Advancements in Lithium–Oxygen Batteries: A Comprehensive Review of Cathode and Anode Materials
Abstract
:1. Introduction
2. Basic Principles and Fundamental Issues of Lithium–Oxygen Batteries
2.1. Basic Principles of Lithium–Oxygen Batteries
2.2. Fundamental Issues in Lithium–Oxygen Batteries
3. Advances in Anode Material Research
3.1. Strategies for Lithium Anode Alternatives (Non-Pure Lithium Anodes)
3.2. Electrolyte Design
3.3. Protective Layers and Membrane Modifications
4. Advances in Cathode Material Research
4.1. Progress in Cathode Material Research
4.1.1. Advances in Carbon-Based Cathode Materials
4.1.2. Noble Metals and Their Oxides

4.1.3. Transition Metal Oxides/Carbides

4.1.4. Perovskite Materials
4.1.5. Emerging Cathode Materials
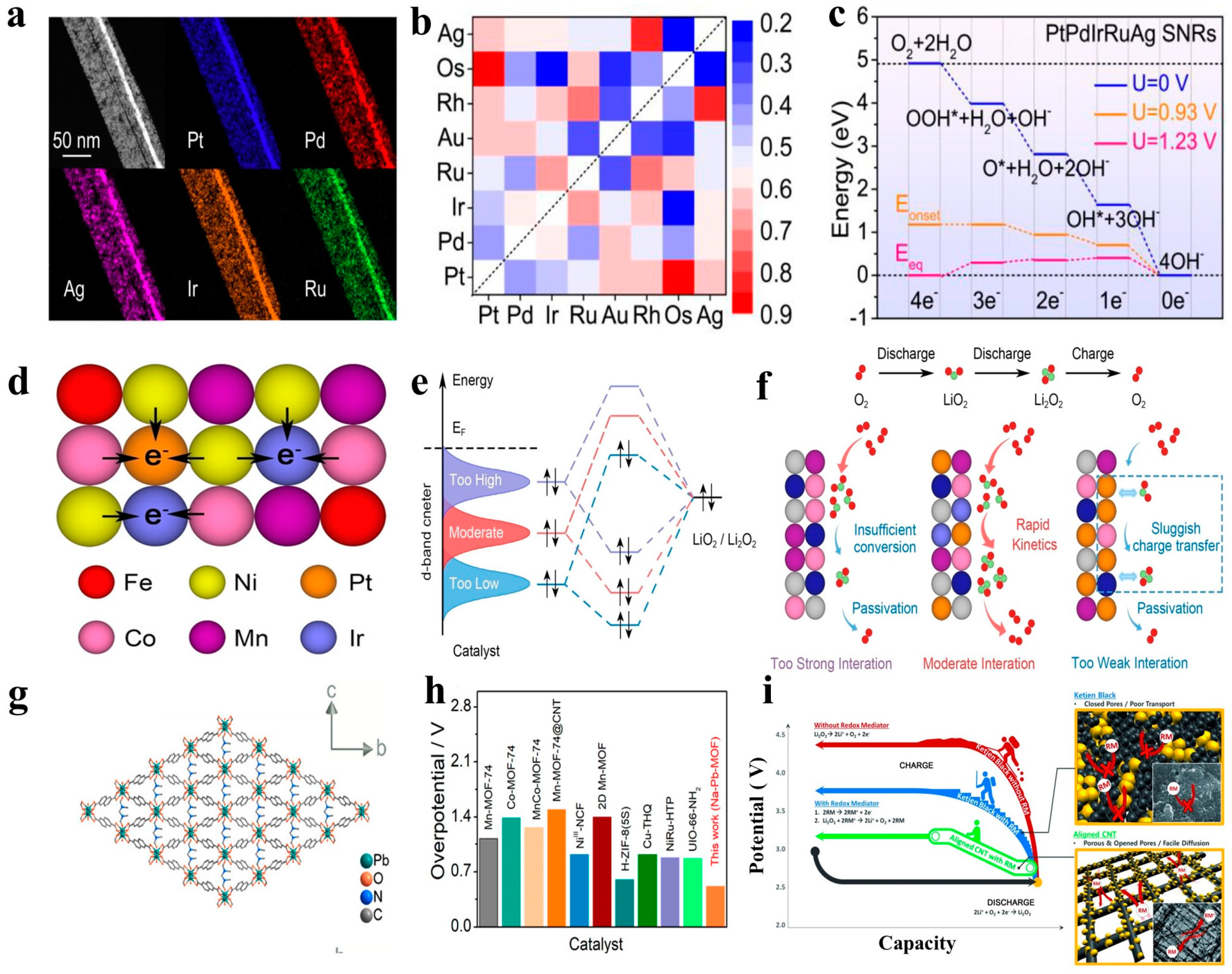
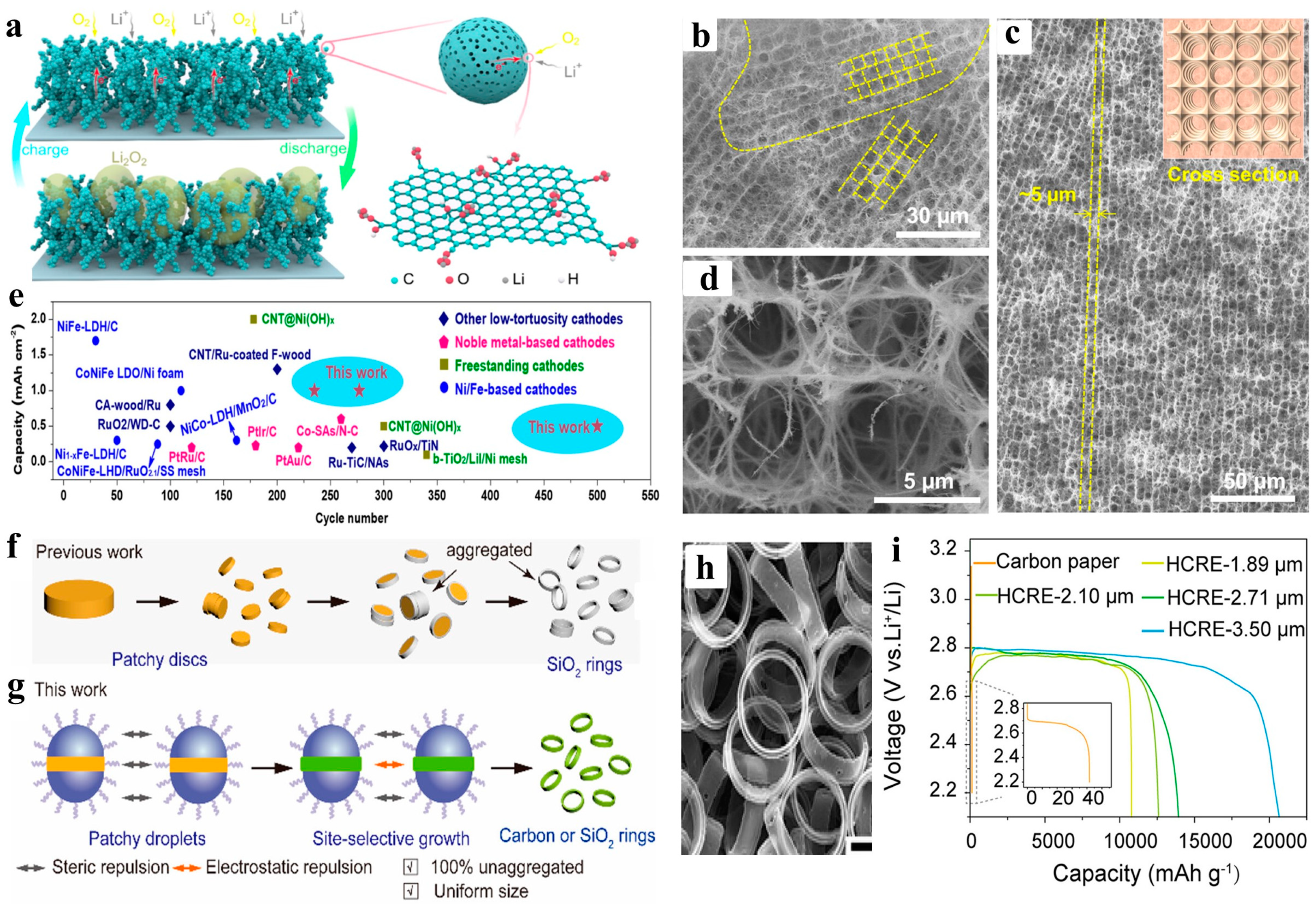
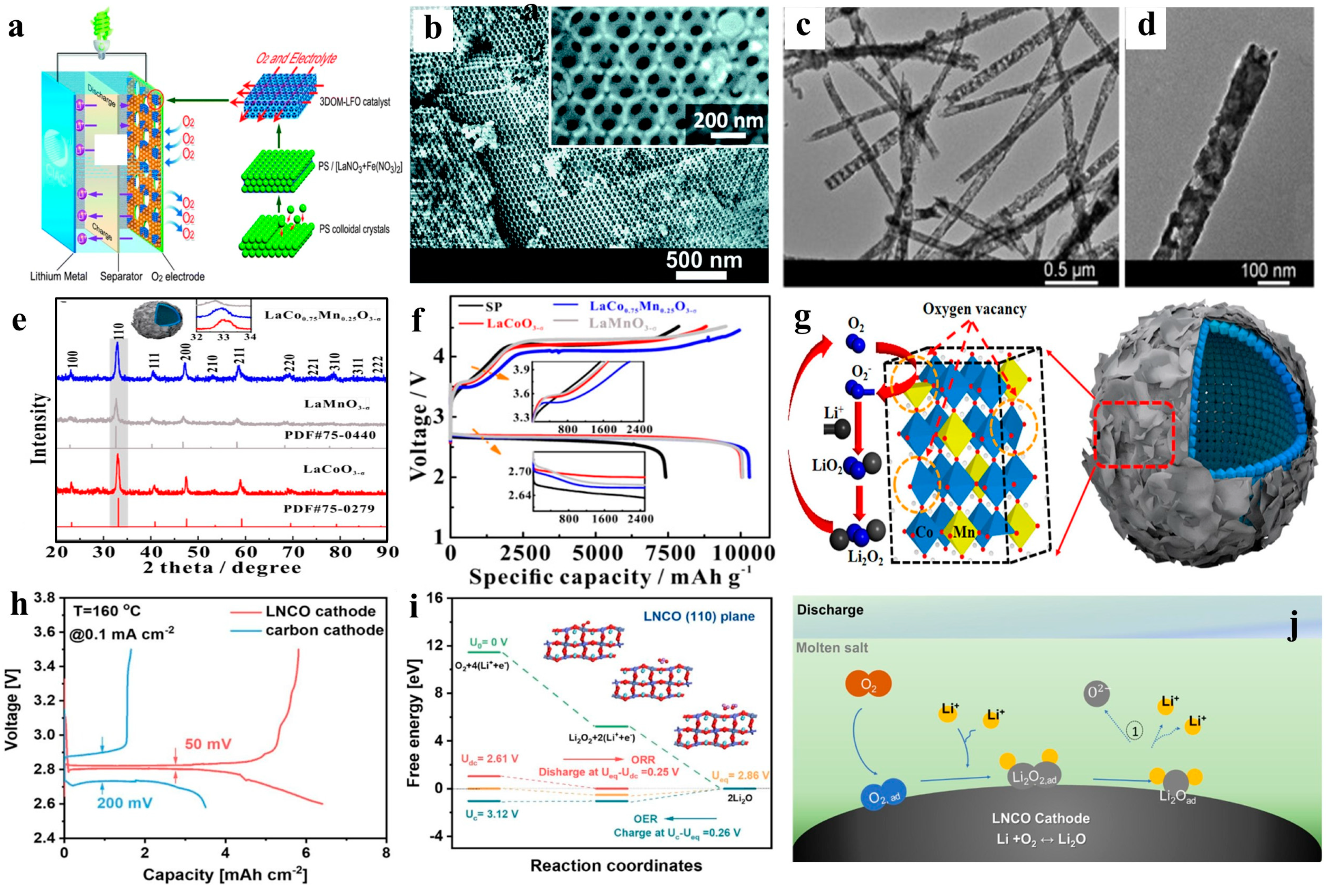
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chi, X.; Li, M.; Di, J.; Bai, P.; Song, L.; Wang, X.; Li, F.; Liang, S.; Xu, J.; Yu, J. A Highly Stable and Flexible Zeolite Electrolyte Solid-State Li-Air Battery. Nature 2021, 592, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The Lithium-Ion Battery: State of the Art and Future Perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical Energy Storage for Transportation—Approaching the Limits of, and Going beyond, Lithium-Ion Batteries. Energy Environ. Sci. 2012, 5, 7854. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Lu, T.; Qian, Y.; Liu, K.; Wu, C.; Li, X.; Xiao, J.; Zeng, X.; Zhang, Y.; Chou, S. Recent Progress of Electrolyte Materials for Solid-State Lithium-Oxygen (Air) Batteries. Adv. Energy Mater. 2024, 14, 2400766. [Google Scholar] [CrossRef]
- Rigoni, A.S.; Breedon, M.; Spencer, M.J.S. Use of Perfluorochemicals in Li-Air Batteries: A Critical Review. ACS Appl. Mater. Interfaces 2024, 16, 26967–26983. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yang, Y.; Zhang, Q.; Xie, Z.; Zhou, Z. Graphitic Carbon Nitride (g-C3N4) Based Photo-Assisted Li–O2 Batteries: Progress, Challenge, and Perspective. Coord. Chem. Rev. 2024, 511, 215879. [Google Scholar] [CrossRef]
- Ge, B.; Hu, L.; Yu, X.; Wang, L.; Fernandez, C.; Yang, N.; Liang, Q.; Yang, Q. Engineering Triple-Phase Interfaces around the Anode toward Practical Alkali Metal–Air Batteries. Adv. Mater. 2024, 36, 2400937. [Google Scholar] [CrossRef] [PubMed]
- Geng, D.; Ding, N.; Hor, T.S.A.; Chien, S.W.; Liu, Z.; Wuu, D.; Sun, X.; Zong, Y. From Lithium-Oxygen to Lithium-Air Batteries: Challenges and Opportunities. Adv. Energy Mater. 2016, 6, 1502164. [Google Scholar] [CrossRef]
- Jung, J.-W.; Cho, S.-H.; Nam, J.S.; Kim, I.-D. Current and Future Cathode Materials for Non-Aqueous Li-Air (O2) Battery Technology—A Focused Review. Energy Storage Mater. 2020, 24, 512–528. [Google Scholar] [CrossRef]
- Kumar, J.; Kumar, B. Development of Membranes and a Study of Their Interfaces for Rechargeable Lithium-Air Battery. J. Power Sources 2009, 194, 1113–1119. [Google Scholar] [CrossRef]
- Chang, Z.; Xu, J.; Liu, Q.; Li, L.; Zhang, X. Recent Progress on Stability Enhancement for Cathode in Rechargeable Non-Aqueous Lithium-Oxygen Battery. Adv. Energy Mater. 2015, 5, 1500633. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, Q.; Chen, J. Strategies toward High-Performance Cathode Materials for Lithium-Oxygen Batteries. Small 2018, 14, 1800078. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Chen, J. Metal-Air Batteries: From Oxygen Reduction Electrochemistry to Cathode Catalysts. Chem. Soc. Rev. 2012, 41, 2172. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Ding, F.; Xiao, J.; Zhang, J.; Xu, W.; Park, S.; Zhang, J.; Wang, Y.; Liu, J. Making Li-Air Batteries Rechargeable: Material Challenges. Adv. Funct. Mater. 2013, 23, 987–1004. [Google Scholar] [CrossRef]
- Xu, K.; Von Cresce, A. Interfacing Electrolytes with Electrodes in Li Ion Batteries. J. Mater. Chem. 2011, 21, 9849. [Google Scholar] [CrossRef]
- Shu, C.; Wang, J.; Long, J.; Liu, H.; Dou, S. Understanding the Reaction Chemistry during Charging in Aprotic Lithium-Oxygen Batteries: Existing Problems and Solutions. Adv. Mater. 2019, 31, 1804587. [Google Scholar] [CrossRef] [PubMed]
- Hirshberg, D.; Sharon, D.; De La Llave, E.; Afri, M.; Frimer, A.A.; Kwak, W.-J.; Sun, Y.-K.; Aurbach, D. Feasibility of Full (Li-Ion)-O2 Cells Comprised of Hard Carbon Anodes. ACS Appl. Mater. Interfaces 2017, 9, 4352–4361. [Google Scholar] [CrossRef]
- Deng, H.; Qiu, F.; Li, X.; Qin, H.; Zhao, S.; He, P.; Zhou, H. A Li-Ion Oxygen Battery with Li-Si Alloy Anode Prepared by a Mechanical Method. Electrochem. Commun. 2017, 78, 11–15. [Google Scholar] [CrossRef]
- Hassoun, J.; Jung, H.-G.; Lee, D.-J.; Park, J.-B.; Amine, K.; Sun, Y.-K.; Scrosati, B. A Metal-Free, Lithium-Ion Oxygen Battery: A Step Forward to Safety in Lithium-Air Batteries. Nano Lett. 2012, 12, 5775–5779. [Google Scholar] [CrossRef] [PubMed]
- Kwak, W.-J.; Shin, H.-J.; Reiter, J.; Tsiouvaras, N.; Hassoun, J.; Passerini, S.; Scrosati, B.; Sun, Y.-K. Understanding Problems of Lithiated Anodes in Lithium Oxygen Full-Cells. J. Mater. Chem. A 2016, 4, 10467–10471. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, K.; Tang, J.; Liao, K.; Bai, S.; Yi, J.; Yamauchi, Y.; Ishida, M.; Zhou, H. A Long-Life Lithium Ion Oxygen Battery Based on Commercial Silicon Particles as the Anode. Energy Environ. Sci. 2016, 9, 3262–3271. [Google Scholar] [CrossRef]
- Lökçü, E.; Anik, M. Synthesis and Electrochemical Performance of Lithium Silicide Based Alloy Anodes for Li-Ion Oxygen Batteries. Int. J. Hydrogen Energy 2021, 46, 10624–10631. [Google Scholar] [CrossRef]
- Elia, G.A.; Bresser, D.; Reiter, J.; Oberhumer, P.; Sun, Y.-K.; Scrosati, B.; Passerini, S.; Hassoun, J. Interphase Evolution of a Lithium-Ion/Oxygen Battery. ACS Appl. Mater. Interfaces 2015, 7, 22638–22643. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Dong, X.; Wang, Y.; Xia, Y. A Lithium Air Battery with a Lithiated Al-Carbon Anode. Chem. Commun. 2015, 51, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Freunberger, S.A.; Peng, Z.; Bardé, F.; Bruce, P.G. Li-O2 Battery with a Dimethylformamide Electrolyte. J. Am. Chem. Soc. 2012, 134, 7952–7957. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Freunberger, S.A.; Peng, Z.; Fontaine, O.; Bruce, P.G. Charging a Li–O2 Battery Using a Redox Mediator. Nat. Chem. 2013, 5, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Bryantsev, V.S.; Giordani, V.; Walker, W.; Uddin, J.; Lee, I.; Van Duin, A.C.T.; Chase, G.V.; Addison, D. Investigation of Fluorinated Amides for Solid-Electrolyte Interphase Stabilization in Li-O2 Batteries Using Amide-Based Electrolytes. J. Phys. Chem. C 2013, 117, 11977–11988. [Google Scholar] [CrossRef]
- Walker, W.; Giordani, V.; Uddin, J.; Bryantsev, V.S.; Chase, G.V.; Addison, D. A Rechargeable Li-O2 Battery Using a Lithium Nitrate/N,N-Dimethylacetamide Electrolyte. J. Am. Chem. Soc. 2013, 135, 2076–2079. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Younesi, R.; Richardson, W.; Liu, J.; Zhu, J.; Edstrom, K.; Gustafsson, T. Increased Cycling Efficiency of Lithium Anodes in Dimethyl Sulfoxide Electrolytes for Use in Li-O2 Batteries. ECS Electrochem. Lett. 2014, 3, A62–A65. [Google Scholar] [CrossRef]
- Zhang, T.; Liao, K.; He, P.; Zhou, H. A Self-Defense Redox Mediator for Efficient Lithium-O2 Batteries. Energy Environ. Sci. 2016, 9, 1024–1030. [Google Scholar] [CrossRef]
- Lim, H.; Song, H.; Kim, J.; Gwon, H.; Bae, Y.; Park, K.; Hong, J.; Kim, H.; Kim, T.; Kim, Y.H.; et al. Superior Rechargeability and Efficiency of Lithium–Oxygen Batteries: Hierarchical Air Electrode Architecture Combined with a Soluble Catalyst. Angew. Chem.-Int. Ed. 2014, 53, 3926–3931. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ren, J.; Zhang, W.; Xie, M.; Li, Y.; Sun, D.; Shen, Y.; Huang, Y. Protecting the Li-Metal Anode in a Li-O2 Battery by Using Boric Acid as an SEI-Forming Additive. Adv. Mater. 2018, 30, 1803270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Guo, Z.; Xu, Y.; Wang, Y.; Peng, H. High-Performance Lithium-Air Battery with a Coaxial-Fiber Architecture. Angew. Chem.-Int. Ed. 2016, 55, 4487–4491. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Geng, J.; Jiang, Z.; Ren, M.; Wen, B.; Chen, J.; Li, F. Solvation Structure with Enhanced Anionic Coordination for Stable Anodes in Lithium-Oxygen Batteries. Angew. Chem. Int. Ed. 2023, 62, e202306236. [Google Scholar] [CrossRef] [PubMed]
- Kichambare, P.; Rodrigues, S.; Kumar, J. Mesoporous Nitrogen-Doped Carbon-Glass Ceramic Cathodes for Solid-State Lithium–Oxygen Batteries. ACS Appl. Mater. Interfaces 2012, 4, 49–52. [Google Scholar] [CrossRef]
- Kitaura, H.; Zhou, H. Electrochemical Performance and Reaction Mechanism of All-Solid-State Lithium-Air Batteries Composed of Lithium, Li1+xAlyGe2−y(PO4)3 Solid Electrolyte and Carbon Nanotube Air Electrode. Energy Environ. Sci. 2012, 5, 9077. [Google Scholar] [CrossRef]
- Yi, J.; Liu, X.; Guo, S.; Zhu, K.; Xue, H.; Zhou, H. Novel Stable Gel Polymer Electrolyte: Toward a High Safety and Long Life Li-Air Battery. ACS Appl. Mater. Interfaces 2015, 7, 23798–23804. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.C.; Ida, S.; Ishihara, T. Surface Coating Layer on Li Metal for Increased Cycle Stability of Li-O2 Batteries. J. Electrochem. Soc. 2014, 161, A821–A826. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Gao, S.; Dou, Y.; Pan, K.; Zhang, Z.; Zhou, Z. Electrospinning-Assisted Porous Skeleton Electrolytes for Semi-Solid Li-O2 Batteries. Chem. Commun. 2024, 60, 5070–5073. [Google Scholar] [CrossRef]
- Amici, J.; Alidoost, M.; Caldera, F.; Versaci, D.; Zubair, U.; Trotta, F.; Francia, C.; Bodoardo, S. PEEK-WC/Nanosponge Membranes for Lithium-Anode Protection in Rechargeable Li-O2 Batteries. ChemElectroChem 2018, 5, 1599–1605. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, T.H.; Lee, Y.J.; Park, H.B.; Lee, Y.J. Graphene Oxide Sieving Membrane for Improved Cycle Life in High-Efficiency Redox-Mediated Li-O2 Batteries. Small 2018, 14, 1801456. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Koo, D.; Ha, S.; Jung, S.C.; Yim, T.; Kim, H.; Oh, S.K.; Kim, D.-M.; Choi, A.; Kang, Y.; et al. Two-Dimensional Phosphorene-Derived Protective Layers on a Lithium Metal Anode for Lithium-Oxygen Batteries. ACS Nano 2018, 12, 4419–4430. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Sayahpour, B.; Abbasi, P.; Ngo, A.T.; Karis, K.; Jokisaari, J.R.; Liu, C.; Narayanan, B.; Gerard, M.; Yasaei, P.; et al. A Lithium-Oxygen Battery with a Long Cycle Life in an Air-like Atmosphere. Nature 2018, 555, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-S.; Kwak, W.-J.; Chae, S.; Wi, S.; Li, L.; Hu, J.; Tao, J.; Wang, C.; Xu, W.; Zhang, J.-G. Stable Solid Electrolyte Interphase Layer Formed by Electrochemical Pretreatment of Gel Polymer Coating on Li Metal Anode for Lithium-Oxygen Batteries. ACS Energy Lett. 2021, 6, 3321–3331. [Google Scholar] [CrossRef]
- Shi, L.; Li, Z.; Li, Y.; Wang, G.; Wu, M.; Wen, Z. Suppressing Redox Shuttle with MXene-Modified Separators for Li-O2 Batteries. ACS Appl. Mater. Interfaces 2021, 13, 30766–30775. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yang, J.; Zhou, J.; Zhang, T.; Li, L.; Wang, J.; Nuli, Y. A Stable Organic-Inorganic Hybrid Layer Protected Lithium Metal Anode for Long-Cycle Lithium-Oxygen Batteries. J. Power Sources 2017, 366, 265–269. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Zou, G.; Liu, D.; Peng, Q. Lithiation MXene Derivative Skeletons for Wide-Temperature Lithium Metal Anodes. Adv. Funct. Mater. 2021, 31, 2101180. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Wang, J.; Liu, D.; Zou, G.; Liu, B.; Tse, J.S.; Li, L.; Ren, L.; Peng, Q. Lithiation MAX Derivative Electrodes with Low Overpotential and Long-Term Cyclability in a Wide-Temperature Range. Energy Storage Mater. 2022, 47, 611–619. [Google Scholar] [CrossRef]
- Liu, J.; Bai, L.; He, J.; Wang, C.; Li, Q.; Song, F.; Hong, Z.; Chen, Y.; Zeng, F.; Cheng, C.; et al. Interfacial Polymerization-Modified Polyetherimide (PEI) Separator for Li-O2 Battery with Boosted Performance. ACS Appl. Polym. Mater. 2022, 4, 5781–5788. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, K.; Zhang, C.; Yu, H.; Wang, X.; Yang, D.; Wang, J.; Huang, G.; Zhang, S. A Novel Material for High-Performance Li-O2 Battery Separator: Polyetherketone Nanofiber Membrane. Small 2022, 18, 2201470. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.M.; Jiang, Z. A Polymer Electrolyte-Based Rechargeable Lithium/Oxygen Battery. J. Electrochem. Soc. 1996, 143, 1–5. [Google Scholar] [CrossRef]
- Luo, L.; Liu, B.; Song, S.; Xu, W.; Zhang, J.-G.; Wang, C. Revealing the Reaction Mechanisms of Li-O2 Batteries Using Environmental Transmission Electron Microscopy. Nat. Nanotechnol. 2017, 12, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Freunberger, S.A.; Hardwick, L.J.; Chen, Y.; Giordani, V.; Bardé, F.; Novák, P.; Graham, D.; Tarascon, J.; Bruce, P.G. Oxygen Reactions in a Non-Aqueous Li+ Electrolyte. Angew. Chem.-Int. Ed. 2011, 50, 6351–6355. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Scheffler, R.; Speidel, A.; Bethune, D.S.; Shelby, R.M.; Luntz, A.C. On the Efficacy of Electrocatalysis in Nonaqueous Li-O2 Batteries. J. Am. Chem. Soc. 2011, 133, 18038–18041. [Google Scholar] [CrossRef]
- Zhang, S.S.; Foster, D.; Read, J. Discharge Characteristic of a Non-Aqueous Electrolyte Li/O2 Battery. J. Power Sources 2010, 195, 1235–1240. [Google Scholar] [CrossRef]
- Adams, B.D.; Radtke, C.; Black, R.; Trudeau, M.L.; Zaghib, K.; Nazar, L.F. Current Density Dependence of Peroxide Formation in the Li-O2 Battery and Its Effect on Charge. Energy Environ. Sci. 2013, 6, 1772. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Speidel, A.; Scheffler, R.; Miller, D.C.; Viswanathan, V.; Hummelshøj, J.S.; Nørskov, J.K.; Luntz, A.C. Twin Problems of Interfacial Carbonate Formation in Nonaqueous Li–O2 Batteries. J. Phys. Chem. Lett. 2012, 3, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Carboni, M.; Marrani, A.G.; Spezia, R.; Brutti, S. 1,2-Dimethoxyethane Degradation Thermodynamics in Li-O2 Redox Environments. Chem.-Eur. J. 2016, 22, 17188–17203. [Google Scholar] [CrossRef] [PubMed]
- Mahne, N.; Schafzahl, B.; Leypold, C.; Leypold, M.; Grumm, S.; Leitgeb, A.; Strohmeier, G.A.; Wilkening, M.; Fontaine, O.; Kramer, D.; et al. Singlet Oxygen Generation as a Major Cause for Parasitic Reactions during Cycling of Aprotic Lithium-Oxygen Batteries. Nat. Energy 2017, 2, 17036. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kannan, A.G.; Woo, H.-S.; Jin, D.-G.; Kim, W.; Ryu, K.; Kim, D.-W. A Bi-Functional Metal-Free Catalyst Composed of Dual-Doped Graphene and Mesoporous Carbon for Rechargeable Lithium-Oxygen Batteries. J. Mater. Chem. A 2015, 3, 18456–18465. [Google Scholar] [CrossRef]
- Lou, P.; Cui, Z.; Guo, X. Achieving Highly Stable Li-O2 Battery Operation by Designing a Carbon Nitride-Based Cathode towards a Stable Reaction Interface. J. Mater. Chem. A 2017, 5, 18207–18213. [Google Scholar] [CrossRef]
- Zhan, Y.; Lu, M.; Yang, S.; Xu, C.; Liu, Z.; Lee, J.Y. Activity of Transition-Metal (Manganese, Iron, Cobalt, and Nickel) Phosphates for Oxygen Electrocatalysis in Alkaline Solution. ChemCatChem 2016, 8, 372–379. [Google Scholar] [CrossRef]
- Ma, S.; Xie, M.; Lou, A.; Dong, Y.; Li, Z.; Lu, Y.; Liu, Q. Air Activation Enhances the Porosity and N,O Synergistic Effect towards an Efficient Metal Free Carbon Cathode for Li-O2 Battery. Electrochim. Acta 2024, 475, 143660. [Google Scholar] [CrossRef]
- Zhao, T.; Yao, Y.; Yuan, Y.; Wang, M.; Wu, F.; Amine, K.; Lu, J. A Universal Method to Fabricating Porous Carbon for Li-O2 Battery. Nano Energy 2021, 82, 105782. [Google Scholar] [CrossRef]
- Zhao, B.; Ye, Z.; Kong, X.; Han, L.; Xia, Z.; Chen, K.; Wang, Q.; Li, M.; Shang, Y.; Cao, A. Orthogonal-Channel, Low-Tortuosity Carbon Nanotube Platforms for High-Performance Li–O2 Batteries. ACS Nano 2023, 17, 18382–18391. [Google Scholar] [CrossRef]
- Shi, S.; Shen, Z.; Li, S.; Wang, Q.; Wen, R.; Liu, B. High-Yield Synthesis of Colloidal Carbon Rings and Their Applications in Self-Standing Electrodes of Li-O2 Batteries. J. Am. Chem. Soc. 2023, 145, 27664–27671. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Lv, W.; Wei, W.; Kang, F.; Zhai, D.; Yang, Q.-H. Oxygen-Enriched Carbon Nanotubes as a Bifunctional Catalyst Promote the Oxygen Reduction/Evolution Reactions in Li-O2 Batteries. Carbon 2019, 141, 561–567. [Google Scholar] [CrossRef]
- Jo, H.-G.; Ahn, H.-J. Accelerating the Oxygen Reduction Reaction and Oxygen Evolution Reaction Activities of N and P Co-Doped Porous Activated Carbon for Li-O2 Batteries. Catalysts 2020, 10, 1316. [Google Scholar] [CrossRef]
- Jiang, Y.; Cheng, J.; Zou, L.; Li, X.; Gong, Y.; Chi, B.; Pu, J.; Li, J. In-Situ Growth of CeO2 Nanoparticles on N-Doped Reduced Graphene Oxide for Anchoring Li2O2 Formation in Lithium-Oxygen Batteries. Electrochim. Acta 2016, 210, 712–719. [Google Scholar] [CrossRef]
- Ma, S.; Wu, Y.; Wang, J.; Zhang, Y.; Zhang, Y.; Yan, X.; Wei, Y.; Liu, P.; Wang, J.; Jiang, K.; et al. Reversibility of Noble Metal-Catalyzed Aprotic Li-O2 Batteries. Nano Lett. 2015, 15, 8084–8090. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.J.; Kim, D.Y.; Kim, D.W.; Park, O.O.; Kang, Y. Facile Synthesis of Palladium Nanodendrites Supported on Graphene Nanoplatelets: An Efficient Catalyst for Low Overpotentials in Lithium-Oxygen Batteries. J. Mater. Chem. A 2016, 4, 578–586. [Google Scholar] [CrossRef]
- Xu, J.-J.; Wang, Z.-L.; Xu, D.; Zhang, L.-L.; Zhang, X.-B. Tailoring Deposition and Morphology of Discharge Products towards High-Rate and Long-Life Lithium-Oxygen Batteries. Nat. Commun. 2013, 4, 2438. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Liu, P.; Li, F.; He, P.; Guo, X.; Chen, M.; Zhou, H. Core-Shell-Structured CNT@RuO2 Composite as a High-Performance Cathode Catalyst for Rechargeable Li-O2 Batteries. Angew. Chem.-Int. Ed. 2014, 53, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Wang, X.; Sun, Y.; Tang, D.; Han, M.; He, P.; Jiang, X.; Zhang, T.; Zhou, H. An Oxygen Cathode with Stable Full Discharge-Charge Capability Based on 2D Conducting Oxide. Energy Environ. Sci. 2015, 8, 1992–1997. [Google Scholar] [CrossRef]
- Zhou, Y.; Yin, K.; Gu, Q.; Tao, L.; Li, Y.; Tan, H.; Zhou, J.; Zhang, W.; Li, H.; Guo, S. Lewis-Acidic PtIr Multipods Enable High-Performance Li-O2 Batteries. Angew. Chem.-Int. Ed. 2021, 60, 26592–26598. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jung Lee, Y.; Luo, X.; Chun Lau, K.; Asadi, M.; Wang, H.-H.; Brombosz, S.; Wen, J.; Zhai, D.; Chen, Z.; et al. A Lithium–Oxygen Battery Based on Lithium Superoxide. Nature 2016, 529, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yi, J.; Wu, S.; Liu, Y.; Yang, S.; He, P.; Zhou, H. Li-CO2 Electrochemistry: A New Strategy for CO2 Fixation and Energy Storage. Joule 2017, 1, 359–370. [Google Scholar] [CrossRef]
- Liu, B.; Sun, Y.; Liu, L.; Chen, J.; Yang, B.; Xu, S.; Yan, X. Recent Advances in Understanding Li-CO2 Electrochemistry. Energy Environ. Sci. 2019, 12, 887–922. [Google Scholar] [CrossRef]
- Han, X.; Liu, Y.; Jia, Z.; Chen, Y.-C.; Wan, J.; Weadock, N.; Gaskell, K.J.; Li, T.; Hu, L. Atomic-Layer-Deposition Oxide Nanoglue for Sodium Ion Batteries. Nano Lett. 2014, 14, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Luo, G.; Zhao, Q.; Wu, D.; Yang, T.; Wen, J.; Wang, R.; Xu, C.; Hu, N. Ru Single Atoms on N-Doped Carbon by Spatial Confinement and Ionic Substitution Strategies for High-Performance Li-O2 Batteries. J. Am. Chem. Soc. 2020, 142, 16776–16786. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Ding, Y.; Ma, J.; Das, P.; Zhang, B.; Wu, Z.-S.; Bao, X. Spatially Confined Sub-Nanometer Pt in RuO2 Nanosheet as Robust Bifunctional Oxygen Electrocatalyst for Stabilizing Li-O2 Batteries. Chem Catal. 2023, 3, 100658. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wu, F.; Zou, G.; Gaumet, J.-J.; Li, J.; Fernandez, C.; Wang, Y.; Peng, Q. Nitrogen-Anchored Boridene Enables Mg-CO2 Batteries with High Reversibility. J. Am. Chem. Soc. 2024, 146, 9967–9974. [Google Scholar] [CrossRef] [PubMed]
- Débart, A.; Bao, J.; Armstrong, G.; Bruce, P.G. An O2 Cathode for Rechargeable Lithium Batteries: The Effect of a Catalyst. J. Power Sources 2007, 174, 1177–1182. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, J.; Zhou, J.; Zang, Y.; Zheng, X.; Zhu, Z.; Liu, B.; Wang, G.; Qian, Y. Tailoring the Adsorption Behavior of Superoxide Intermediates on Nickel Carbide Enables High-Rate Li–O2 Batteries. eScience 2022, 2, 389–398. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, S. Recent Advances in Cathode Catalyst Architecture for Lithium–Oxygen Batteries. eScience 2023, 3, 100123. [Google Scholar] [CrossRef]
- Débart, A.; Paterson, A.J.; Bao, J.; Bruce, P.G. α-MnO2 Nanowires: A Catalyst for the O2 Electrode in Rechargeable Lithium Batteries. Angew. Chem.-Int. Ed. 2008, 47, 4521–4524. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhu, G.; Qiu, Z.; Wang, Y.; Xia, Y. High Performance Li-O2 Battery Using γ-MnOOH Nanorods as a Catalyst in an Ionic-Liquid Based Electrolyte. Electrochem. Commun. 2012, 25, 26–29. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zheng, J.P.; Liang, R.; Zhang, C.; Wang, B.; Au, M.; Hendrickson, M.; Plichta, E.J. α-MnO2/Carbon Nanotube/Carbon Nanofiber Composite Catalytic Air Electrodes for Rechargeable Lithium-Air Batteries. J. Electrochem. Soc. 2011, 158, A822. [Google Scholar] [CrossRef]
- Jin, L.; Xu, L.; Morein, C.; Chen, C.; Lai, M.; Dharmarathna, S.; Dobley, A.; Suib, S.L. Titanium Containing γ-MnO2 (TM) Hollow Spheres: One-Step Synthesis and Catalytic Activities in Li/Air Batteries and Oxidative Chemical Reactions. Adv. Funct. Mater. 2010, 20, 3373–3382. [Google Scholar] [CrossRef]
- Cao, Y.; Wei, Z.; He, J.; Zang, J.; Zhang, Q.; Zheng, M.; Dong, Q. α-MnO2 Nanorods Grown in Situ on Graphene as Catalysts for Li-O2 Batteries with Excellent Electrochemical Performance. Energy Environ. Sci. 2012, 5, 9765. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.-A.; Hu, X. Nanostructured Hydrotreating Catalysts for Electrochemical Hydrogen Evolution. Chem. Soc. Rev. 2014, 43, 6555. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Li, S.; Zhang, X.; Zhang, J.; Wang, R.; Zhang, H.; Pan, B.; Xie, Y. Atomically-Thin Molybdenum Nitride Nanosheets with Exposed Active Surface Sites for Efficient Hydrogen Evolution. Chem. Sci. 2014, 5, 4615–4620. [Google Scholar] [CrossRef]
- Michalsky, R.; Zhang, Y.-J.; Peterson, A.A. Trends in the Hydrogen Evolution Activity of Metal Carbide Catalysts. ACS Catal. 2014, 4, 1274–1278. [Google Scholar] [CrossRef]
- Ma, L.; Ting, L.R.L.; Molinari, V.; Giordano, C.; Yeo, B.S. Efficient Hydrogen Evolution Reaction Catalyzed by Molybdenum Carbide and Molybdenum Nitride Nanocatalysts Synthesized via the Urea Glass Route. J. Mater. Chem. A 2015, 3, 8361–8368. [Google Scholar] [CrossRef]
- Zhao, Y.; Kamiya, K.; Hashimoto, K.; Nakanishi, S. In Situ CO2-Emission Assisted Synthesis of Molybdenum Carbonitride Nanomaterial as Hydrogen Evolution Electrocatalyst. J. Am. Chem. Soc. 2015, 137, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Sakaushi, K.; Clavel, G.; Shalom, M.; Antonietti, M.; Fellinger, T.-P. A General Salt-Templating Method to Fabricate Vertically Aligned Graphitic Carbon Nanosheets and Their Metal Carbide Hybrids for Superior Lithium Ion Batteries and Water Splitting. J. Am. Chem. Soc. 2015, 137, 5480–5485. [Google Scholar] [CrossRef]
- Wan, C.; Leonard, B.M. Iron-Doped Molybdenum Carbide Catalyst with High Activity and Stability for the Hydrogen Evolution Reaction. Chem. Mater. 2015, 27, 4281–4288. [Google Scholar] [CrossRef]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.-Y.; Lou, X.W. Porous Molybdenum Carbide Nano-Octahedrons Synthesized via Confined Carburization in Metal-Organic Frameworks for Efficient Hydrogen Production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wu, H.B.; Xia, B.Y.; Xu, C.; Lou, X.W. Hierarchical β-Mo2C Nanotubes Organized by Ultrathin Nanosheets as a Highly Efficient Electrocatalyst for Hydrogen Production. Angew. Chem. Int. Ed. 2015, 54, 15395–15399. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zheng, R.; Du, D.; Ren, L.; Wen, X.; Wang, X.; Tian, G.; Shu, C. Adjusting the 3d Orbital Occupation of Ti in Ti3C2 MXene via Nitrogen Doping to Boost Oxygen Electrode Reactions in Li-O2 Battery. Small 2023, 19, 2206611. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, M.; Wang, X.; Huang, H. Theoretical Prediction of Catalytic Activity of Ti2C MXene as Cathode for Li-O2 Batteries. J. Phys. Chem. C 2019, 123, 17466–17471. [Google Scholar] [CrossRef]
- Li, J.; Han, K.; Huang, J.; Li, G.; Peng, S.; Li, N.; Wang, J.; Zhang, W.; Du, Y.; Fan, Y.; et al. Polarized Nucleation and Efficient Decomposition of Li2O2 for Ti2C MXene Cathode Catalyst under a Mixed Surface Condition in Lithium-Oxygen Batteries. Energy Storage Mater. 2021, 35, 669–678. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Y.; Zhang, J.; Zhou, N.; Li, M.; Liu, H.; Huo, B.; Chao, M.; Zeng, K. Interfacial Oxygen Bridge Bonding with Mo-O-Ti Units in MoOx@Ti3C2 MXene Harness Efficient Li-O2 Battery at High Rate. Appl. Catal. B Environ. Energy 2024, 351, 123984. [Google Scholar] [CrossRef]
- Li, J.; Zhou, H.; Jian, Z.; Li, H.; Guan, X.; Xing, Y.; Zhang, S.; Xu, H. Improved Electrocatalytic Activity of Three-Dimensional Open-Structured Co3O4@MnO2 Bifunctional Catalysts of Li-O2 Batteries by Inducing the Oriented Growth of Li2O2. ACS Sustain. Chem. Eng. 2021, 9, 5334–5344. [Google Scholar] [CrossRef]
- Ma, Z.; Yuan, X.; Li, L.; Ma, Z.-F. The Double Perovskite Oxide Sr2CrMoO6−δ as an Efficient Electrocatalyst for Rechargeable Lithium Air Batteries. Chem. Commun. 2014, 50, 14855–14858. [Google Scholar] [CrossRef]
- Yang, W.; Salim, J.; Li, S.; Sun, C.; Chen, L.; Goodenough, J.B.; Kim, Y. Perovskite Sr0.95Ce0.05CoO3−δ Loaded with Copper Nanoparticles as a Bifunctional Catalyst for Lithium-Air Batteries. J. Mater. Chem. 2012, 22, 18902. [Google Scholar] [CrossRef]
- Zhang, D.; Song, Y.; Du, Z.; Wang, L.; Li, Y.; Goodenough, J.B. Active LaNi1−xFexO3 Bifunctional Catalysts for Air Cathodes in Alkaline Media. J. Mater. Chem. A 2015, 3, 9421–9426. [Google Scholar] [CrossRef]
- Xu, J.-J.; Wang, Z.-L.; Xu, D.; Meng, F.-Z.; Zhang, X.-B. 3D Ordered Macroporous LaFeO3 as Efficient Electrocatalyst for Li–O2 Batteries with Enhanced Rate Capability and Cyclic Performance. Energy Environ. Sci. 2014, 7, 2213. [Google Scholar] [CrossRef]
- Xu, J.; Xu, D.; Wang, Z.; Wang, H.; Zhang, L.; Zhang, X. Synthesis of Perovskite-Based Porous La0.75Sr0.25MnO3 Nanotubes as a Highly Efficient Electrocatalyst for Rechargeable Lithium–Oxygen Batteries. Angew. Chem.-Int. Ed. 2013, 52, 3887–3890. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qian, Z.; Han, G.; Sun, B.; Zuo, P.; Du, C.; Ma, Y.; Huo, H.; Lou, S.; Yin, G. Perovskite LaCoxMn1−xO3−σ with Tunable Defect and Surface Structures as Cathode Catalysts for Li–O2 Batteries. ACS Appl. Mater. Interfaces 2020, 12, 10452–10460. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Pan, Z.-Z.; Yao, P.; Yuan, J.; Xia, C.; Zhao, Y.; Li, Y. A 98.2% Energy Efficiency Li-O2 Battery Using a LaNi0.5Co0.5O3 Perovskite Cathode with Extremely Fast Oxygen Reduction and Evolution Kinetics. Chem. Eng. J. 2023, 452, 139608. [Google Scholar] [CrossRef]
- Tao, L.; Sun, M.; Zhou, Y.; Luo, M.; Lv, F.; Li, M.; Zhang, Q.; Gu, L.; Huang, B.; Guo, S. A General Synthetic Method for High-Entropy Alloy Subnanometer Ribbons. J. Am. Chem. Soc. 2022, 144, 10582–10590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hui, X.; Nie, Y.; Wang, R.; Wang, C.; Zhang, Z.; Yin, L. New Conceptual Catalyst on Spatial High-Entropy Alloy Heterostructures for High-Performance Li-O2 Batteries. Small 2023, 19, 2206742. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Rao, Y.; Shi, W.; Yang, J.; Ning, W.; Li, H.; Yao, Y.; Zhou, H.; Guo, S. Sabatier Relations in Electrocatalysts Based on High-Entropy Alloys with Wide-Distributed d-Band Centers for Li-O2 Batteries. Angew. Chem.-Int. Ed. 2023, 62, e202310894. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gu, Q.; Xin, Y.; Tang, X.; Wu, H.; Guo, S. Orbital Coupling of PbO7 Node in Single-Crystal Metal–Organic Framework Enhances Li-O2 Battery Electrocatalysis. Nano Lett. 2023, 23, 10600–10607. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Zhang, Z.; Dou, Y.; Li, M.; Wu, J.; Zhang, Z.; Zhou, Z. An Efficient Multifunctional Soluble Catalyst for Li-O2 Batteries. CCS Chem. 2024, 6, 1810–1820. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Meng, X.; Wang, Q.; Zhang, Y.; Yan, S.; Luo, S. Advancements in Lithium–Oxygen Batteries: A Comprehensive Review of Cathode and Anode Materials. Batteries 2024, 10, 260. https://doi.org/10.3390/batteries10080260
Guo J, Meng X, Wang Q, Zhang Y, Yan S, Luo S. Advancements in Lithium–Oxygen Batteries: A Comprehensive Review of Cathode and Anode Materials. Batteries. 2024; 10(8):260. https://doi.org/10.3390/batteries10080260
Chicago/Turabian StyleGuo, Jing, Xue Meng, Qing Wang, Yahui Zhang, Shengxue Yan, and Shaohua Luo. 2024. "Advancements in Lithium–Oxygen Batteries: A Comprehensive Review of Cathode and Anode Materials" Batteries 10, no. 8: 260. https://doi.org/10.3390/batteries10080260



