Operando Fabricated Quasi-Solid-State Electrolyte Hinders Polysulfide Shuttles in an Advanced Li-S Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the Quasi-Solid Electrolyte Precursor
- PETEA: Pentaerythritol tetraacrylate (Sigma-Aldrich)
- LS2: 0.7 M LiNO3 + 0.3 M LiTFSI in DOL/DME (1:1; v/v)
- LiTFSI: bis(trifluoromethane)sulfonimide lithium salt (Aldrich, USA anhydrous 99.99%)
- LiNO3: Lithium nitrate (Alfa Aesar, Ward Hill, MA, USA anhydrous 99%).
- DOL: 1,3-dioxolane (DOL, Aldrich, anhydrous 99.8%)
- DME: 1,2-dimethoxyethane (DME, Aldrich, anhydrous 99.5%)
2.2. Preparation of the S/C Cathode
2.3. Fabrication of the Quasi-Solid Li–S Battery
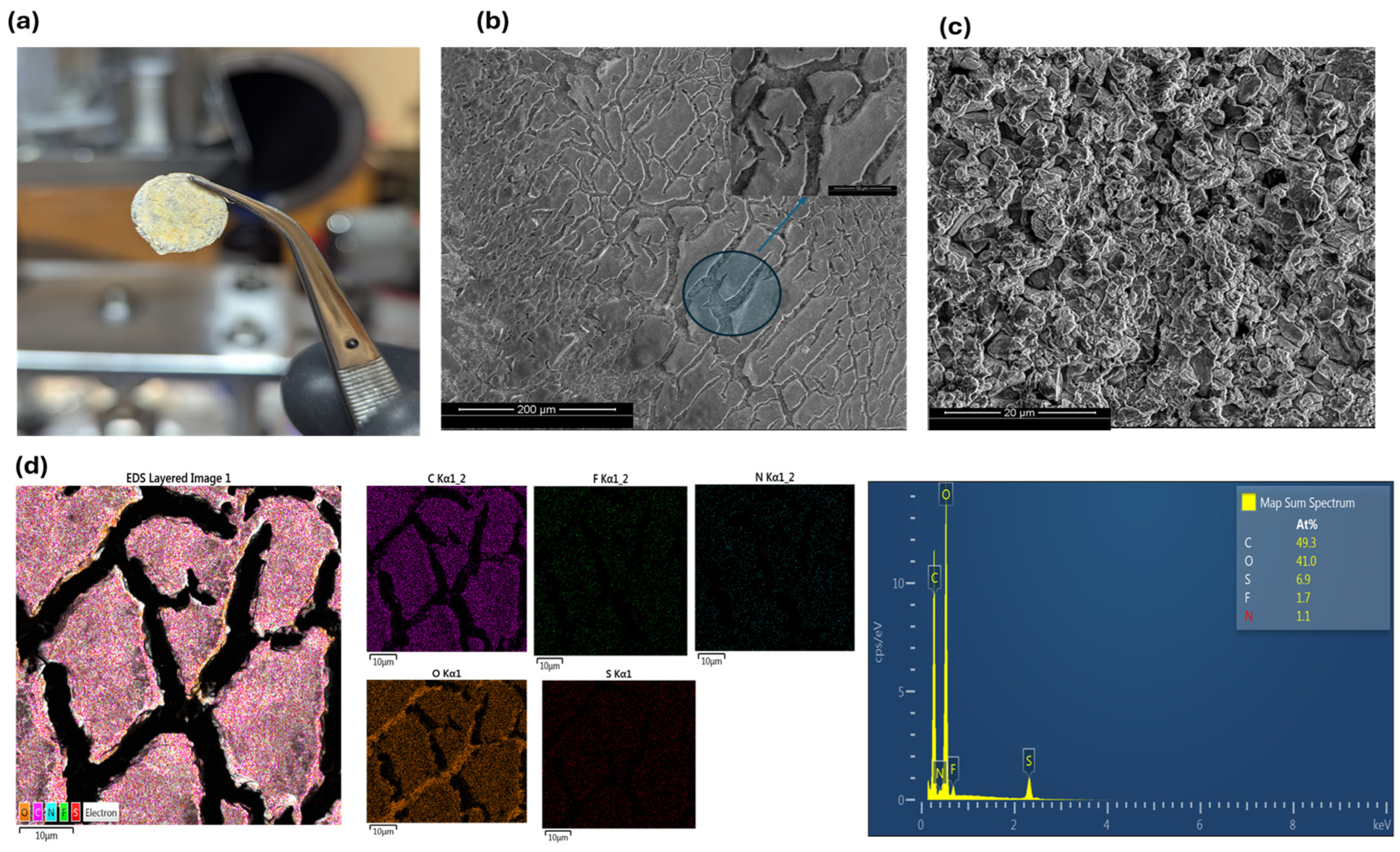
2.4. Materials Characterization
2.5. Electrochemical Measurements
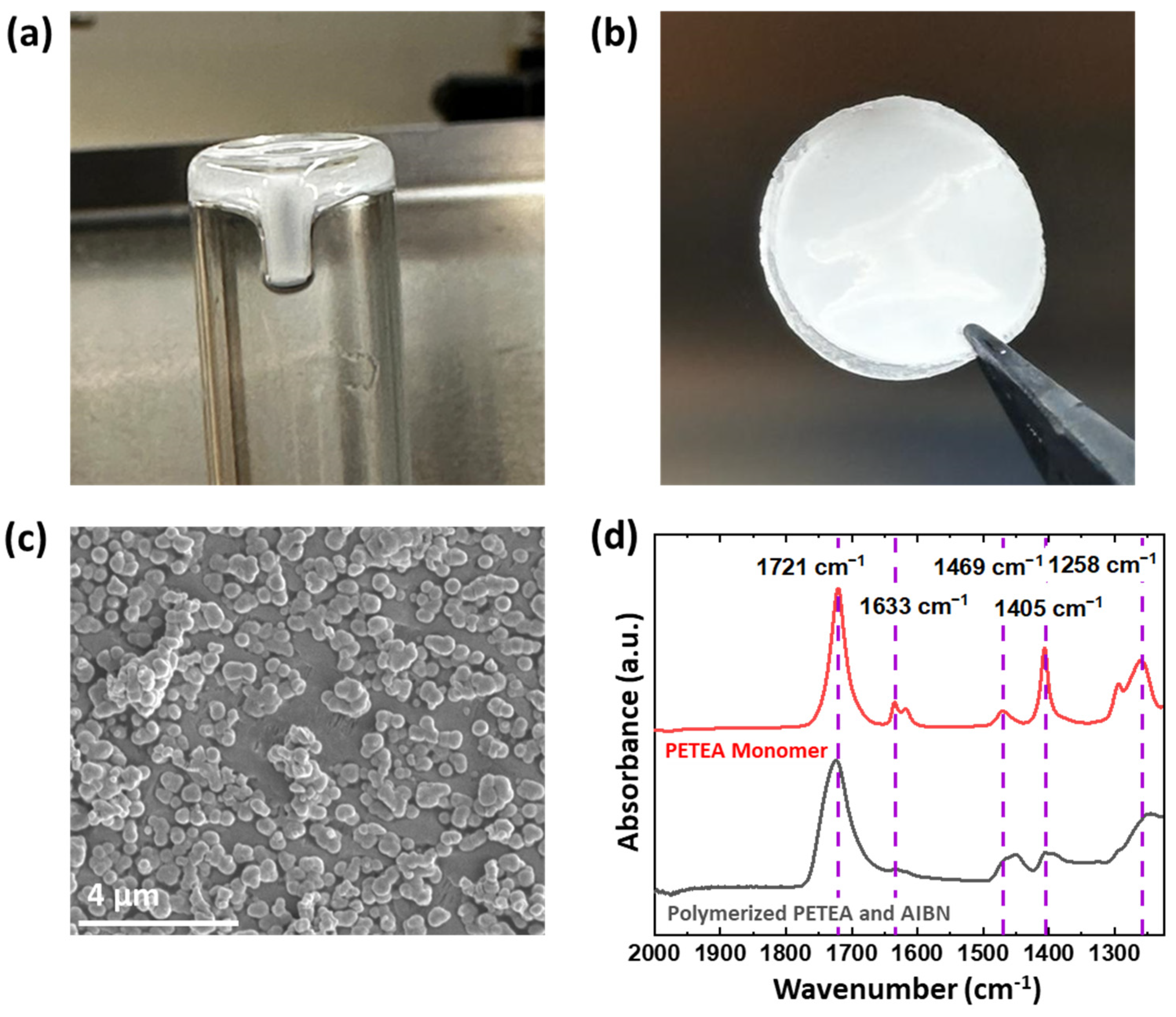
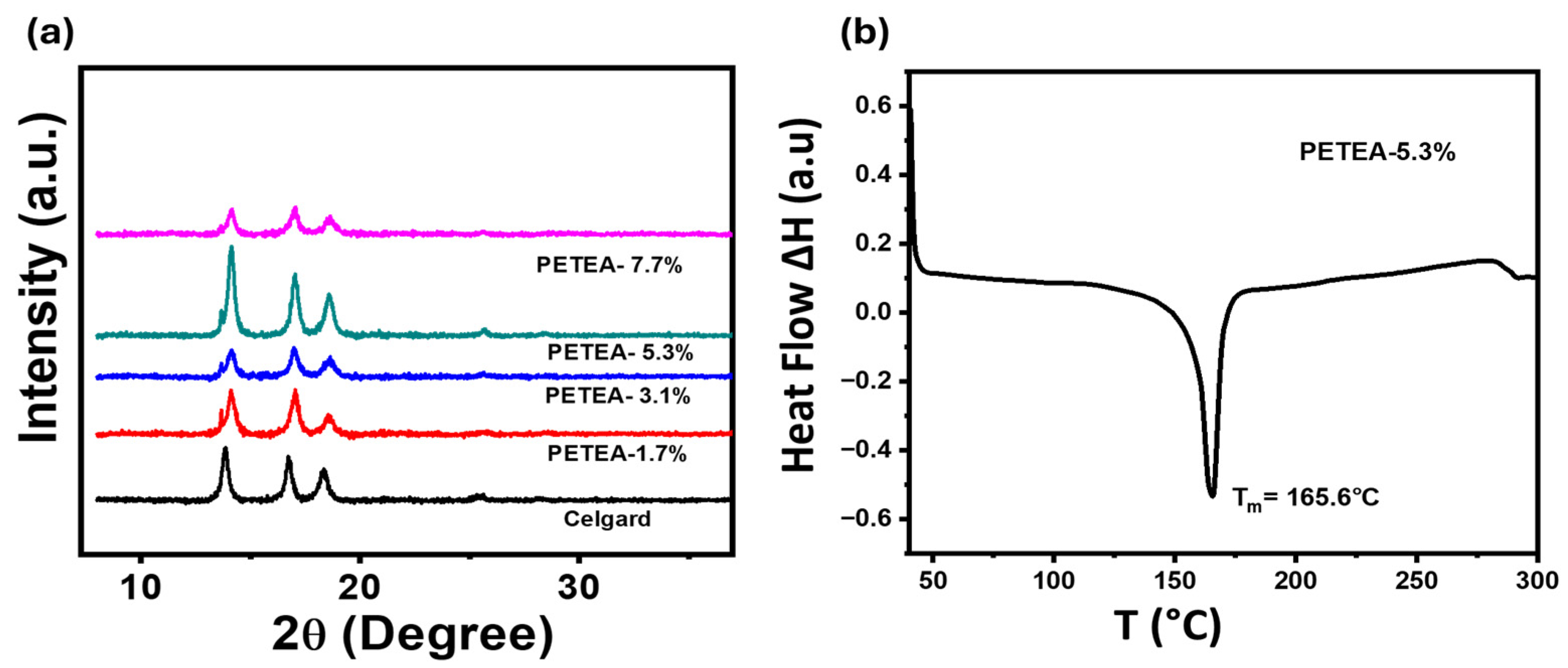
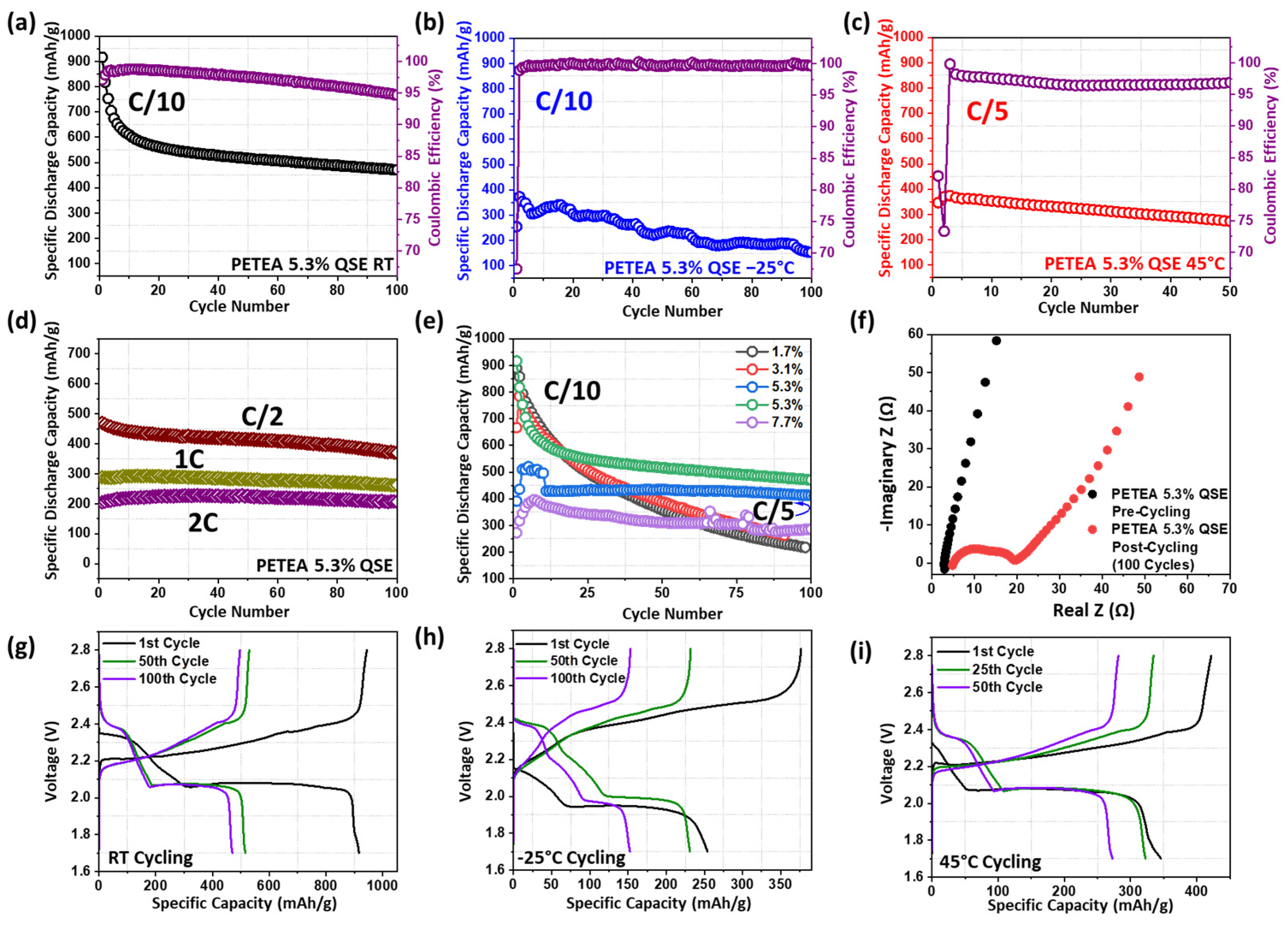
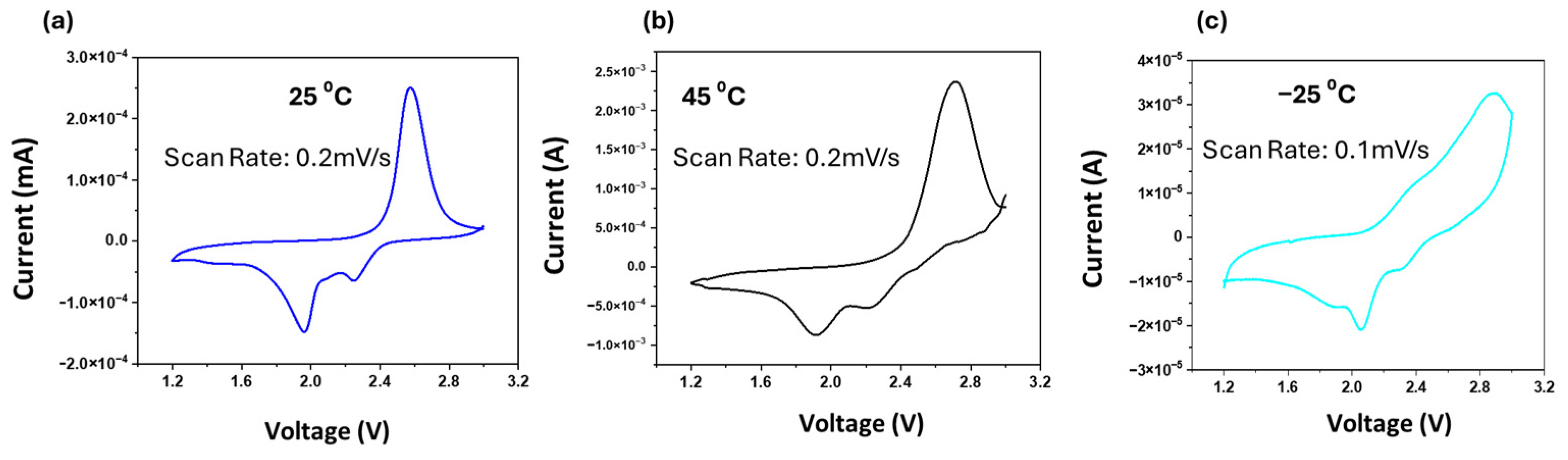
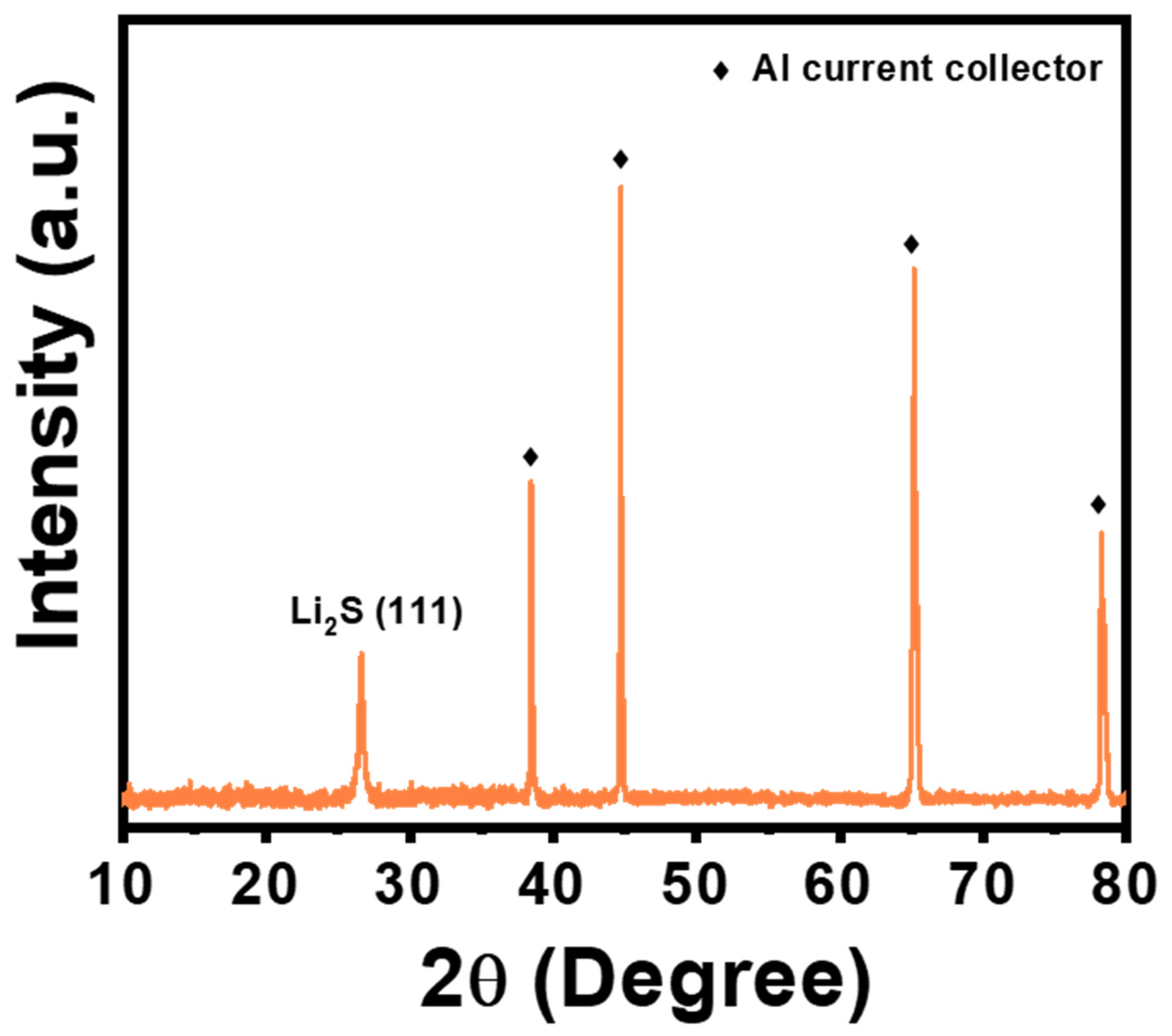
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, J.; Patel, R.; Saxena, R.; Nair, A. Thermal analysis of high specific energy NCM-21700 Li-ion battery cell under hybrid battery thermal management system for EV applications. J. Energy Storage 2024, 88, 111567. [Google Scholar] [CrossRef]
- Rahmani, E.; Fattahi, A.; Panahi, E.; Mahmoudi, Y. Thermal management improvement for a pack of cylindrical batteries using nanofluids and topological modifications. J. Power Sources 2023, 564, 232876. [Google Scholar] [CrossRef]
- Fang, X.; Peng, H. A Revolution in Electrodes: Recent Progress in Rechargeable Lithium–Sulfur Batteries. Small 2015, 11, 1488–1511. [Google Scholar] [CrossRef]
- Cutting cobalt. Nat. Energy 2020, 5, 825. [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267–278. [Google Scholar] [CrossRef]
- Sun, J.; Wang, T.; Gao, Y.; Pan, Z.; Hu, R.; Wang, J. Will lithium-sulfur batteries be the next beyond-lithium ion batteries and even much better? InfoMat 2022, 4, 12359. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.; Guo, Z. Challenges and future perspectives on sodium and potassium ion batteries for grid-scale energy storage. Mater. Today Proc. 2021, 50, 400–417. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.-H.; Zu, C.; Su, Y.-S. Rechargeable Lithium–Sulfur Batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Yin, Y.; Xin, S.; Guo, Y.; Wan, L. Lithium–Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem. Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Benveniste, G.; Sánchez, A.; Rallo, H.; Corchero, C.; Amante, B. Comparative life cycle assessment of Li-Sulphur and Li-ion batteries for electric vehicles. Resour. Conserv. Recycl. Adv. 2022, 15, 200086. [Google Scholar] [CrossRef]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide Shuttle Study in the Li/S Battery System. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, L.; Wei, Z.; Huang, Q.; Deng, Y.; Zheng, Z. Cracking-Controlled Slurry Coating of Mosaic Electrode for Flexible and High-Performance Lithium–Sulfur Battery. Adv. Energy Mater. 2023, 13, 2203621. [Google Scholar] [CrossRef]
- Sun, K.; Wu, Q.; Tong, X.; Gan, H. Electrolyte with Low Polysulfide Solubility for Li–S Batteries. ACS Appl. Energy Mater. 2018, 1, 2608–2618. [Google Scholar] [CrossRef]
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. Lithium-Sulfur Batteries: Attaining the Critical Metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Wang, Y.; Zhong, W. Development of Electrolytes towards Achieving Safe and High-Performance Energy-Storage Devices: A Review. ChemElectroChem 2015, 2, 22–36. [Google Scholar] [CrossRef]
- Judez, X.; Martinez-Ibañez, M.; Santiago, A.; Armand, M.; Zhang, H.; Li, C. Quasi-solid-state electrolytes for lithium sulfur batteries: Advances and perspectives. J. Power Sources 2019, 438, 226985. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, D.; He, Y.-B.; Fu, Y.; Qin, X.; Miao, C.; Du, H.; Li, B.; Yang, Q.-H.; Lin, Z.; et al. Novel gel polymer electrolyte for high-performance lithium–sulfur batteries. Nano Energy 2016, 22, 278–289. [Google Scholar] [CrossRef]
- Bhardwaj, R.K.; Zitoun, D. Recent Progress in Solid Electrolytes for All-Solid-State Metal(Li/Na)–Sulfur Batteries. Batteries 2023, 9, 110. [Google Scholar] [CrossRef]
- Han, D.-D.; Liu, S.; Liu, Y.-T.; Zhang, Z.; Li, G.-R.; Gao, X.-P. Lithiophilic gel polymer electrolyte to stabilize the lithium anode for a quasi-solid-state lithium–sulfur battery. J. Mater. Chem. A 2018, 6, 18627–18634. [Google Scholar] [CrossRef]
- Ren, Y.; Manthiram, A. A Dual-Phase Electrolyte for High-Energy Lithium–Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2202566. [Google Scholar] [CrossRef]
- Qin, S.; Yu, Y.; Zhang, J.; Ren, Y.; Sun, C.; Zhang, S.; Zhang, L.; Hu, W.; Yang, H.; Yang, D. Separator-Free In Situ Dual-Curing Solid Polymer Electrolytes with Enhanced Interfacial Contact for Achieving Ultrastable Lithium-Metal Batteries. Adv. Energy Mater. 2023, 13, 2301470. [Google Scholar] [CrossRef]
- Koralalage, M.K.; Shreyas, V.; Arnold, W.R.; Akter, S.; Thapa, A.; Narayanan, B.; Wang, H.; Sumanasekera, G.U.; Jasinski, J.B. Functionalization of Cathode–Electrolyte Interface with Ionic Liquids for High-Performance Quasi-Solid-State Lithium–Sulfur Batteries: A Low-Sulfur Loading Study. Batteries 2024, 10, 155. [Google Scholar] [CrossRef]
- Gupta, A.; Bhargav, A.; Jones, J.-P.; Bugga, R.V.; Manthiram, A. Influence of Lithium Polysulfide Clustering on the Kinetics of Electrochemical Conversion in Lithium–Sulfur Batteries. Chem. Mater. 2020, 32, 2070–2077. [Google Scholar] [CrossRef]
- Gupta, A.; Manthiram, A. Unifying the clustering kinetics of lithium polysulfides with the nucleation behavior of Li2S in lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 13242–13251. [Google Scholar] [CrossRef]
- Kim, S.; Jung, J.; Kim, I.; Kwon, H.; Cho, H.; Kim, H.-T. Tuning of electrolyte solvation structure for low-temperature operation of lithium–sulfur batteries. Energy Storage Mater. 2023, 59, 102763. [Google Scholar] [CrossRef]
- Bai, Z.; Fan, K.; Guo, M.; Wang, M.; Yang, T.; Wang, N. Rational Design of a Cost-Effective Biomass Carbon Framework for High-Performance Lithium Sulfur Batteries. Batteries 2023, 9, 594. [Google Scholar] [CrossRef]
- Huang, L.; Lu, T.; Xu, G.; Zhang, X.; Jiang, Z.; Zhang, Z.; Wang, Y.; Han, P.; Cui, G.; Chen, L. Thermal runaway routes of large-format lithium-sulfur pouch cell batteries. Joule 2022, 6, 906–922. [Google Scholar] [CrossRef]
- Qu, W.; Xia, J.; Luo, C.; Zhang, C.; Chen, R.; Lv, W.; Yang, Q. Lithium-Sulfur Batteries at Extreme Temperatures: Challenges, Strategies and Prospects. Energy Environ. Mater. 2023, 6, 12444. [Google Scholar] [CrossRef]
- Shen, Z.; Shen, Z.; Zhong, J.; Zhong, J.; Jiang, S.; Jiang, S.; Xie, W.; Xie, W.; Zhan, S.; Zhan, S.; et al. Polyacrylonitrile Porous Membrane-Based Gel Polymer Electrolyte by In Situ Free-Radical Polymerization for Stable Li Metal Batteries. ACS Appl. Mater. Interfaces 2022, 14, 41022–41036. [Google Scholar] [CrossRef]
- Nguyen, A.; Verma, R.; Song, G.; Kim, J.; Park, C. In Situ Polymerization on a 3D Ceramic Framework of Composite Solid Electrolytes for Room-Temperature Solid-State Batteries. Adv. Sci. 2023, 10, e2207744. [Google Scholar] [CrossRef]
- Pal, P.; Ghosh, A. Robust Succinonitrile Plastic Crystal-Based Ionogel for All-Solid-State Li-Ion and Dual-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 4295–4304. [Google Scholar] [CrossRef]
- Evans, J.; Vincent, C.A.; Bruce, P.G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 1987, 28, 2324–2328. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, Y.; Chen, Y.; Zhang, J.; Jiang, H.; Chen, X.; Zhang, Z.; Zeng, Y.; Sa, B.; Wei, Q.; et al. Reducing polarization of lithium-sulfur batteries via ZnS/reduced graphene oxide accelerated lithium polysulfide conversion. Mater. Today Energy 2020, 18, 100519. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, H.; Zhu, X.; He, P.; Zhou, H. A stable quasi-solid electrolyte improves the safe operation of highly efficient lithium-metal pouch cells in harsh environments. Nat. Commun. 2022, 13, 1510. [Google Scholar] [CrossRef]
- Li, W.; Pang, Y.; Zhu, T.; Wang, Y.; Xia, Y. A gel polymer electrolyte based lithium-sulfur battery with low self-discharge. Solid State Ionics 2018, 318, 82–87. [Google Scholar] [CrossRef]
- Zhu, S.; Ma, F.; Wang, Y.; Yan, W.; Sun, D.; Jin, Y. New small molecule gel electrolyte with high ionic conductivity for Li–S batteries. J. Mater. Sci. 2017, 52, 4086–4095. [Google Scholar] [CrossRef]
- Jean-Fulcrand, A.; Jeon, E.J.; Karimpour, S.; Garnweitner, G. Cross-Linked Solid Polymer-Based Catholyte for Solid-State Lithium-Sulfur Batteries. Batteries 2023, 9, 341. [Google Scholar] [CrossRef]
- Hu, C.; Chen, H.; Shen, Y.; Lu, D.; Zhao, Y.; Lu, A.-H.; Wu, X.; Lu, W.; Chen, L. In situ wrapping of the cathode material in lithium-sulfur batteries. Nat. Commun. 2017, 8, 479. [Google Scholar] [CrossRef]
- Xue, L.; Li, Y.; Hu, A.; Zhou, M.; Chen, W.; Lei, T.; Yan, Y.; Huang, J.; Yang, C.; Wang, X.; et al. In Situ/Operando Raman Techniques in Lithium–Sulfur Batteries. Small Struct. 2022, 3, 2100170. [Google Scholar] [CrossRef]
- Hagen, M.; Schiffels, P.; Hammer, M.; Dörfler, S.; Tübke, J.; Hoffmann, M.J.; Althues, H.; Kaskel, S. In-Situ Raman Investigation of Polysulfide Formation in Li-S Cells. J. Electrochem. Soc. 2013, 160, A1205–A1214. [Google Scholar] [CrossRef]
- Yeon, J.-T.; Jang, J.-Y.; Han, J.-G.; Cho, J.; Lee, K.T.; Choi, N.-S. Raman Spectroscopic and X-ray Diffraction Studies of Sulfur Composite Electrodes during Discharge and Charge. J. Electrochem. Soc. 2012, 159, A1308–A1314. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Capacity Fade Analysis of Sulfur Cathodes in Lithium–Sulfur Batteries. Adv. Sci. 2016, 3, 1600101. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Gupta, K.N.; Choi, A.; Pol, V. Operando Fabricated Quasi-Solid-State Electrolyte Hinders Polysulfide Shuttles in an Advanced Li-S Battery. Batteries 2024, 10, 349. https://doi.org/10.3390/batteries10100349
Das S, Gupta KN, Choi A, Pol V. Operando Fabricated Quasi-Solid-State Electrolyte Hinders Polysulfide Shuttles in an Advanced Li-S Battery. Batteries. 2024; 10(10):349. https://doi.org/10.3390/batteries10100349
Chicago/Turabian StyleDas, Sayan, Krish Naresh Gupta, Austin Choi, and Vilas Pol. 2024. "Operando Fabricated Quasi-Solid-State Electrolyte Hinders Polysulfide Shuttles in an Advanced Li-S Battery" Batteries 10, no. 10: 349. https://doi.org/10.3390/batteries10100349
APA StyleDas, S., Gupta, K. N., Choi, A., & Pol, V. (2024). Operando Fabricated Quasi-Solid-State Electrolyte Hinders Polysulfide Shuttles in an Advanced Li-S Battery. Batteries, 10(10), 349. https://doi.org/10.3390/batteries10100349








