Synthesis Methods of Si/C Composite Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Synthesis Methods
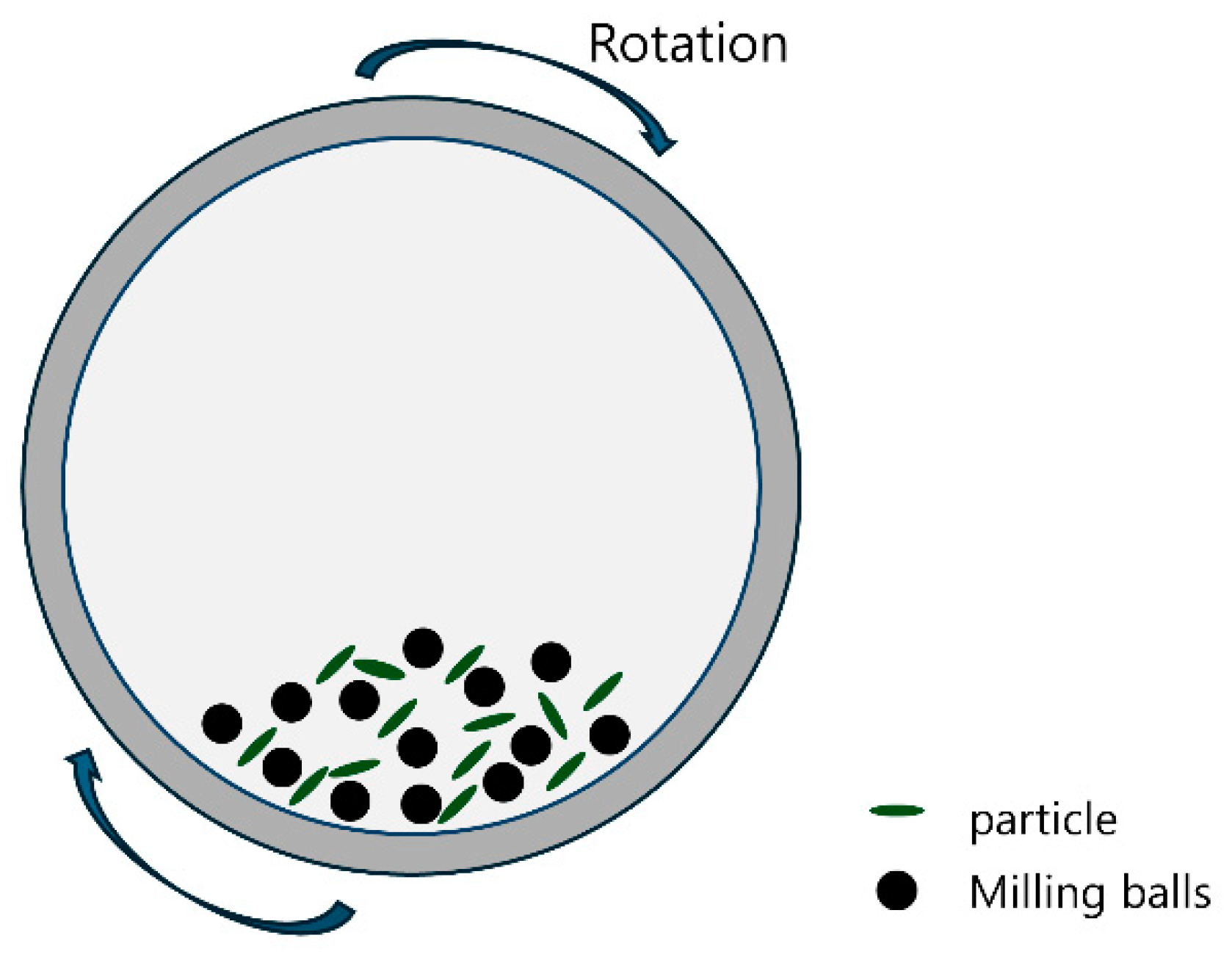
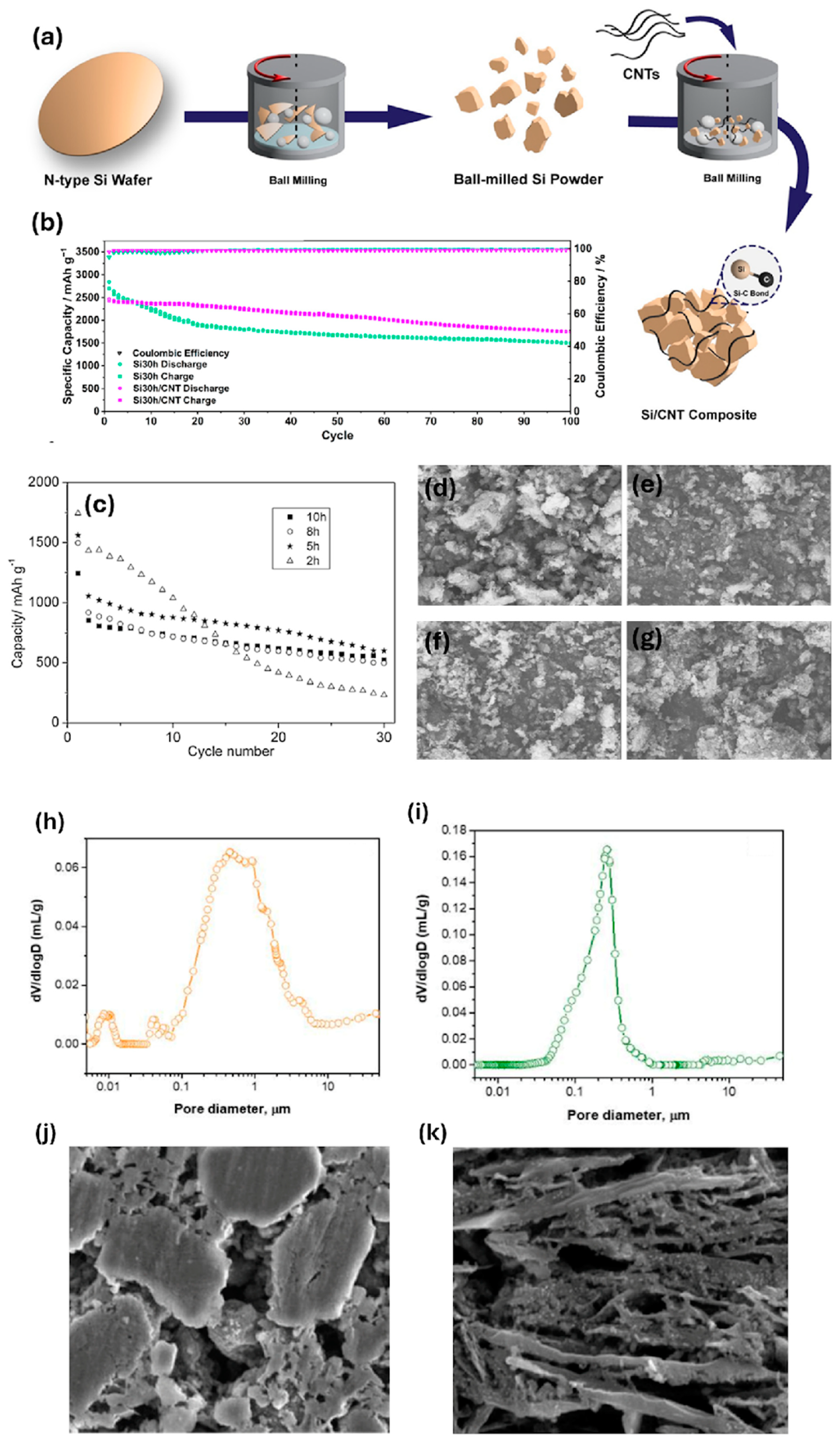
2.1. Ball Milling
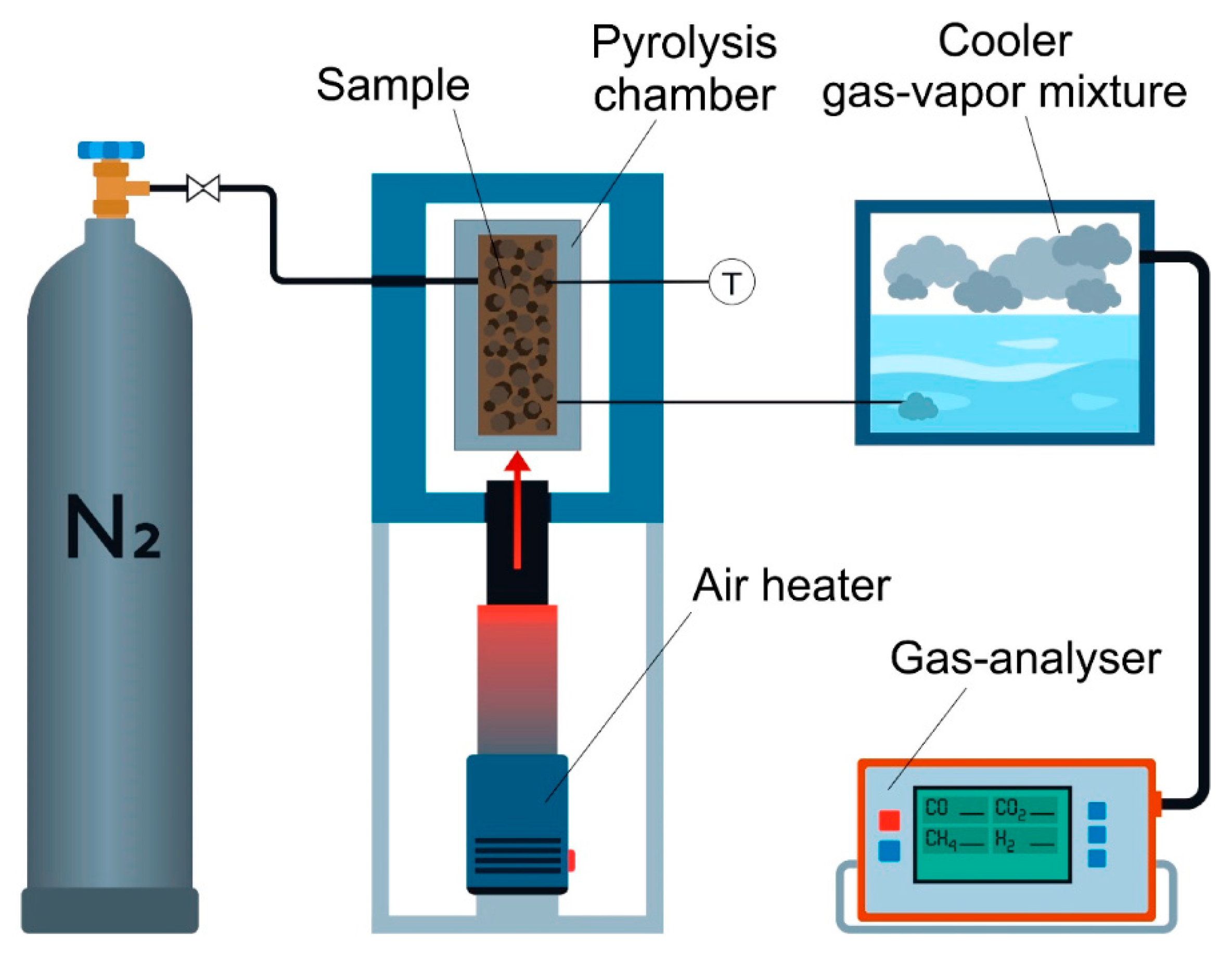
2.2. Pyrolysis
2.3. Spray Drying
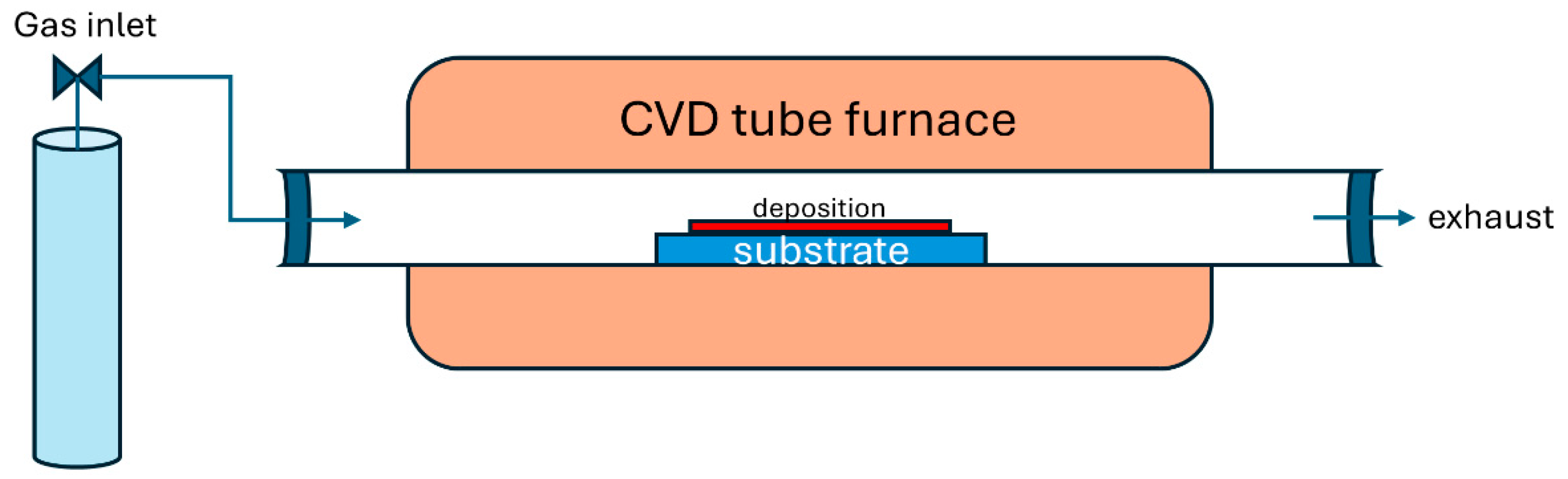
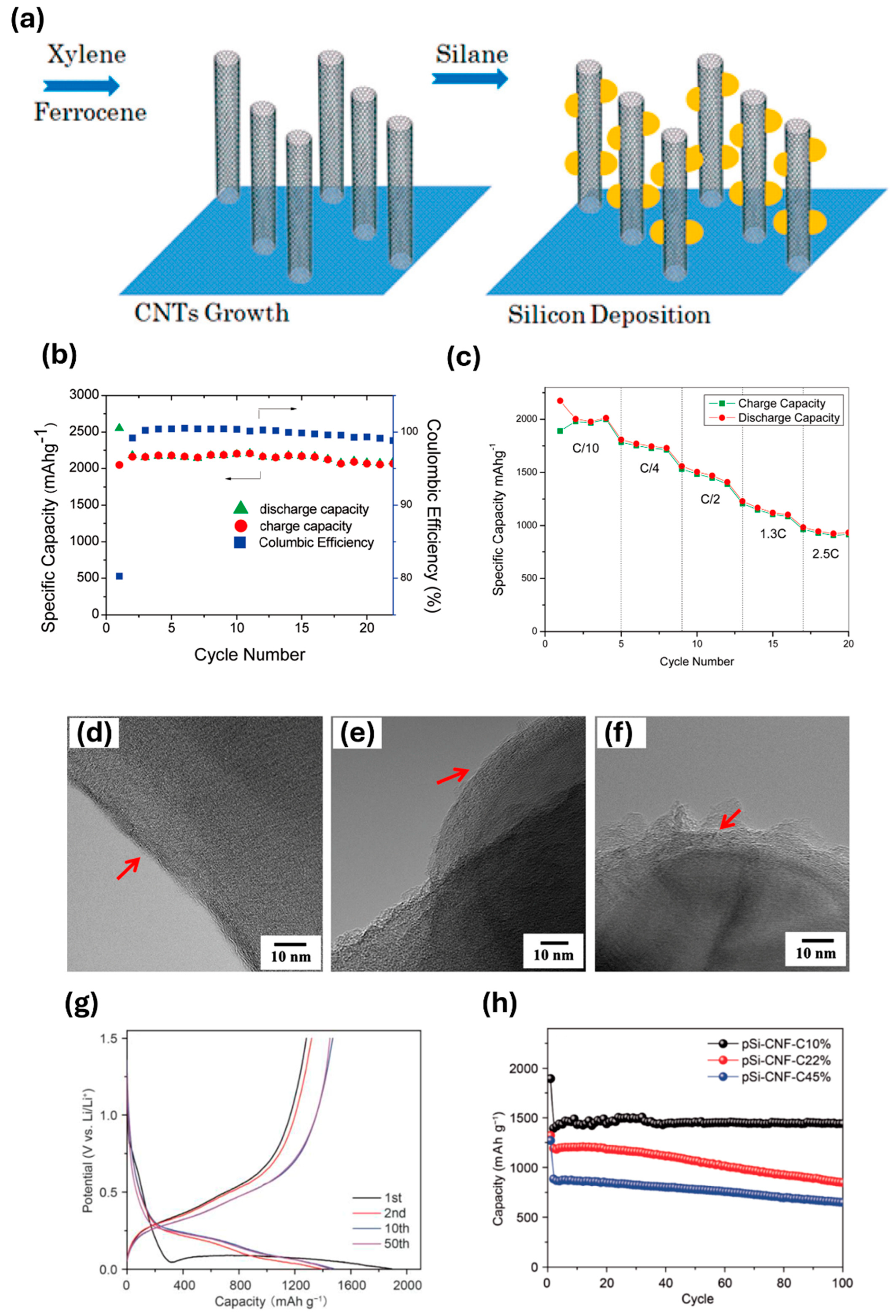
2.4. Chemical Vapor Deposition
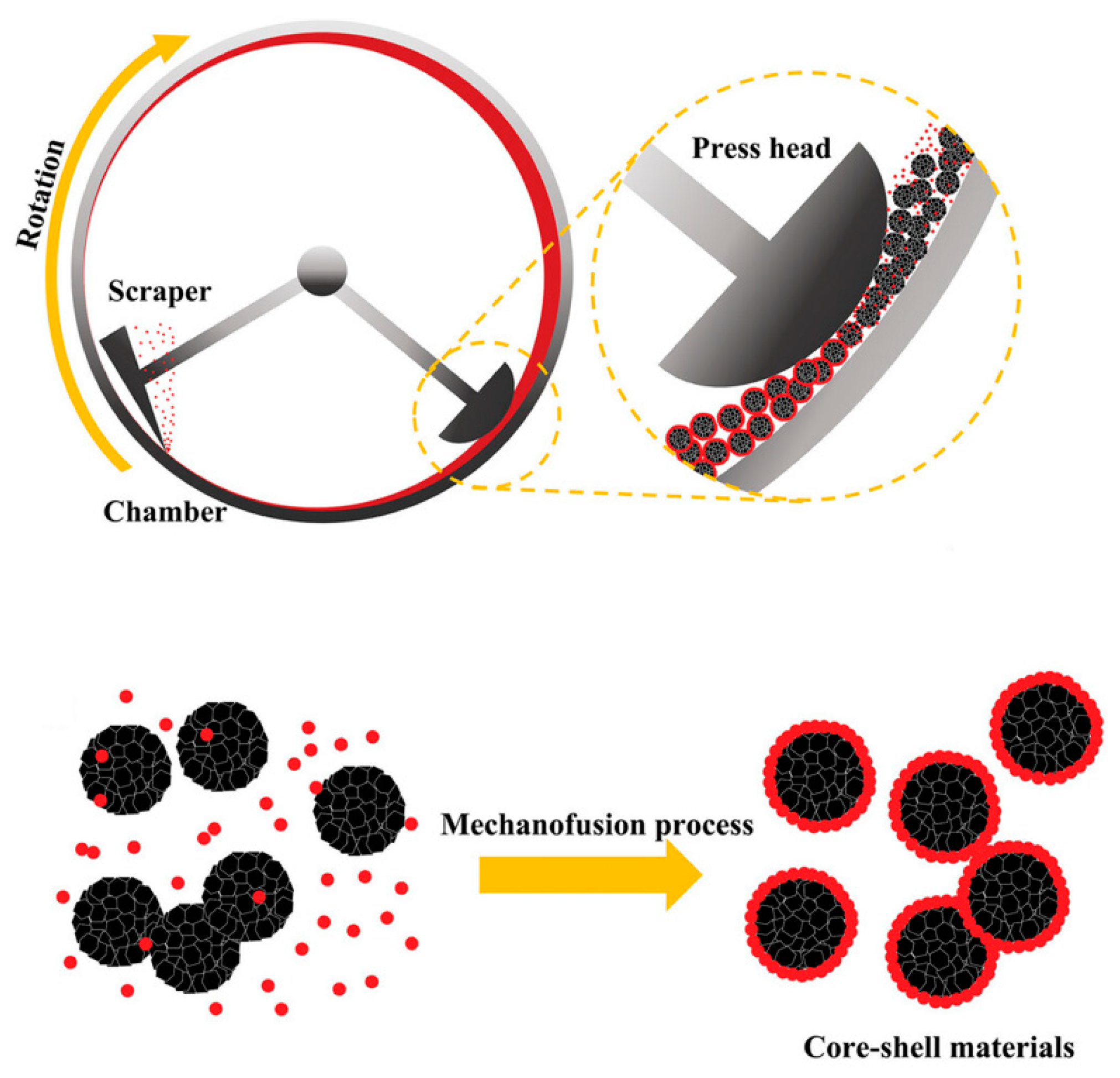
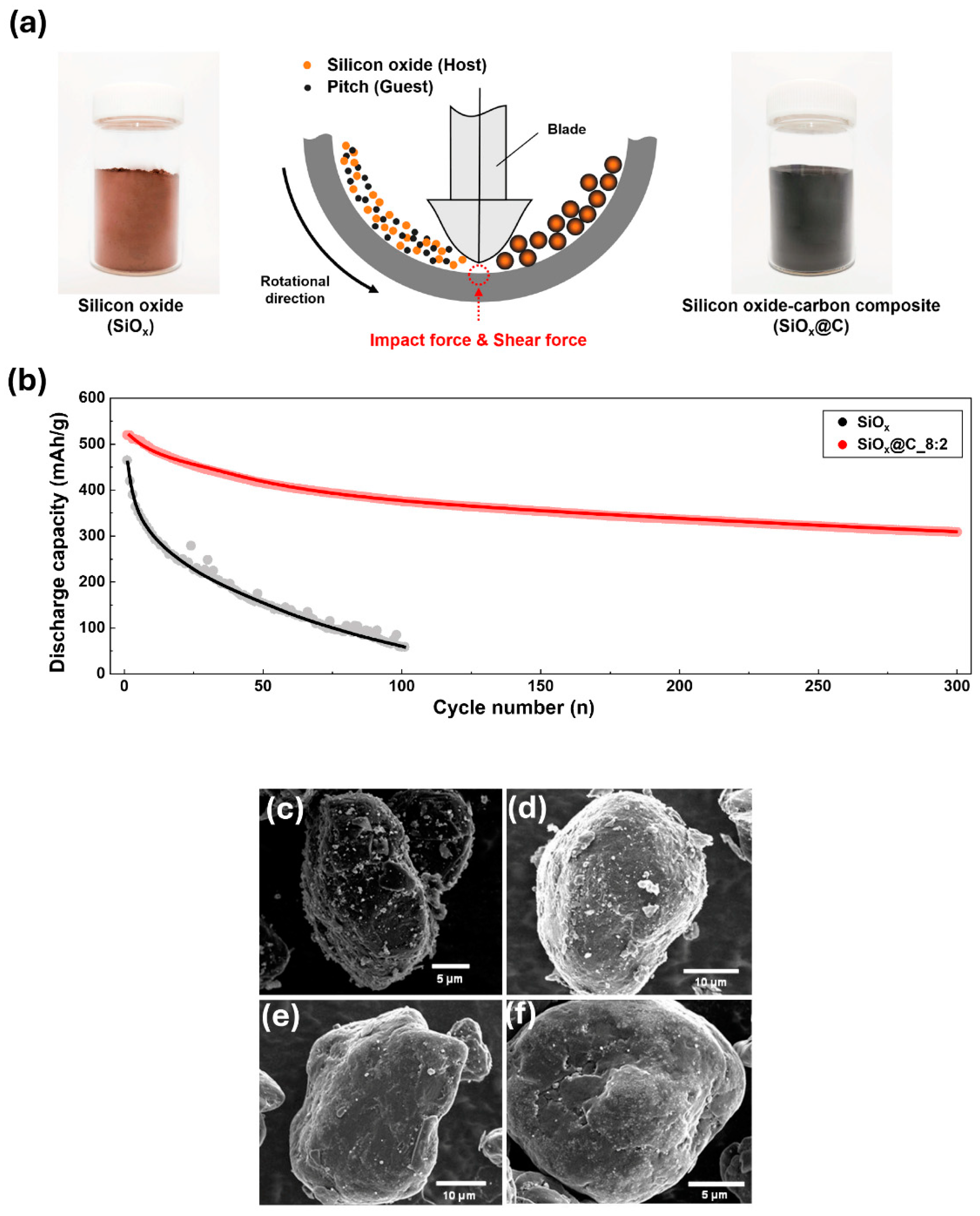
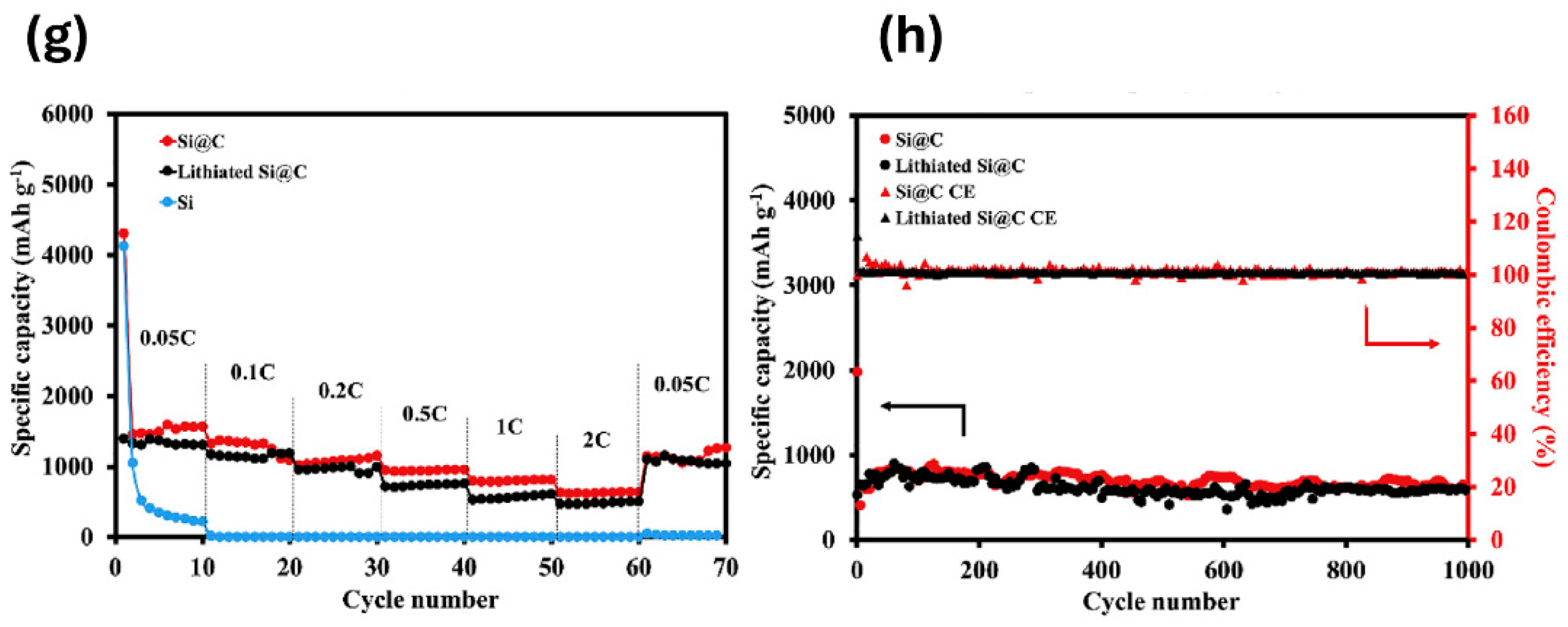
2.5. Mechanofusion
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dou, F.; Shi, L.Y.; Chen, G.R.; Zhang, D.S. Silicon/Carbon Composite Anode Materials for Lithium-Ion Batteries. Electrochem. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.W.; Amine, K. 30 Years of Lithium-Ion Batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, S.; Jung, D.S.; Roh, K.C.; Seo, J.; Kim, J.; Kim, K.; Kim, P.J.; Choi, J. Synergistically enhanced LiF-rich protective layer for highly stable silicon anodes. Appl. Surf. Sci. 2024, 661, 160023. [Google Scholar] [CrossRef]
- Lee, H.; Yoon, T.; Chae, O. Strategies for Enhancing the Stability of Lithium Metal Anodes in Solid-State Electrolytes. Micromachines 2024, 15, 453. [Google Scholar] [CrossRef]
- Su, H.P.; Barragan, A.A.; Geng, L.X.; Long, D.H.; Ling, L.C.; Bozhilov, K.N.; Mangolini, L.; Guo, J.C. Colloidal Synthesis of Silicon-Carbon Composite Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2017, 56, 10780–10785. [Google Scholar] [CrossRef]
- Nzereogu, P.U.; Omah, A.D.; Ezema, F.I.; Iwuoha, E.I.; Nwanya, A.C. Anode materials for lithium-ion batteries: A review. Appl. Surf. Sci. Adv. 2022, 9, 100233. [Google Scholar] [CrossRef]
- Ko, J.; So, S.; Kim, M.; Kim, I.; Ahn, Y.N.; Hur, J. Promoting Zn2+ migration through polar perovskite dielectric layer on Zn metal anode for the enhanced aqueous Zn-ion batteries. Chem. Eng. J. 2023, 462, 142308. [Google Scholar] [CrossRef]
- Nam, G.; Hwang, J.; Kang, D.H.; Oh, S.; Chae, S.; Yoon, M.; Ko, M.S. Mechanical densification synthesis of single-crystalline Ni-rich cathode for high-energy lithium-ion batteries. J. Energy Chem. 2023, 79, 562–568. [Google Scholar] [CrossRef]
- Song, W.; Chae, O.B.; Ryu, J.H. Surface Nitridation of Nano-sized Anatase TiO2 using Urea and Thiourea for Enhanced Electrochemical Performance in Lithium-ion Batteries. J. Electrochem. Sci. Technol. 2024, 15, 512–520. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, T.; Chae, O.B. Behavior of NO3−-Based Electrolyte Additive in Lithium Metal Batteries. Batteries 2024, 10, 135. [Google Scholar] [CrossRef]
- Phan Nguyen, T.; Thi Giang, T.; Tae Kim, I. Restructuring NiO to LiNiO2: Ultrastable and reversible anodes for lithium-ion batteries. Chem. Eng. J. 2022, 437, 135292. [Google Scholar] [CrossRef]
- Sui, D.; Liu, J. Constriction-susceptible lithium support for fast cycling of solid-state lithium metal battery. Chin. Chem. Lett. 2024, 110417. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Chae, O.B.; Kim, G.; Peterson, A.A.; Wu, M.; Jung, H.T. Long-Range Uniform Deposition of Ag Nanoseed on Cu Current Collector for High-Performance Lithium Metal Batteries. Small 2024, 20, e2307200. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Myeong, S.; Lee, D.; Yoo, H.E.; Kim, J.; Kim, C.; Kim, J.; Sun, S.; Kwon, J.; Kim, S.C.; et al. Improved Li-ion kinetics of the anode by kneading process of binder for lithium-ion batteries with high energy density. Electrochim. Acta 2023, 464, 142900. [Google Scholar] [CrossRef]
- de las Casas, C.; Li, W.Z. A review of application of carbon nanotubes for lithium ion battery anode material. J. Power Sources 2012, 208, 74–85. [Google Scholar] [CrossRef]
- Chan, C.K.; Peng, H.L.; Liu, G.; McIlwrath, K.; Zhang, X.F.; Huggins, R.A.; Cui, Y. High-performance lithium battery anodes using silicon nanowires. Nat. Nanotechnol. 2008, 3, 31–35. [Google Scholar] [CrossRef]
- Chae, O.B.; Rynearson, L.; Lucht, B.L. Distance-Dependent Solid Electrolyte Interphase Control by Electrochemical Pretreatment. ACS Energy Lett. 2022, 7, 3087–3094. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Self-Assembled Few-Layered MoS on SnO Anode for Enhancing Lithium-Ion Storage. Nanomaterials 2020, 10, 2558. [Google Scholar] [CrossRef]
- Lee, G.; Kim, I.; Hur, J. Highly conductive and robust telluride-carbon hybrid matrix for enhanced copper diphosphide anode in Li-ion batteries. J. Alloys Compd. 2023, 950, 169914. [Google Scholar] [CrossRef]
- So, S.; Ko, J.; Ahn, Y.N.; Kim, I.; Hur, J. Unraveling improved electrochemical kinetics of In2Te3-based anodes embedded in hybrid matrix for Li-ion batteries. Chem. Eng. J. 2022, 429, 132395. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Liu, W.W.; Kashkooli, A.G.; Xiao, X.C.; Cai, M.; Chen, Z.W. Silicon-Based Anodes for Lithium-Ion Batteries: From Fundamentals to Practical Applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Uono, H.; Satou, T.; Fuse, T.; Ishihara, T.; Ue, M.; Senna, M. Cyclic properties of Si-Cu/carbon nanocomposite anodes for Li-ion secondary batteries. J. Electrochem. Soc. 2005, 152, A523–A526. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wu, B.R.; Mu, G.; Ma, C.W.; Mu, D.B.; Wu, F. Recent progress and perspectives on silicon anode: Synthesis and prelithiation for LIBs energy storage. J. Energy Chem. 2022, 64, 615–650. [Google Scholar] [CrossRef]
- Quilty, C.D.; Wu, D.R.; Li, W.Z.; Bock, D.C.; Wang, L.; Housel, L.M.; Abraham, A.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S. Electron and Ion Transport in Lithium and Lithium-Ion Battery Negative and Positive Composite Electrodes. Chem. Rev. 2023, 123, 1327–1363. [Google Scholar] [CrossRef]
- Choi, W.S.; Kim, M.; Kim, I.T. Te-rP-C Anodes Prepared Using a Scalable Milling Process for High-Performance Lithium-Ion Batteries. Micromachines 2023, 14, 2156. [Google Scholar] [CrossRef]
- Kidanu, W.G.; Hur, J.; Kim, I.T. Gallium-Indium-Tin Eutectic as a Self-Healing Room-Temperature Liquid Metal Anode for High-Capacity Lithium-Ion Batteries. Materials 2022, 15, 168. [Google Scholar] [CrossRef]
- Luo, F.; Liu, B.N.; Zheng, J.Y.; Chu, G.; Zhong, K.F.; Li, H.; Huang, X.J.; Chen, L.Q. Review-Nano-Silicon/Carbon Composite Anode Materials Towards Practical Application for Next Generation Li-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2509–A2528. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Bärmann, P.; Diehl, M.; Göbel, L.; Ruttert, M.; Nowak, S.; Winter, M.; Placke, T. Impact of the silicon particle size on the pre-lithiation behavior of silicon/carbon composite materials for lithium ion batteries. J. Power Sources 2020, 464, 228224. [Google Scholar] [CrossRef]
- Shi, C.; Wang, T.; Liao, X.; Qie, B.; Yang, P.; Chen, M.; Wang, X.; Srinivasan, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136–142. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Li, P.; Kim, H.; Myung, S.T.; Sun, Y.K. Diverting Exploration of Silicon Anode into Practical Way: A Review Focused on Silicon-Graphite Composite for Lithium Ion Batteries. Energy Storage Mater. 2021, 35, 550–576. [Google Scholar] [CrossRef]
- Gonzalez, A.F.; Yang, N.H.; Liu, R.S. Silicon Anode Design for Lithium-Ion Batteries: Progress and Perspectives. J. Phys. Chem. C 2017, 121, 27775–27787. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.Y.; Fukuda, K.; Umeno, T.; Dimov, N.; Ogumi, Z. Carbon-coated Si as a lithium-ion battery anode material. J. Electrochem. Soc. 2002, 149, A1598–A1603. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.X.; Shao, R.; Wu, J.; Jiang, R.Y.; Jin, Z. Recent progress and future perspective on practical silicon anode-based lithium ion batteries. Energy Storage Mater. 2022, 46, 482–502. [Google Scholar] [CrossRef]
- Preman, A.N.; Vo, T.N.; Choi, S.; Lee, H.; Lim, Y.E.; Kim, I.; Ahn, S.K. Self-Healable Poly(Acrylic Acid) Binder toward Optimized Electrochemical Performance for Silicon Anodes: Importance of Balanced Properties. ACS Appl. Energy Mater. 2024, 7, 749–759. [Google Scholar] [CrossRef]
- Gu, M.; He, Y.; Zheng, J.M.; Wang, C.M. Nanoscale silicon as anode for Li-ion batteries: The fundamentals, promises, and challenges. Nano Energy 2015, 17, 366–383. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.X.; Yan, Z.H.; Cheng, F.Y.; Chen, J. Structure design and mechanism analysis of silicon anode for lithium-ion batteries. Sci. China Mater. 2019, 62, 1515–1536. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhu, X.J.; Pan, D. Solutions for the problems of silicon-carbon anode materials for lithium-ion batteries. R. Soc. Open Sci. 2018, 5, 172370. [Google Scholar] [CrossRef]
- Park, E.; So, S.; Hur, J. Carbon-free hydrated cobalt vanadium oxide as a promising anode for lithium-ion batteries. Appl. Surf. Sci. 2022, 579, 152182. [Google Scholar] [CrossRef]
- Li, P.; Zhao, G.Q.; Zheng, X.B.; Xu, X.; Yao, C.H.; Sun, W.P.; Dou, S.X. Recent progress on silicon-based anode materials for practical lithium-ion battery applications. Energy Storage Mater. 2018, 15, 422–446. [Google Scholar] [CrossRef]
- Ozanam, F.; Rosso, M. Silicon as anode material for Li-ion batteries. Mater. Sci. Eng. B-Adv. 2016, 213, 2–11. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.D.; Liu, N.; Zhu, J. Challenges and Recent Progress in the Development of Si Anodes for Lithium-Ion Battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Shen, T.; Yao, Z.J.; Xia, X.H.; Wang, X.L.; Gu, C.D.; Tu, J.P. Rationally Designed Silicon Nanostructures as Anode Material for Lithium-Ion Batteries. Adv. Eng. Mater. 2018, 20, 1700591. [Google Scholar] [CrossRef]
- Szczech, J.R.; Jin, S. Nanostructured silicon for high capacity lithium battery anodes. Energy Environ. Sci. 2011, 4, 56–72. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing nanostructured Si anodes for high energy lithium ion batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Rahman, M.A.; Song, G.S.; Bhatt, A.I.; Wong, Y.C.; Wen, C.E. Nanostructured Silicon Anodes for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 647–678. [Google Scholar] [CrossRef]
- Teki, R.; Datta, M.K.; Krishnan, R.; Parker, T.C.; Lu, T.M.; Kumta, P.N.; Koratkar, N. Nanostructured Silicon Anodes for Lithium Ion Rechargeable Batteries. Small 2009, 5, 2236–2242. [Google Scholar] [CrossRef]
- Cao, Z.; Zheng, X.Y.; Qu, Q.T.; Huang, Y.H.; Zheng, H.H. Electrolyte Design Enabling a High-Safety and High-Performance Si Anode with a Tailored Electrode-Electrolyte Interphase. Adv. Mater. 2021, 33, 2103178. [Google Scholar] [CrossRef]
- Zhang, S.; He, M.N.; Su, C.C.; Zhang, Z.C. Advanced electrolyte/additive for lithium-ion batteries with silicon anode. Curr. Opin. Chem. Eng. 2016, 13, 24–35. [Google Scholar] [CrossRef]
- Xu, C.; Lindgren, F.; Philippe, B.; Gorgoi, M.; Björefors, F.; Edström, K.; Gustafsson, T. Improved Performance of the Silicon Anode for Li-Ion Batteries: Understanding the Surface Modification Mechanism of Fluoroethylene Carbonate as an Effective Electrolyte Additive. Chem. Mat. 2015, 27, 2591–2599. [Google Scholar] [CrossRef]
- Liu, H.B.; Sun, Q.; Zhang, H.Q.; Cheng, J.; Li, Y.Y.; Zeng, Z.; Zhang, S.; Xu, X.; Ji, F.J.; Li, D.P.; et al. The application road of silicon-based anode in lithium-ion batteries: From liquid electrolyte to solid-state electrolyte. Energy Storage Mater. 2023, 55, 244–263. [Google Scholar] [CrossRef]
- He, Y.; Jiang, L.; Chen, T.W.; Xu, Y.B.; Jia, H.P.; Yi, R.; Xue, D.C.; Song, M.; Genc, A.; Bouchet-Marquis, C.; et al. Progressive growth of the solid-electrolyte interphase towards the Si anode interior causes capacity fading. Nat. Nanotechnol. 2021, 16, 1113–1120. [Google Scholar] [CrossRef]
- Xu, Z.X.; Yang, J.; Li, H.P.; Nuli, Y.N.; Wang, J.L. Electrolytes for advanced lithium ion batteries using silicon-based anodes. J. Mater. Chem. A 2019, 7, 9432–9446. [Google Scholar] [CrossRef]
- Li, Z.H.; Zhang, Y.P.; Liu, T.F.; Gao, X.H.; Li, S.Y.; Ling, M.; Liang, C.D.; Zheng, J.C.; Lin, Z. Silicon Anode with High Initial Coulombic Efficiency by Modulated Trifunctional Binder for High-Areal-Capacity Lithium-Ion Batteries. Adv. Energy Mater. 2020, 10, 1903110. [Google Scholar] [CrossRef]
- Gao, S.L.; Sun, F.Y.; Brady, A.; Pan, Y.Y.; Erwin, A.; Yang, D.D.; Tsukruk, V.; Stack, A.G.; Saito, T.; Yang, H.B.; et al. Ultra-efficient polymer binder for silicon anode in high-capacity lithium-ion batteries. Nano Energy 2020, 73, 104804. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward Efficient Binders for Li-Ion Battery Si-Based Anodes: Polyacrylic Acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Y.; Tan, R.; Tian, L.L.; Liu, Y.D.; Chen, H.B.; Pan, F. Novel conductive binder for high-performance silicon anodes in lithium ion batteries. Nano Energy 2017, 36, 206–212. [Google Scholar] [CrossRef]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.; Saito, T.; Ahn, S.K. Progress of 3D network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Luo, W.; Chen, X.Q.; Xia, Y.; Chen, M.; Wang, L.J.; Wang, Q.Q.; Li, W.; Yang, J.P. Surface and Interface Engineering of Silicon-Based Anode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1701083. [Google Scholar] [CrossRef]
- Jiang, C.L.; Xiang, L.; Miao, S.J.; Shi, L.; Xie, D.H.; Yan, J.X.; Zheng, Z.J.; Zhang, X.M.; Tang, Y.B. Flexible Interface Design for Stress Regulation of a Silicon Anode toward Highly Stable Dual-Ion Batteries. Adv. Mater. 2020, 32, e1908470. [Google Scholar] [CrossRef]
- Lv, Y.Y.; Han, Z.B.; Jia, R.R.; Shi, L.Y.; Yuan, S. Porous interface for fast charging silicon anode. Battery Energy 2022, 1, 20220009. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.J.; Li, S.Y.; Xi, F.S.; Tong, Z.Q.; Chen, X.H.; Wei, K.X.; Ma, W.H. Controllable Interface Engineering for the Preparation of High Rate Silicon Anode. Adv. Funct. Mater. 2024, 34, 2403574. [Google Scholar] [CrossRef]
- Wang, L.; Yu, J.; Li, S.Y.; Xi, F.S.; Ma, W.H.; Wei, K.X.; Lu, J.J.; Tong, Z.Q.; Liu, B.; Luo, B. Recent advances in interface engineering of silicon anodes for enhanced lithium-ion battery performance. Energy Storage Mater. 2024, 66, 103243. [Google Scholar] [CrossRef]
- Zuo, X.X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Andersen, H.F.; Foss, C.E.L.; Voje, J.; Tronstad, R.; Mokkelbost, T.; ErikVullum, P.; Ulvestad, A.; Kirkengen, M.; Mæhlen, J.P. Silicon-Carbon composite anodes from industrial battery grade silicon. Sci. Rep. 2019, 9, 14814. [Google Scholar] [CrossRef]
- Liu, Y.; Hanai, K.; Yang, J.; Imanishi, N.; Hirano, A.; Takeda, Y. Silicon/carbon composites as anode materials for Li-ion batteries. Electrochem. Solid-State Lett. 2004, 7, A369–A372. [Google Scholar] [CrossRef]
- Wang, J.Z.; Zhong, C.; Chou, S.L.; Liu, H.K. Flexible free-standing graphene-silicon composite film for lithium-ion batteries. Electrochem. Commun. 2010, 12, 1467–1470. [Google Scholar] [CrossRef]
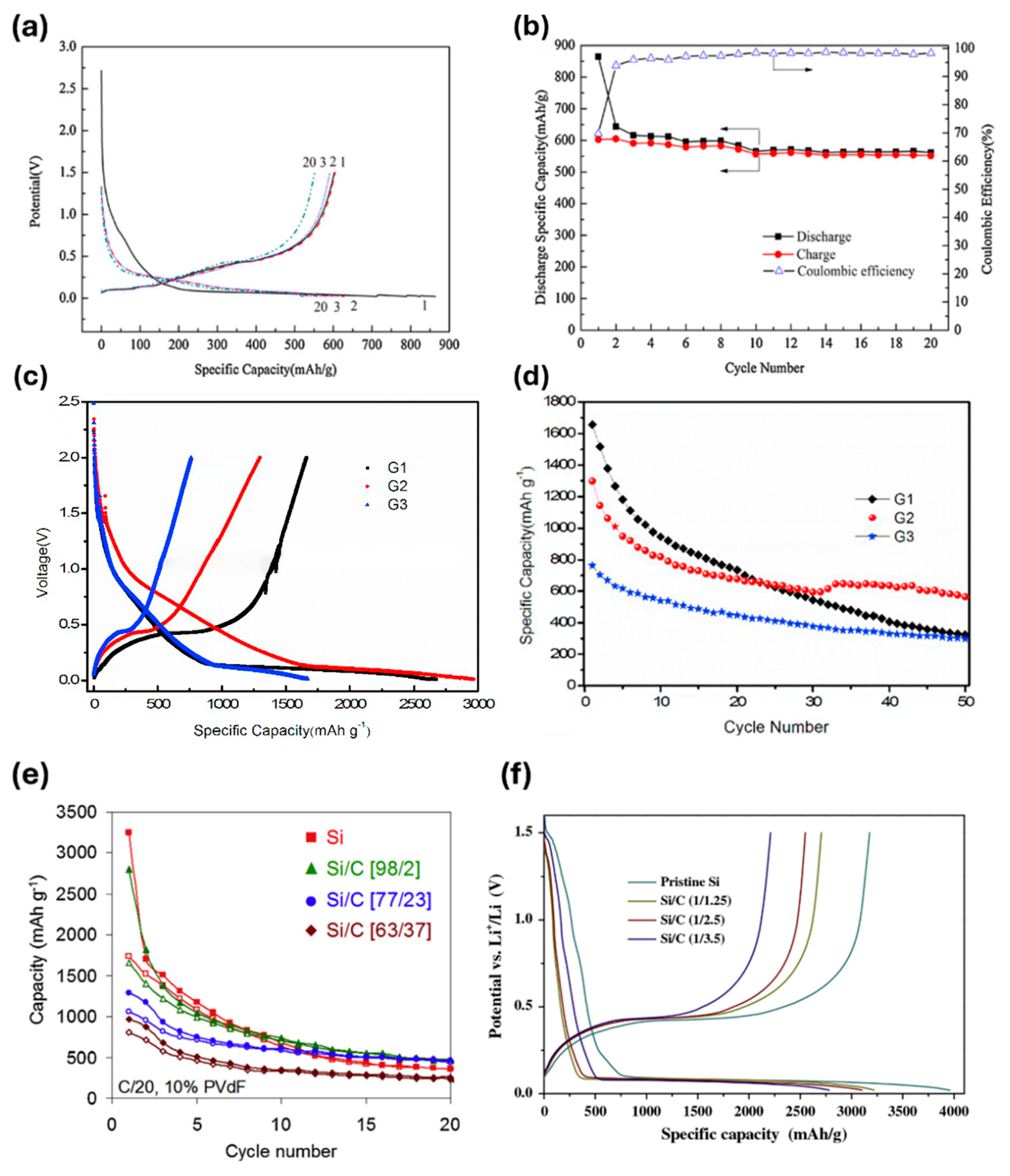
- Mhadhbi, M. Modelling of the High-Energy Ball Milling Process. Adv. Mat. Phys. Chem. 2021, 11, 31–44. [Google Scholar] [CrossRef]
- Yu, S.X.; Guo, B.B.; Zeng, T.B.; Qu, H.Q.; Yang, J.L.; Bai, J.M. Graphene-based lithium-ion battery anode materials manufactured by mechanochemical ball milling process: A review and perspective. Compos. Pt. B-Eng. 2022, 246, 110232. [Google Scholar] [CrossRef]
- Bhagyaraj, S.M.; Oluwafemi, O.S.; Kalarikkal, N.; Thomas, S. Synthesis of Inorganic Nanomaterials: Advances and Key Technologies; Woodhead Publishing: Duxford, UK; Cambridge, MA, USA, 2018; p. 12. 300p. [Google Scholar]
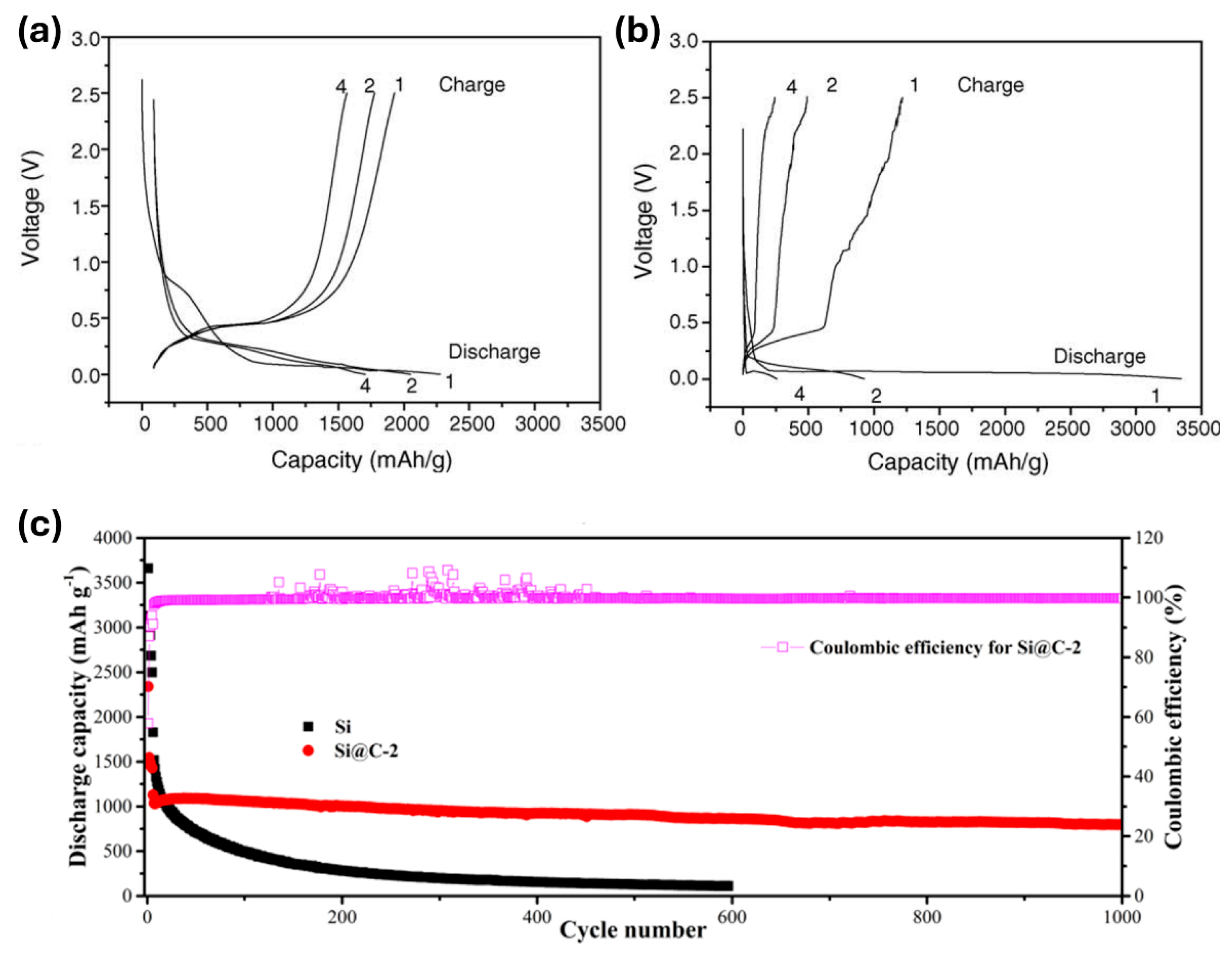
- Zhang, Y.; Cheng, Y.; Song, J.; Zhang, Y.; Shi, Q.; Wang, J.; Tian, F.; Yuan, S.; Su, Z.; Zhou, C.; et al. Functionalization-assistant ball milling towards Si/graphene anodes in high performance Li-ion batteries. Carbon 2021, 181, 300–309. [Google Scholar] [CrossRef]
- Wang, D.; Gao, M.; Pan, H.; Wang, J.; Liu, Y. High performance amorphous-Si@SiOx/C composite anode materials for Li-ion batteries derived from ball-milling and in situ carbonization. J. Power Sources 2014, 256, 190–199. [Google Scholar] [CrossRef]
- Qian, L.; Lan, J.-L.; Xue, M.; Yu, Y.; Yang, X. Two-step ball-milling synthesis of a Si/SiOx/C composite electrode for lithium ion batteries with excellent long-term cycling stability. RSC Adv. 2017, 7, 36697–36704. [Google Scholar] [CrossRef]
- Koraag, P.Y.E.; Firdaus, A.M.; Hawari, N.H.; Refino, A.D.; Dempwolf, W.; Iskandar, F.; Peiner, E.; Wasisto, H.S.; Sumboja, A. Covalently Bonded Ball-Milled Silicon/CNT Nanocomposite as Lithium-Ion Battery Anode Material. Batteries 2022, 8, 165. [Google Scholar] [CrossRef]
- Cu, P.; Cai, R.; Zhou, Y.K.; Shao, Z.P. Si/C composite lithium-ion battery anodes synthesized from coarse silicon and citric acid through combined ball milling and thermal pyrolysis. Electrochim. Acta 2010, 55, 3876–3883. [Google Scholar]
- Cabello, M.; Gucciardi, E.; Herrán, A.; Carriazo, D.; Villaverde, A.; Rojo, T. Towards a High-Power Si@graphite Anode for Lithium Ion Batteries through a Wet Ball Milling Process. Molecules 2020, 25, 2494. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.G.; Zhang, H.L.; Zhao, Z.G.; Li, F.; Liu, C.; Cheng, H.M. Composite anode material of silicon/graphite/carbon nanotubes for Li-ion batteries. Electrochim. Acta 2006, 51, 4994–5000. [Google Scholar] [CrossRef]
- Han, J.; Zhao, C.C.; Wang, L.; Song, J.; Yang, D.; Tian, Q.H. Simple ball milling-assisted method enabling N-doped carbon embedded Si for high performance lithium-ion battery anode. J. Alloys Compd. 2023, 966, 171668. [Google Scholar] [CrossRef]
- Gorshkov, A.; Berezikov, N.; Kaltaev, A.; Yankovsky, S.; Slyusarsky, K.; Tabakaev, R.; Larionov, K. Analysis of the Physicochemical Characteristics of Biochar Obtained by Slow Pyrolysis of Nut Shells in a Nitrogen Atmosphere. Energies 2021, 14, 8075. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Chapter 8—Thermochemical Route for Biohydrogen Production. In Biohydrogen, 2nd ed.; Pandey, A., Mohan, S.V., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 187–218. [Google Scholar]
- Zhang, G.W.; He, Y.Q.; Feng, Y.; Wang, H.F.; Zhang, T.; Xie, W.N.; Zhu, X.N. Enhancement in liberation of electrode materials derived from spent lithium-ion battery by pyrolysis. J. Clean. Prod. 2018, 199, 62–68. [Google Scholar] [CrossRef]
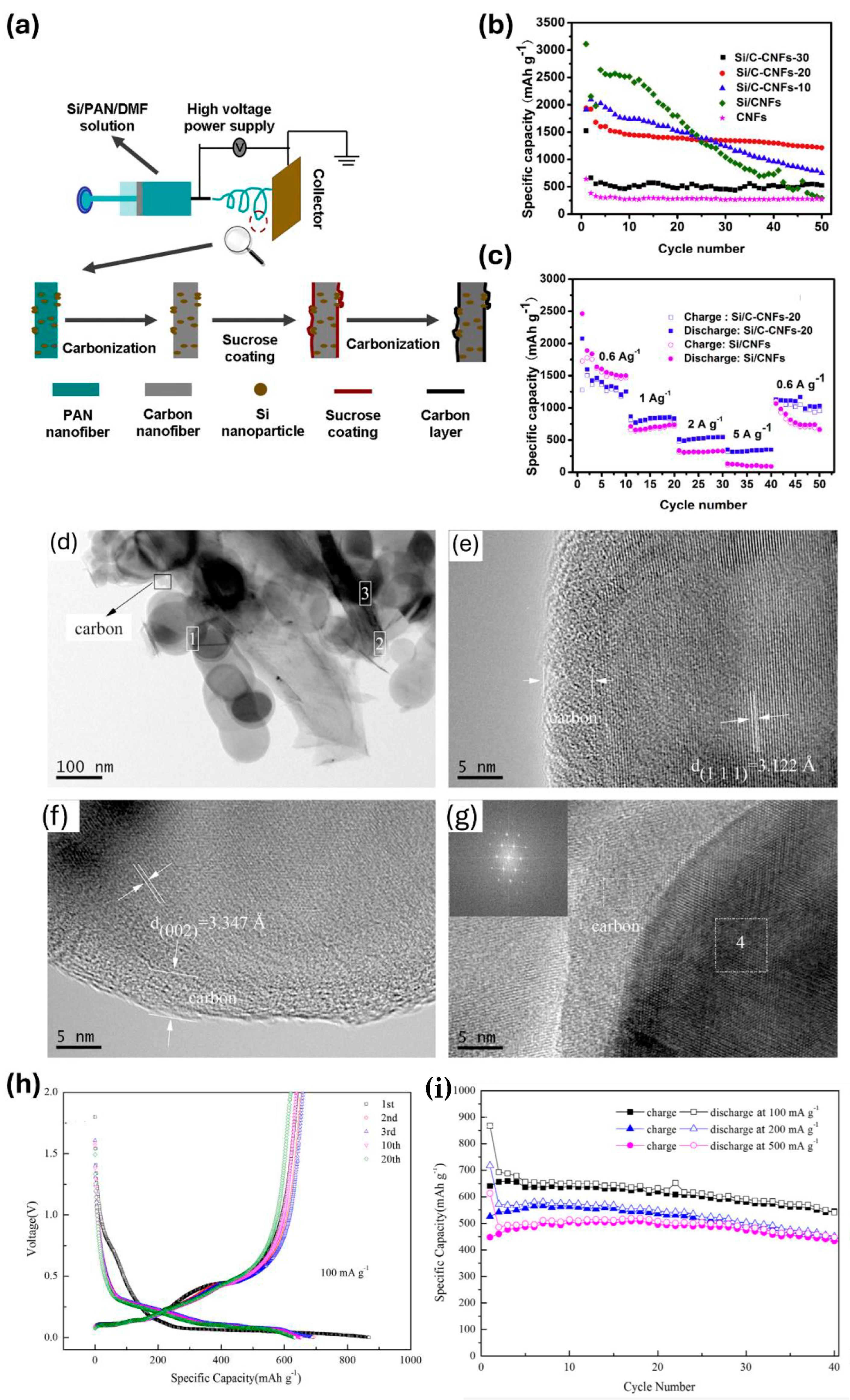
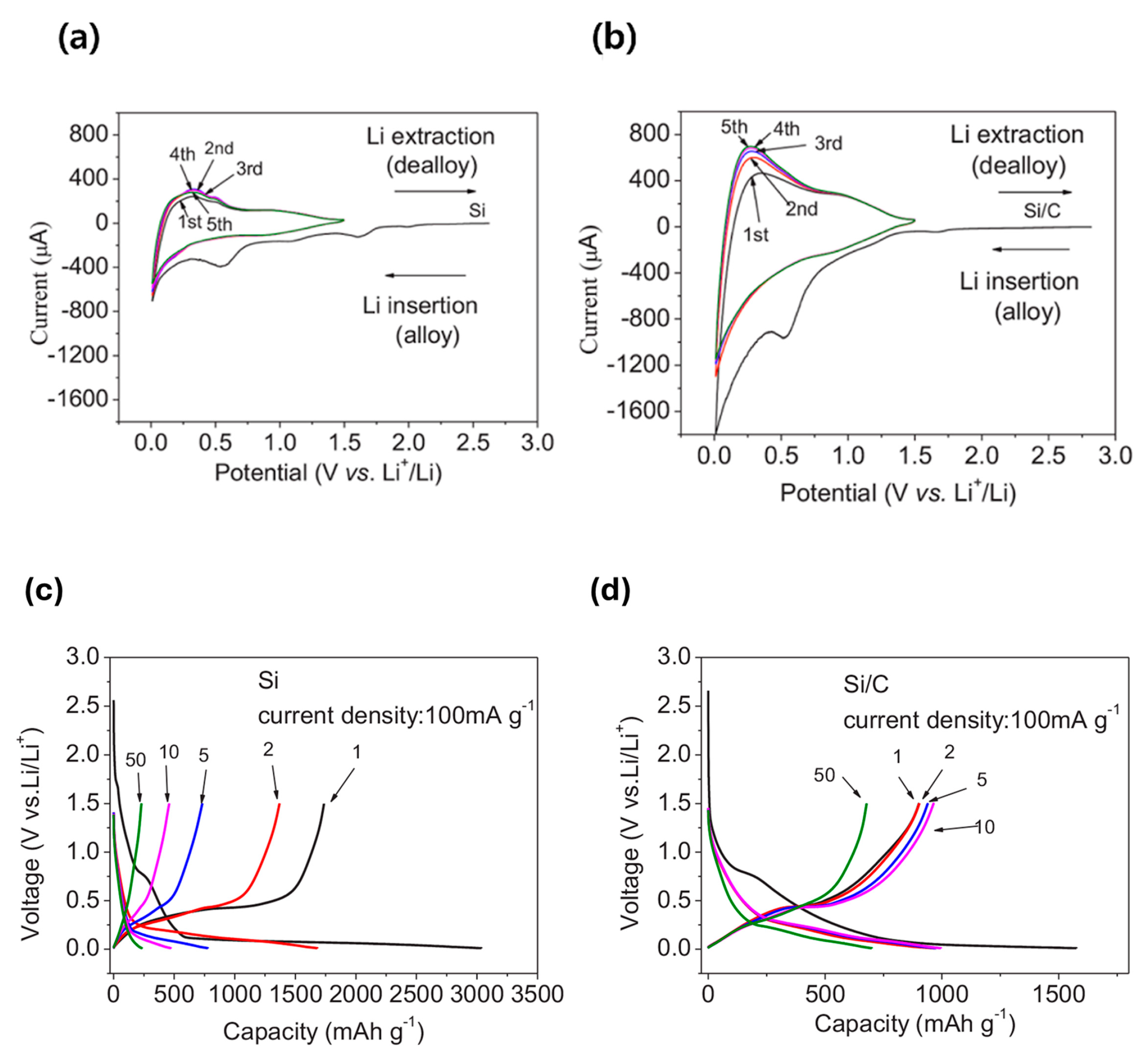
- Chen, Y.L.; Hu, Y.; Shao, J.Z.; Shen, Z.; Chen, R.Z.; Zhang, X.W.; He, X.; Song, Y.Z.; Xing, X.L. Pyrolytic carbon-coated silicon/carbon nanofiber composite anodes for high-performance lithium-ion batteries. J. Power Sources 2015, 298, 130–137. [Google Scholar] [CrossRef]
- Yang, Z.W.; Yang, Y.; Guo, H.J.; Wang, Z.X.; Li, X.H.; Zhou, Y.; Wang, J.X. Compact structured silicon/carbon composites as high-performance anodes for lithium ion batteries. Ionics 2018, 24, 3405–3411. [Google Scholar] [CrossRef]
- Hu, J.Z.; Wang, M.; Meyer, A.W.; Huang, X.S.; Cheng, Y.T. Oxidative Pyrolysis of Si/Polyacrylonitrile Composites as an Unconventional Approach to Fabricate High Performance Lithium Ion Battery Negative Electrodes. J. Electrochem. Soc. 2019, 166, A3716–A3722. [Google Scholar] [CrossRef]
- Su, M.R.; Wang, Z.X.; Guo, H.J.; Li, X.H.; Huang, S.L.; Gan, L. Silicon, flake graphite and phenolic resin-pyrolyzed carbon based Si/C composites as anode material for lithium-ion batteries. Adv. Powder Technol. 2013, 24, 921–925. [Google Scholar] [CrossRef]
- Wang, M.S.; Fan, L.Z. Silicon/carbon nanocomposite pyrolyzed from phenolic resin as anode materials for lithium-ion batteries. J. Power Sources 2013, 244, 570–574. [Google Scholar] [CrossRef]
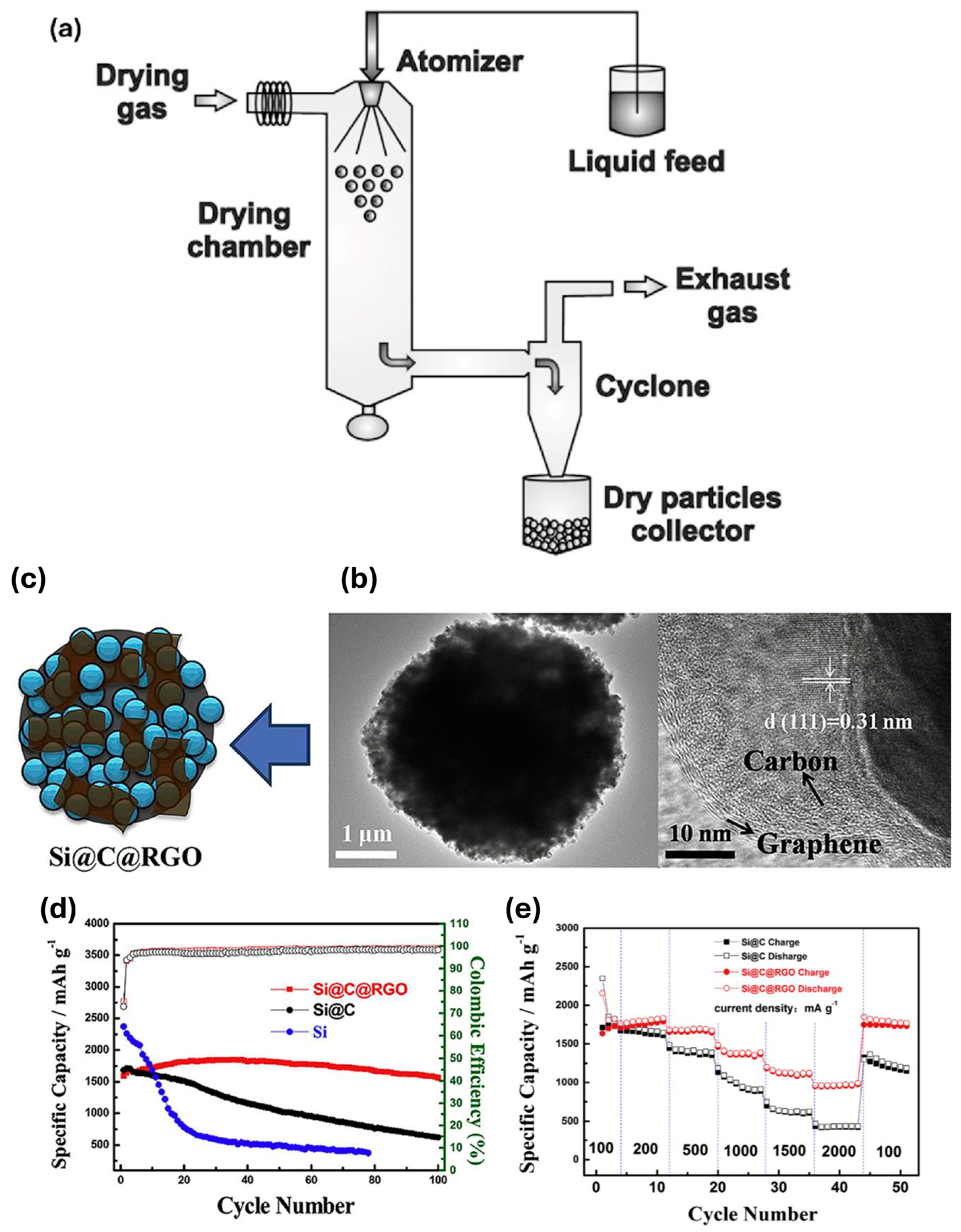
- Campbell, H.R.; Alsharif, F.M.; Marsac, P.J.; Lodder, R.A. The Development of a Novel Pharmaceutical Formulation of D-Tagatose for Spray-Drying. J. Pharm. Innov. 2022, 17, 194–206. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Pan, Q.R.; Zuo, P.J.; Lou, S.F.; Mu, T.S.; Du, C.Y.; Cheng, X.Q.; Ma, Y.L.; Gao, Y.Z.; Yin, G.P. Micro-sized spherical silicon@carbon@graphene prepared by spray drying as anode material for lithium-ion batteries. J. Alloys Compd. 2017, 723, 434–440. [Google Scholar] [CrossRef]
- Lai, J.; Guo, H.J.; Wang, Z.X.; Li, X.H.; Zhang, X.P.; Wu, F.X.; Yue, P. Preparation and characterization of flake graphite/silicon/carbon spherical composite as anode materials for lithium-ion batteries. J. Alloys Compd. 2012, 530, 30–35. [Google Scholar] [CrossRef]
- Su, M.R.; Liu, S.; Tao, L.; Tang, Y.P.; Dou, A.C.; Lv, J.; Liu, Y.J. Silicon@graphene composite prepared by spray-drying method as anode for lithium ion batteries. J. Electroanal. Chem. 2019, 844, 86–90. [Google Scholar] [CrossRef]
- Paireau, C.; Jouanneau, S.; Ammar, M.R.; Simon, P.; Béguin, F.; Raymundo-Piñero, E. Si/C composites prepared by spray drying from cross-linked polyvinyl alcohol as Li-ion batteries anodes. Electrochim. Acta 2015, 174, 361–368. [Google Scholar] [CrossRef]
- Wang, D.S.; Gao, M.X.; Pan, H.G.; Liu, Y.F.; Wang, J.H.; Li, S.Q.; Ge, H.W. Enhanced cycle stability of micro-sized Si/C anode material with low carbon content fabricated via spray drying and in situ carbonization. J. Alloys Compd. 2014, 604, 130–136. [Google Scholar] [CrossRef]
- Shi, Q.T.; Zhou, J.H.; Ullah, S.; Yang, X.Q.; Tokarska, K.; Trzebicka, B.; Ta, H.Q.; Rümmeli, M.H. A review of recent developments in Si/C composite materials for Li-ion batteries. Energy Storage Mater. 2021, 34, 735–754. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, Y.; Li, J.; Tian, S.; Fei, T.; Li, H. Effects of deposition temperature and time on HfC nanowires synthesized by CVD on SiC-coated C/C composites. Ceram. Int. 2016, 42, 5623–5628. [Google Scholar] [CrossRef]
- Shukrullah, S.; Mohamed, N.; Shaharun, M.; Saheed, M.; Irshad, M. Effect of CVD process temperature on activation energy and structural growth of MWCNTs. Metall. Mater. Trans. A 2016, 47, 1413–1424. [Google Scholar] [CrossRef]
- Wang, W.; Kumta, P.N. Nanostructured Hybrid Silicon/Carbon Nanotube Heterostructures: Reversible High-Capacity Lithium-Ion Anodes. ACS Nano 2010, 4, 2233–2241. [Google Scholar] [CrossRef]
- Fu, K.; Xue, L.G.; Yildiz, O.; Li, S.L.; Lee, H.; Li, Y.; Xu, G.J.; Zhou, L.; Bradford, P.D.; Zhang, X.W. Effect of CVD carbon coatings on Si@CNF composite as anode for lithium-ion batteries. Nano Energy 2013, 2, 976–986. [Google Scholar] [CrossRef]
- Jin, H.C.; Sun, Q.; Wang, J.T.; Ma, C.; Ling, L.C.; Qiao, W.M. Preparation and electrochemical properties of novel silicon-carbon composite anode materials with a core-shell structure. New Carbon Mater. 2021, 36, 390–398. [Google Scholar] [CrossRef]
- Liu, B.; Huang, P.; Xie, Z.Y.; Huang, Q.Z. Large-Scale Production of a Silicon Nanowire/Graphite Composites Anode via the CVD Method for High-Performance Lithium-Ion Batteries. Energy Fuels 2021, 35, 2758–2765. [Google Scholar] [CrossRef]
- Yu, J.L.; Yang, J.; Feng, X.J.; Jia, H.; Wang, J.L.; Lu, W. Uniform Carbon Coating on Silicon Nanoparticles by Dynamic CVD Process for Electrochemical Lithium Storage. Ind. Eng. Chem. Res. 2014, 53, 12697–12704. [Google Scholar] [CrossRef]
- Chen, W.L.; Dave, R.N.; Pfeffer, R.; Walton, O. Numerical simulation of Mechanofusion system. Powder Technol. 2004, 146, 121–136. [Google Scholar] [CrossRef]
- Alonso, M.; Satoh, M.; Miyanami, K. Mechanism of the combined coating-mechanofusion processing of powders. Powder Technol. 1989, 59, 45–52. [Google Scholar] [CrossRef]
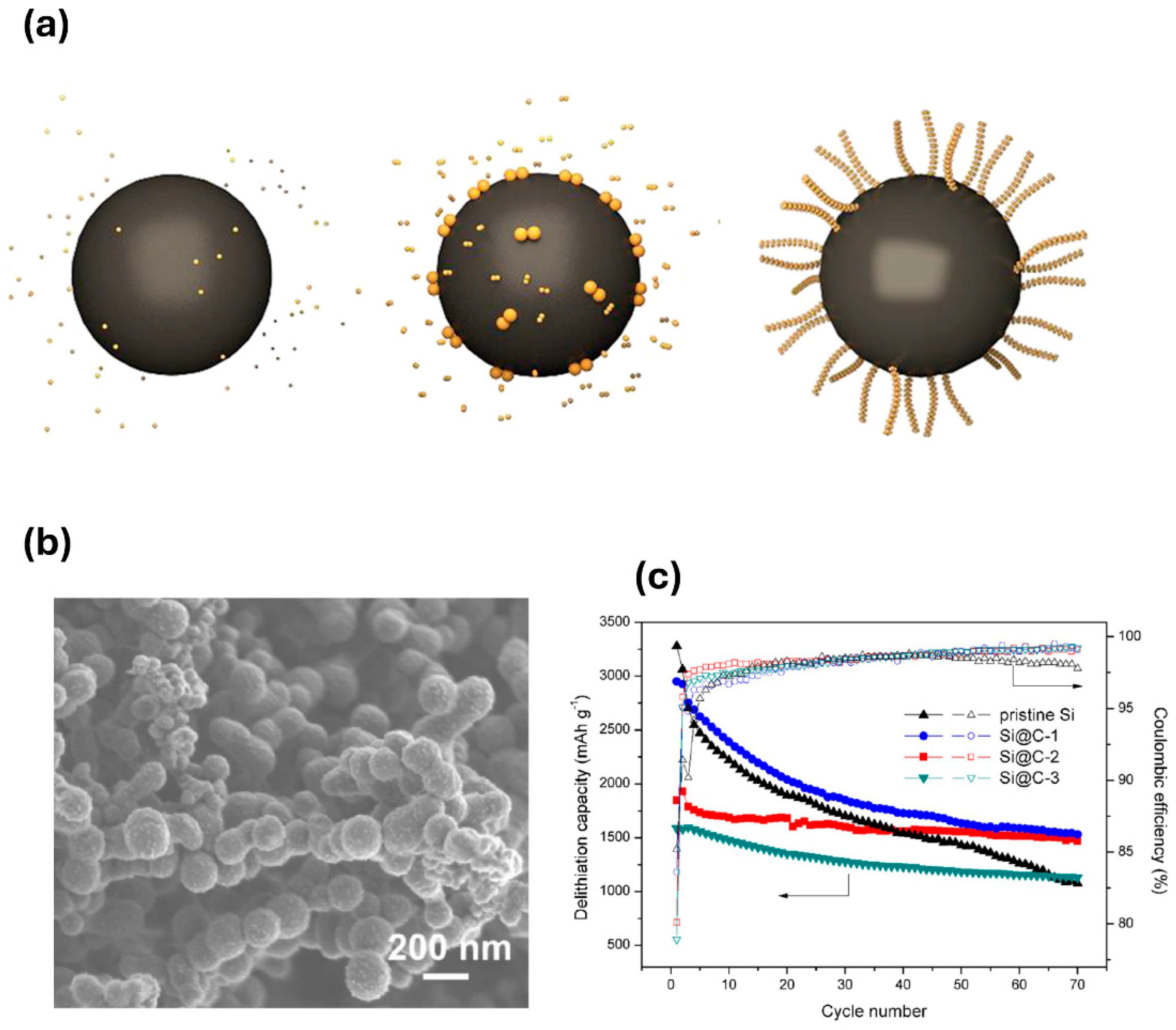
- Tubtimkuna, S.; Danilov, D.L.; Sawangphruk, M.; Notten, P.H.L. Review of the Scalable Core-Shell Synthesis Methods: The Improvements of Li-Ion Battery Electrochemistry and Cycling Stability. Small Methods 2023, 7, e2300345. [Google Scholar] [CrossRef]
- Kim, S.J.; Ha, S.J.; Lee, J.U.; Jeon, Y.P.; Hong, J.Y.; Bedia, J.; Vedyagin, A.A. Preparation of Silicon Oxide-Carbon Composite with Tailored Electrochemical Properties for Anode in Lithium-Ion Batteries. C-J. Carbon Res. 2023, 9, 114. [Google Scholar] [CrossRef]
- Cao, Y.D.; Hatchard, T.D.; Dunlap, R.A.; Obrovac, M.N. Mechanofusion-derived Si-alloy/graphite composite electrode materials for Li-ion batteries. J. Mater. Chem. A 2019, 7, 8335–8343. [Google Scholar] [CrossRef]
- Jiang, H.R.; Salehabadi, M.; Yasmin, S.; Wang, J.; Obrovac, M.N. Nano-Si Filled Graphite Anode Particles by Mechanofusion. J. Electrochem. Soc. 2023, 170, 120511. [Google Scholar] [CrossRef]
- Wutthiprom, J.; Phattharasupakun, N.; Tomon, C.; Sawangphruk, M. Scalable solvent-free mechanofusion and magnesiothermic reduction processes for obtaining carbon nanospheres-encapsulated crystalline silicon anode for Li-ion batteries. Electrochim. Acta 2020, 352, 136457. [Google Scholar] [CrossRef]
| Synthesis Method | Advantages | Disadvantages | Materials | Capacity | Ref. |
|---|---|---|---|---|---|
| Ball milling | Large-scale production | Non-uniform particles | Si/CNT | 1760 mAh/g after 100 cycles | [76] |
| Pyrolysis | High yield | High energy | Si/C | 1555 mAh/g after 100 cycles | [86] |
| Spray Drying | Spherical particle, simple process | Bulky equipment | Si/C | 1041 mAh/g after 50 cycles | [91] |
| CVD | Uniform particle, various particle design | Expensive process | pSi-CNF | 848 mAh/g after 100 cycles | [101] |
| Mechanofusion | Spherical particle | Expensive process | Si/C | 600 mAh/g after 1000 cycles | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.; Lee, H.; Chae, O.B. Synthesis Methods of Si/C Composite Materials for Lithium-Ion Batteries. Batteries 2024, 10, 381. https://doi.org/10.3390/batteries10110381
Park I, Lee H, Chae OB. Synthesis Methods of Si/C Composite Materials for Lithium-Ion Batteries. Batteries. 2024; 10(11):381. https://doi.org/10.3390/batteries10110381
Chicago/Turabian StylePark, Inkyu, Hanbyeol Lee, and Oh B. Chae. 2024. "Synthesis Methods of Si/C Composite Materials for Lithium-Ion Batteries" Batteries 10, no. 11: 381. https://doi.org/10.3390/batteries10110381
APA StylePark, I., Lee, H., & Chae, O. B. (2024). Synthesis Methods of Si/C Composite Materials for Lithium-Ion Batteries. Batteries, 10(11), 381. https://doi.org/10.3390/batteries10110381








