Conductive Zinc-Based Metal–Organic Framework Nanorods as Cathodes for High-Performance Zn-Ion Capacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
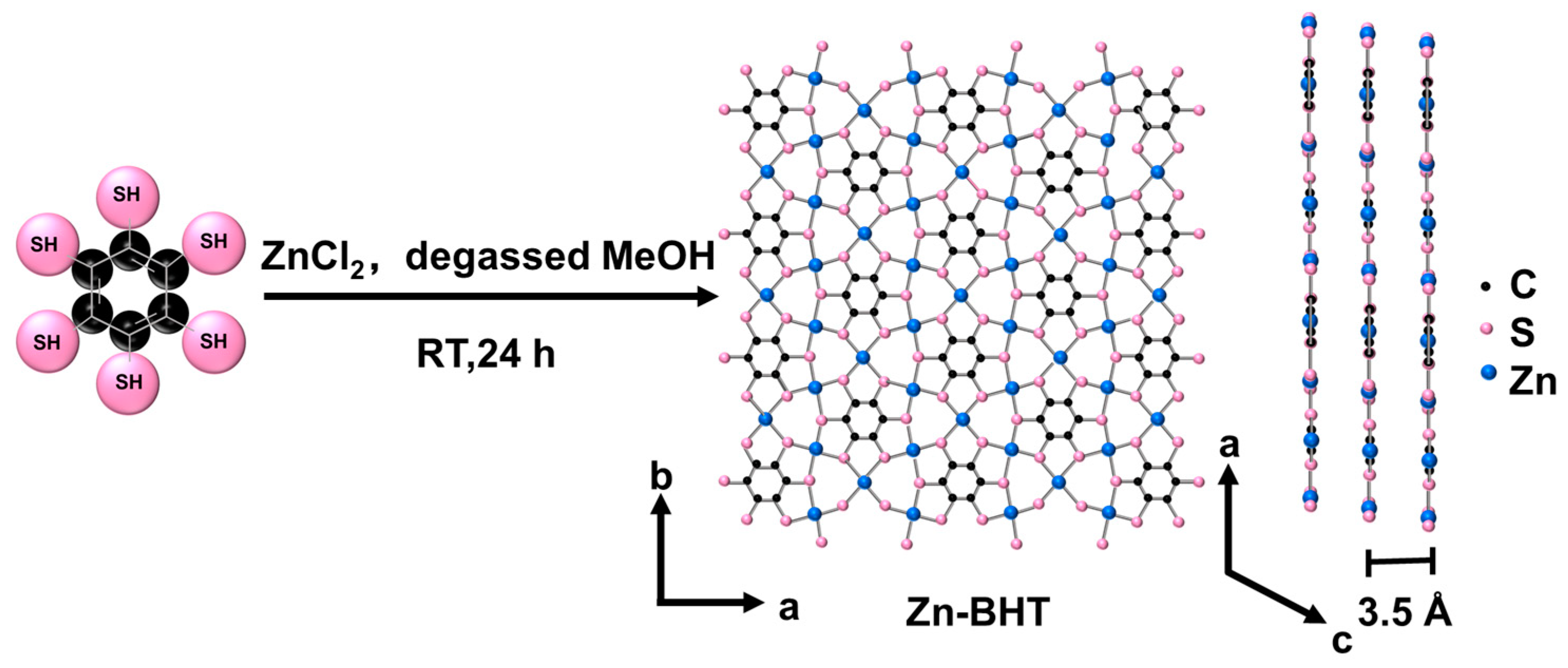
2.2. Synthesis of Zn-BHT and PC
2.3. Material Characterization
2.4. Electrode Preparation and Electrochemical Measurement
3. Results
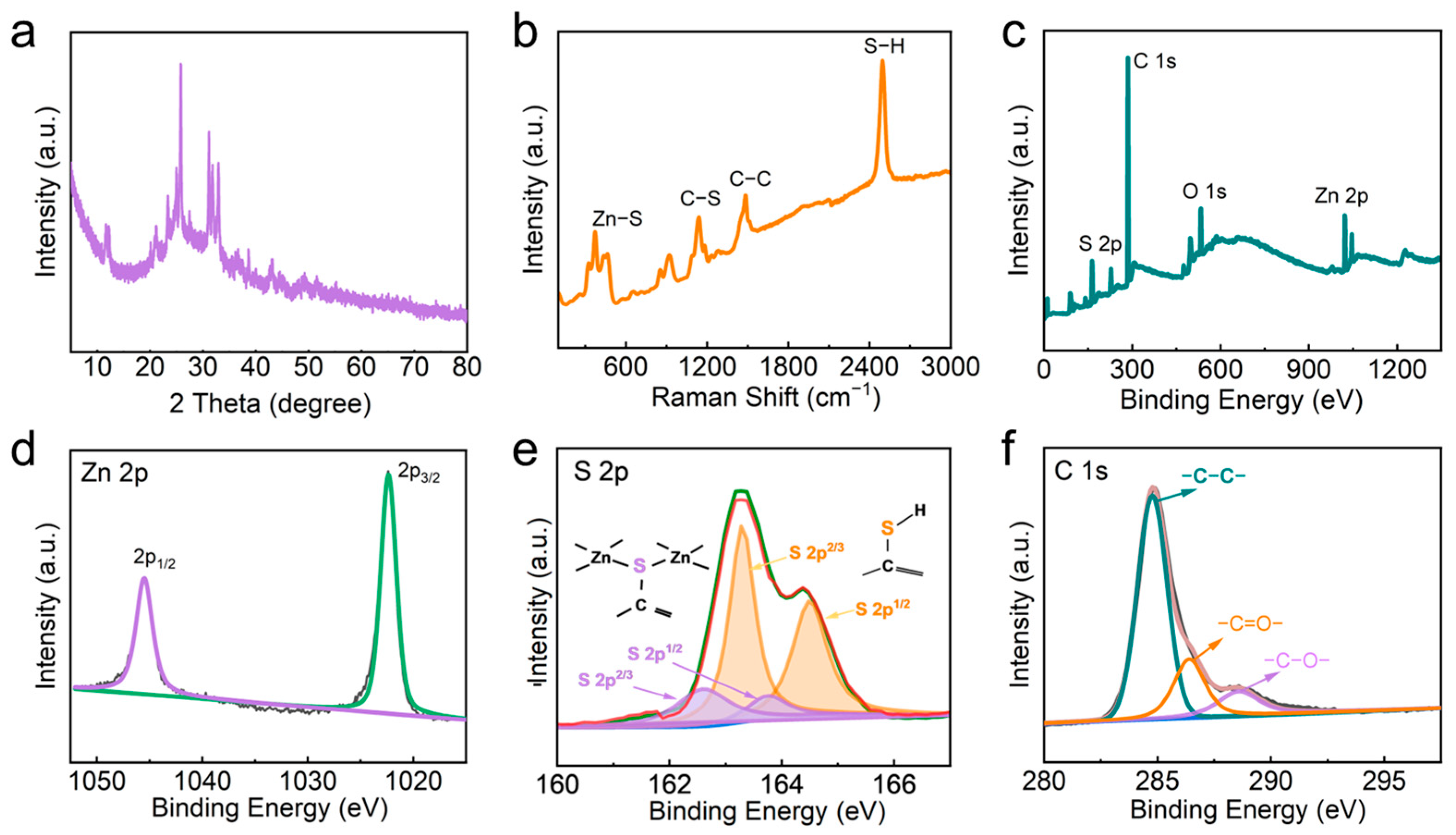
3.1. Structure Characterization of Zn-BHT
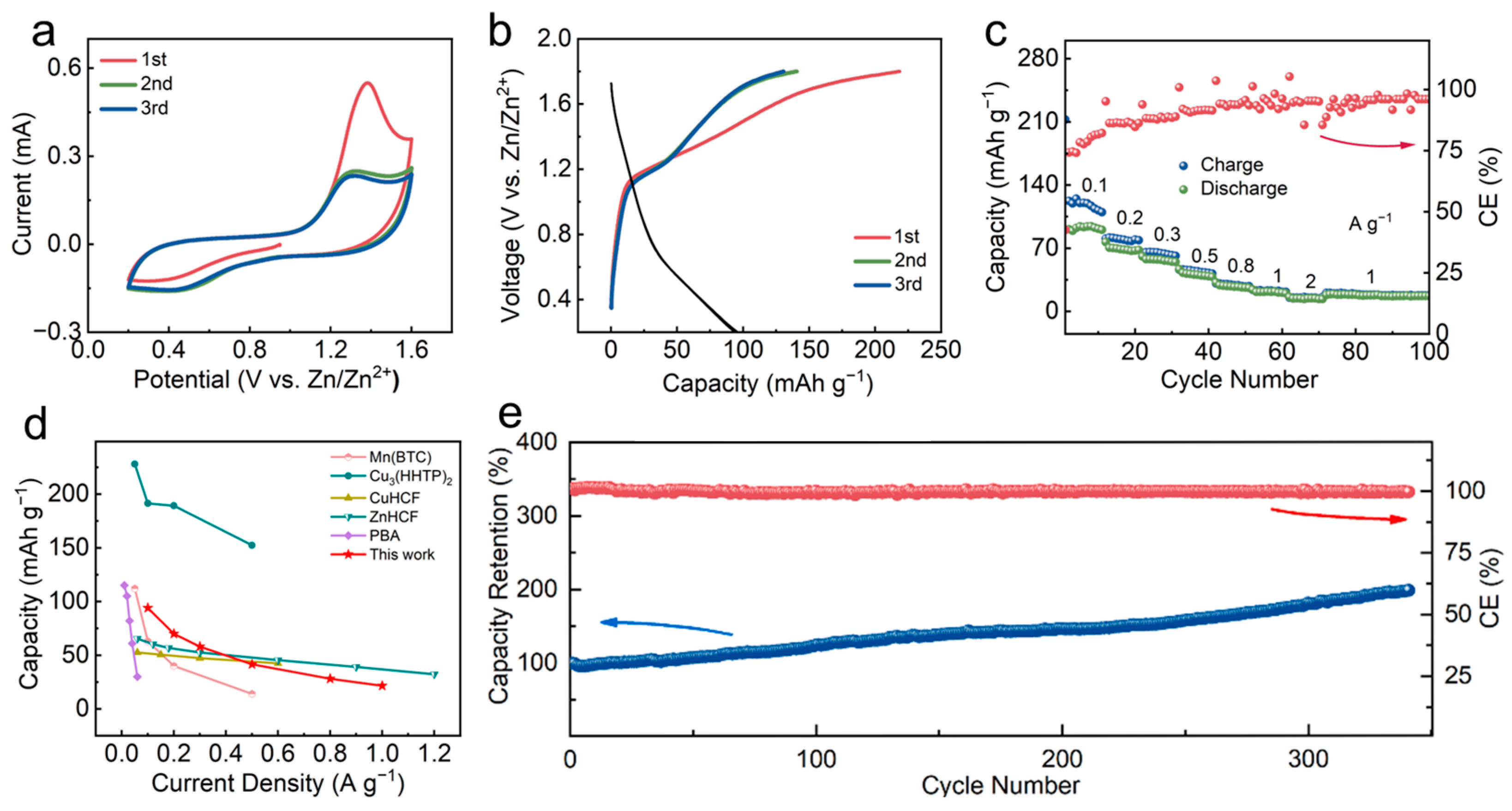
3.2. Electrochemical Properties of Zn-BHT
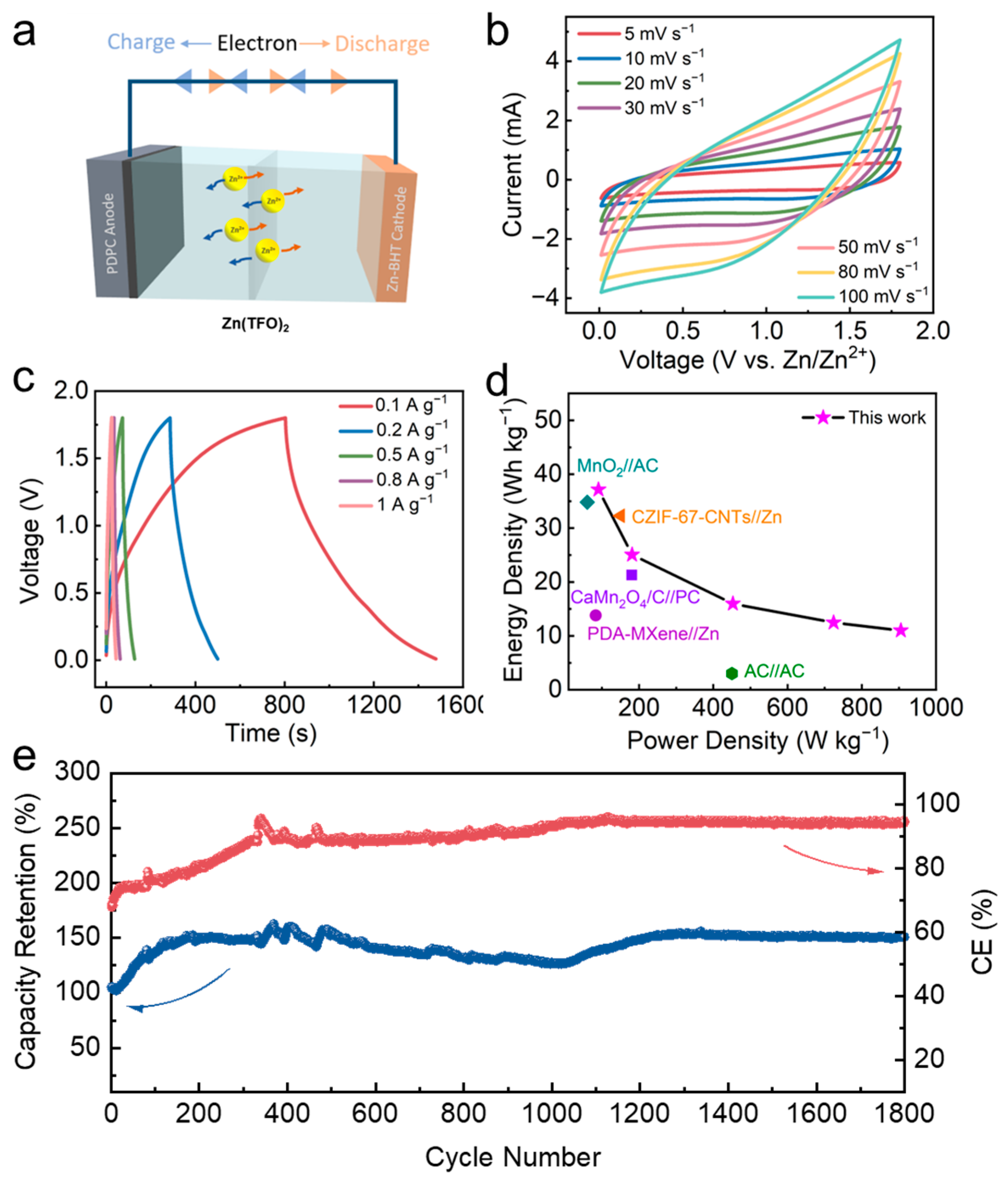
3.3. Electrochemical Properties of Fabricated ZIC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Blanc, L.E.; Kundu, D.; Nazar, L.F. Scientific challenges for the implementation of Zn-ion batteries. Joule 2020, 4, 771–799. [Google Scholar] [CrossRef]
- Sun, J.; Liu, C.; Song, X.; Zhang, J.; Liu, Y.; Liang, L.; Jiang, R.; Yuan, C. Electrochemical energy storage devices under particular service environments: Achievements, challenges, and perspective. Appl. Phys. Rev. 2022, 9, 031301. [Google Scholar] [CrossRef]
- Liu, Z.; Huang, Y.; Huang, Y.; Yang, Q.; Li, X.; Huang, Z.; Zhi, C. Voltage issue of aqueous rechargeable metal-ion batteries. Chem. Soc. Rev. 2020, 49, 180–232. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 1–16. [Google Scholar] [CrossRef]
- Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 2020, 11, 1550. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Li, W.; Dahn, J.R.; Wainwright, D.S. Rechargeable lithium batteries with aqueous electrolytes. Science 1994, 264, 1115–1118. [Google Scholar] [CrossRef]
- Luo, J.; Cui, W.; He, P.; Xia, Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef]
- Deng, Q.; Liu, F.; Wu, X.; Li, C.; Zhou, W.; Long, B. An aqueous BiI3-Zn battery with dual mechanisms of Zn2+ (de)intercalation and I−/I2 redox. J. Energy Chem. 2024, 89, 670–678. [Google Scholar] [CrossRef]
- Zhou, H.; Li, X.; Li, Y.; Zheng, M.; Pang, H. Applications of MxSey (M = Fe, Co, Ni) and their composites in electrochemical energy storage and conversion. Nano-micro Lett. 2019, 11, 40. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, F.; Huang, S.; Wang, S.; Niu, Z.; Chen, J. A chemically self-charging aqueous zinc-ion battery. Nat. Commun. 2020, 11, 2199. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. Infomat. 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Chen, L.; Liu, F.; Dong, Y.; Pei, Y.; Wang, X.; Wu, X.; Zheng, X.; He, W.; Long, B. Anion-induced facet-selective etching of Zn metal anode for long cycle aqueous Zn-ion batteries. J. Energy Storage 2024, 87, 111529. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, J.; Dong, L.; Liu, W.; Mou, J.; Zhao, L.; Wang, J.; Ren, D.; Wu, J.; Xu, C.; et al. Multivalent ion storage towards high-performance aqueous zinc-ion hybrid supercapacitors. Energy Storage Mater. 2019, 20, 335–342. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, L.; Wu, Z.; Guo, X.; Fang, G.; Liang, S. Investigation of V2O5 as a low-cost rechargeable aqueous zinc ion battery cathode. Chem. Commun. 2018, 54, 4457–4460. [Google Scholar] [CrossRef]
- Jia, D.; Zheng, K.; Song, M.; Tan, H.; Zhang, A.; Wang, L.; Yue, L.; Li, D.; Li, C.; Liu, J. VO2·0.2H2O nanocuboids anchored onto graphene sheets as the cathode material for ultrahigh capacity aqueous zinc ion batteries. Nano Res. 2020, 13, 215–224. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, Z.; Ma, T.; Zhang, J.; Zhang, Y.; Ma, R.; Zhang, D.; Yan, T. In situ electrochemical Mn vacancies in CoMnHCF for a high level of zinc storage. Chem. Commun. 2024, 60, 4080–4083. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Wu, T.; Bergh, W.V.D.; Stefik, M.; Huang, K. Reversible molecular and ionic storage mechanisms in high-performance Zn0.1V2O5·nH2O xerogel cathode for aqueous Zn-ion batteries. ACS Nano 2021, 15, 10678–10688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Duan, T.; Xiao, M.; Pei, Y.; Wang, X.; Zhi, C.; Wu, X.; Long, B.; Wu, Y. BiOI nanopaper as a high-capacity, long-life and insertion-type anode for a flexible quasi-solid-state Zn-ion battery. ACS Appl. Mater. Interfaces 2022, 14, 25516–25523. [Google Scholar] [CrossRef]
- Wang, W.; Tang, Y.; Liu, J.; Li, H.; Wang, R.; Zhang, L.; Liang, F.; Bai, W.; Zhang, L.; Zhang, C. Boosting the zinc storage of a small-molecule organic cathode by a desalinization strategy. Chem. Sci. 2023, 14, 9033–9040. [Google Scholar] [CrossRef]
- Kim, S.J.; Zhu, Y.; Wu, D.R.; Sadique, N.; Quilty, C.D.; Wu, L.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Unraveling the dissolution-mediated reaction mechanism of α-MnO2 cathodes for aqueous Zn-ion batteries. Small 2020, 16, 2005406. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, L.; Cao, B.; Du, H.; Lu, H.; Ma, Y.; Wang, H.; Guo, H.; Huang, Q.; Xu, B.; et al. Van der Waals interaction-driven self-assembly of V2O5 nanoplates and MXene for high-performing zinc-ion batteries by suppressing vanadium dissolution. ACS Nano 2022, 16, 14539–14548. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sun, J.; Zhang, W.; Hou, L.; Liang, L.; Liu, Y.; Yuan, C. Bottom-up fabrication of 1D Cu-based conductive metal–organic framework nanowires as a high-rate anode towards efficient lithium storage. ChemSusChem 2019, 12, 5051–5058. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, L.; Zhou, X.; Liu, Z. Towards high-voltage aqueous metal-ion batteries beyond 1.5 V: The zinc/zinc hexacyanoferrate system. Adv. Energy. Mater. 2015, 5, 1400930. [Google Scholar] [CrossRef]
- Sheberla, D.; Bachman, J.C.; Elias, J.S.; Sun, C.; Shao-Horn, Y.; Dinca, M. Conductive MOF electrodes for stable supercapacitors with high areal capacitance. Nat. Mater. 2017, 16, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sun, J.; Wei, J.; Liu, Y.; Hou, L.; Yuan, C. Conductive metal-organic frameworks: Recent advances in electrochemical energy-related applications and perspectives. Carbon Energy 2020, 2, 203–222. [Google Scholar] [CrossRef]
- Li, W.; Ding, K.; Tian, H.; Yao, M.; Nath, B.; Deng, W.; Wang, Y.; Xu, G. Conductive metal-organic framework nanowire array electrodes for high-performance solid-state supercapacitors. Adv. Funct. Mater. 2017, 27, 1702067. [Google Scholar] [CrossRef]
- Feng, D.; Lei, T.; Lukatskaya, M.; Park, J.; Huang, Z.; Lee, M.; Shaw, L.; Chen, S.; Yakovenko, A.; Kulkarni, A.; et al. Robust and conductive two-dimensional metal−organic frameworks with exceptionally high volumetric and areal capacitance. Nat. Energy 2018, 3, 30–36. [Google Scholar] [CrossRef]
- Nam, K.W.; Park, S.S.; Reis, R.D.; Dravid, V.P.; Kim, H.; Mirkin, C.A.; Stoddart, J.F. Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries. Nat. Commun. 2019, 10, 4948. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Jin, D.; Zhang, Y.; Cai, Y.; Ma, J.; Zhang, L. Engineering layer structure of MoS2-graphene composites with robust and fast lithium storage for high-performance Li-ion capacitors. Energy Storage Mater. 2017, 9, 195–205. [Google Scholar] [CrossRef]
- Banda, H.; Dou, J.; Chen, T.; Libretto, N.J.; Chaudhary, M.; Bernard, G.M.; Miller, J.T.; Michaelis, V.K.; Dinca, M. High-capacitance pseudocapacitors from Li+ ion intercalation in nonporous, electrically conductive 2D coordination polymers. J. Am. Chem. Soc. 2021, 143, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Sakamoto, R.; Hoshiko, K.; Takada, K.; Miyachi, M.; Ryu, J.H.; Sasaki, S.; Kim, J.; Nakazato, K.; Takata, M.; et al. π-Conjugated nickel bis(dithiolene) complex nanosheet. J. Am. Chem. Soc. 2013, 135, 2462–2465. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Lu, C.; Su, W. Highly conductive 2D metal-organic framework thin film fabricated by liquid-liquid interfacial reaction using one-pot-synthesized benzenehexathiol. Langmuir 2018, 34, 15754–15762. [Google Scholar] [CrossRef]
- Wang, Y.; Chiang, C.; Chang, C.; Maeda, H.; Fukui, N.; Wang, I.; Wen, C.; Lu, K.; Huang, S.; Jian, W.; et al. Two-dimensional bis(dithiolene)iron(II) self-powered UV photodetectors with ultrahigh air stability. Adv. Sci. 2021, 8, 2100564. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium-sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Guo, L.; Sun, X.; Zhang, J.; Liu, Y.; Hou, L.; Yuan, C. Conductive Co-based metal–organic framework nanowires: A competitive high-rate anode towards advanced Li-ion capacitors. J. Mater. Chem. A 2019, 7, 24788–24791. [Google Scholar] [CrossRef]
- Trócoli, R.; Mantia, F.L. An aqueous zinc-ion battery based on copper hexacyanoferrate. ChemSusChem 2015, 8, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Pulletikurthi, G.; Endres, F. A Prussian blue/zinc secondary battery with a bio-ionic liquid–water mixture as electrolyte. ACS Appl. Mater. Interfaces 2016, 8, 12158–12164. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.; Jiang, B.; Wang, X.; Liu, W.; Dong, L.; Kang, F.; Xu, C. High-performance aqueous zinc-ion batteries realized by MOF materials. Nano-Micro Lett. 2020, 12, 152. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, Z.Y.; Wang, Y.; Tian, W.; Yi, Z.; Cai, C.; Jiang, Y.; Hu, L. Ultrafast zinc-ion diffusion ability observed in 6.0-nanometer spinel nanodots. ACS Nano 2019, 13, 10376–10385. [Google Scholar] [CrossRef]
- Wang, X.; Xi, B.; Ma, X.; Feng, Z.; Jia, Y.; Feng, J.; Qian, Y.; Xiong, S. Boosting zinc-ion storage capability by effectively suppressing vanadium dissolution based on robust layered barium vanadate. Nano Lett. 2020, 20, 2899–2906. [Google Scholar] [CrossRef]
- He, B.; Zhang, Q.; Man, P.; Zhou, Z.; Li, C.; Li, Q.; Xie, L.; Wang, X.; Pang, H.; Yao, Y. Self-sacrificed synthesis of conductive vanadium-based metal–organic framework nanowire-bundle arrays as binder-free cathodes for high-rate and high-energy-density wearable Zn-ion batteries. Nano Energy 2019, 64, 103935. [Google Scholar] [CrossRef]
- Hu, P.; Yan, M.; Zhu, T.; Wang, X.; Wei, X.; Li, J.; Zhou, L.; Li, Z.; Chen, L.; Mai, L. Zn/V2O5 aqueous hybrid-ion battery with high voltage platform and long cycle life. ACS Appl. Mater. Interfaces 2017, 9, 42717–42722. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Yu, J.; Xiao, T.; Peng, L.; Fan, L.; Wang, C.; Shen, Q.; Wu, W. Tailoring the grain size of Bi-layer graphene by pulsed laser deposition. Nanomater. 2018, 8, 885. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Liu, Y.; Tan, Z.; Zhang, J.; Hou, L.; Yuan, C. High-yield and in situ fabrication of high-content nitrogen-doped graphene nanoribbons@Co/CoOOH as an integrated sulfur host towards Li–S batteries. J. Mater. Chem. A 2020, 8, 3048–3059. [Google Scholar] [CrossRef]
- Ding, L.; Gao, Q.; Yuan, C. Hierarchical CaMn2O4/C network framework toward aqueous Zn ion hybrid capacitors as competitive cathodes. Batteries 2023, 9, 586. [Google Scholar] [CrossRef]
- Dai, J.; Zhu, C.; Fan, Y.; Wen, L.; Zhao, Y.; Huang, Z.; Zeng, W.; Wang, S. Zinc-ion supercapacitor with stress sensing function based on CZIF-67-CNTs cathode. J. Energy Storage 2024, 83, 110670. [Google Scholar] [CrossRef]
- Peng, M.; Wang, L.; Li, L.; Tang, X.; Huang, B.; Hu, T.; Yuan, K.; Chen, Y. Manipulating the interlayer spacing of 3D MXenes with improved stability and zinc-ion storage capability. Adv. Funct. Mater. 2022, 32, 2109524. [Google Scholar] [CrossRef]
- Gao, H.; Wang, J.; Zhang, R.; Wu, L.; Tao, C.; Deng, G. An aqueous hybrid lithium ion capacitor based on activated graphene and modified LiFePO4 with high specific capacitance. Mater. Res. Express 2019, 6, 045509. [Google Scholar] [CrossRef]
- Lu, K.; Li, D.; Gao, X.; Dai, H.; Wang, N.; Ma, H. An advanced aqueous sodium-ion supercapacitor with a manganous hexacyanoferrate cathode and a Fe3O4/rGO anode. J. Mater. Chem. A 2015, 3, 16013–16019. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Zhang, Q.; Liu, C.; Zhang, A.; Hou, L.; Yuan, C. Conductive Zinc-Based Metal–Organic Framework Nanorods as Cathodes for High-Performance Zn-Ion Capacitors. Batteries 2024, 10, 222. https://doi.org/10.3390/batteries10070222
Sun J, Zhang Q, Liu C, Zhang A, Hou L, Yuan C. Conductive Zinc-Based Metal–Organic Framework Nanorods as Cathodes for High-Performance Zn-Ion Capacitors. Batteries. 2024; 10(7):222. https://doi.org/10.3390/batteries10070222
Chicago/Turabian StyleSun, Jinfeng, Qian Zhang, Chanjuan Liu, Anning Zhang, Linrui Hou, and Changzhou Yuan. 2024. "Conductive Zinc-Based Metal–Organic Framework Nanorods as Cathodes for High-Performance Zn-Ion Capacitors" Batteries 10, no. 7: 222. https://doi.org/10.3390/batteries10070222







