Designing a Stable Alloy Interlayer on Li Metal Anodes for Fast Charging of All-Solid-State Li Metal Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Sputter Deposition of Tin on Li Metal Surface
2.2. Plasma Cleaning of Sn-Coated Li Metal Anodes
2.3. Deposition of a Polymer/Ceramic Layer on Sn-Coated Li Metal Anodes
2.4. Preparation of LiFePO4 Electrodes
2.5. Preparation of the Solid Polymer Electrolyte
2.6. Assembling of Pouch Cells and Electrochemical Tests
2.7. Characterizations
3. Results and Discussion
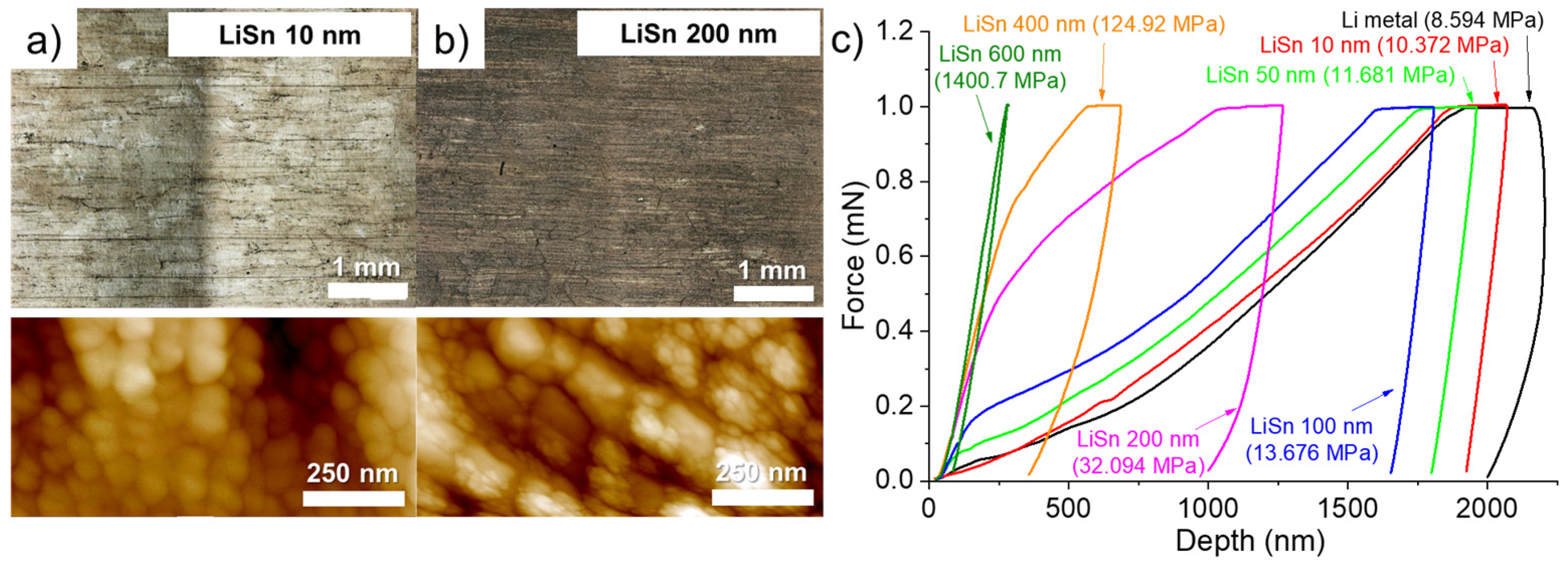
3.1. Formation of an Alloy Layer on Li Surface during Deposition of Sn
3.2. Evolution of the Deposited Layer during Battery Assembly and Cycling
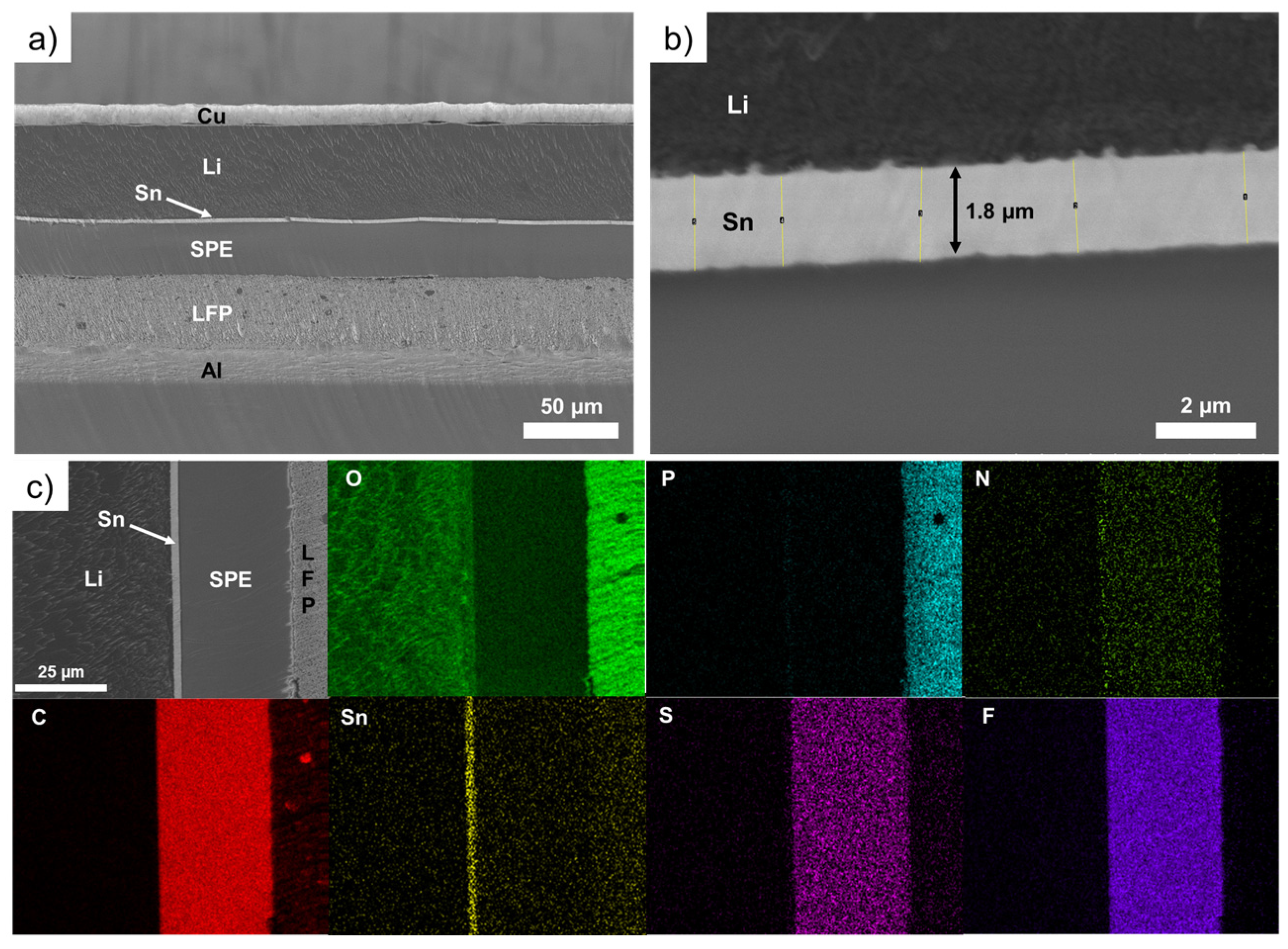
3.2.1. LFP/SPE/Li Battery Stack after Assembling
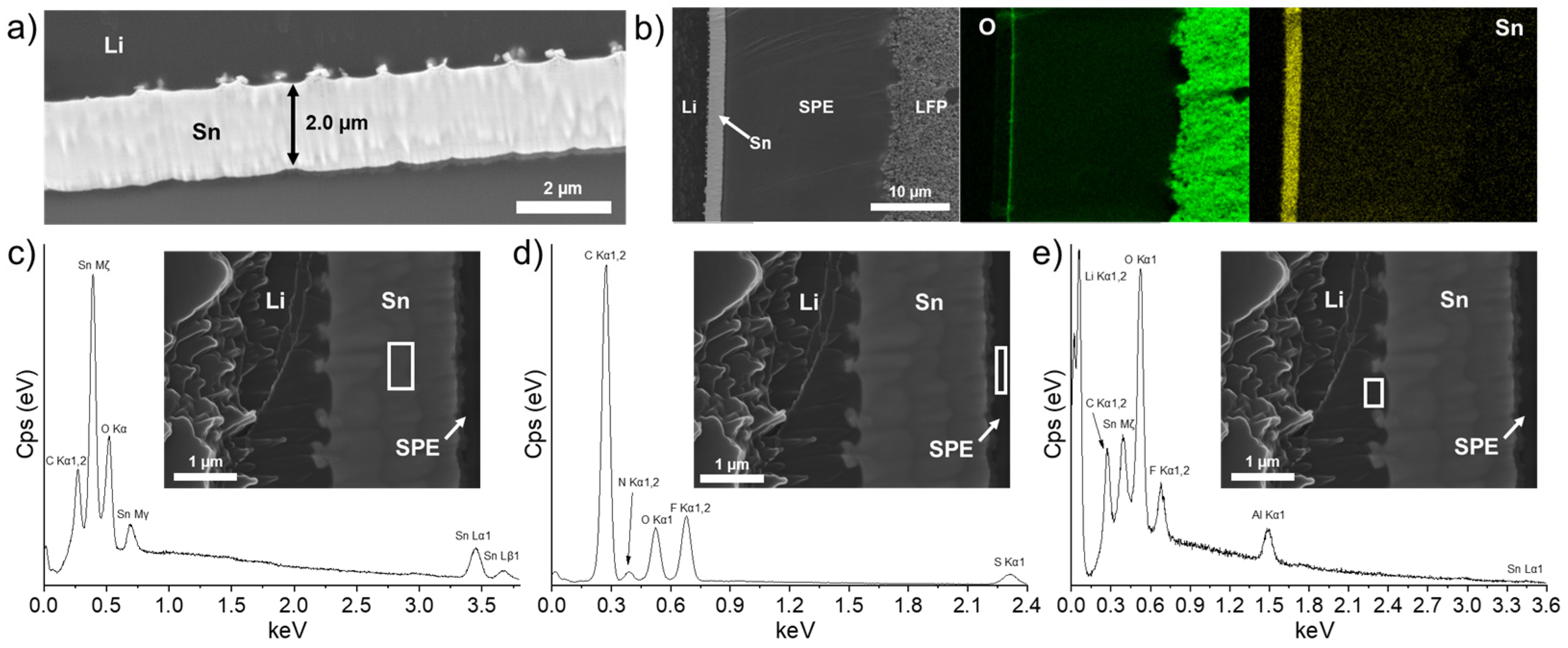
3.2.2. LFP/SPE/Li Battery Stack after One C/40 Charge
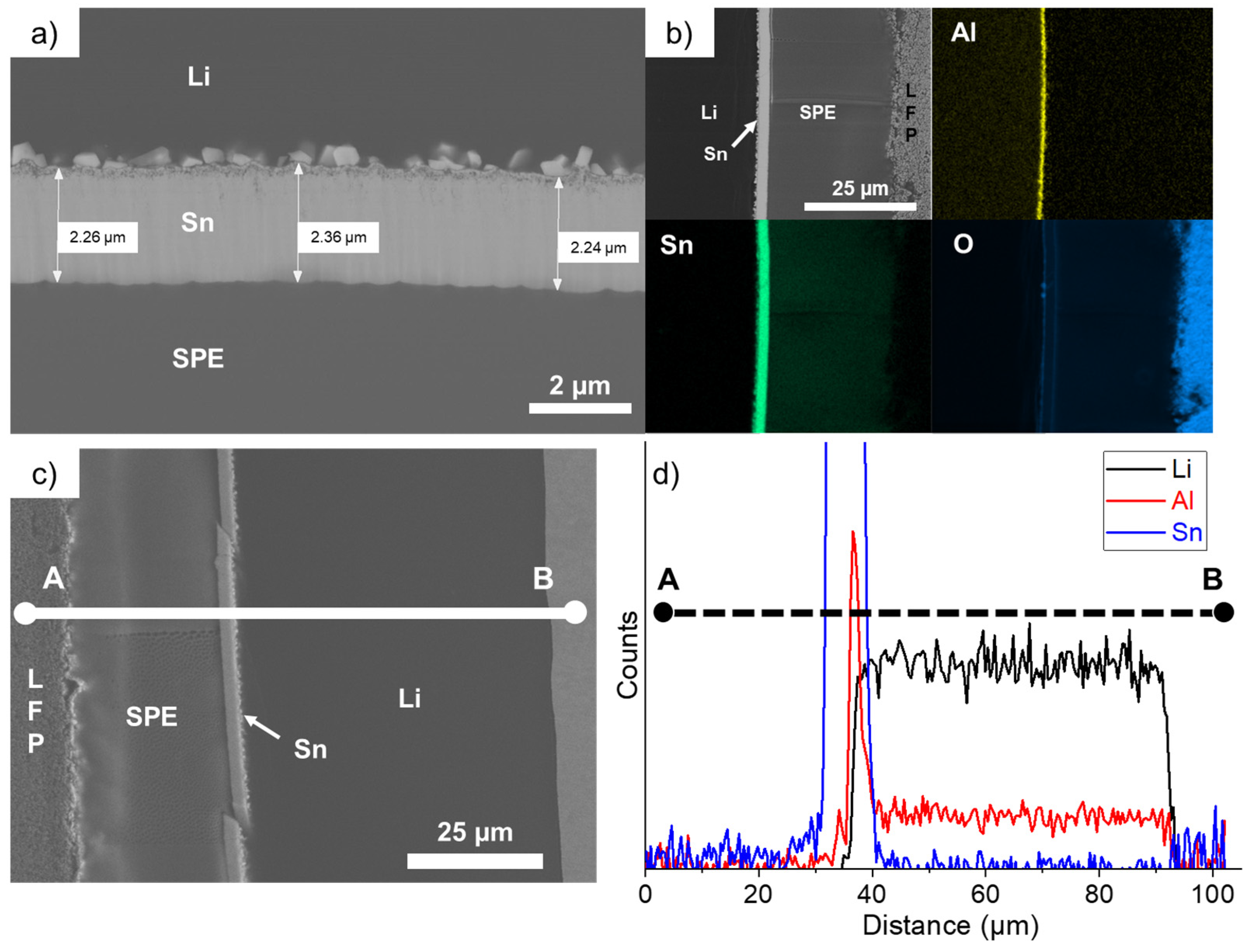
3.2.3. LFP/SPE/Li Battery Stack after Long-Cycling at 1C in Charge
3.3. Overcoating of a Polymer/Ceramic Layer on the Surface of the Li–Sn Foil
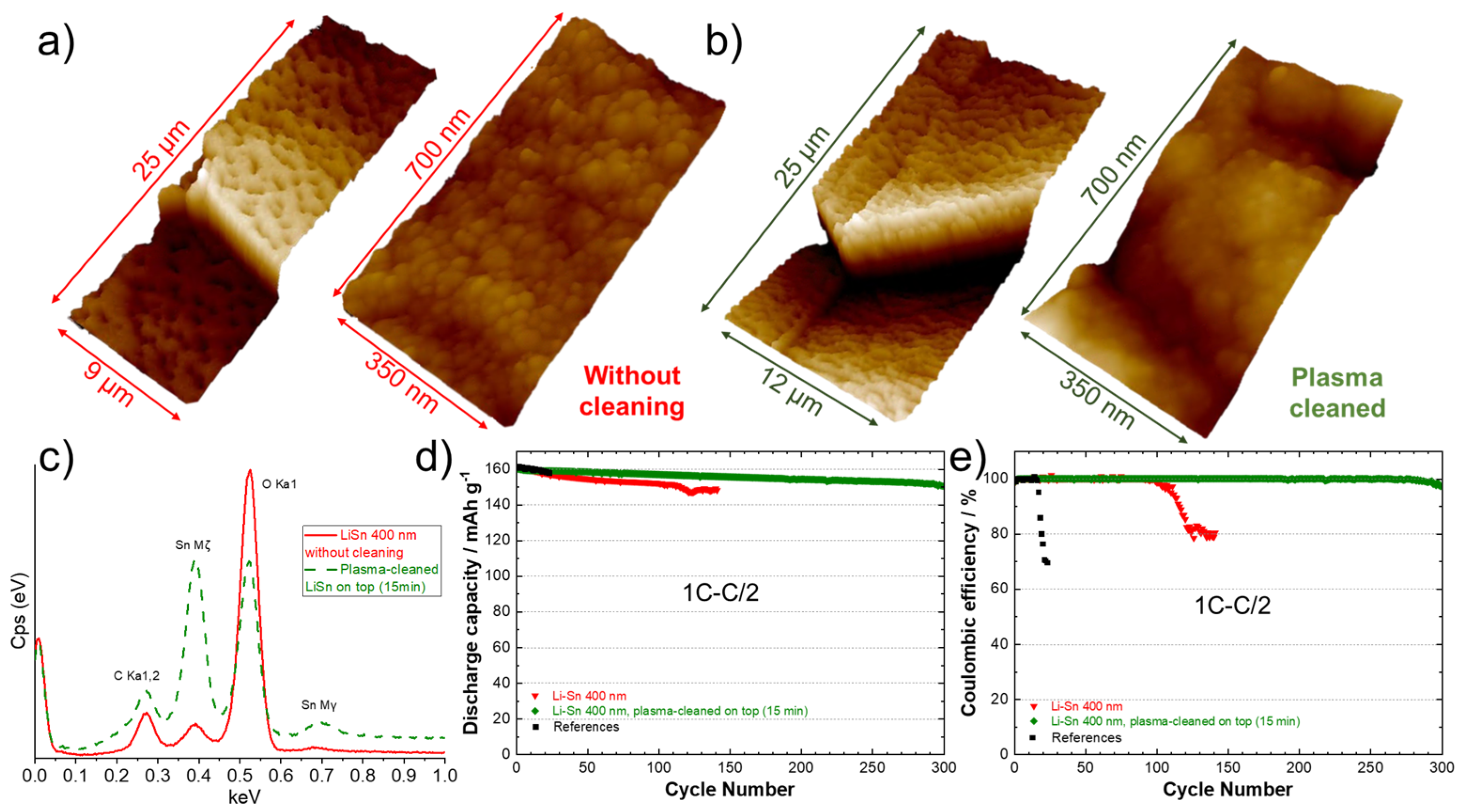
3.4. Effect of Plasma Cleaning of Sn Surface
4. Conclusions
- -
- The process must be easily transposable at the industrial level, fast, clean, reproducible, cheap (Sn metal), and performed in a well-controlled environment.
- -
- Ultra-thin Li metal anode can also be modified with this technique.
- -
- The layer must be dense and thin to not impact the energy density of the electrochemical system.
- -
- The in-situ generation of a 3D Li-rich alloy favors the fast Li+ ion transfer at the SPE/Sn interface [40].
- -
- The chemical and electrochemical stabilities of the artificial layer permit to cycle the battery at high C-rates for several hundreds of cycles.
- -
- The higher electrochemical potential of the alloy layer in comparison to Li metal reduces the reactivity with the electrolyte [41].
- -
- Post-modifications can be applied on the alloy layer to respond to various applications (e.g., reduce the SPE thickness by deposing a thin ceramic-rich polymer layer, overcoating of a SPE, deposition of a non-electronic-conductive layer, etc.).
- -
- The electrodeposition of Li+ ions must occur uniformly underneath the artificial layer and on the Li metal side.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Huang, J.; Xie, Y.; Wei, D.; Chen, J.; Shi, Z. Solid Electrolyte Interphase on Lithium Metal Anodes. ChemSusChem 2024, 17, e202301777. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lee, H.; Song, J.; Ryou, M.-H.; Lee, Y.M.; Kim, H.-T.; Park, J.-K. Composite protective layer for Li metal anode in high-performance lithium–oxygen batteries. Electrochem. Commun. 2014, 40, 45–48. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, S.; Zhou, J.; Jin, Y.; Liu, Y.; Qin, Y.; Shen, C.; Han, W.; Wang, D. Volumetric variation confinement: Surface protective structure for high cyclic stability of lithium metal electrodes. J. Mater. Chem. A 2016, 4, 2427–2432. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, J.; Jin, J.; Yang, S.; Li, G. Nanoscale surface modification of polymer nanofibers enables uniform lithium nucleation and deposition for stable lithium metal anodes. Chem. Eng. J. 2022, 430, 132865. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Liang, Z.; Zhao, J.; Yan, K.; Cui, Y. Lithium-coated polymeric matrix as a minimum volume-change and dendrite-free lithium metal anode. Nat. Commun. 2016, 7, 10992. [Google Scholar] [CrossRef]
- Bai, M.; Xie, K.; Hong, B.; Yuan, K.; Li, Z.; Huang, Z.; Shen, C.; Lai, Y. An artificial Li3PO4 solid electrolyte interphase layer to achieve petal-shaped deposition of lithium. Solid State Ion. 2019, 333, 101–104. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Zhao, Y.; Goncharova, L.V.; Wang, G.; Adair, K.R.; Wang, C.; Li, R.; Zhu, Y.; Qian, Y.; et al. In Situ Li3PS4 Solid-State Electrolyte Protection Layers for Superior Long-Life and High-Rate Lithium-Metal Anodes. Adv. Mater. 2018, 30, 1804684. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626–632. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Lee, Y.M.; Lee, Y.; Winter, M.; Bieker, P. Mechanical Surface Modification of Lithium Metal: Towards Improved Li Metal Anode Performance by Directed Li Plating. Adv. Funct. Mater. 2015, 25, 834–841. [Google Scholar] [CrossRef]
- Kazyak, E.; Wood, K.N.; Dasgupta, N.P. Improved Cycle Life and Stability of Lithium Metal Anodes through Ultrathin Atomic Layer Deposition Surface Treatments. Chem. Mater. 2015, 27, 6457–6462. [Google Scholar] [CrossRef]
- Kozen, A.C.; Lin, C.-F.; Pearse, A.J.; Schroeder, M.A.; Han, X.; Hu, L.; Lee, S.-B.; Rubloff, G.W.; Noked, M. Next-Generation Lithium Metal Anode Engineering via Atomic Layer Deposition. ACS Nano 2015, 9, 5884–5892. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, K.-S.; Chen, X.; Ramirez, G.; Huang, Z.; Geise, N.R.; Steinrück, H.-G.; Fisher, B.L.; Shahbazian-Yassar, R.; Toney, M.F.; et al. Novel ALD Chemistry Enabled Low-Temperature Synthesis of Lithium Fluoride Coatings for Durable Lithium Anodes. ACS Appl. Mater. Interfaces 2018, 10, 26972–26981. [Google Scholar] [CrossRef] [PubMed]
- Alaboina, P.K.; Rodrigues, S.; Rottmayer, M.; Cho, S.-J. In Situ Dendrite Suppression Study of Nanolayer Encapsulated Li Metal Enabled by Zirconia Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2018, 10, 32801–32808. [Google Scholar] [CrossRef] [PubMed]
- Bela, M.M.; Schmidt, C.; Neuhaus, K.; Hering, T.; Stan, M.C.; Winter, M.; Börner, M. Tunable LiZn-Intermetallic Coating Thickness on Lithium Metal and Its Effect on Morphology and Performance in Lithium Metal Batteries. Adv. Mater. Interfaces 2024, 11, 2300836. [Google Scholar] [CrossRef]
- Stan, M.C.; Becking, J.; Kolesnikov, A.; Wankmiller, B.; Frerichs, J.E.; Hansen, M.R.; Bieker, P.; Kolek, M.; Winter, M. Sputter coating of lithium metal electrodes with lithiophilic metals for homogeneous and reversible lithium electrodeposition and electrodissolution. Mater. Today 2020, 39, 137–145. [Google Scholar] [CrossRef]
- Delaporte, N.; Perea, A.; Collin-Martin, S.; Léonard, M.; Matton, J.; Gariepy, V.; Demers, H.; Clément, D.; Rivard, E.; Vijh, A. High Performance Lithium Metal Anode with a Nanolayer of LiZn Alloy for All-Solid-State Batteries, Batter. Supercaps 2022, 5, e202200245. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, S.-M.; Ko, E.-H.; Kim, T.-H.; Nah, Y.-C.; Lee, S.-J.; Lee, J.H.; Kim, H.-K. Roll-to-Roll sputtered ITO/Cu/ITO multilayer electrode for flexible, transparent thin film heaters and electrochromic applications. Sci. Rep. 2016, 6, 33868. [Google Scholar] [CrossRef]
- Tao, X.; Stuart, B.W.; Assender, H.E. Roll-to-roll manufacture of flexible thin-film thermoelectric generators using flexography with vacuum vapour deposition. Surf. Coat. Technol. 2022, 447, 128826. [Google Scholar] [CrossRef]
- Delaporte, N.; Lajoie, G.; Darwiche, A.; Vigeant, M.-J.; Collin-Martin, S.; Clément, D. Stabilization of lithium anode with ceramic-rich interlayer for all solid-state batteries. RSC Adv. 2022, 12, 15493–15507. [Google Scholar] [CrossRef]
- Fujiwara, S.; Tamura, Y.; Maki, H.; Azuma, N.; Takeuchi, Y. Development of New High-Purity Alumina. Available online: https://www.sumitomo-chem.co.jp/english/rd/report/files/docs/20070102_fth.pdf (accessed on 14 July 2024).
- Hovington, P.; Timoshevskii, V.; Burgess, S.; Demers, H.; Statham, P.; Gauvin, R.; Zaghib, K. Can We Detect Li K X-ray in Lithium Compounds Using Energy Dispersive Spectroscopy? Scanning 2016, 38, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Ohshita, R.; Fujimoto, M.; Kamino, M.; Fujitani, S. Advanced Structures in Electrodeposited Tin Base Negative Electrodes for Lithium Secondary Batteries. J. Electrochem. Soc. 2003, 150, A679. [Google Scholar] [CrossRef]
- Wang, J.; Raistrick, I.D.; Huggins, R.A. Behavior of Some Binary Lithium Alloys as Negative Electrodes in Organic Solvent-Based Electrolytes. J. Electrochem. Soc. 1986, 133, 457–460. [Google Scholar] [CrossRef]
- Serikkazyyeva, A.; Mashekova, A.; Uzakbaiuly, B.; Bakenov, Z.; Mukanova, A. Novel Li/LixSny thin film designed as an anode for lithium-ion microbatteries. J. Alloys Compd. 2023, 965, 171381. [Google Scholar] [CrossRef]
- Li, M.; Zhou, D.; Wang, C.; Weng, W.; Jiang, M.; Liu, G.; Yao, X.; He, H. In Situ Formed Li–Ag Alloy Interface Enables Li10GeP2S12-Based All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2021, 13, 50076–50082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J. Lithium insertion/extraction mechanism in alloy anodes for lithium-ion batteries. J. Power Sources 2011, 196, 877–885. [Google Scholar] [CrossRef]
- Joachin, H.; Kaun, T.D.; Zaghib, K.; Prakash, J. Electrochemical and Thermal Studies of Carbon-Coated LiFePO4 Cathode. J. Electrochem. Soc. 2009, 156, A401–A406. [Google Scholar] [CrossRef]
- Sakuma, M.; Suzuki, K.; Hirayama, M.; Kanno, R. Reactions at the electrode/electrolyte interface of all-solid-state lithium batteries incorporating Li–M (M = Sn, Si) alloy electrodes and sulfide-based solid electrolytes. Solid State Ion. 2016, 285, 101–105. [Google Scholar] [CrossRef]
- Li, X.; Liang, J.; Yang, X.; Adair, K.R.; Wang, C.; Zhao, F.; Sun, X. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 2020, 13, 1429–1461. [Google Scholar] [CrossRef]
- Riegger, L.M.; Schlem, R.; Sann, J.; Zeier, W.G.; Janek, J. Lithium-Metal Anode Instability of the Superionic Halide Solid Electrolytes and the Implications for Solid-State Batteries. Angew. Chem. Int. Ed. 2021, 60, 6718–6723. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, Q.; Yu, C.; Zhang, S.; Adair, K.; Wang, S.; Liu, Y.; Zhao, Y.; Liang, J.; Wang, C.; et al. Ultrastable Anode Interface Achieved by Fluorinating Electrolytes for All-Solid-State Li Metal Batteries. ACS Energy Lett. 2020, 5, 1035–1043. [Google Scholar] [CrossRef]
- Zhang, H.; Song, X.; Pu, Y.; Ding, M.; Zhang, W.; Cao, P. In-situ polymerisation of fluorene to achieve theoretical capacity in LiFePO4 cells. Electrochim. Acta 2024, 483, 143999. [Google Scholar] [CrossRef]
- Homann, G.; Stolz, L.; Neuhaus, K.; Winter, M.; Kasnatscheew, J. Effective Optimization of High Voltage Solid-State Lithium Batteries by Using Poly(ethylene oxide)-Based Polymer Electrolyte with Semi-Interpenetrating Network. Adv. Funct. Mater. 2020, 30, 2006289. [Google Scholar] [CrossRef]
- Homann, G.; Stolz, L.; Nair, J.; Laskovic, I.C.; Winter, M.; Kasnatscheew, J. Poly(Ethylene Oxide)-based Electrolyte for Solid-State-Lithium-Batteries with High Voltage Positive Electrodes: Evaluating the Role of Electrolyte Oxidation in Rapid Cell Failure. Sci. Rep. 2020, 10, 4390. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lin, D.; Yuen, P.Y.; Liu, K.; Xie, J.; Dauskardt, R.H.; Cui, Y. An Artificial Solid Electrolyte Interphase with High Li-Ion Conductivity, Mechanical Strength, and Flexibility for Stable Lithium Metal Anodes. Adv. Mater. 2017, 29, 1605531. [Google Scholar] [CrossRef]
- Jing, H.-K.; Kong, L.-L.; Liu, S.; Li, G.-R.; Gao, X.-P. Protected lithium anode with porous Al2O3 layer for lithium–sulfur battery. J. Mater. Chem. A 2015, 3, 12213–12219. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, F.; Gao, J.; Zhou, W.; Huo, H.; Zhou, J.; Li, L. Dendrite-Free Lithium Plating Induced by In Situ Transferring Protection Layer from Separator. Adv. Funct. Mater. 2020, 30, 1907020. [Google Scholar] [CrossRef]
- Yang, C.; Liu, B.; Jiang, F.; Zhang, Y.; Xie, H.; Hitz, E.; Hu, L. Garnet/polymer hybrid ion-conducting protective layer for stable lithium metal anode. Nano Res. 2017, 10, 4256–4265. [Google Scholar] [CrossRef]
- Liang, X.; Pang, Q.; Kochetkov, I.R.; Sempere, M.S.; Huang, H.; Sun, X.; Nazar, L.F. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2017, 2, 17119. [Google Scholar] [CrossRef]
- Zhu, Y.; He, X.; Mo, Y. Origin of Outstanding Stability in the Lithium Solid Electrolyte Materials: Insights from Thermodynamic Analyses Based on First-Principles Calculations. ACS Appl. Mater. Interfaces 2015, 7, 23685–23693. [Google Scholar] [CrossRef]



| Thickness of Sn Deposited (nm) | Thickness of LixSny Layer Measured (nm) | Condition of Assembling/Cycling | Expansion (%) |
|---|---|---|---|
| 400 | 1.4–1.5 | No assembling | Up to 380 |
| 600 | 1.8 | No assembling | 300 |
| 600 | 1.8 | After cell assembling | 300 |
| 600 | 2.0 | After one charge at C/40 | 330 |
| 600 | 2.3 | After 225 cycles at 1C–C/2 | 380 |
| - | - | - | 380 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delaporte, N.; Perea, A.; Collin-Martin, S.; Léonard, M.; Matton, J.; Demers, H.; Clément, D.; Gariépy, V.; Zhu, W. Designing a Stable Alloy Interlayer on Li Metal Anodes for Fast Charging of All-Solid-State Li Metal Batteries. Batteries 2024, 10, 253. https://doi.org/10.3390/batteries10070253
Delaporte N, Perea A, Collin-Martin S, Léonard M, Matton J, Demers H, Clément D, Gariépy V, Zhu W. Designing a Stable Alloy Interlayer on Li Metal Anodes for Fast Charging of All-Solid-State Li Metal Batteries. Batteries. 2024; 10(7):253. https://doi.org/10.3390/batteries10070253
Chicago/Turabian StyleDelaporte, Nicolas, Alexis Perea, Steve Collin-Martin, Mireille Léonard, Julie Matton, Hendrix Demers, Daniel Clément, Vincent Gariépy, and Wen Zhu. 2024. "Designing a Stable Alloy Interlayer on Li Metal Anodes for Fast Charging of All-Solid-State Li Metal Batteries" Batteries 10, no. 7: 253. https://doi.org/10.3390/batteries10070253
APA StyleDelaporte, N., Perea, A., Collin-Martin, S., Léonard, M., Matton, J., Demers, H., Clément, D., Gariépy, V., & Zhu, W. (2024). Designing a Stable Alloy Interlayer on Li Metal Anodes for Fast Charging of All-Solid-State Li Metal Batteries. Batteries, 10(7), 253. https://doi.org/10.3390/batteries10070253







