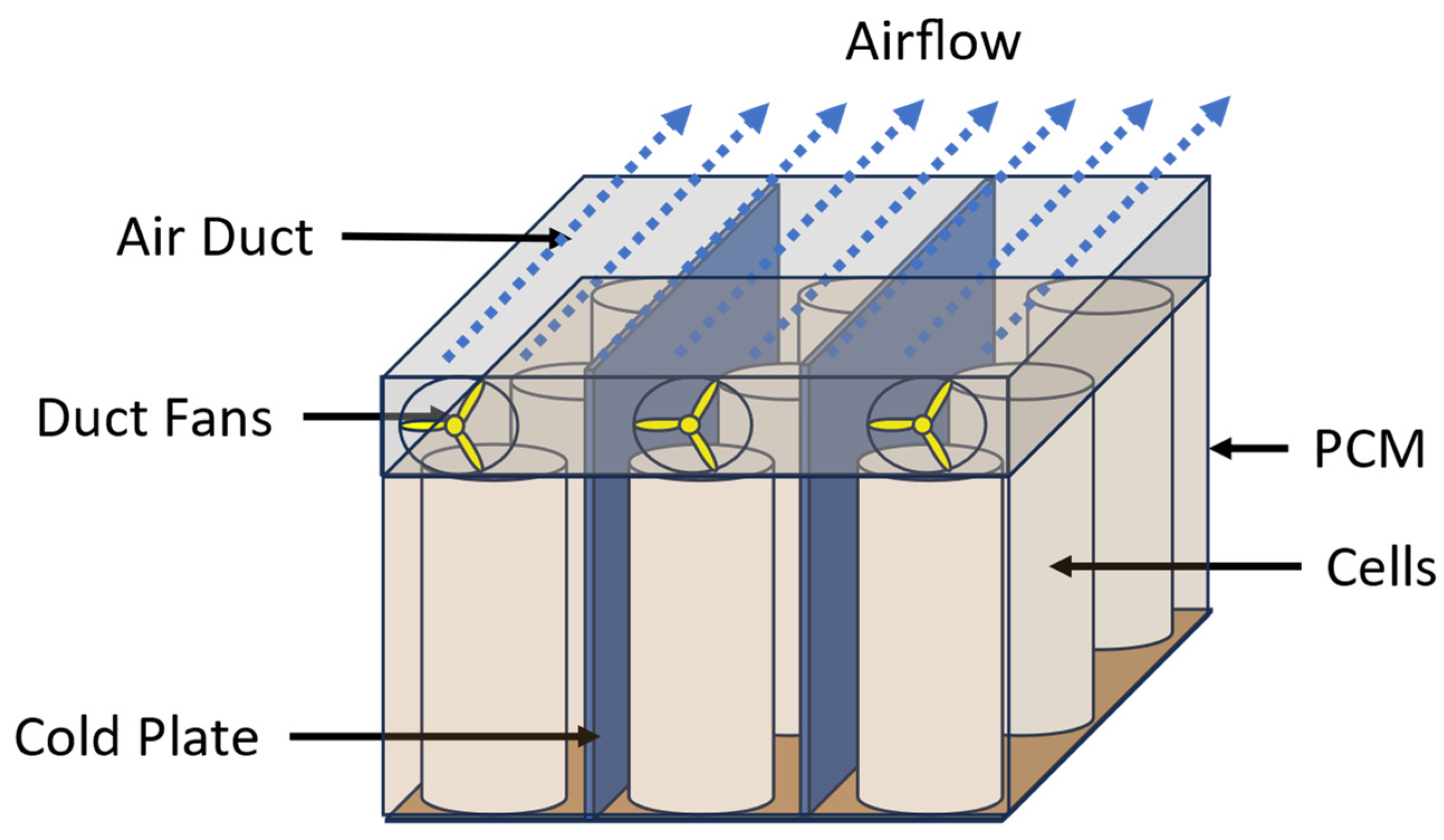
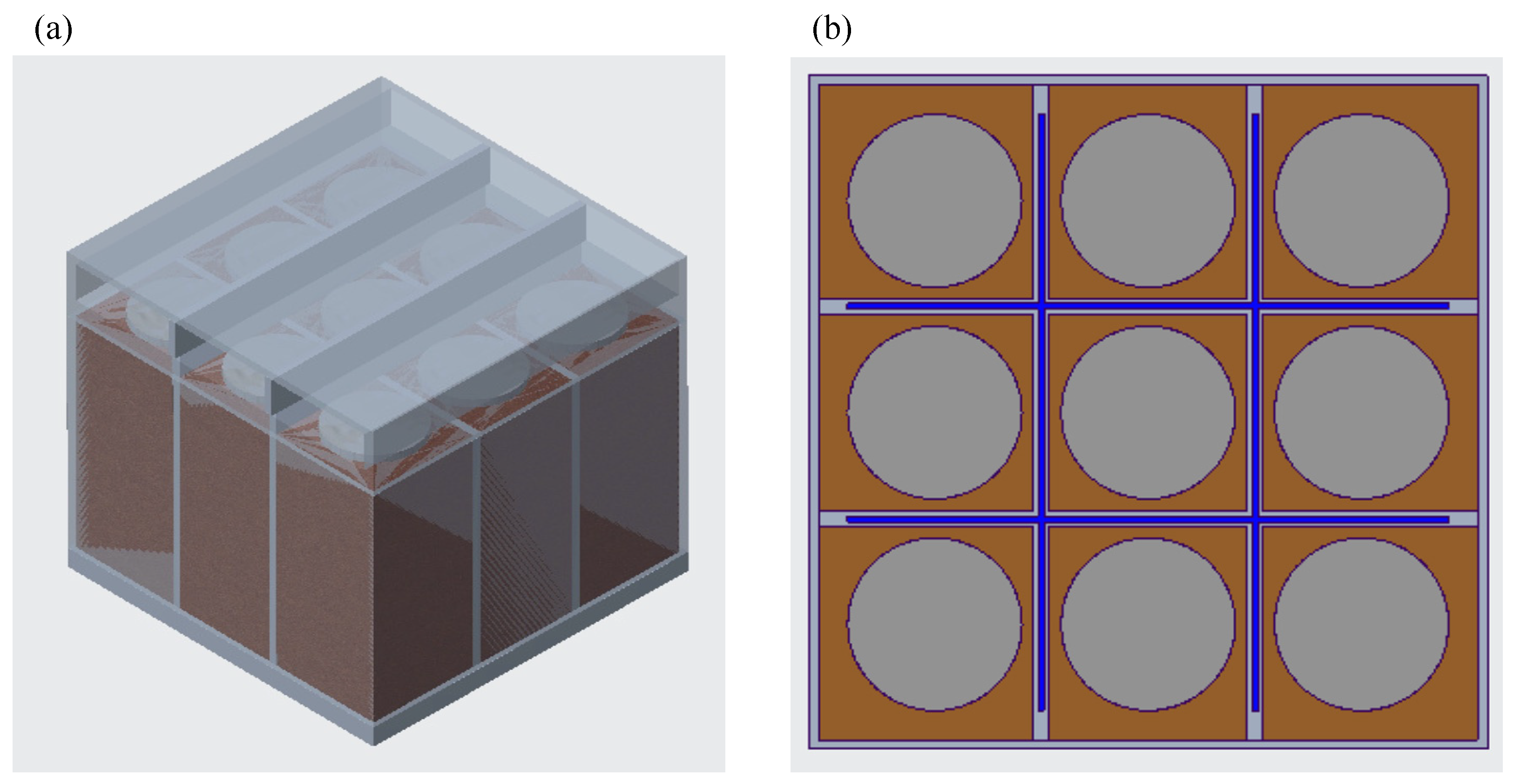
2.1. Battery Module Configuration
- The battery module should have a high temperature uniformity.
- The fluid pumping power should be eliminated.
- It should have modularity for scalability.
2.2. Increasing the Thermal Conductivity of Water through Nanoparticles
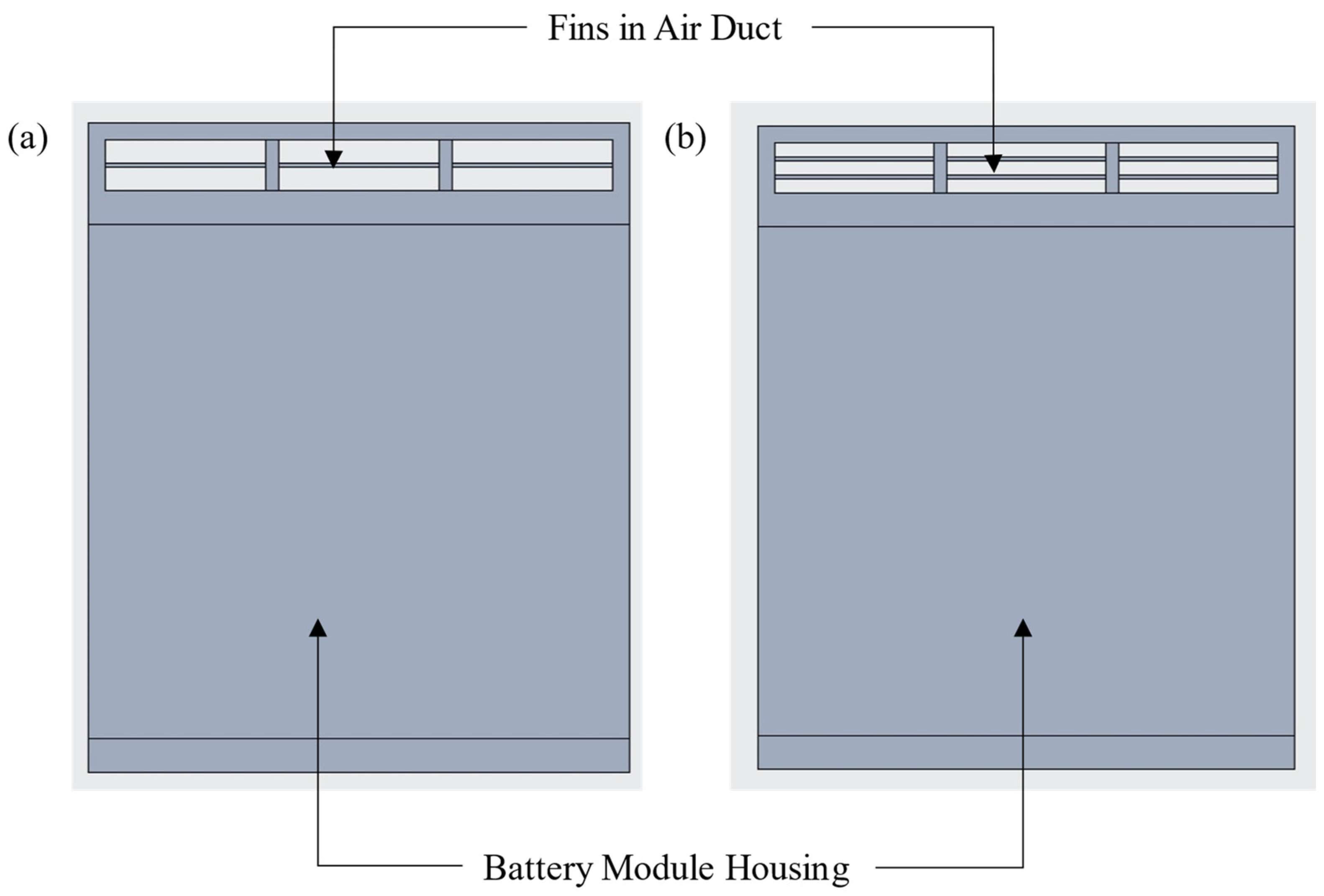
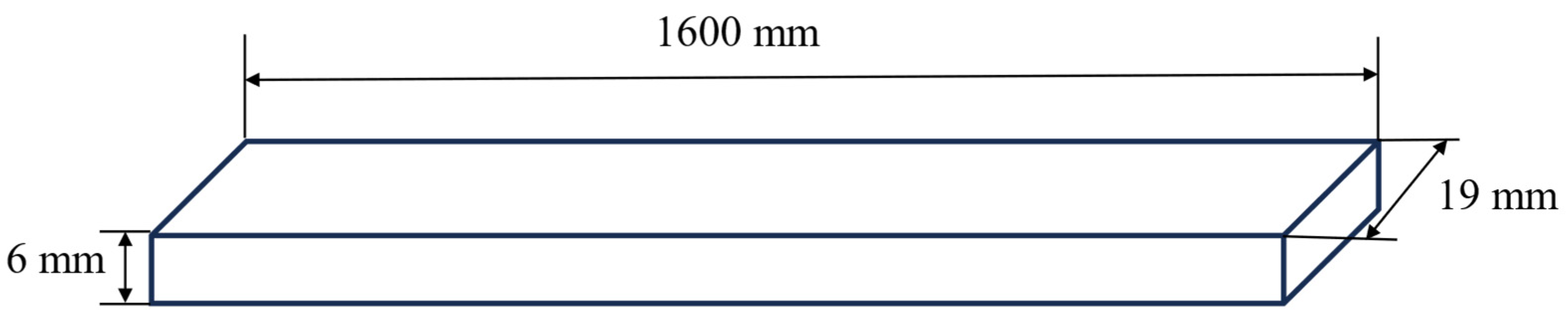
2.3. Addition of Fins in the Battery Module to Improve Heat Transfer
2.4. Scalability of the Battery Module
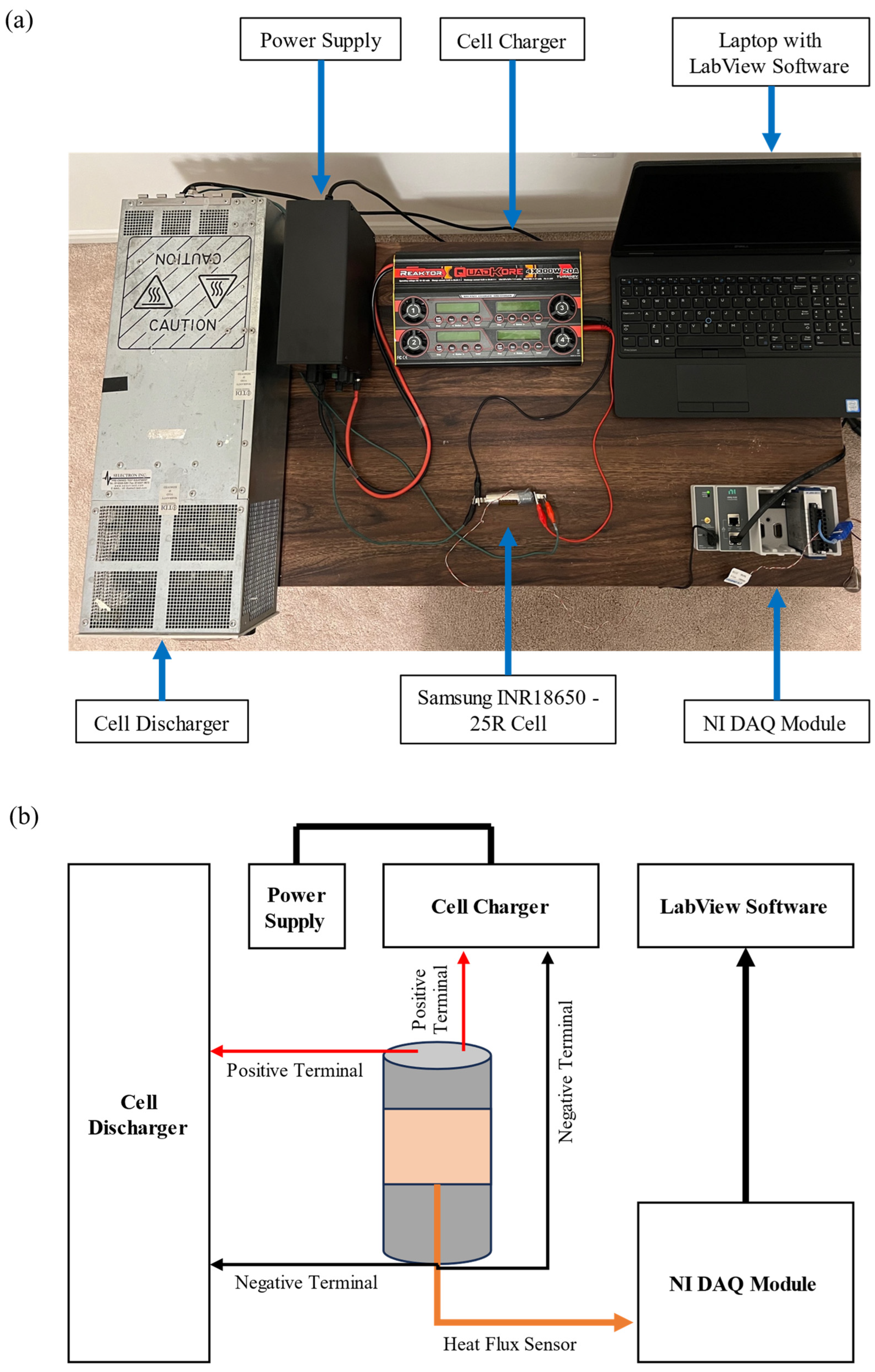
3. Experimental Setup and Procedure
3.1. Experiment I—Heat Flux Measurement
3.2. Experiment II—Temperature Measurement
4. Numerical Modeling
4.1. Governing Equations
- Airflow through the duct is incompressible and the temperature of the air at the battery module inlet is at an ambient temperature.
- The fluid in the liquid cooling is incompressible and the temperature of the fluid at the start of the discharge process is at an ambient temperature.
- There is no leakage of fluid from the liquid cooling components.
- The battery module is kept at an atmospheric pressure.
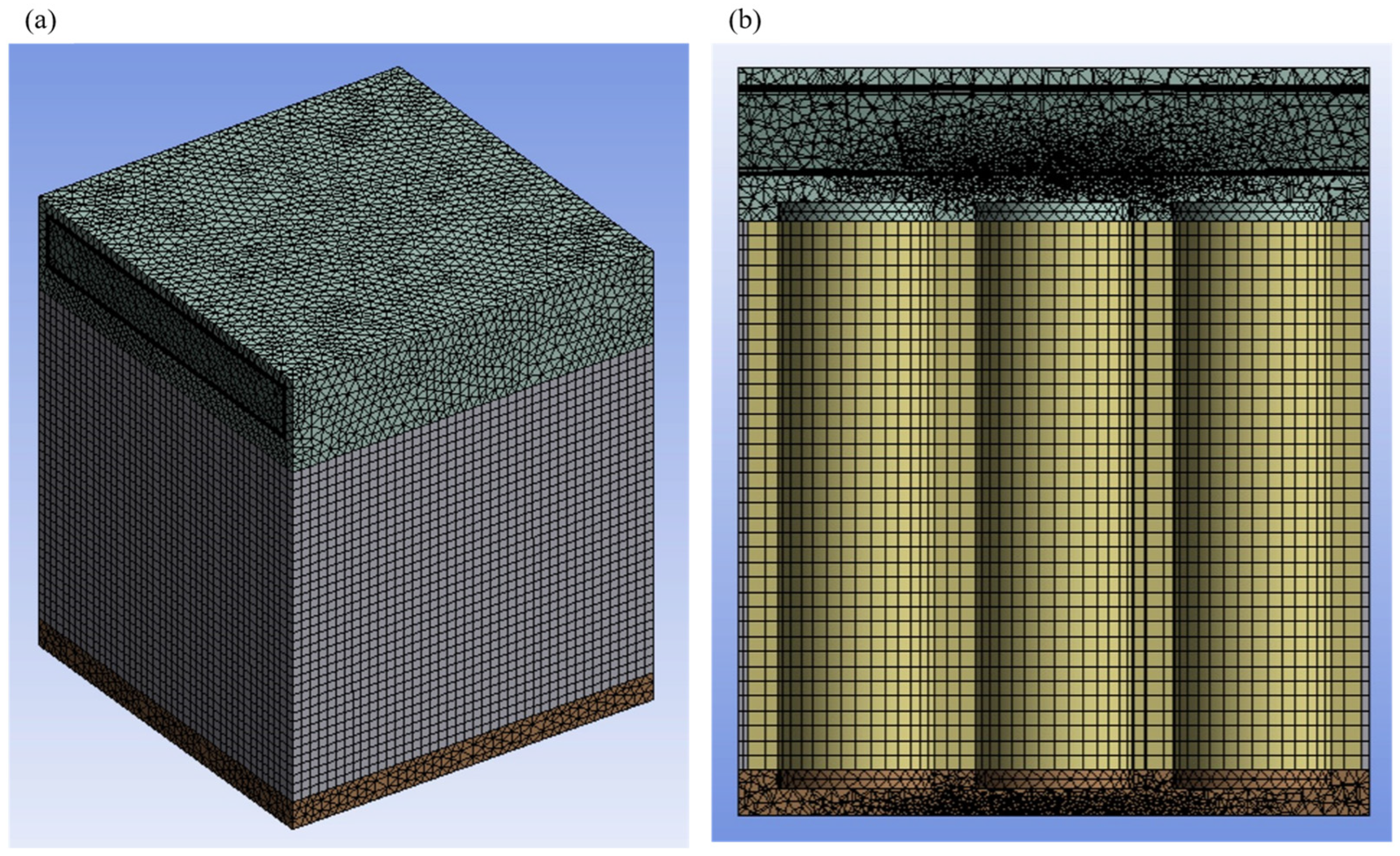
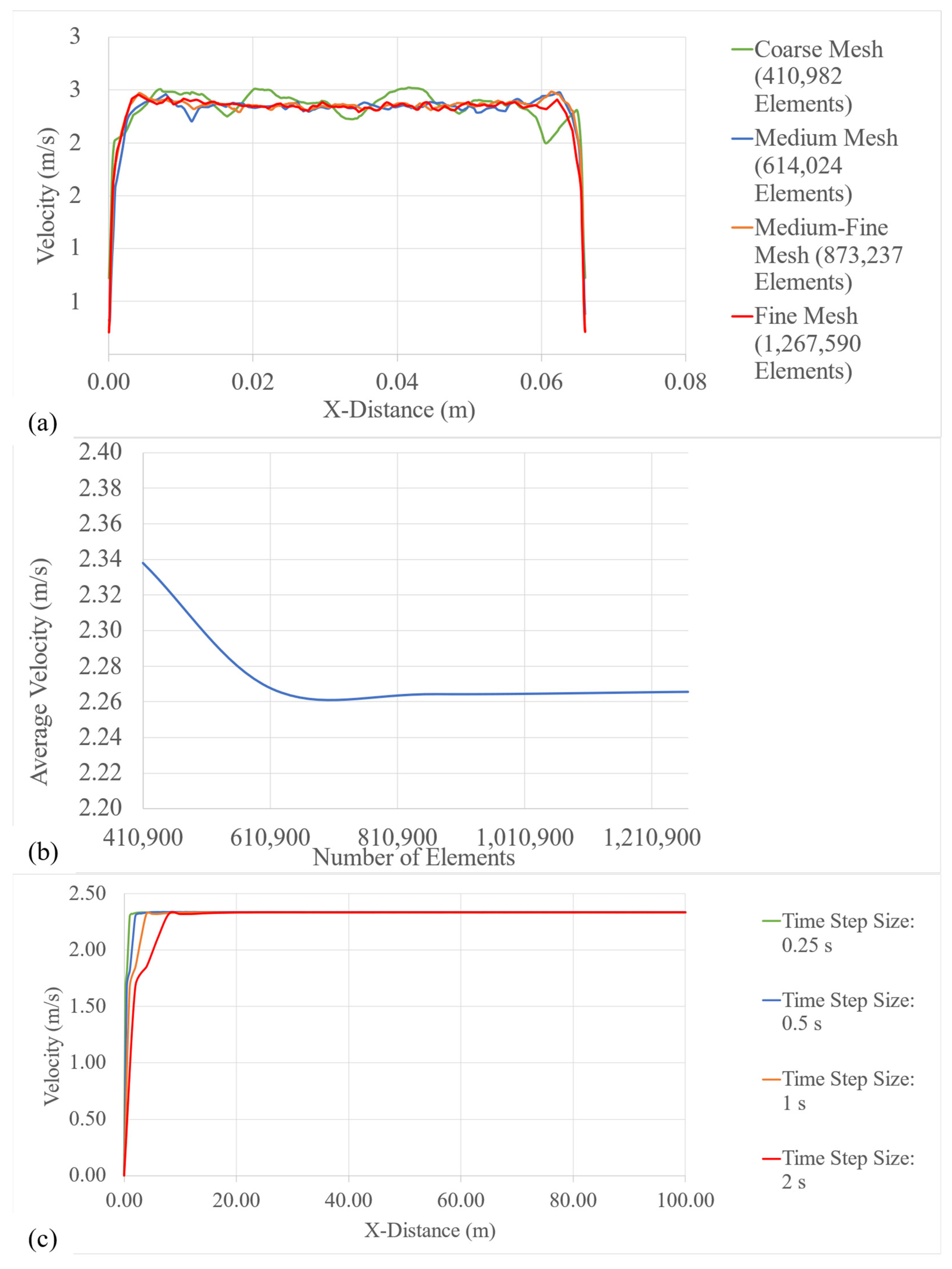
4.2. Mesh-Independence and Time-Independence Studies
4.3. Boundary Conditions and Simulation Procedure
5. Results and Discussion
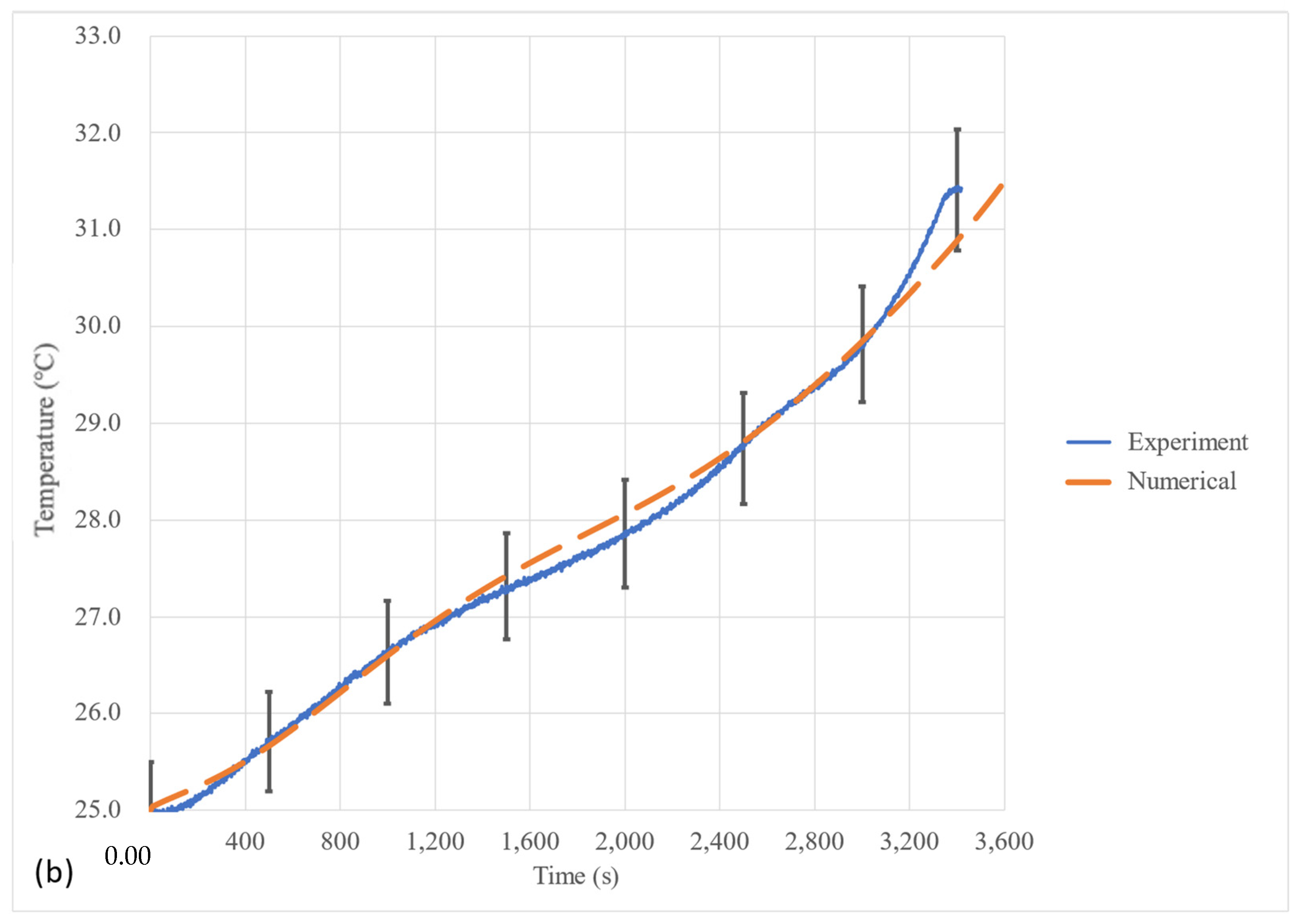
5.1. Heat Flux Profile and Numerical Model Validation
5.2. Thermal Analysis of the Proposed Hybrid Strategy
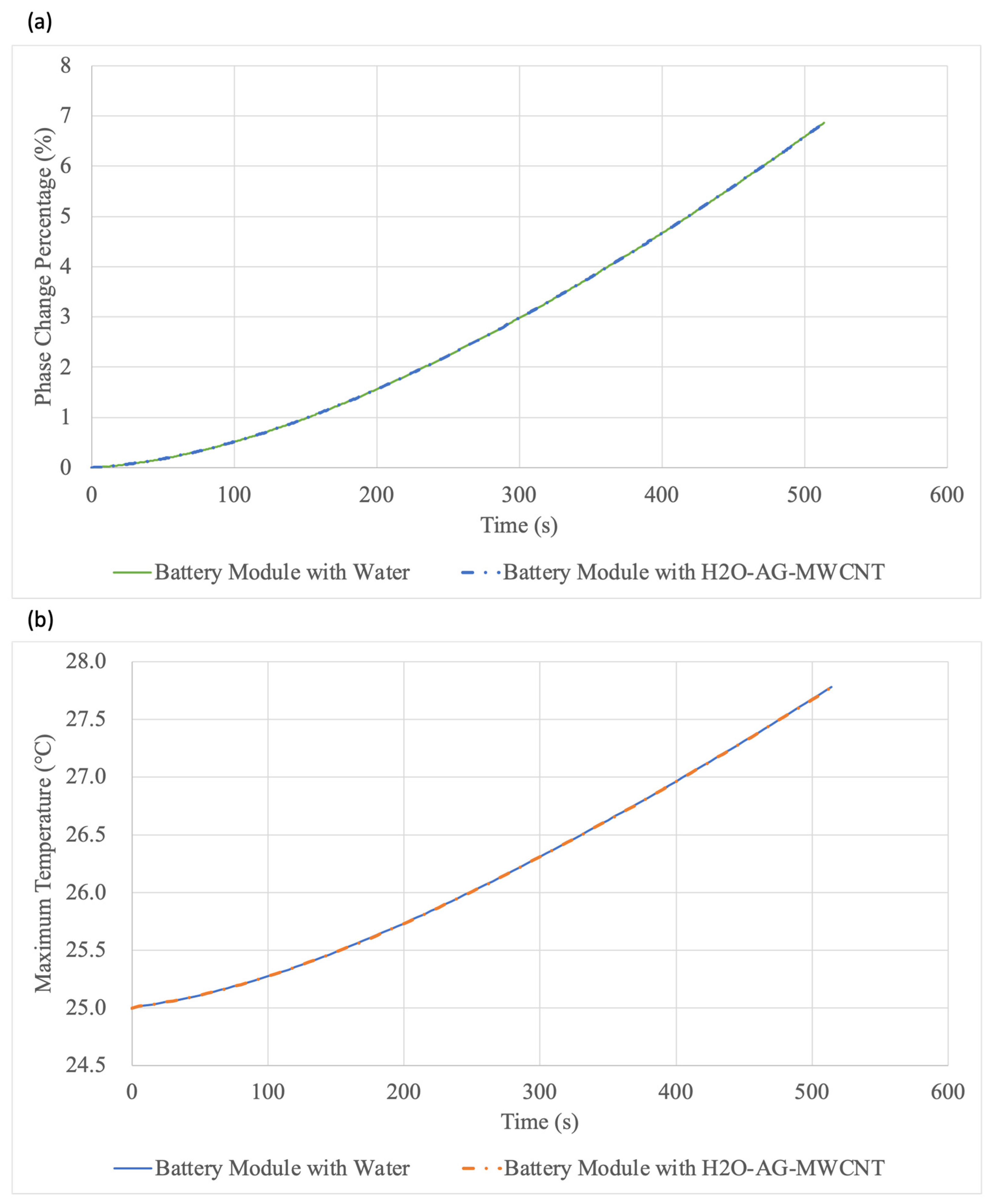
5.3. Increasing the Thermal Conductivity of Water through Nanoparticles
5.4. Addition of Fins in the Battery Module to Improve the Heat Transfer
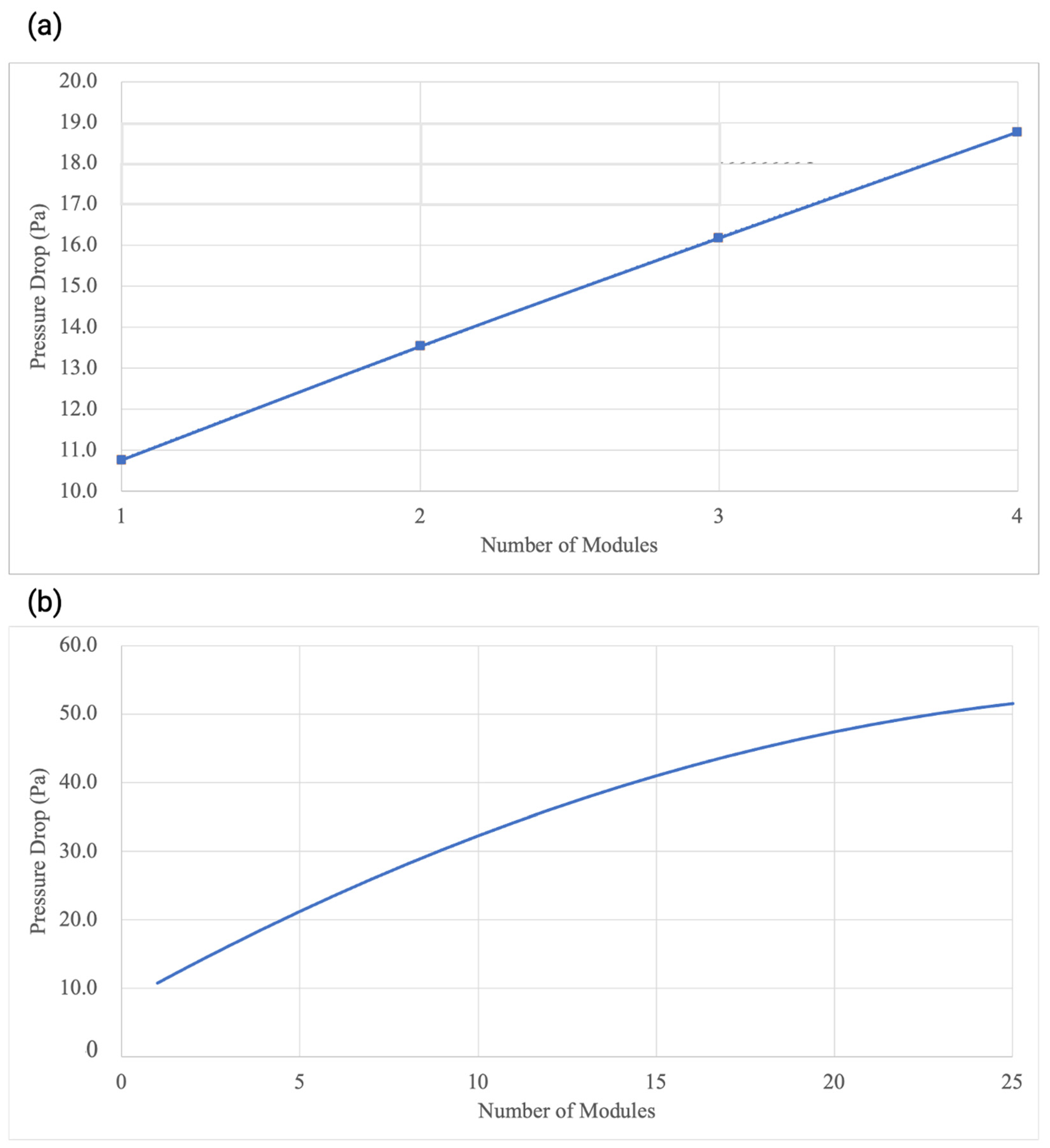
5.5. Scalability of the Battery Module
5.6. Comparison with the Open Literature
6. Conclusions
7. Limitations and Future Research Outlook
- The research was conducted on 18650 Li-ion cells and it is not applicable to larger cells without further investigation.
- The research was conducted on cylindrical Li-ion cells and it is not applicable to different cell geometries without further investigation.
- The research is not applicable to discharge rates higher than 7 C without further investigation.
- The structural integrity of various developed components has not been investigated.
- Experiments should be conducted to obtain heat flux profiles of lithium-ion cells at high charging rates and at various drive cycles, and used to obtain and improve the thermal performance of the hybrid battery module.
- Experiments should be conducted by developing a prototype of the scaled-up battery pack to improve the numerical simulations for the scaled battery pack and further validate it.
- A study should be conducted for the developed hybrid battery module with larger lithium-ion cells, such as the 26650 and 42120 cylindrical cells.
- An experimental and numerical study should be conducted to evaluate the performance of the developed hybrid thermal management strategies and battery module in instances of thermal runaway.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| density (kg m−3) | |
| t | time (s) |
| velocity (m s−1) | |
| ϵ | is a small number (0.001) to prevent division by zero |
| characteristic length (m) | |
| user-defined function for the continuity equation | |
| P | pressure (Pa) |
| stress tensor | |
| Amush | is the mushy zone constant |
| gravitational acceleration (9.81 m s−2) | |
| external forces, porous-media source terms, and user-defined sources | |
| dynamic viscosity (Pa s) | |
| T | temperature (K) |
| unit tensor | |
| energy (J) | |
| effective thermal conductivity (W m−1 K−1) | |
| H | enthalpy (J) |
| h | sensible enthalpy (J) |
| ∆H | latent heat (J) |
| liquid fraction | |
| specific heat capacity (J kg−1 K−1) | |
| m | mass (kg) |
| Q | heat generation (W) |
| Re | Reynolds number (dimensionless) |
| v | volume (m3) |
References
- US EPA. Fast Facts on Transportation Greenhouse Gas Emission. Available online: https://www.epa.gov/greenvehicles/fast-facts-transportation-greenhouse-gas-emissions (accessed on 4 August 2023).
- Omar, N.; Monem, M.A.; Firouz, Y.; Salminen, J.; Smekens, J.; Hegazy, O.; Gaulous, H.; Mulder, G.; Bossche, P.V.D.; Coosemans, T.; et al. Lithium iron phosphate based battery—Assessment of the aging parameters and development of cycle life model. Appl. Energy 2014, 113, 1575–1585. [Google Scholar] [CrossRef]
- Sefidan, A.M.; Sojoudi, A.; Saha, S.C. Nanofluid-based cooling of cylindrical lithium-ion battery packs employing forced air flow. Int. J. Therm. Sci. 2017, 117, 44–58. [Google Scholar] [CrossRef]
- Saw, L.H.; Poon, H.M.; Thiam, H.S.; Cai, Z.; Chong, W.T.; Pambudi, N.A.; King, Y.J. Novel thermal management system using mist cooling for lithium-ion battery packs. Appl. Energy 2018, 223, 146–158. [Google Scholar] [CrossRef]
- Rao, Z.; Wang, S.; Zhang, G. Simulation and experiment of thermal energy management with phase change material for ageing LiFePO4 power battery. Energy Convers. Manag. 2011, 52, 3408–3414. [Google Scholar] [CrossRef]
- Wei, Y.; Agelin-Chaab, M. Development and experimental analysis of a hybrid cooling concept for electric vehicle battery packs. J. Energy Storage 2019, 25, 100906. [Google Scholar] [CrossRef]
- Shahid, S.; Chea, B.; Agelin-Chaab, M. Development of a hybrid cooling concept for cylindrical li-ion cells. J. Energy Storage 2022, 50, 104214. [Google Scholar] [CrossRef]
- Park, S.; Jung, D. Battery cell arrangement and heat transfer fluid effects on the parasitic power consumption and the cell temperature distribution in a hybrid electric vehicle. J. Power Sources 2013, 227, 191–198. [Google Scholar] [CrossRef]
- Patil, M.S.; Seo, J.-H.; Lee, M.-Y. A novel dielectric fluid immersion cooling technology for Li-ion battery thermal management. Energy Convers. Manag. 2020, 229, 113715. [Google Scholar] [CrossRef]
- Dubey, P.; Pulugundla, G.; Srouji, A.K. Direct Comparison of Immersion and Cold-Plate Based Cooling for Automotive Li-Ion Battery Modules. Energies 2021, 14, 1259. [Google Scholar] [CrossRef]
- Shahid, S.; Agelin-Chaab, M. A review of thermal runaway prevention and mitigation strategies for lithium-ion batteries. Energy Convers. Manag. X 2022, 16, 100310. [Google Scholar] [CrossRef]
- Panchal, S.; Khasow, R.; Dincer, I.; Agelin-Chaab, M.; Fraser, R.; Fowler, M. Thermal design and simulation of mini-channel cold plate for water cooled large sized prismatic lithium-ion battery. Appl. Therm. Eng. 2017, 122, 80–90. [Google Scholar] [CrossRef]
- Panchal, S.; Mathewson, S.; Fraser, R.; Culham, R.; Fowler, M. Thermal Management of Lithium-Ion Pouch Cell with Indirect Liquid Cooling using Dual Cold Plates Approach. SAE Int. J. Altern. Powertrains 2015, 4, 1184. [Google Scholar] [CrossRef]
- Zhao, J.; Rao, Z.; Li, Y. Thermal performance of mini-channel liquid cooled cylinder based battery thermal management for cylindrical lithium-ion power battery. Energy Convers. Manag. 2015, 103, 157–165. [Google Scholar] [CrossRef]
- Zhao, C.; Sousa, A.C.M.; Jiang, F. Minimization of thermal non-uniformity in lithium-ion battery pack cooled by channeled liquid flow. Int. J. Heat Mass. Transf. 2019, 129, 660–670. [Google Scholar] [CrossRef]
- Rao, Z.; Qian, Z.; Kuang, Y.; Li, Y. Thermal performance of liquid cooling based thermal management system for cylindrical lithium-ion battery module with variable contact surface. Appl. Therm. Eng. 2017, 123, 1514–1522. [Google Scholar] [CrossRef]
- He, F.; Ams, A.A.; Roosien, Y.; Tao, W.; Geist, B.; Singh, K. Reduced-order thermal modeling of liquid-cooled lithium-ion battery pack for EVs and HEVs. In Proceedings of the IEEE Transportation and Electrification Conference and Expo, Chicago, IL, USA, 14 June 2017. [Google Scholar] [CrossRef]
- Al-Hallaj, S.; Selman, J.R. A Novel Thermal Management System for Electric Vehicle Batteries Using Phase-Change Material. J. Electrochem. Soc. 2000, 147, 3231. [Google Scholar] [CrossRef]
- Javani, N.; Dincer, I.; Naterer, G.F. Numerical Modeling of Submodule Heat Transfer with Phase Change Material for Thermal Management of Electric Vehicle Battery Packs. J. Therm. Sci. Eng. Appl. 2015, 7, 031005. [Google Scholar] [CrossRef]
- Jiang, G.; Huang, J.; Liu, M.; Cao, M. Experiment and simulation of thermal management for a tube-shell Li-ion battery pack with composite phase change material. Appl. Therm. Eng. 2017, 120, 1–9. [Google Scholar] [CrossRef]
- He, F.; Li, X.; Zhang, G.; Zhong, G.; He, J. Experimental investigation of thermal management system for lithium ion batteries module with coupling effect by heat sheets and phase change materials. Int. J. Energy Res. 2018, 42, 3279–3288. [Google Scholar] [CrossRef]
- Hussain, A.; Abidi, I.H.; Tso, C.Y.; Chan, K.C.; Luo, Z.; Chao, C.Y.H. Thermal management of lithium ion batteries using graphene coated nickel foam saturated with phase change materials. Int. J. Therm. Sci. 2018, 124, 23–35. [Google Scholar] [CrossRef]
- Ye, G.; Zhang, G.; Jiang, L.; Yang, X. Temperature control of battery modules through composite phase change materials with dual operating temperature regions. Chem. Eng. J. 2022, 449, 137733. [Google Scholar] [CrossRef]
- Wu, W.; Ye, G.; Zhang, G.; Yang, X. Composite phase change material with room-temperature-flexibility for battery thermal management. Chem. Eng. J. 2022, 428, 131116. [Google Scholar] [CrossRef]
- Faraji, H.; El-Alami, M.; Arshad, A.; Hariti, Y. Numerical Survey on Performance of Hybrid NePCM for Cooling of Electronics: Effect of Heat Source Position and Heat Sink Inclination. J. Therm. Sci. Eng. Appl. 2021, 13, 051010. [Google Scholar] [CrossRef]
- Arshad, A.; Jabbal, M.; Faraji, H.; Talebizadehsardari, P.; Bashir, M.A.; Yan, Y. Numerical study of nanocomposite phase change material-based heat sink for the passive cooling of electronic components. Heat Mass. Transf. 2021, 1–15. [Google Scholar] [CrossRef]
- Faraji, H.; Yıldız, Ç.; Arshad, A.; Arıcı, M.; Choukairy, K.; El-Alami, M. Passive thermal management strategy for cooling multiple portable electronic components: Hybrid nanoparticles enhanced phase change materials as an innovative solution. J. Energy Storage 2023, 70, 108087. [Google Scholar] [CrossRef]
- Wei, Y.; Agelin-Chaab, M. Experimental investigation of a novel hybrid cooling method for lithium-ion batteries. Appl. Therm. Eng. 2018, 136, 375–387. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Li, Y.Z.; Mao, Y.; Zhang, Y.; Guo, W.; Zhong, M. A forced gas cooling circle packaging with liquid cooling plate for the thermal management of Li-ion batteries under space environment. Appl. Therm. Eng. 2017, 123, 929–939. [Google Scholar] [CrossRef]
- Shahid, S.; Agelin-Chaab, M. Development of hybrid thermal management techniques for battery packs. Appl. Therm. Eng. 2021, 186, 116542. [Google Scholar] [CrossRef]
- Kumar, M.D.; Bhowmik, C.; Bhowmik, S.; Murari, P.K. Property-enhanced paraffin-based composite phase change material for thermal energy storage: A review. Environ. Sci. Pollut. Res. 2022, 29, 43556–43587. [Google Scholar] [CrossRef]
- ANSYS. ANSYS Fluent Theory Guide; ANSYS, Inc.: Canonsburg, PA, USA, 2020. [Google Scholar]
- Paraffin 42-44, in Block Form 8002-74-2. Available online: https://www.sigmaaldrich.com/CA/en/product/mm/107150 (accessed on 24 August 2023).
- Amburi, P.K.; Senthilkumar, G.; Nithya, A. Novel use of CuO nanoparticles additive for improving thermal conductivity of MgO/water and MWCNT/water nanofluids. J. Therm. Anal. Calorim. 2023, 148, 10389–10398. [Google Scholar] [CrossRef]
- Bioucas, F.E.B.; Köhn, C.; Jean-Fulcrand, A.; Garnweitner, G.; Koller, T.M.; Fröba, A.P. Effective Thermal Conductivity of Nanofluids Containing Silicon Dioxide or Zirconium Dioxide Nanoparticles Dispersed in a Mixture of Water and Glycerol. Int. J. Thermophys. 2022, 43, 167. [Google Scholar] [CrossRef]
- Pourrajab, R.; Noghrehabadi, A.; Behbahani, M.; Hajidavalloo, E. An efficient enhancement in thermal conductivity of water-based hybrid nanofluid containing MWCNTs-COOH and Ag nanoparticles: Experimental study. J. Therm. Analy Calorim. 2021, 143, 3331–3343. [Google Scholar] [CrossRef]
- Corasaniti, S.; Bovesecchi, G.; Gori, F. Experimental Thermal Conductivity of Alumina Nanoparticles in Water with and without Sonication. Int. J. Thermophys. 2021, 42, 23. [Google Scholar] [CrossRef]
- Jabbari, F.; Rajabpour, A.; Saedodin, S. Thermal conductivity of CNT–water nanofluid at different temperatures, volume fractions, and diameters: Experimental investigation and molecular dynamics simulations. Microfluid. Nanofluidics 2021, 25, 102. [Google Scholar] [CrossRef]
- Ghasemi, M.; Niknejadi, M.; Toghraie, D. Direct effect of nanoparticles on the thermal conductivity of CuO-water nanofluid in a phase transition phenomenon using molecular dynamics simulation. J. Therm. Analy Calorim. 2021, 144, 2483–2495. [Google Scholar] [CrossRef]
- Tesla Model S/X Pack—75 kWh/214 Ah/350 V. Available online: https://www.secondlife-evbatteries.com/products/tesla-75kw-pack (accessed on 27 September 2023).
- Introduction of INR18650-25R. Available online: https://www.powerstream.com/p/INR18650-25R-datasheet.pdf (accessed on 17 November 2023).
- Moffat, R.J. Describing the uncertainties in experimental results. Exp. Therm. Fluid. Sci. 1988, 1, 3–17. [Google Scholar] [CrossRef]
- Zeng, W.; Niu, Y.; Li, S.; Hu, S.; Mao, B.; Zhang, Y. Cooling performance and optimization of a new hybrid thermal management system of cylindrical battery. Appl. Therm. Eng. 2022, 217, 119171. [Google Scholar] [CrossRef]
- Liu, Z.; Cao, M.; Zhang, Y.; Li, J.; Jiang, G.; Shi, H. Thermal management of cylindrical battery pack based on a combination of silica gel composite phase change material and copper tube liquid cooling. J. Energy Storage 2023, 71, 108205. [Google Scholar] [CrossRef]
- Xu, Q.; Xie, Y.; Huang, Y.; Li, X.; Huang, H.; Bei, S.; Wang, H.; Zheng, K.; Wang, X.; Li, L. Enhancement of thermal management for cylindrical battery module based on a novel wrench-shaped design for the cold plate. Sust. Energy Technol. Assess. 2023, 59, 103421. [Google Scholar] [CrossRef]
- Xiong, X.; Wang, Z.; Fan, Y.; Wang, H. Numerical analysis of cylindrical lithium-ion battery thermal management system based on bionic flow channel structure. Therm. Sci. Eng. Prog. 2023, 42, 101879. [Google Scholar] [CrossRef]
- Ling, L.; Li, L.; Xie, Y.; Wang, T.; Zheng, K.; Shan, S.; Zhang, L.; Bei, S.; Xu, Q. Optimal Design of Minichannel Cold Plate for the Thermal Management of Cylindrical Battery Modules. Energy Technol. 2023, 11, 2201484. [Google Scholar] [CrossRef]










| Materials | Density (kg/m3) | Specific Heat (J/kg K) | Thermal Conductivity (W/m K) | Melting Heat Capacity (J/kg) | Phase Change Temperature (°C) |
|---|---|---|---|---|---|
| Aluminum | 2719 | 871 | 202.4 | - | - |
| Wood | 700 | 2310 | 0.173 | - | - |
| Air | 1.225 | 1006 | 0.0242 | - | - |
| Water | 998.2 | 4182 | 0.6 | 334,000 | 100 |
| Paraffin | 880 | 2150 | 0.21 | 245,000 | 42–44 |
| Paraffin with Copper Foam | 880 | 2150 | 3.11 | 170,400 | 42–43 |
| Battery Module Configuration | Number of Fins | Inlet Velocity (m/s) |
|---|---|---|
| Configuration 1 | 1 | 5.93 |
| Configuration 2 | 2 | 9.28 |
| Parameters | Reference Value (W/m2) | Absolute Bias Error (W/m2) | Relative Bias Error (%) | Relative Precision Error (%) | Total Uncertainty (%) |
|---|---|---|---|---|---|
| Min. Heat Flux | 18.99 | 1.27 | 6.69 | 1.90 | 6.95 |
| Max. Heat Flux | 584.16 | 1.27 | 0.22 | 0.05 | 0.22 |
| Parameters | Reference Value (°C) | Absolute Bias Error (°C) | Relative Bias Error (%) | Relative Precision Error (%) | Total Uncertainty (%) |
|---|---|---|---|---|---|
| Min. Temp. | 25 | 1 | 4 | 2.22 | 4.57 |
| Max. Temp. | 47 | 1 | 2.13 | 1.49 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahid, S.; Agelin-Chaab, M. Investigation of Heat Transfer Enhancement Techniques on a Scalable Novel Hybrid Thermal Management Strategy for Lithium-Ion Battery Packs. Batteries 2024, 10, 32. https://doi.org/10.3390/batteries10010032
Shahid S, Agelin-Chaab M. Investigation of Heat Transfer Enhancement Techniques on a Scalable Novel Hybrid Thermal Management Strategy for Lithium-Ion Battery Packs. Batteries. 2024; 10(1):32. https://doi.org/10.3390/batteries10010032
Chicago/Turabian StyleShahid, Seham, and Martin Agelin-Chaab. 2024. "Investigation of Heat Transfer Enhancement Techniques on a Scalable Novel Hybrid Thermal Management Strategy for Lithium-Ion Battery Packs" Batteries 10, no. 1: 32. https://doi.org/10.3390/batteries10010032
APA StyleShahid, S., & Agelin-Chaab, M. (2024). Investigation of Heat Transfer Enhancement Techniques on a Scalable Novel Hybrid Thermal Management Strategy for Lithium-Ion Battery Packs. Batteries, 10(1), 32. https://doi.org/10.3390/batteries10010032









