Environmental Aspects and Recycling of Solid-State Batteries: A Comprehensive Review
Abstract
1. Introduction
2. Environmental Impact of SSB Manufacture
2.1. Raw Material Extraction and Processing
2.2. Manufacturing Process of SSBs
2.3. Potential Environmental Hazards Associated with Novel Materials Used in SSBs
3. Usage and Operational Environmental Impact
3.1. Energy Efficiency of SSBs in Application
3.2. Comparison of the Operational Environmental Footprint with Traditional Battery Technologies
3.3. Life Cycle Analysis and Overall Carbon Footprint during Operational Phase
4. End of Life and Disposal of SSBs
4.1. Challenges in the Disposal of SSBs
4.2. Environmental Risks Associated with Landfills and Incineration
4.3. Regulations and Policies Governing Battery Disposal
5. Recycling and Reuse of SSBs
5.1. Overview of Existing Recycling Methods for Batteries
5.2. Innovations in Recycling: Emerging Technologies and Methodologies
5.3. Case Studies and Real-World Examples
5.4. Analysis of Successful Implementations of Recycling and Sustainable Practices in SSB Life Cycle Management
5.5. Lessons Learned and Best Practices
6. Future Directions and Research Needs
6.1. Identification of Gaps in Current Research and Technology
6.2. Potential Avenues for Future Innovations in Recycling and Reducing Environmental Impact
6.3. The Role of Interdisciplinary Research in Advancing Sustainable SSB Technologies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SSB | solid-state battery |
| EV | electric vehicle |
| Pb-A | lead-acid |
| LIB | lithium-ion battery |
| GHG | greenhouse gas |
| CED | cumulative energy demand |
| GWP100 | global warning potential for 100-year time horizon |
| CSP | cold sintering process |
| LCA | life cycle analysis |
| RCRA | Resource Conservation and Recovery Act |
| MIIT | Ministry of Industry and Information Technology |
| TEPCO | Tokyo Electric Power Company |
| CASIP | China All-Solid-State Battery Innovation Collaboration Platform |
| LCO | lithium cobalt oxide (LiCoO2) |
| LFP | lithium iron phosphate (LiFePO4/C) |
| NCM | lithium nickel cobalt manganese oxide (LiNiCoMnO2) |
| PV-EV | photovoltaic electric vehicle |
| SPE | solid polymer electrolyte |
| LiTFSI | lithium bis(trifluoromethanesulfonyl)imide |
| NMF | N-methylformamide |
| LLZTO | Li6.5La3Zr1.5Ta0.5O12 garnet electrolyte |
| MOF | metal–organic framework |
| OEM | original equipment manufacturer |
| CE | circular economy |
| CBM | circular business model |
| EU | European Union |
| BIGP | battery identity global passport |
References
- Zeng, D.; Yao, J.; Zhang, L.; Xu, R.; Wang, S.; Yan, X.; Yu, C.; Wang, L. Promoting Favorable Interfacial Properties in Lithium-Based Batteries Using Chlorine-Rich Sulfide Inorganic Solid-State Electrolytes. Nat. Commun. 2022, 13, 1909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Feng, Q.; Lei, Y.; Tang, S.; Xu, L.; Xiong, Y.; Fang, G.; Wang, Y.; Yang, P.; Liu, J.; et al. Quasi-Solid-State Zn-Air Batteries with an Atomically Dispersed Cobalt Electrocatalyst and Organohydrogel Electrolyte. Nat. Commun. 2022, 13, 3689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Z.; Wu, Y.; Ji, S.; Yuan, Z.; Liu, J.; Zhu, M. In Situ Construction a Stable Protective Layer in Polymer Electrolyte for Ultralong Lifespan Solid-State Lithium Metal Batteries. Adv. Sci. 2022, 9, 2104277. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Rong, X.; Gao, A.; Liu, Y.; Li, J.; Mao, M.; Qi, X.; Chai, G.; Zhang, Q.; Suo, L.; et al. Rational Design of a Topological Polymeric Solid Electrolyte for High-Performance All-Solid-State Alkali Metal Batteries. Nat. Commun. 2022, 13, 4181. [Google Scholar] [CrossRef] [PubMed]
- Minnmann, P.; Strauss, F.; Bielefeld, A.; Ruess, R.; Adelhelm, P.; Burkhardt, S.; Dreyer, S.L.; Trevisanello, E.; Ehrenberg, H.; Brezesinski, T.; et al. Designing Cathodes and Cathode Active Materials for Solid-State Batteries. Adv. Energy Mater. 2022, 12, 2201425. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, F.; Shen, L.; Deng, S.; Wang, Z.; Li, M.; Yao, X. 20 μm-Thick Li6.4La3Zr1.4Ta0.6O12-Based Flexible Solid Electrolytes for All-Solid-State Lithium Batteries. Energy Mater. Adv. 2022, 2022, 9753506. [Google Scholar] [CrossRef]
- Albertus, P.; Anandan, V.; Ban, C.; Balsara, N.; Belharouak, I.; Buettner-Garrett, J.; Chen, Z.; Daniel, C.; Doeff, M.; Dudney, N.J.; et al. Challenges for and Pathways toward Li-Metal-Based All-Solid-State Batteries. ACS Energy Lett. 2021, 6, 1399–1404. [Google Scholar] [CrossRef]
- Castillo, J.; Qiao, L.; Santiago, A.; Judez, X.; De Buruaga, A.S.; Jimenez, G.; Armand, M.; Zhang, H.; Li, C. Perspective of Polymer-Based Solid-State Li-S Batteries. Energy Mater. 2022, 2, 200003. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Meng, Y.S.; Jang, J. Scaling up High-Energy-Density Sulfidic Solid-State Batteries: A Lab-to-Pilot Perspective. Joule 2022, 6, 1755–1769. [Google Scholar] [CrossRef]
- Chi, X.; Zhang, Y.; Hao, F.; Kmiec, S.; Dong, H.; Xu, R.; Zhao, K.; Ai, Q.; Terlier, T.; Wang, L.; et al. An Electrochemically Stable Homogeneous Glassy Electrolyte Formed at Room Temperature for All-Solid-State Sodium Batteries. Nat. Commun. 2022, 13, 2854. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, K.; Kim, S.; Yoon, K.; Han, S.; Lee, M.H.; Ko, Y.; Noh, J.H.; Kim, W.; Kang, K. Design of a Lithiophilic and Electron-Blocking Interlayer for Dendrite-Free Lithium-Metal Solid-State Batteries. Sci. Adv. 2022, 8, eabq0153. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Teo, J.H.; Walther, F.; Ma, Y.; Zhang, R.; Mazilkin, A.; Tang, Y.; Goonetilleke, D.; Janek, J.; Bianchini, M.; et al. Advanced Nanoparticle Coatings for Stabilizing Layered Ni-Rich Oxide Cathodes in Solid-State Batteries. Adv. Funct. Mater. 2022, 32, 2111829. [Google Scholar] [CrossRef]
- Hu, J.; Lai, C.; Chen, K.; Wu, Q.; Gu, Y.; Wu, C.; Li, C. Dual Fluorination of Polymer Electrolyte and Conversion-Type Cathode for High-Capacity All-Solid-State Lithium Metal Batteries. Nat. Commun. 2022, 13, 7914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Han, W.-Q. From Liquid to Solid-State Lithium Metal Batteries: Fundamental Issues and Recent Developments. Nano-Micro Lett. 2024, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Kalnaus, S.; Dudney, N.J.; Westover, A.S.; Herbert, E.; Hackney, S. Solid-State Batteries: The Critical Role of Mechanics. Science 2023, 381, eabg5998. [Google Scholar] [CrossRef] [PubMed]
- Janek, J.; Zeier, W.G. Challenges in Speeding up Solid-State Battery Development. Nat. Energy 2023, 8, 230–240. [Google Scholar] [CrossRef]
- Liang, X.; Tan, F.; Wei, F.; Du, J. Research progress of all solid-state thin film lithium battery. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012138. [Google Scholar] [CrossRef]
- Wu, C.; Lou, J.; Zhang, J.; Chen, Z.; Kakar, A.; Emley, B.; Ai, Q.; Guo, H.; Liang, Y.; Lou, J.; et al. Current Status and Future Directions of All-Solid-State Batteries with Lithium Metal Anodes, Sulfide Electrolytes, and Layered Transition Metal Oxide Cathodes. Nano Energy 2021, 87, 106081. [Google Scholar] [CrossRef]
- Al Shaqsi, A.Z.; Sopian, K.; Al-Hinai, A. Review of Energy Storage Services, Applications, Limitations, and Benefits. Energy Rep. 2020, 6, 288–306. [Google Scholar] [CrossRef]
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy Storage Systems: A Review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Zhang, H.; Judez, X.; Adenusi, H.; Armand, M.; Passerini, S.; Figgemeier, E. Production of High-Energy Li-Ion Batteries Comprising Silicon-Containing Anodes and Insertion-Type Cathodes. Nat. Commun. 2021, 12, 5459. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; He, Z.; Li, Y.; Mao, J.; Dai, K.; Yan, C.; Zheng, J. An Advance Review of Solid-State Battery: Challenges, Progress and Prospects. Sustain. Mater. Technol. 2021, 29, e00297. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Shuai, H.; Luo, Z.; Wang, B.; Fang, S.; Zou, G.; Hou, H.; Peng, H.; Ji, X. Recent Advances of Composite Electrolytes for Solid-State Li Batteries. J. Energy Chem. 2022, 67, 524–548. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Wu, Y.; Li, H.; Yang, J.; Liao, Y.; Qu, R.; Zhang, Z. Liquid Electrolytes for Low-Temperature Lithium Batteries: Main Limitations, Current Advances, and Future Perspectives. Energy Storage Mater. 2023, 56, 642–663. [Google Scholar] [CrossRef]
- Zachmann, N.; Petranikova, M.; Ebin, B. Electrolyte Recovery from Spent Lithium-Ion Batteries Using a Low Temperature Thermal Treatment Process. J. Ind. Eng. Chem. 2023, 118, 351–361. [Google Scholar] [CrossRef]
- Raabe, D. The Materials Science behind Sustainable Metals and Alloys. Chem. Rev. 2023, 123, 2436–2608. [Google Scholar] [CrossRef] [PubMed]
- Boaretto, N.; Garbayo, I.; Valiyaveettil-SobhanRaj, S.; Quintela, A.; Li, C.; Casas-Cabanas, M.; Aguesse, F. Lithium Solid-State Batteries: State-of-the-Art and Challenges for Materials, Interfaces and Processing. J. Power Sources 2021, 502, 229919. [Google Scholar] [CrossRef]
- Igogo, T.; Awuah-Offei, K.; Newman, A.; Lowder, T.; Engel-Cox, J. Integrating Renewable Energy into Mining Operations: Opportunities, Challenges, and Enabling Approaches. Appl. Energy 2021, 300, 117375. [Google Scholar] [CrossRef]
- Stampatori, D.; Raimondi, P.P.; Noussan, M. Li-Ion Batteries: A Review of a Key Technology for Transport Decarbonization. Energies 2020, 13, 2638. [Google Scholar] [CrossRef]
- Ferrari, S.; Falco, M.; Muñoz-García, A.B.; Bonomo, M.; Brutti, S.; Pavone, M.; Gerbaldi, C. Solid-State Post Li Metal Ion Batteries: A Sustainable Forthcoming Reality? Adv. Energy Mater. 2021, 11, 2100785. [Google Scholar] [CrossRef]
- Murdock, B.E.; Toghill, K.E.; Tapia-Ruiz, N. A Perspective on the Sustainability of Cathode Materials Used in Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2102028. [Google Scholar] [CrossRef]
- Dehghani-Sanij, A.R.; Tharumalingam, E.; Dusseault, M.B.; Fraser, R. Study of Energy Storage Systems and Environmental Challenges of Batteries. Renew. Sustain. Energy Rev. 2019, 104, 192–208. [Google Scholar] [CrossRef]
- Porzio, J.; Scown, C.D. Life-Cycle Assessment Considerations for Batteries and Battery Materials. Adv. Energy Mater. 2021, 11, 2100771. [Google Scholar] [CrossRef]
- Schnell, J.; Tietz, F.; Singer, C.; Hofer, A.; Billot, N.; Reinhart, G. Prospects of Production Technologies and Manufacturing Costs of Oxide-Based All-Solid-State Lithium Batteries. Energy Environ. Sci. 2019, 12, 1818–1833. [Google Scholar] [CrossRef]
- Degen, F.; Schütte, M. Life Cycle Assessment of the Energy Consumption and GHG Emissions of State-of-the-Art Automotive Battery Cell Production. J. Clean. Prod. 2022, 330, 129798. [Google Scholar] [CrossRef]
- Lai, X.; Gu, H.; Chen, Q.; Tang, X.; Zhou, Y.; Gao, F.; Han, X.; Guo, Y.; Bhagat, R.; Zheng, Y. Investigating Greenhouse Gas Emissions and Environmental Impacts from the Production of Lithium-Ion Batteries in China. J. Clean. Prod. 2022, 372, 133756. [Google Scholar] [CrossRef]
- Keshavarzmohammadian, A.; Cook, S.M.; Milford, J.B. Cradle-to-Gate Environmental Impacts of Sulfur-Based Solid-State Lithium Batteries for Electric Vehicle Applications. J. Clean. Prod. 2018, 202, 770–778. [Google Scholar] [CrossRef]
- Chen, J.; Wu, J.; Wang, X.; Zhou, A.; Yang, Z. Research Progress and Application Prospect of Solid-State Electrolytes in Commercial Lithium-Ion Power Batteries. Energy Storage Mater. 2021, 35, 70–87. [Google Scholar] [CrossRef]
- Balaish, M.; Gonzalez-Rosillo, J.C.; Kim, K.J.; Zhu, Y.; Hood, Z.D.; Rupp, J.L.M. Processing Thin but Robust Electrolytes for Solid-State Batteries. Nat. Energy 2021, 6, 227–239. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Wang, D.; Adair, K.; Liang, J.; Sun, X. Development of the Cold Sintering Process and Its Application in Solid-State Lithium Batteries. J. Power Sources 2018, 393, 193–203. [Google Scholar] [CrossRef]
- Zaman, W.; Hatzell, K.B. Processing and Manufacturing of next Generation Lithium-Based All Solid-State Batteries. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101003. [Google Scholar] [CrossRef]
- Raj, V.; Aetukuri, N.P.B.; Nanda, J. Solid State Lithium Metal Batteries—Issues and Challenges at the Lithium-Solid Electrolyte Interface. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100999. [Google Scholar] [CrossRef]
- Gnanavendan, S.; Selvaraj, S.K.; Dev, S.J.; Mahato, K.K.; Swathish, R.S.; Sundaramali, G.; Accouche, O.; Azab, M. Challenges, Solutions and Future Trends in EV-Technology: A Review. IEEE Access 2024, 12, 17242–17260. [Google Scholar] [CrossRef]
- Boopathi, S. Implementation of Green Manufacturing Practices in Automobile Fields: A Review. In Sustainable Machining and Green Manufacturing; Thirumalai Kumaran, S., Ko, T.J., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 221–248. ISBN 978-1-394-19783-5. [Google Scholar]
- Karpagaraj, A.; Gopikrishnan, T.; Singh, S.K. Reuse and Recycling of Electronic Waste from a Global Solution Perspective. In Electronic Waste Management; Kumar, S., Kumar, V., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 104–123. ISBN 978-1-119-89151-2. [Google Scholar]
- Berrueta, A.; Ursua, A.; Martin, I.S.; Eftekhari, A.; Sanchis, P. Supercapacitors: Electrical Characteristics, Modeling, Applications, and Future Trends. IEEE Access 2019, 7, 50869–50896. [Google Scholar] [CrossRef]
- Kunde, G.B.; Sehgal, B. Industrial Innovation Through Sustainable Materials. In Handbook of Smart Materials, Technologies, and Devices; Hussain, C.M., Di Sia, P., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–42. ISBN 978-3-030-58675-1. [Google Scholar]
- Yang, X.; Adair, K.R.; Gao, X.; Sun, X. Recent Advances and Perspectives on Thin Electrolytes for High-Energy-Density Solid-State Lithium Batteries. Energy Environ. Sci. 2021, 14, 643–671. [Google Scholar] [CrossRef]
- Fan, E.; Li, L.; Wang, Z.; Lin, J.; Huang, Y.; Yao, Y.; Chen, R.; Wu, F. Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 2020, 120, 7020–7063. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yuan, X. The Advances and Opportunities of Developing Solid-State Battery Technology: Based on the Patent Information Relation Matrix. Energy 2024, 296, 131178. [Google Scholar] [CrossRef]
- Wang, L.; Zhong, Y.; Wang, H.; Malyi, O.I.; Wang, F.; Zhang, Y.; Hong, G.; Tang, Y. New Emerging Fast Charging Microscale Electrode Materials. Small 2024, 20, 2307027. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Nie, C.; Guo, W.; Liu, D.; Chen, Y.; Ju, Z.; Zhuang, Q. Inorganic Cathode Materials for Potassium Ion Batteries. Mater. Today Energy 2022, 25, 100982. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Solid Electrolytes for High-Temperature Stable Batteries and Supercapacitors. Adv. Energy Mater. 2021, 11, 2002869. [Google Scholar] [CrossRef]
- Ye, T.; Li, L.; Zhang, Y. Recent Progress in Solid Electrolytes for Energy Storage Devices. Adv. Funct. Mater. 2020, 30, 2000077. [Google Scholar] [CrossRef]
- Mauger, A.; Julien, C.M.; Paolella, A.; Armand, M.; Zaghib, K. Zaghib Building Better Batteries in the Solid State: A Review. Materials 2019, 12, 3892. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Ibn-Mohammed, T.; Koh, L.; Reaney, I.M. Life Cycle Assessment of Functional Materials and Devices: Opportunities, Challenges, and Current and Future Trends. J. Am. Ceram. Soc. 2019, 102, 7037–7064. [Google Scholar] [CrossRef]
- Thonemann, N.; Schulte, A.; Maga, D. How to Conduct Prospective Life Cycle Assessment for Emerging Technologies? A Systematic Review and Methodological Guidance. Sustainability 2020, 12, 1192. [Google Scholar] [CrossRef]
- Padgett, E.; Papageorgopoulos, D. System-level Constraints on Fuel Cell Materials and Electrocatalysts. In Electrocatalysis for Membrane Fuel Cells; Alonso-Vante, N., Di Noto, V., Eds.; Wiley: Hoboken, NJ, USA, 2023; pp. 1–22. ISBN 978-3-527-34837-4. [Google Scholar]
- Fonseca, N.; Thummalapalli, S.V.; Jambhulkar, S.; Ravichandran, D.; Zhu, Y.; Patil, D.; Thippanna, V.; Ramanathan, A.; Xu, W.; Guo, S.; et al. 3D Printing-Enabled Design and Manufacturing Strategies for Batteries: A Review. Small 2023, 19, 2302718. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.S.; Wagh, N.K.; Kim, S.; Lee, J. Li, Na, K, Mg, Zn, Al, and Ca Anode Interface Chemistries Developed by Solid-State Electrolytes. Adv. Sci. 2023, 10, 2304235. [Google Scholar] [CrossRef] [PubMed]
- Zor, C.; Turrell, S.J.; Uyanik, M.S.; Afyon, S. Lithium Plating and Stripping: Toward Anode-Free Solid-State Batteries. Adv. Energy Sustain. Res. 2023, 2300001. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Habibi, S. Review of the Li-Ion Battery, Thermal Management, and AI-Based Battery Management System for EV Application. Energies 2022, 16, 185. [Google Scholar] [CrossRef]
- Mohammed, A.G.; Elfeky, K.E.; Wang, Q. Recent Advancement and Enhanced Battery Performance Using Phase Change Materials Based Hybrid Battery Thermal Management for Electric Vehicles. Renew. Sustain. Energy Rev. 2022, 154, 111759. [Google Scholar] [CrossRef]
- Akasapu, U.; Hehenberger, P. A Design Process Model for Battery Systems Based on Existing Life Cycle Assessment Results. J. Clean. Prod. 2023, 407, 137149. [Google Scholar] [CrossRef]
- Lie, K.W.; Synnevåg, T.A.; Lamb, J.J.; Lien, K.M. The Carbon Footprint of Electrified City Buses: A Case Study in Trondheim, Norway. Energies 2021, 14, 770. [Google Scholar] [CrossRef]
- Zhang, S.S. Challenges and Strategies for Fast Charge of Li-Ion Batteries. ChemElectroChem 2020, 7, 3569–3577. [Google Scholar] [CrossRef]
- Soini, M.C.; Parra, D.; Patel, M.K. Impact of Prosumer Battery Operation on the Cost of Power Supply. J. Energy Storage 2020, 29, 101323. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Dühnen, S.; Betz, J.; Kolek, M.; Schmuch, R.; Winter, M.; Placke, T. Toward Green Battery Cells: Perspective on Materials and Technologies. Small Methods 2020, 4, 2000039. [Google Scholar] [CrossRef]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Shen, H.; Yi, E.; Cheng, L.; Amores, M.; Chen, G.; Sofie, S.W.; Doeff, M.M. Solid-state electrolyte considerations for electric vehicle batteries. Sustain. Energy Fuels 2019, 3, 1647–1659. [Google Scholar] [CrossRef]
- Tan, D.H.S.; Xu, P.; Yang, H.; Kim, M.; Nguyen, H.; Wu, E.A.; Doux, J.-M.; Banerjee, A.; Meng, Y.S.; Chen, Z. Sustainable Design of Fully Recyclable All Solid-State Batteries. MRS Energy Sustain. 2020, 7, 23. [Google Scholar] [CrossRef]
- Kasemchainan, J.; Bruce, P.G. All-Solid-State Batteries and Their Remaining Challenges: A Potential Route towards Safer, Higher Performing Batteries. Johns. Matthey Technol. Rev. 2018, 62, 177–180. [Google Scholar] [CrossRef]
- Winslow, K.M.; Laux, S.J.; Townsend, T.G. A Review on the Growing Concern and Potential Management Strategies of Waste Lithium-Ion Batteries. Resour. Conserv. Recycl. 2018, 129, 263–277. [Google Scholar] [CrossRef]
- Beghi, M.; Braghin, F.; Roveda, L. Enhancing Disassembly Practices for Electric Vehicle Battery Packs: A Narrative Comprehensive Review. Designs 2023, 7, 109. [Google Scholar] [CrossRef]
- Beaudet, A.; Larouche, F.; Amouzegar, K.; Bouchard, P.; Zaghib, K. Key Challenges and Opportunities for Recycling Electric Vehicle Battery Materials. Sustainability 2020, 12, 5837. [Google Scholar] [CrossRef]
- Melzack, N. Advancing battery design based on environmental impacts using an aqueous Al-ion cell as a case study. Sci. Rep. 2022, 12, 8911. [Google Scholar] [CrossRef] [PubMed]
- Noudeng, V.; Quan, N.V.; Xuan, T.D. A Future Perspective on Waste Management of Lithium-Ion Batteries for Electric Vehicles in Lao PDR: Current Status and Challenges. Int. J. Environ. Res. Public Health 2022, 19, 16169. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, W.; Rajaeifar, M.A.; Heidrich, O.; Christensen, P. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries. Energy Environ. Sci. 2021, 14, 6099–6121. [Google Scholar] [CrossRef]
- Patel, A.N.; Lander, L.; Ahuja, J.; Bulman, J.; Lum, J.K.H.; Pople, J.O.D.; Hales, A.; Patel, Y.; Edge, J.S. Lithium-ion battery second life: Pathways, challenges and outlook. Front. Chem. 2024, 12, 1358417. [Google Scholar] [CrossRef]
- Rarotra, S.; Sahu, S.; Kumar, P.; Kim, K.; Tsang, Y.F.; Kumar, V.; Kumar, P.; Srinivasan, M.; Veksha, A.; Lisak, G. Progress and Challenges on Battery Waste Management: A Critical Review. ChemistrySelect 2020, 5, 6182–6193. [Google Scholar] [CrossRef]
- Harper, G.D.J.; Kendrick, E.; Anderson, P.A.; Mrozik, W.; Christensen, P.; Lambert, S.; Greenwood, D.; Das, P.K.; Ahmeid, M.; Milojevic, Z.; et al. Roadmap for a Sustainable Circular Economy in Lithium-Ion and Future Battery Technologies. J. Phys. Energy 2023, 5, 021501. [Google Scholar] [CrossRef]
- Lei, J.; Fu, H.-Z.; Ho, Y.-S. A Global Perspective of Bioaccumulation Research Using Bibliometric Analysis. COLLNET J. Scientometr. Inf. Manag. 2018, 12, 327–341. [Google Scholar] [CrossRef]
- Bird, R.; Baum, Z.J.; Yu, X.; Ma, J. The Regulatory Environment for Lithium-Ion Battery Recycling. ACS Energy Lett. 2022, 7, 736–740. [Google Scholar] [CrossRef]
- Thompson, D.L.; Hartley, J.M.; Lambert, S.M.; Shiref, M.; Harper, G.D.J.; Kendrick, E.; Anderson, P.; Ryder, K.S.; Gaines, L.; Abbott, A.P. The importance of design in lithium ion battery recycling—A critical review. Green Chem. 2020, 22, 7585–7603. [Google Scholar] [CrossRef]
- Zanoletti, A.; Carena, E.; Ferrara, C.; Bontempi, E. A Review of Lithium-Ion Battery Recycling: Technologies, Sustainability, and Open Issues. Batteries 2024, 10, 38. [Google Scholar] [CrossRef]
- Crocce Romano Espinosa, D.; Moura Bernardes, A.; Alberto Soares Tenório, J. Brazilian Policy on Battery Disposal and Its Practical Effects on Battery Recycling. J. Power Sources 2004, 137, 134–139. [Google Scholar] [CrossRef]
- Gaines, L. Lithium-ion battery recycling processes: Research towards a sustainable course. Sustain. Mater. Technol. 2018, 17, e00068. [Google Scholar] [CrossRef]
- Resource Conservation and Recovery Act (RCRA) Laws and Regulations 2024. Available online: https://www.epa.gov/rcra (accessed on 14 February 2024).
- Universal Waste. Available online: https://www.epa.gov/hw/universal-waste (accessed on 6 May 2024).
- ReCell Center. Available online: https://www.recellcenter.org (accessed on 6 May 2024).
- Pacios, R.; Villaverde, A.; Martínez-Ibañez, M.; Casas-Cabanas, M.; Aguesse, F.; Kvasha, A. Roadmap for Competitive Production of Solid-State Batteries: How to Convert a Promise into Reality. Adv. Energy Mater. 2023, 13, 2301018. [Google Scholar] [CrossRef]
- Ahuis, M.; Doose, S.; Vogt, D.; Michalowski, P.; Zellmer, S.; Kwade, A. Recycling of Solid-State Batteries. Nat. Energy 2024, 9, 373–385. [Google Scholar] [CrossRef]
- Azhari, L.; Bong, S.; Ma, X.; Wang, Y. Recycling for All Solid-State Lithium-Ion Batteries. Matter 2020, 3, 1845–1861. [Google Scholar] [CrossRef]
- Wu, X.; Ji, G.; Wang, J.; Zhou, G.; Liang, Z. Toward Sustainable All Solid-State Li–Metal Batteries: Perspectives on Battery Technology and Recycling Processes. Adv. Mater. 2023, 35, 2301540. [Google Scholar] [CrossRef]
- Ajith, K.; Selvin, P.C.; Abhilash, K.P.; Palaniyandy, N.; Helen, P.A.; Somasundharam, G. Recycling of All-Solid-State Lithium-Ion Batteries. In Solid State Batteries; Palaniyandy, N., Abhilash, K.P., Nalini, B., Eds.; Advances in Material Research and Technology; Springer International Publishing: Cham, Switzerland, 2022; pp. 245–274. ISBN 978-3-031-12469-3. [Google Scholar]
- Zhang, Q.; Gao, X.-W.; Liu, X.; Gu, Q.; Mu, J.; Luo, W.-B. Economical and Ecofriendly Lithium-Ion Battery Recycling: Material Flow and Energy Flow. ACS Sustain. Chem. Eng. 2024, 12, 2511–2530. [Google Scholar] [CrossRef]
- Alessia, A.; Alessandro, B.; Maria, V.-G.; Carlos, V.-A.; Francesca, B. Challenges for Sustainable Lithium Supply: A Critical Review. J. Clean. Prod. 2021, 300, 126954. [Google Scholar] [CrossRef]
- Kastanaki, E.; Giannis, A. Dynamic Estimation of End-of-Life Electric Vehicle Batteries in the EU-27 Considering Reuse, Remanufacturing and Recycling Options. J. Clean. Prod. 2023, 393, 136349. [Google Scholar] [CrossRef]
- Jacob, M.; Wissel, K.; Clemens, O. Recycling of Solid-State Batteries—Challenge and Opportunity for a Circular Economy? Mater. Futures 2024, 3, 012101. [Google Scholar] [CrossRef]
- Ali, H.; Khan, H.A.; Pecht, M. Preprocessing of Spent Lithium-Ion Batteries for Recycling: Need, Methods, and Trends. Renew. Sustain. Energy Rev. 2022, 168, 112809. [Google Scholar] [CrossRef]
- Kaya, M. State-of-the-Art Lithium-Ion Battery Recycling Technologies. Circ. Econ. 2022, 1, 100015. [Google Scholar] [CrossRef]
- Kader, Z.A.; Marshall, A.; Kennedy, J. A Review on Sustainable Recycling Technologies for Lithium-Ion Batteries. Emergent Mater. 2021, 4, 725–735. [Google Scholar] [CrossRef]
- Jung, J.; Sui, P.-C.; Zhang, J. Hydrometallurgical Recycling of Lithium-Ion Battery Materials, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; ISBN 978-1-00-326920-5. [Google Scholar]
- Larouche, F.; Demopoulos, G.P.; Amouzegar, K.; Bouchard, P.; Zaghib, K. Recycling of Li-Ion and Li-Solid State Batteries: The Role of Hydrometallurgy. In Extraction 2018; Davis, B.R., Moats, M.S., Wang, S., Gregurek, D., Kapusta, J., Battle, T.P., Schlesinger, M.E., Alvear Flores, G.R., Jak, E., Goodall, G., et al., Eds.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2018; pp. 2541–2553. ISBN 978-3-319-95021-1. [Google Scholar]
- Tanhaei, M.; Beiramzadeh, Z.; Kholghi Eshkalak, S.; Katal, R. Recycling and Management of Lithium Battery as Electronic Waste. In Handbook of Solid Waste Management; Baskar, C., Ramakrishna, S., Baskar, S., Sharma, R., Chinnappan, A., Sehrawat, R., Eds.; Springer Nature Singapore: Singapore, 2022; pp. 1605–1634. ISBN 9789811642296. [Google Scholar]
- Ji, H.; Wang, J.; Ma, J.; Cheng, H.-M.; Zhou, G. Fundamentals, Status and Challenges of Direct Recycling Technologies for Lithium Ion Batteries. Chem. Soc. Rev. 2023, 52, 8194–8244. [Google Scholar] [CrossRef] [PubMed]
- Piątek, J.; Afyon, S.; Budnyak, T.M.; Budnyk, S.; Sipponen, M.H.; Slabon, A. Sustainable Li-Ion Batteries: Chemistry and Recycling. Adv. Energy Mater. 2021, 11, 2003456. [Google Scholar] [CrossRef]
- Bai, Y.; Muralidharan, N.; Sun, Y.-K.; Passerini, S.; Stanley Whittingham, M.; Belharouak, I. Energy and Environmental Aspects in Recycling Lithium-Ion Batteries: Concept of Battery Identity Global Passport. Mater. Today 2020, 41, 304–315. [Google Scholar] [CrossRef]
- Gong, S.; Dong, E.; Bingguo, L.; Yuwen, C.; Niu, Y.; Ji, G.; Chen, W.; Hou, K.; Zhang, L.; Guo, S. Eco-Friendly Closed-Loop Recycling of Nickel, Cobalt, Manganese, and Lithium from Spent Lithium-Ion Battery Cathodes. Sep. Purif. Technol. 2024, 348, 127771. [Google Scholar]
- Wang, T.-W.; Liu, T.; Sun, H. Direct Recycling for Advancing Sustainable Battery Solutions. Mater. Today Energy 2023, 38, 101434. [Google Scholar] [CrossRef]
- Sheth, R.P.; Ranawat, N.S.; Chakraborty, A.; Mishra, R.P.; Khandelwal, M. The Lithium-Ion Battery Recycling Process from a Circular Economy Perspective—A Review and Future Directions. Energies 2023, 16, 3228. [Google Scholar] [CrossRef]
- Waidha, A.I.; Salihovic, A.; Jacob, M.; Vanita, V.; Aktekin, B.; Brix, K.; Wissel, K.; Kautenburger, R.; Janek, J.; Ensinger, W.; et al. Recycling of All-Solid-State Li-ion Batteries: A Case Study of the Separation of Individual Components within a System Composed of LTO, LLZTO and NMC. ChemSusChem 2023, 16, e202202361. [Google Scholar] [CrossRef] [PubMed]
- Ali Nowroozi, M.; Iqbal Waidha, A.; Jacob, M.; Van Aken, P.A.; Predel, F.; Ensinger, W.; Clemens, O. Towards Recycling of LLZO Solid Electrolyte Exemplarily Performed on LFP/LLZO/LTO Cells. ChemistryOpen 2022, 11, e202100274. [Google Scholar] [CrossRef] [PubMed]
- Spacenet: European Pattent Office. Available online: https://worldwide.espacenet.com (accessed on 5 May 2024).
- Karkar, Z.; Houache, M.S.E.; Yim, C.-H.; Abu-Lebdeh, Y. An Industrial Perspective and Intellectual Property Landscape on Solid-State Battery Technology with a Focus on Solid-State Electrolyte Chemistries. Batteries 2024, 10, 24. [Google Scholar] [CrossRef]
- Nomoto, K.; Miyazaki, A. Solid State Battery. Patent WO2021177212A1, 10 September 2021. [Google Scholar]
- Sasaki, I.; Fujinoki, N.; Miyazaki, A.; Shiotani, S.; Sugiyama, T. Positive Electrode Material and Battery. Patent WO2021157361A1, 12 August 2021. [Google Scholar]
- Morita, K.; Yada, C.; Suzuki, Y. Method for Producing All Solid State State Battery 2022. Patent JP2022052690A, 4 April 2022. [Google Scholar]
- Takeuchi, K.; Shiotani, S.; Otomo, T. Solid Electrolyte. Patent JP2020061326A, 16 April 2020. [Google Scholar]
- Li, X. All Solid-State Battery and Manufacturing Method for All Solid-State Battery 2022. Patent EP4117058A1, 5 July 2022. [Google Scholar]
- Nagano, A. Method of Evaluating All-Solid-State Battery. Patent JP2022076604A, 10 May 2022. [Google Scholar]
- Emerging Technology News. Available online: https://etn.news/buzz/toyota-ev-battery-reduce-reuse-recycling-initiatives-global-markets (accessed on 5 May 2024).
- Jera’s Action. Available online: https://www.jera.co.jp/en/action/discover/009 (accessed on 4 May 2024).
- Tepco. Available online: https://www.tepco.co.jp/en/hd/newsroom/press/archives/2023/20230529_01.html (accessed on 3 May 2024).
- Fleutot, B.; Zhang, X.; Garitte, E. Solid Electrolytes Comprising an Ionic Bifunctional Molecule and Use Thereof in Electrochemistry. Patent WO2023/133642, 1 July 2023. [Google Scholar]
- Delaporte, N.; Morizur, V.; Lajoie, G. Anodeless Coating Layer for All-Solid-State Battery and All-Solid-State Battery Incuding Anodeless Coating Layer. Patent WO2023/141720, 2 August 2023. [Google Scholar]
- Amouzegar, K.; Leblanc, D.; Paolella, A.; Guerfi, A.; Kaboli, S. Process for Producing an Anode for Lithium Batteries 2023. Patent WO2023/133627, 1 July 2023. [Google Scholar]
- Hydro-Québec. Available online: https://news.hydroquebec.com/en/press-releases/1317/hydro-quebec-licenses-dongshi-kingpower-science-and-technology-to-use-its-solid-state-lithium-battery-technology/ (accessed on 3 May 2024).
- Lithion. Available online: https://www.lithiontechnologies.com/en/solutions/recycle-your-batteries/ (accessed on 3 May 2024).
- Electrive. Available online: https://www.electrive.com/2024/02/13/chinas-car-and-battery-industry-launches-solid-state-battery-offensive/ (accessed on 5 May 2024).
- Catl. Available online: https://www.catl.com/en/solution/recycling/ (accessed on 2 May 2024).
- CATL and Volvo Cars Join Hands for Sustainable Development. Available online: https://www.catl.com/en/news/6234.html (accessed on 5 May 2024).
- Basf. Available online: https://catalysts.basf.com/industries/recycling/lithium-ion-battery-recycling (accessed on 12 May 2024).
- Svolt. Available online: https://www.svolt-eu.com/en/our-heartbeat/ (accessed on 11 May 2024).
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Sloop, S.; Crandon, L.; Allen, M.; Koetje, K.; Reed, L.; Gaines, L.; Sirisaksoontorn, W.; Lerner, M. A Direct Recycling Case Study from a Lithium-Ion Battery Recall. Sustain. Mater. Technol. 2020, 25, e00152. [Google Scholar] [CrossRef]
- Van Hoof, G.; Robertz, B.; Verrecht, B. Towards Sustainable Battery Recycling: A Carbon Footprint Comparison between Pyrometallurgical and Hydrometallurgical Battery Recycling Flowsheets. Metals 2023, 13, 1915. [Google Scholar] [CrossRef]
- Umicore. Available online: https://brs.umicore.com/en/recycling/ (accessed on 9 May 2024).
- Tedjar, F.F.J.C. Method for the Mixed Recycling of Lithium Based Anode Batteries and Cells. Patent US7820317B2, 26 October 2010. [Google Scholar]
- Lan, Y.; Li, X.; Zhou, G.; Yao, W.; Cheng, H.; Tang, Y. Direct Regenerating Cathode Materials from Spent Lithium-Ion Batteries. Adv. Sci. 2024, 11, 2304425. [Google Scholar] [CrossRef] [PubMed]
- Wegener, K.; Chen, W.H.; Dietrich, F.; Dröder, K.; Kara, S. Robot Assisted Disassembly for the Recycling of Electric Vehicle Batteries. In Proceedings of the 22nd CIRP Conference on Life Cycle Engineering, Sydney, Australia, 7–9 April 2015; Volume 29, pp. 716–721. [Google Scholar] [CrossRef]
- ReLib. Available online: https://www.faraday.ac.uk/research/lithium-ion/recycle-reuse/ (accessed on 3 May 2024).
- Argonne National Lab. Available online: https://www.anl.gov/topic/battery-recycling (accessed on 5 May 2024).
- Fraunhofer Institute. Available online: https://www.iwks.fraunhofer.de/en/competencies/Energy-Materials/Batteries-and-PV-modules.html (accessed on 2 May 2024).
- Aizat Razali, A.; Norazli, S.N.; Sum, W.S.; Yeo, S.Y.; Dolfi, A.; Srinivasan, G. State-of-the-Art of Solid-State Electrolytes on the Road Map of Solid-State Lithium Metal Batteries for E-Mobility. ACS Sustain. Chem. Eng. 2023, 11, 7927–7964. [Google Scholar] [CrossRef]
- Byeon, Y.-W.; Kim, H. Review on Interface and Interphase Issues in Sulfide Solid-State Electrolytes for All-Solid-State Li-Metal Batteries. Electrochem 2021, 2, 452–471. [Google Scholar] [CrossRef]
- Niu, B.; Xu, Z.; Xiao, J.; Qin, Y. Recycling Hazardous and Valuable Electrolyte in Spent Lithium-Ion Batteries: Urgency, Progress, Challenge, and Viable Approach. Chem. Rev. 2023, 123, 8718–8735. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, M.; Zhao, Z.; Tong, B.; Fan, Y.; Hua, Z. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review. ACS Sustain. Chem. Eng. 2018, 6, 13611–13627. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Zhuang, Z.; Liang, Z.; Jia, K.; Ji, G.; Zhou, G.; Cheng, H.-M. Toward Direct Regeneration of Spent Lithium-Ion Batteries: A Next-Generation Recycling Method. Chem. Rev. 2024, 124, 2839–2887. [Google Scholar] [CrossRef] [PubMed]
- Wickerts, S.; Arvidsson, R.; Nordelöf, A.; Svanström, M.; Johansson, P. Prospective Life Cycle Assessment of Lithium-Sulfur Batteries for Stationary Energy Storage. ACS Sustain. Chem. Eng. 2023, 11, 9553–9563. [Google Scholar] [CrossRef]
- Freitas Gomes, I.S.; Perez, Y.; Suomalainen, E. Coupling Small Batteries and PV Generation: A Review. Renew. Sustain. Energy Rev. 2020, 126, 109835. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, P.; Li, X.; Wang, Z.; Qin, X.; Shao, M.; Zhang, L.; Zhou, W. Rational Design of F-Modified Polyester Electrolytes for Sustainable All-Solid-State Lithium Metal Batteries. J. Am. Chem. Soc. 2024, 146, 5940–5951. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Méndez, S. Toward Sustainable Solid Polymer Electrolytes for Lithium-Ion Batteries. ACS Omega 2022, 7, 14457–14464. [Google Scholar] [CrossRef] [PubMed]
- Poizot, P.; Gaubicher, J.; Renault, S.; Dubois, L.; Liang, Y.; Yao, Y. Opportunities and Challenges for Organic Electrodes in Electrochemical Energy Storage. Chem. Rev. 2020, 120, 6490–6557. [Google Scholar] [CrossRef] [PubMed]
- Whang, G.; Zeier, W.G. Transition Metal Sulfide Conversion: A Promising Approach to Solid-State Batteries. ACS Energy Lett. 2023, 8, 5264–5274. [Google Scholar] [CrossRef]
- Wissel, K.; Riegger, L.M.; Schneider, C.; Waidha, A.I.; Famprikis, T.; Ikeda, Y.; Grabowski, B.; Dinnebier, R.E.; Lotsch, B.V.; Janek, J.; et al. Dissolution and Recrystallization Behavior of Li 3 PS 4 in Different Organic Solvents with a Focus on N -Methylformamide. ACS Appl. Energy Mater. 2023, 6, 7790–7802. [Google Scholar] [CrossRef]
- Chen, Q.; Pan, P.; Zhang, M.; Hu, Y.; Fu, K. A Three-Dimensional Fiber-Network-Reinforced Composite Solid-State Electrolyte from Waste Acrylic Fibers for Flexible All-Solid-State Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2023, 15, 38507–38521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, A.; Zhang, X.; Tian, R.; Yang, S.; Xu, S.; Song, D.; Yang, Y. Green Synthesis for Battery Materials: A Case Study of Making Lithium Sulfide via Metathetic Precipitation. ACS Appl. Mater. Interfaces 2023, 15, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Piątek, J.; Budnyak, T.M.; Monti, S.; Barcaro, G.; Gueret, R.; Grape, E.S.; Jaworski, A.; Inge, A.K.; Rodrigues, B.V.M.; Slabon, A. Toward Sustainable Li-Ion Battery Recycling: Green Metal–Organic Framework as a Molecular Sieve for the Selective Separation of Cobalt and Nickel. ACS Sustain. Chem. Eng. 2021, 9, 9770–9778. [Google Scholar] [CrossRef]
- Rajaeifar, M.A.; Ghadimi, P.; Raugei, M.; Wu, Y.; Heidrich, O. Challenges and Recent Developments in Supply and Value Chains of Electric Vehicle Batteries: A Sustainability Perspective. Resour. Conserv. Recycl. 2022, 180, 106144. [Google Scholar] [CrossRef]
- Neumann, J.; Petranikova, M.; Meeus, M.; Gamarra, J.D.; Younesi, R.; Winter, M.; Nowak, S. Recycling of Lithium-Ion Batteries—Current State of the Art, Circular Economy, and Next Generation Recycling. Adv. Energy Mater. 2022, 12, 2102917. [Google Scholar] [CrossRef]
- Albertsen, L.; Richter, J.L.; Peck, P.; Dalhammar, C.; Plepys, A. Circular Business Models for Electric Vehicle Lithium-Ion Batteries: An Analysis of Current Practices of Vehicle Manufacturers and Policies in the EU. Resour. Conserv. Recycl. 2021, 172, 105658. [Google Scholar] [CrossRef]
- Islam, M.T.; Iyer-Raniga, U. Lithium-Ion Battery Recycling in the Circular Economy: A Review. Recycling 2022, 7, 33. [Google Scholar] [CrossRef]
- Mayyas, A.; Steward, D.; Mann, M. The Case for Recycling: Overview and Challenges in the Material Supply Chain for Automotive Li-Ion Batteries. Sustain. Mater. Technol. 2019, 19, e00087. [Google Scholar] [CrossRef]
- Rey, I.; Vallejo, C.; Santiago, G.; Iturrondobeitia, M.; Lizundia, E. Environmental Impacts of Graphite Recycling from Spent Lithium-Ion Batteries Based on Life Cycle Assessment. ACS Sustain. Chem. Eng. 2021, 9, 14488–14501. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Macias-Garbett, R.; Malacara-Becerra, A.; Iqbal, H.M.N.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Environmental Impact of Emerging Contaminants from Battery Waste: A Mini Review. Case Stud. Chem. Environ. Eng. 2021, 3, 100104. [Google Scholar] [CrossRef]
- Ciez, R.E.; Whitacre, J.F. Examining Different Recycling Processes for Lithium-Ion Batteries. Nat. Sustain. 2019, 2, 148–156. [Google Scholar] [CrossRef]
- Slattery, M.; Dunn, J.; Kendall, A. Transportation of Electric Vehicle Lithium-Ion Batteries at End-of-Life: A Literature Review. Resour. Conserv. Recycl. 2021, 174, 105755. [Google Scholar] [CrossRef]
- Melin, H.E.; Rajaeifar, M.A.; Ku, A.Y.; Kendall, A.; Harper, G.; Heidrich, O. Global Implications of the EU Battery Regulation. Science 2021, 373, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Miatto, A.; Wolfram, P.; Reck, B.K.; Graedel, T.E. Uncertain Future of American Lithium: A Perspective until 2050. Environ. Sci. Technol. 2021, 55, 16184–16194. [Google Scholar] [CrossRef] [PubMed]
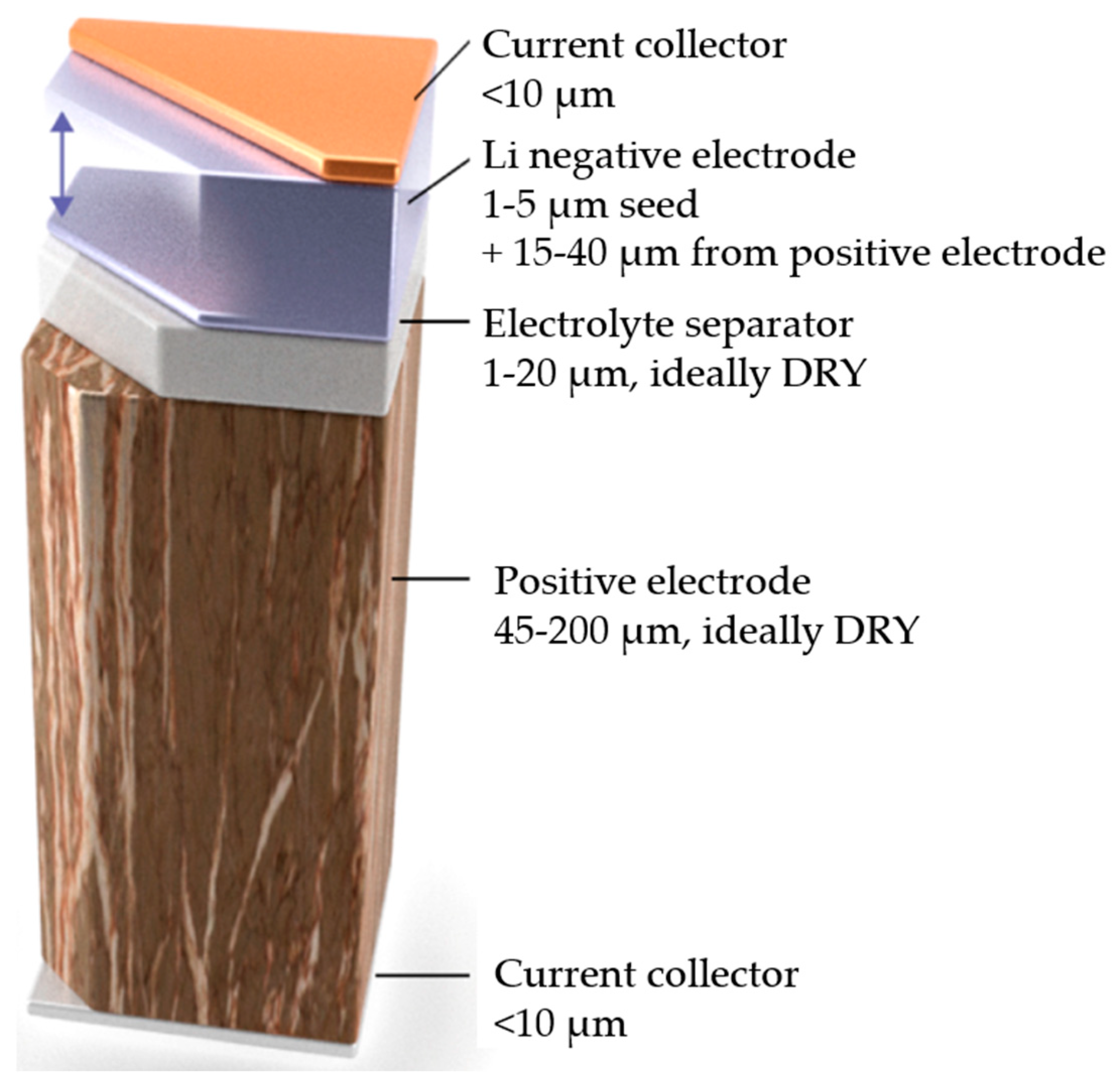
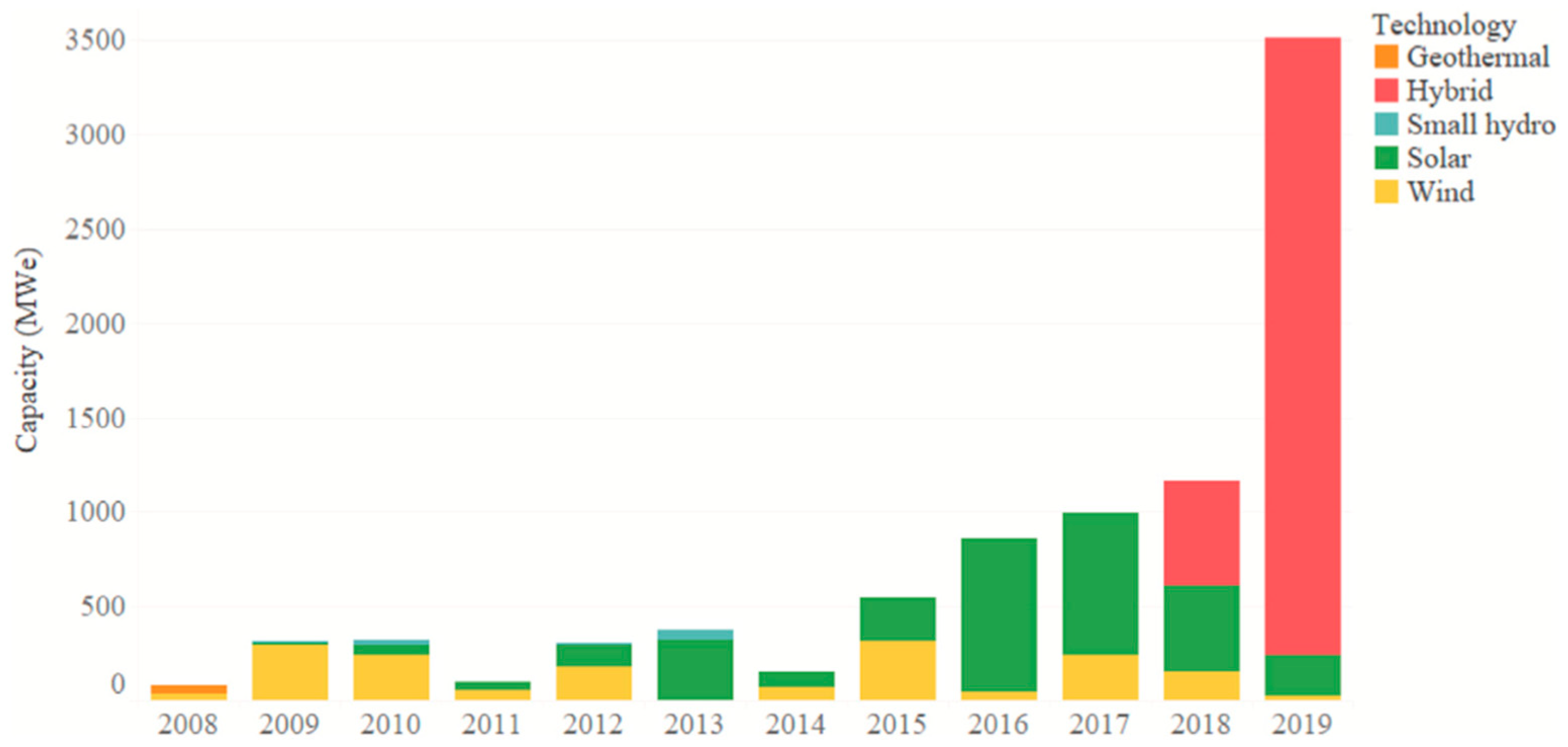


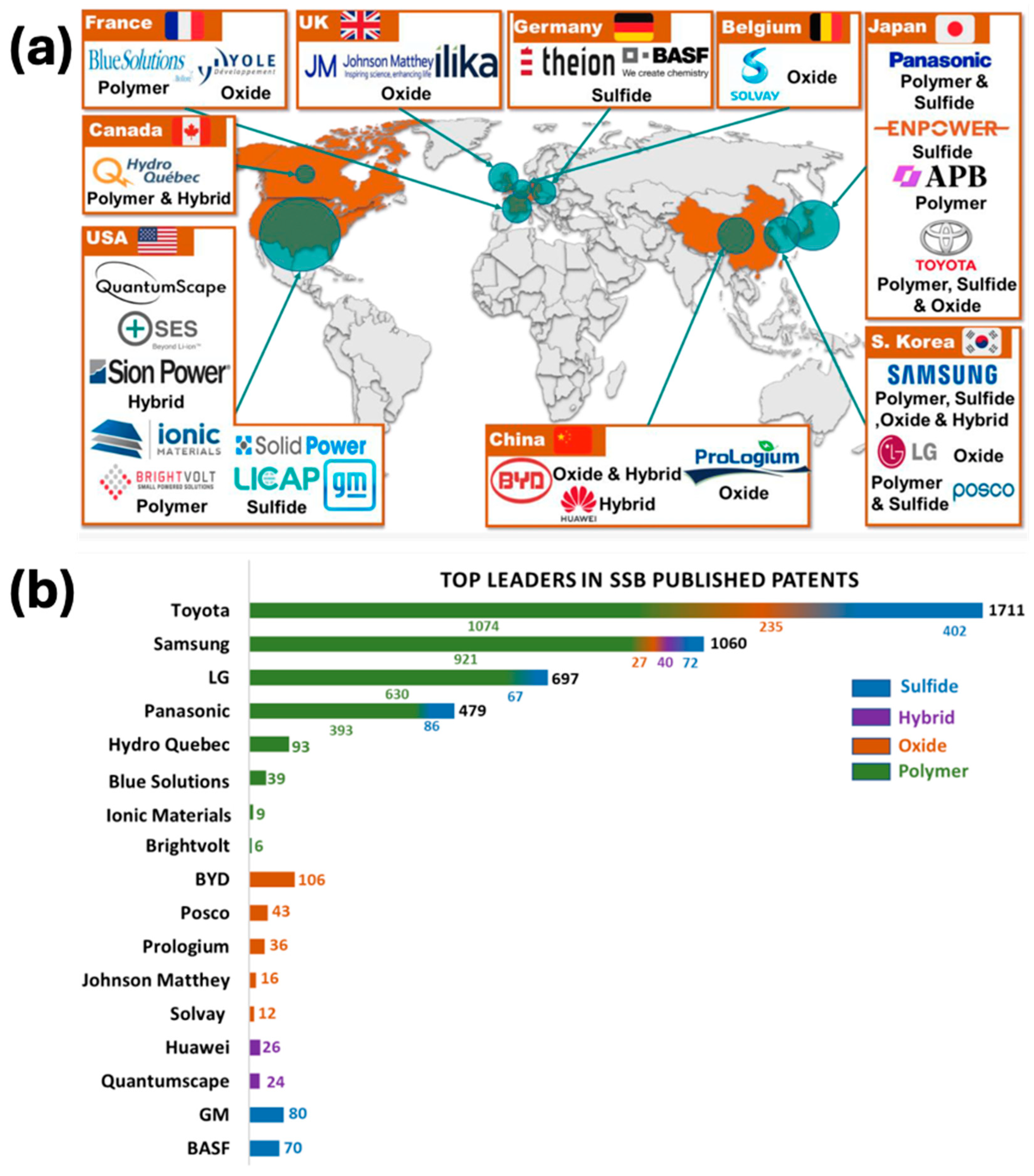
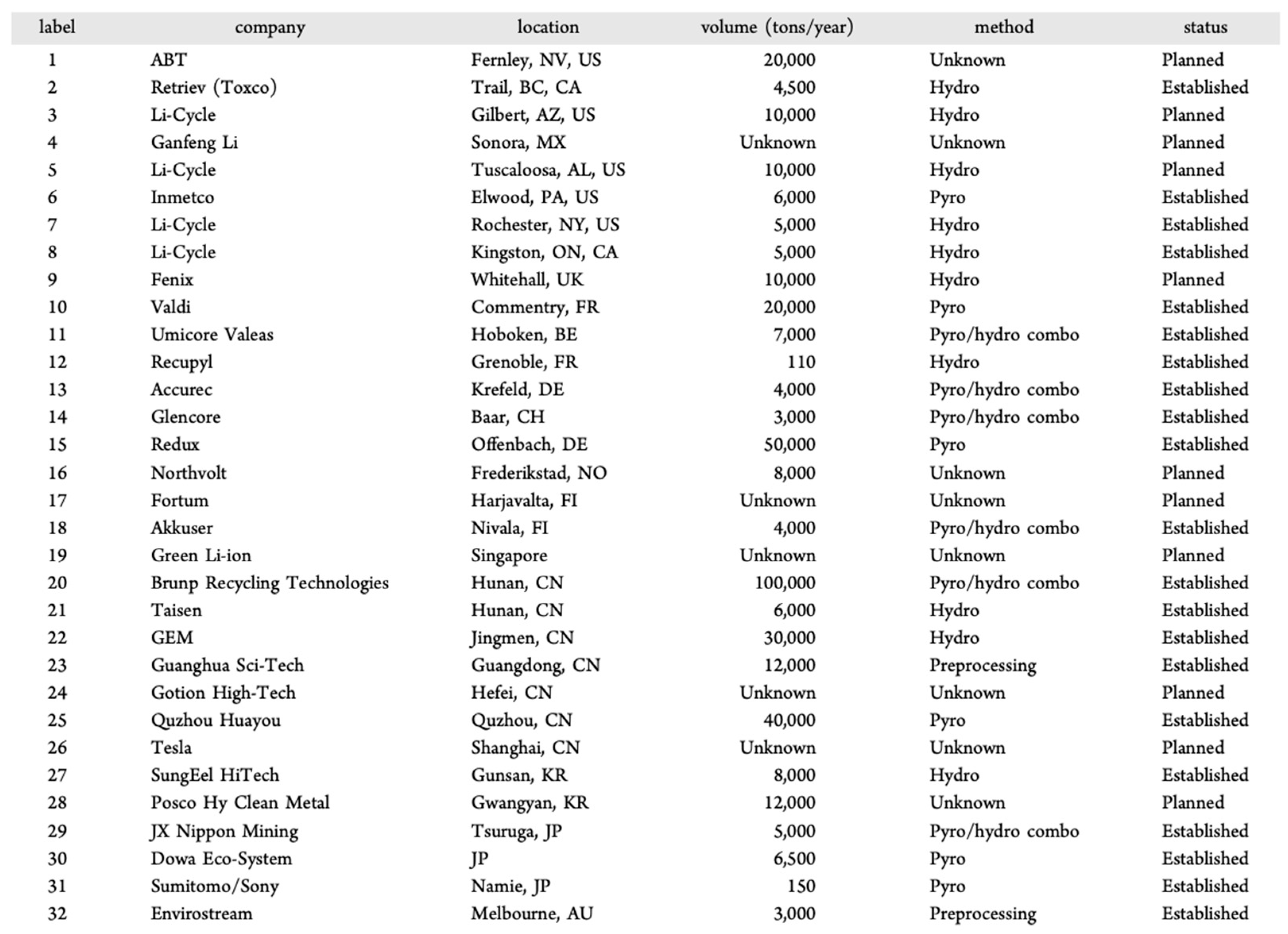
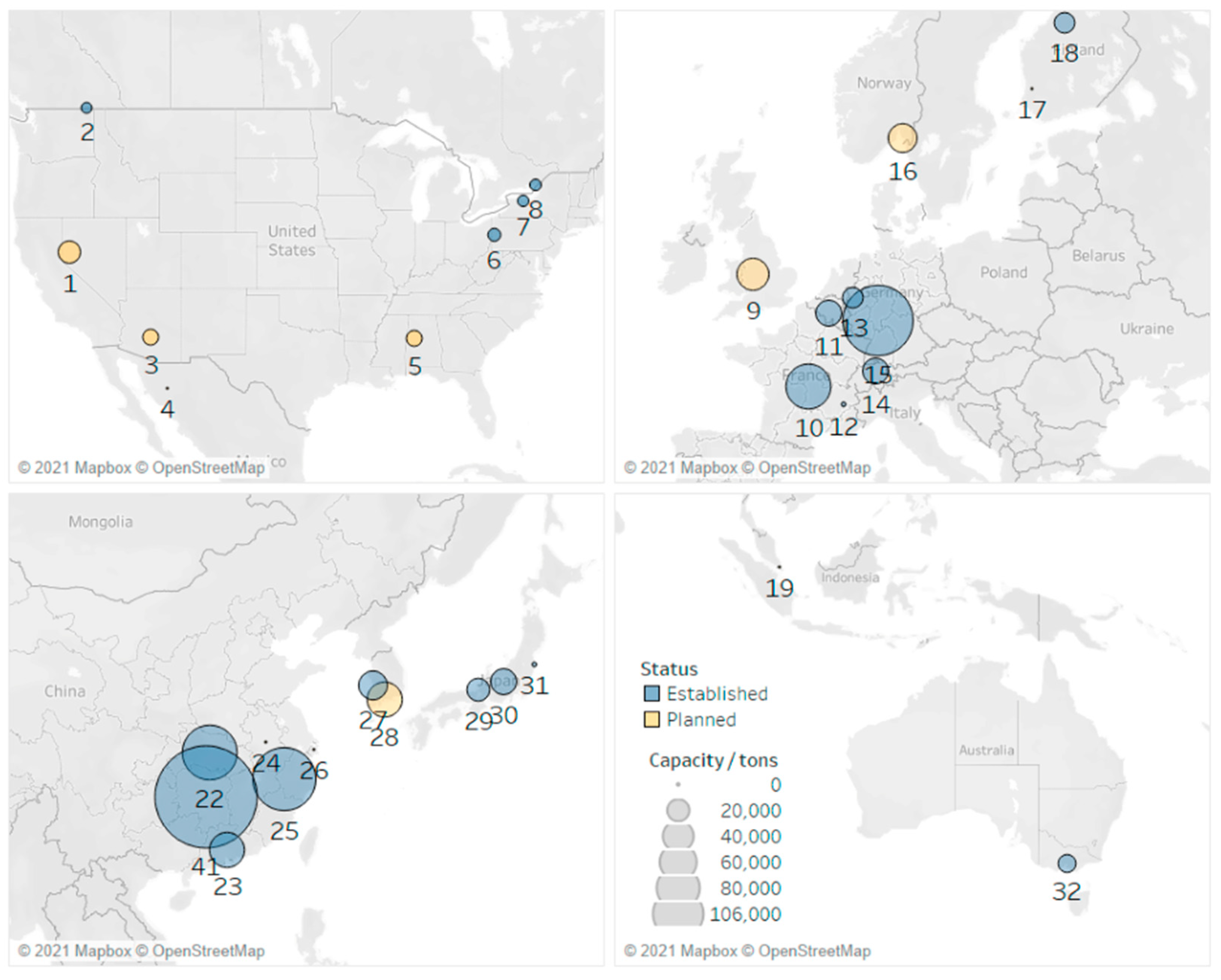
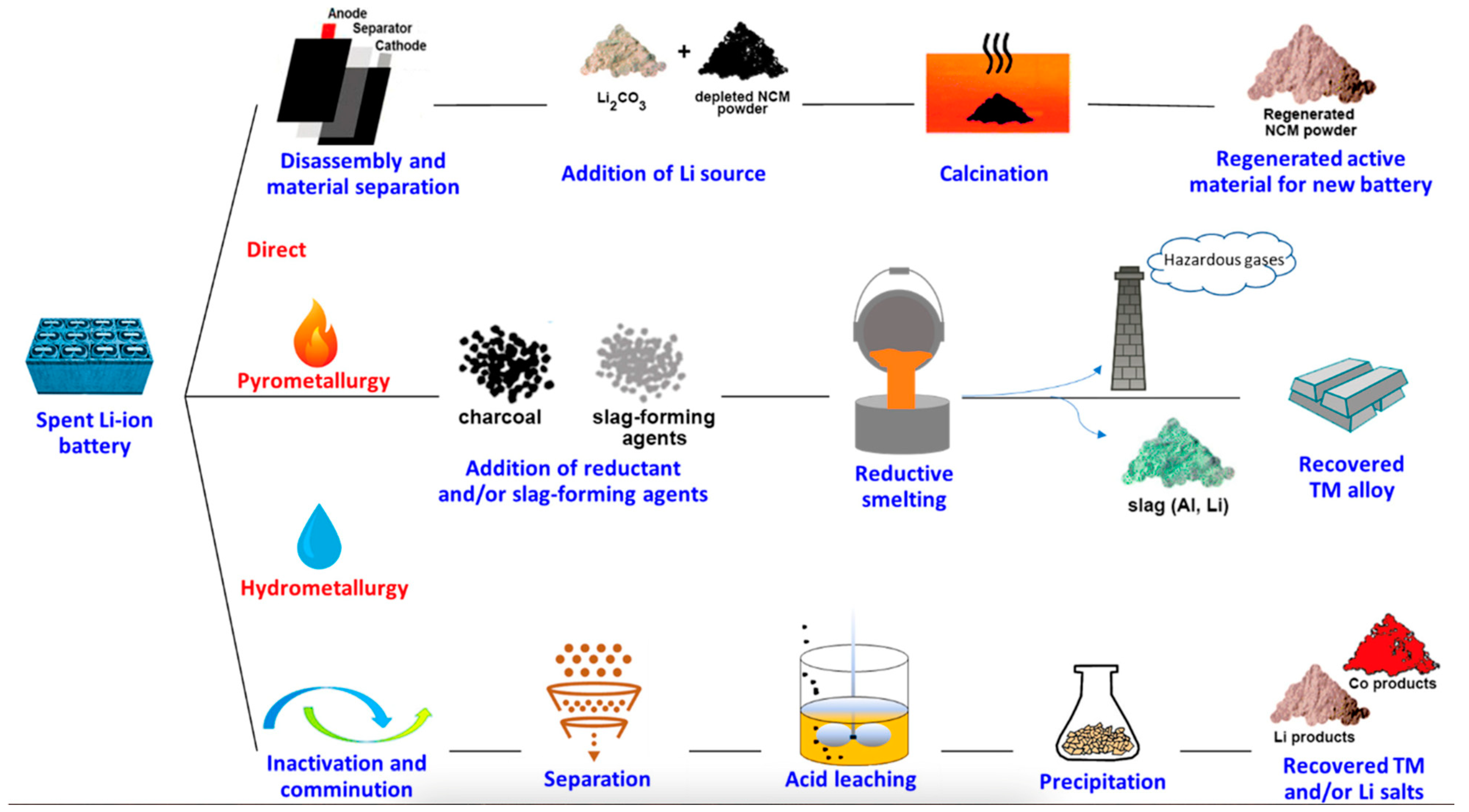
| Solid-State Batteries (SSBs) | Conventional Lithium-Ion Batteries (LIBs) | |
|---|---|---|
| Technology | SSBs represent a significant advancement over LIBs by replacing traditional liquid or gel electrolytes with solid alternatives. This transition allows for greater design flexibility and potential for higher energy densities. | LIBs use liquid electrolytes (typically lithium salts in organic solvents) to facilitate ion movement between electrodes. They have been foundational in portable electronics and the electric vehicle industry for decades. |
| Advantages | Higher energy density: Solid electrolytes enable the use of more active materials, resulting in batteries that can store more energy per unit volume or weight. | Established infrastructure: LIBs benefit from established manufacturing processes and infrastructure for large-scale production. They also have well-developed recycling methods, supporting their economic and environmental sustainability. |
| Improved safety: solid-state design reduces the risk of electrolyte leakage, thermal runaway, and fire hazards associated with liquid electrolytes. | ||
| Longer lifespan: enhanced chemical stability of solid electrolytes contributes to longer cycle life and durability. | ||
| Efficiency across conditions: solid-state architecture enhances battery efficiency across a wider range of temperatures and operating conditions. | ||
| Potential for faster charging: the solid-state design allows for faster ion transport, potentially enabling quicker charging times compared to LIBs. | ||
| Challenges | Interface dynamics: solid electrolytes require precise engineering to maintain stable interfaces with electrodes, which can affect battery performance and longevity. | Safety concerns: liquid electrolytes are prone to leakage and pose fire risks under certain conditions. |
| Material science innovation: continued research is needed to optimize solid electrolyte materials for performance, cost, and scalability. | Lower energy density: limited by the capacity of liquid electrolytes to store ions, which restricts energy storage capacity relative to SSBs. | |
| Environmental impact: concerns include the ecological footprint of raw material extraction (e.g., lithium, cobalt, nickel) for solid electrolytes and the energy-intensive manufacturing processes for solid electrolytes. | Operating efficiency: LIBs may exhibit lower efficiency and performance variability across different temperature ranges compared to solid-state designs. | |
| Environmental Impact | Material extraction: mining of materials like lithium, cobalt, and nickel for solid electrolytes can have significant environmental impacts, including habitat disruption, water pollution, and carbon emissions. | Material extraction: extraction of lithium and other materials for liquid electrolytes can involve environmentally sensitive mining practices. |
| Energy intensity: manufacturing processes for solid-state batteries, such as high-temperature sintering for solid electrolytes, contribute to their carbon footprint. | Production processes: energy-intensive manufacturing processes contribute to LIBs’ carbon footprint and environmental impact. | |
| Recycling challenges: developing effective recycling methods for SSBs is crucial to minimize waste and recover valuable materials due to the complex composition of solid-state battery cells. | Recycling and disposal: LIBs have established recycling infrastructure, but challenges remain in efficiently recovering materials and reducing waste in their end-of-life management. |
| Manufacturing Process | Solid-State Batteries (SSBs) | Conventional Lithium-Ion Batteries (LIBs) |
|---|---|---|
| Electrode Preparation | Often involves the use of dry processes to avoid solvent interactions with the solid electrolyte. Coating and compressing techniques need to account for the brittleness of solid electrolytes. | Typically involves slurry casting processes where active materials, binders, and conductive additives are mixed in a solvent. |
| Electrolyte Integration | Solid electrolytes are integrated either as a separate layer or combined with electrodes in a composite structure. Processes include physical vapor deposition, sintering, or cold pressing. | Liquid electrolytes are added after assembling the cell components, allowing for impregnation into the porous electrode structure. |
| Cell Assembly | Requires careful handling to prevent damage to solid electrolyte layers. Layers are laminated under heat and pressure to ensure good contact and ionic conductivity. | Assembly in dry environments to prevent moisture interaction; electrodes and separators are stacked and rolled. |
| Sealing and Encapsulation | High-integrity sealing is critical to prevent moisture ingress, which can degrade the solid electrolyte. Often requires advanced laser welding techniques. | Sealing is important, but less critical compared to SSBs; typically uses crimping and sealing with adhesives or polymers. |
| Formation and Conditioning | May require specific thermal treatment to enhance ionic conductivity and interface stability between electrodes and electrolytes. | Involves initial charging cycles at controlled rates to form a solid–electrolyte interphase (SEI) on the negative electrode. |
| Scaling and Production Issues | Scaling is challenging due to the precision required in handling and layering brittle materials. Higher initial capital for setup due to specialized equipment. | Well-established manufacturing lines with extensive scalability. Lower initial setup costs due to mature technology. |
| Material Compatibility | Requires materials that are mechanically and chemically stable with each other; issues like interface instability need to be managed. | Compatibility mainly revolves around thermal and chemical stability of the liquid electrolyte with electrode materials. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machín, A.; Cotto, M.C.; Díaz, F.; Duconge, J.; Morant, C.; Márquez, F. Environmental Aspects and Recycling of Solid-State Batteries: A Comprehensive Review. Batteries 2024, 10, 255. https://doi.org/10.3390/batteries10070255
Machín A, Cotto MC, Díaz F, Duconge J, Morant C, Márquez F. Environmental Aspects and Recycling of Solid-State Batteries: A Comprehensive Review. Batteries. 2024; 10(7):255. https://doi.org/10.3390/batteries10070255
Chicago/Turabian StyleMachín, Abniel, María C. Cotto, Francisco Díaz, José Duconge, Carmen Morant, and Francisco Márquez. 2024. "Environmental Aspects and Recycling of Solid-State Batteries: A Comprehensive Review" Batteries 10, no. 7: 255. https://doi.org/10.3390/batteries10070255
APA StyleMachín, A., Cotto, M. C., Díaz, F., Duconge, J., Morant, C., & Márquez, F. (2024). Environmental Aspects and Recycling of Solid-State Batteries: A Comprehensive Review. Batteries, 10(7), 255. https://doi.org/10.3390/batteries10070255










