Optimization of the Operating Voltage of Cobalt-Free Nickel-in-Medium Cathodes for High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. Material Preparation
2.2. Analytical Techniques
2.3. Electrochemical Measurements
3. Results and Discussion
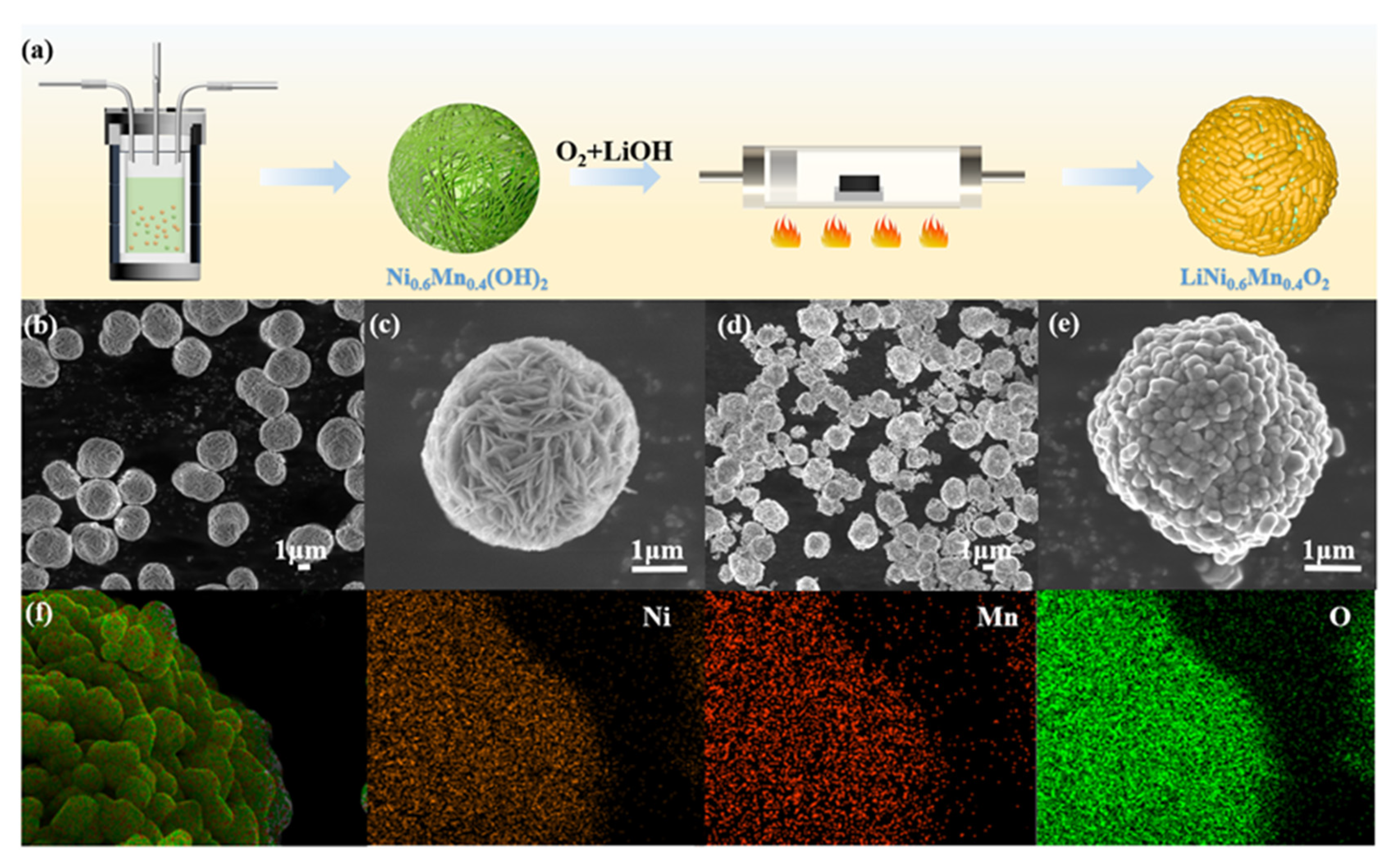
3.1. Morphological and Structural Characterization
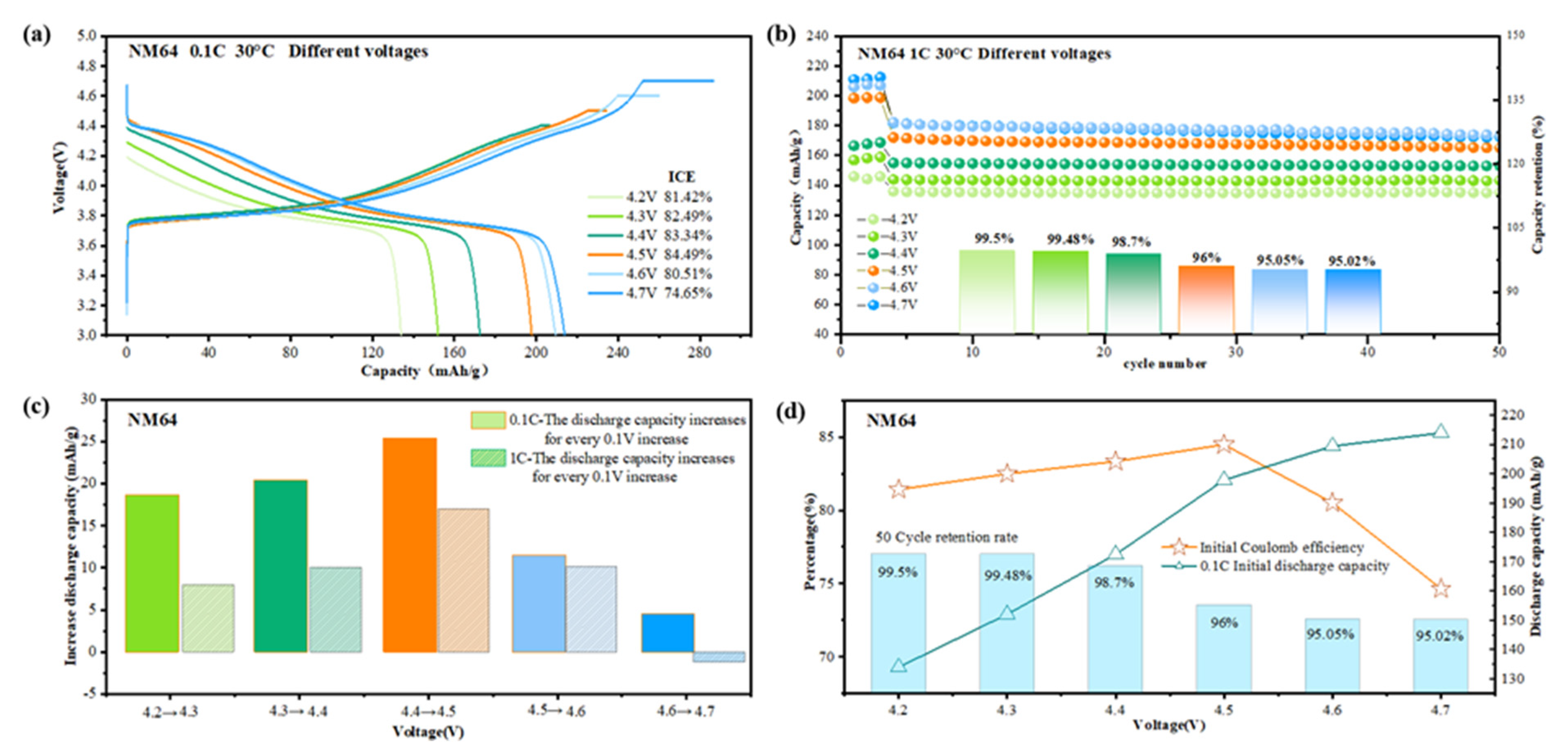
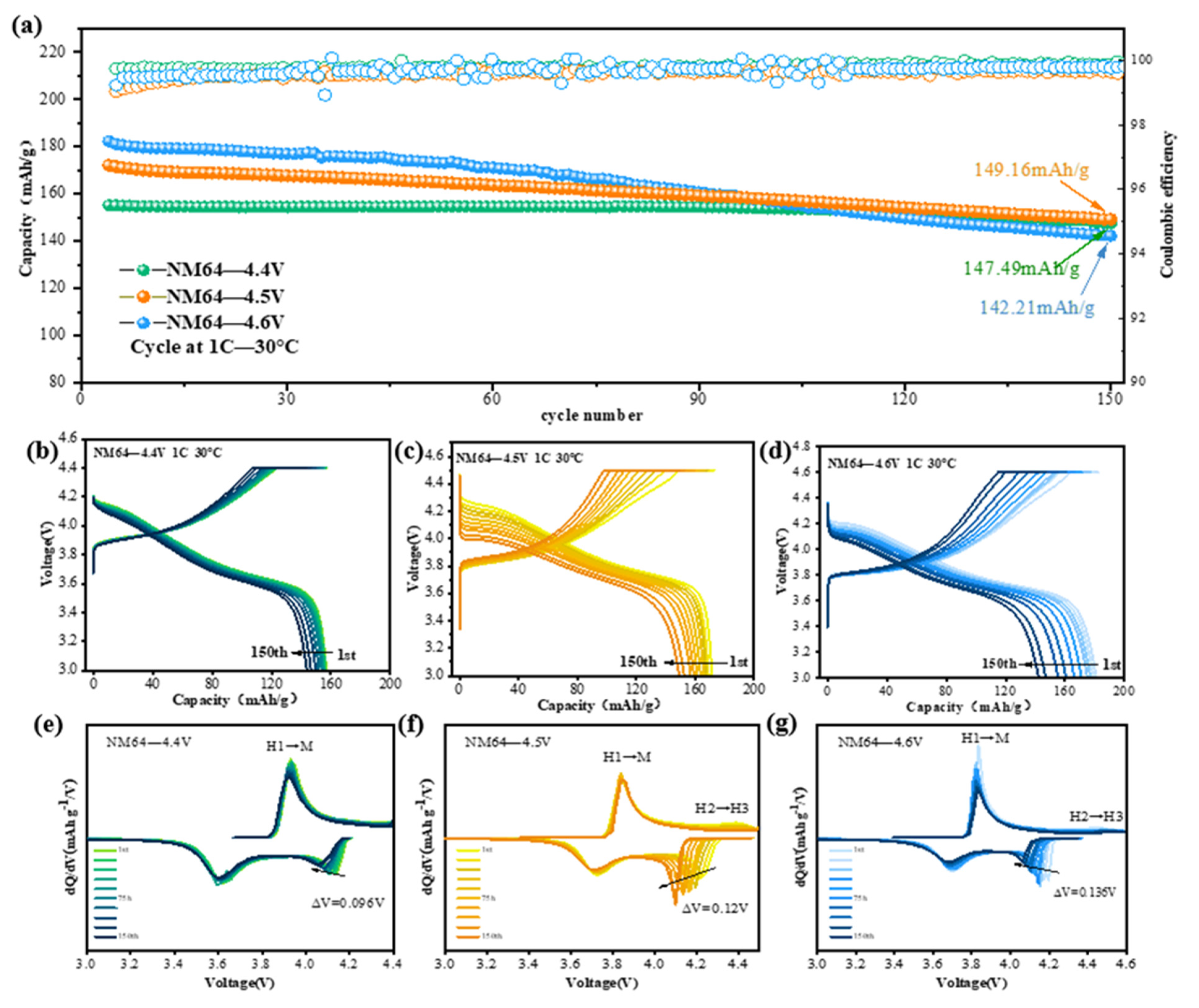
3.2. Electrochemical Performance Analysis
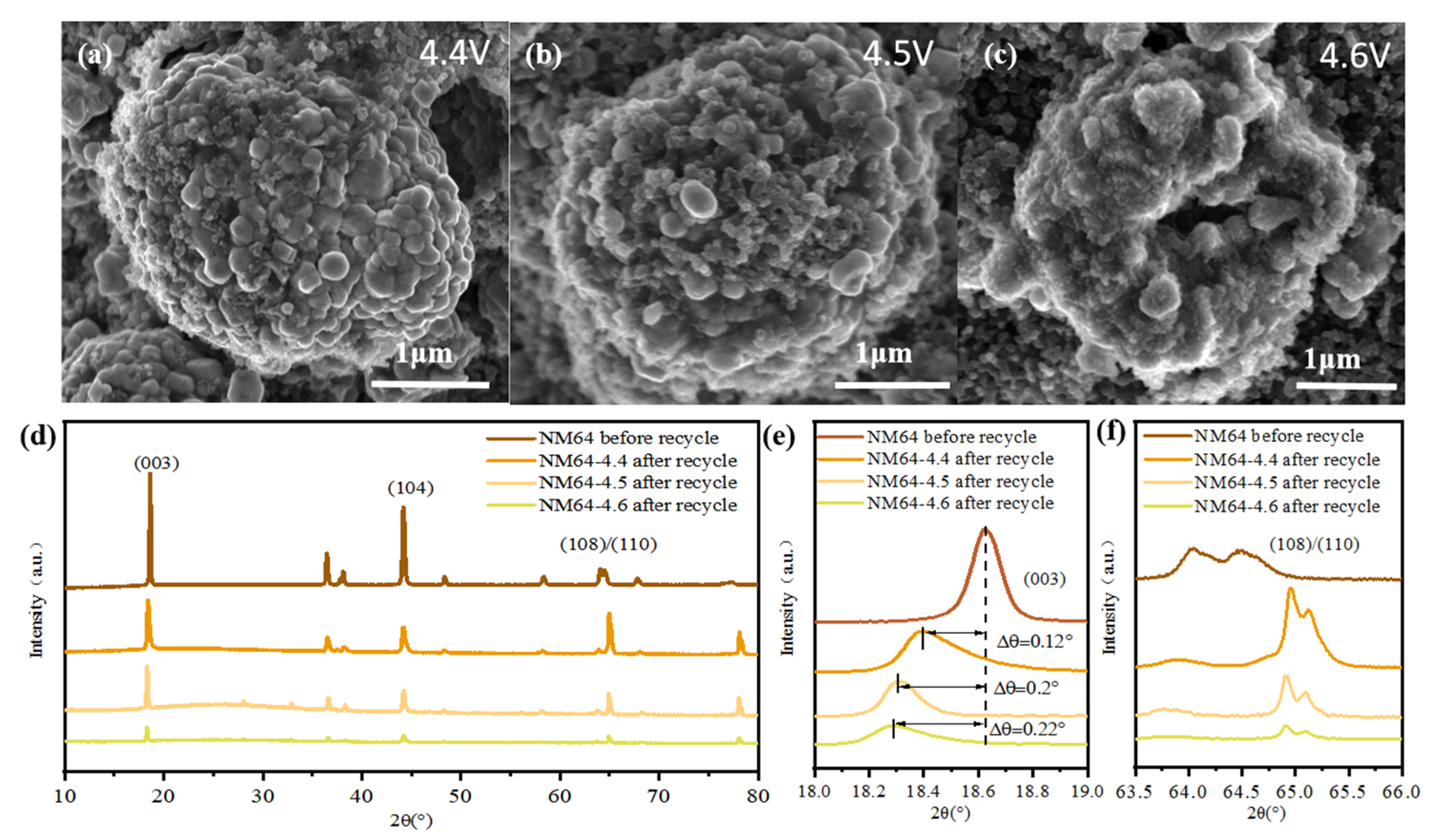
3.3. Post-Cycle Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Ding, Y.-L.; Deng, Y.-P.; Chen, Z. Ni-Rich/Co-Poor Layered Cathode for Automotive Li-Ion Batteries: Promises and Challenges. Adv. Energy Mater. 2020, 10, 1903864. [Google Scholar] [CrossRef]
- Li, H.; Cormier, M.; Zhang, N.; Inglis, J.; Li, J.; Dahn, J.R. Is Cobalt Needed in Ni-Rich Positive Electrode Materials for Lithium Ion Batteries? J. Electrochem. Soc. 2019, 166, A429. [Google Scholar] [CrossRef]
- Olivetti, E.A.; Ceder, G.; Gaustad, G.G.; Fu, X. Lithium-Ion Battery Supply Chain Considerations: Analysis of Potential Bottlenecks in Critical Metals. Joule 2017, 1, 229. [Google Scholar] [CrossRef]
- Shi, C.; Wang, T.; Liao, X.; Qie, B.; Yang, P.; Chen, M.; Wang, X.; Srinivasan, A.; Cheng, Q.; Ye, Q.; et al. Accordion-like stretchable Li-ion batteries with high energy density. Energy Storage Mater. 2019, 17, 136. [Google Scholar] [CrossRef]
- Bessette, S.; Paolella, A.; Kim, C.; Zhu, W.; Hovington, P.; Gauvin, R.; Zaghib, K. Nanoscale Lithium Quantification in LiXNiyCowMnZO2 as Cathode for Rechargeable Batteries. Sci. Rep. 2018, 8, 17575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-N.; Li, Q.; Ouyang, C.; Yu, X.; Ge, M.; Huang, X.; Hu, E.; Ma, C.; Li, S.; Xiao, R.; et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat. Energy 2019, 4, 594. [Google Scholar] [CrossRef]
- Tsai, P.-C.; Wen, B.; Wolfman, M.; Choe, M.-J.; Pan, M.S.; Su, L.; Thornton, K.; Cabana, J.; Chiang, Y.-M. Single-particle measurements of electrochemical kinetics in NMC and NCA cathodes for Li-ion batteries. Energy Environ. Sci. 2018, 11, 860. [Google Scholar] [CrossRef]
- Luo, Y.-h.; Wei, H.-x.; Tang, L.-b.; Huang, Y.-d.; Wang, Z.-y.; He, Z.-j.; Yan, C.; Mao, J.; Dai, K.; Zheng, J.-c. Nickel-rich and cobalt-free layered oxide cathode materials for lithium ion batteries. Energy Storage Mater. 2022, 50, 274. [Google Scholar] [CrossRef]
- Hu, E.; Yu, X.; Lin, R.; Bi, X.; Lu, J.; Bak, S.; Nam, K.-W.; Xin, H.L.; Jaye, C.; Fischer, D.A.; et al. Evolution of redox couples in Li- and Mn-rich cathode materials and mitigation of voltage fade by reducing oxygen release. Nat. Energy 2018, 3, 690. [Google Scholar] [CrossRef]
- Liu, T.; Dai, A.; Lu, J.; Yuan, Y.; Xiao, Y.; Yu, L.; Li, M.; Gim, J.; Ma, L.; Liu, J.; et al. Correlation between manganese dissolution and dynamic phase stability in spinel-based lithium-ion battery. Nat. Commun. 2019, 10, 4721. [Google Scholar] [CrossRef]
- Tong, H.; Yuan, X.; Qin, N.; Han, Y.; Cheng, Y.; Ji, F.; Tuo, R.; Liang, C.; Wang, Y.; Tong, Q.; et al. Plane-controlled growth strategy improves electrochemical performance of cobalt-free LiNi0.9Mn0.1O2 cathode. Prog. Nat. Sci.-Mater. Int. 2024, 34, 569. [Google Scholar] [CrossRef]
- Yi, M.C.; Dolocan, A.; Manthiram, A. Stabilizing the Interphase in Cobalt-Free, Ultrahigh-Nickel Cathodes for Lithium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2213164. [Google Scholar] [CrossRef]
- Liu, X.J.; Chen, M.Y.; Ma, J.J.; Liang, J.Q.; Li, C.S.; Chen, C.J.; He, H.B. Advances in the synthesis strategies of carbon⁃based single⁃atom catalysts and their electrochemical applications. China Powder Sci. Technol. 2024, 30, 35. [Google Scholar]
- Yan, P.; Zheng, J.; Liu, J.; Wang, B.; Cheng, X.; Zhang, Y.; Sun, X.; Wang, C.; Zhang, J.-G. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 2018, 3, 600. [Google Scholar] [CrossRef]
- Mu, L.; Yang, Z.; Tao, L.; Waters, C.K.; Xu, Z.; Li, L.; Sainio, S.; Du, Y.; Xin, H.L.; Nordlund, D.; et al. The sensitive surface chemistry of Co-free, Ni-rich layered oxides: Identifying experimental conditions that influence characterisation results. J. Mater. Chem. A 2020, 8, 17487. [Google Scholar] [CrossRef]
- Kaboli, S.; Demers, H.; Paolella, A.; Darwiche, A.; Dontigny, M.; Clément, D.; Guerfi, A.; Trudeau, M.L.; Goodenough, J.B.; Zaghib, K. Behaviour of Solid Electrolyte in Li-Polymer Battery with NMC Cathode via in-Situ Scanning Electron Microscopy. Nano Lett. 2020, 20, 1607. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Zhang, Y.; Zeng, J.; Fang, Z.; Cao, Y.; Peng, Z.; Tan, X.; Du, K. Mo-F Co-Doping LiNi0.83Co0.11Mn0.06O2 Stabilizes the Structure and Induces Compact Primary Particle To Improve the Electrochemical Performance. ACS Appl. Energy Mater. 2023, 6, 3834. [Google Scholar] [CrossRef]
- Kim, Y.; Seong, W.M.; Manthiram, A. Cobalt-free, high-nickel layered oxide cathodes for lithium-ion batteries: Progress, challenges, and perspectives. Energy Storage Mater. 2021, 34, 250. [Google Scholar] [CrossRef]
- Park, G.-T.; Namkoong, B.; Kim, S.-B.; Liu, J.; Yoon, C.S.; Sun, Y.-K. Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries. Nat. Energy 2022, 7, 946. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Zou, P.; Lin, R.; Ma, L.; Yin, L.; Li, T.; Xu, W.; Jia, H.; Li, Q.; et al. Compositionally complex doping for zero-strain zero-cobalt layered cathodes. Nature 2022, 610, 67. [Google Scholar] [CrossRef]
- de Biasi, L.; Schwarz, B.; Brezesinski, T.; Hartmann, P.; Janek, J.; Ehrenberg, H. Chemical, Structural, and Electronic Aspects of Formation and Degradation Behaviour on Different Length Scales of Ni-Rich NCM and Li-Rich HE-NCM Cathode Materials in Li-Ion Batteries. Adv. Mater. 2019, 31, 1900985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S. Problems and their origins of Ni-rich layered oxide cathode materials. Energy Storage Mater. 2020, 24, 247. [Google Scholar] [CrossRef]
- Liu, T.; Yu, L.; Liu, J.; Lu, J.; Bi, X.; Dai, A.; Li, M.; Li, M.; Hu, Z.; Ma, L.; et al. Understanding Co roles towards developing Co-free Ni-rich cathodes for rechargeable batteries. Nat. Energy 2021, 6, 277. [Google Scholar] [CrossRef]
- Fan, X.; Ou, X.; Zhao, W.; Liu, Y.; Zhang, B.; Zhang, J.; Zou, L.; Seidl, L.; Li, Y.; Hu, G.; et al. In situ inorganic conductive network formation in high-voltage single-crystal Ni-rich cathodes. Nat. Commun. 2021, 12, 5320. [Google Scholar] [CrossRef]
- Jung, S.-K.; Gwon, H.; Hong, J.; Park, K.-Y.; Seo, D.-H.; Kim, H.; Hyun, J.; Yang, W.; Kang, K. Understanding the Degradation Mechanisms of LiNi0.5Co0.2Mn0.3O2 Cathode Material in Lithium Ion Batteries. Adv. Energy Mater. 2014, 4, 1300787. [Google Scholar] [CrossRef]
- Fan, X.; Hu, G.; Zhang, B.; Ou, X.; Zhang, J.; Zhao, W.; Jia, H.; Zou, L.; Li, P.; Yang, Y. Crack-free single-crystalline Ni-rich layered NCM cathode enable superior cycling performance of lithium-ion batteries. Nano Energy 2020, 70, 104450. [Google Scholar] [CrossRef]
- Betz, J.; Brinkmann, J.-P.; Nölle, R.; Lürenbaum, C.; Kolek, M.; Stan, M.C.; Winter, M.; Placke, T. Cross Talk between Transition Metal Cathode and Li Metal Anode: Unravelling Its Influence on the Deposition/ Dissolution Behaviour and Morphology of Lithium. Adv. Energy Mater. 2019, 9, 1900574. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Li, W.; Xu, H.; Zhang, C.; Qiu, X. Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte. Nat. Commun. 2020, 11, 3629. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Shimizu, R.; Cheng, D.; Nguyen, H.; Paulsen, J.; Kumakura, S.; Zhang, M.; Meng, Y.S. Elucidating the Effect of Borate Additive in High-Voltage Electrolyte for Li-Rich Layered Oxide Materials. Adv. Energy Mater. 2022, 12, 2103033. [Google Scholar] [CrossRef]
- Deng, F.; Wang, Y.L.; Zhang, Y.F.; Li, J.X.; Guan, R.; Li, K.Q.; Su, J.Z.; Sun, H.R.; Han, H.L.; Yuan, Z.G.; et al. Prepared of porous magnesium oxide crystal with hydromagnesite method. China Powder Sci. Technol. 2024, 30, 138. [Google Scholar]
- Tang, B.; Zhang, N.; Alter, E.; Eldesoky, A.; Dahn, J.R. Transition Metal Dissolution from Single Crystal Li[Ni1−x−yMnxCoy]O2 and Li[Ni1−xMnx]O2 Positive Electrodes Subjected to Aggresive Conditions. J. Electrochem. Soc. 2024, 171, 010518. [Google Scholar]
- Garayt, M.D.L.; Zhang, N.; Yu, S.; Abraham, J.J.; Murphy, A.; Omessi, R.; Ye, Z.; Azam, S.; Johnson, M.B.; Yang, C.; et al. Single Crystal Li1+x[Ni0.6Mn0.4]1−xO2 Made by All-Dry Synthesis. J. Electrochem. Soc. 2023, 170, 060529. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Z.; Manthiram, A. Insights into the Crossover Effects in Cells with High-Nickel Layered Oxide Cathodes and Silicon/Graphite Composite Anodes. Adv. Energy Mater. 2022, 12, 2103611. [Google Scholar] [CrossRef]
- Zhao, W.; Zou, L.; Zhang, L.; Fan, X.; Zhang, H.; Pagani, F.; Brack, E.; Seidl, L.; Ou, X.; Egorov, K.; et al. Assessing Long-Term Cycling Stability of Single-Crystal Versus Polycrystalline Nickel-Rich NCM in Pouch Cells with 6 mAh cm−2 Electrodes. Small 2022, 18, 2107357. [Google Scholar]
- Wang, B.; Zhang, F.-l.; Zhou, X.-a.; Wang, P.; Wang, J.; Ding, H.; Dong, H.; Liang, W.-B.; Zhang, N.-S.; Li, S.-Y. Which of the nickel-rich NCM and NCA is structurally superior as a cathode material for lithium-ion batteries? J. Mater. Chem. A 2021, 9, 13540. [Google Scholar] [CrossRef]
- Wu, H.E.; Fei, G.T.; Gao, X.D.; Guo, X.; Gong, X.X.; Ma, X.L.; Wang, Q.; Xu, S.H. Research progress on preparation and application of polyaniline and its composite materials. China Powder Sci. Technol. 2023, 29, 70. [Google Scholar]
- Li, L.; Fu, L.; Li, M.; Wang, C.; Zhao, Z.; Xie, S.; Lin, H.; Wu, X.; Liu, H.; Zhang, L.; et al. B-doped and La4NiLiO8-coated Ni-rich cathode with enhanced structural and interfacial stability for lithium-ion batteries. J. Energy Chem. 2022, 71, 588. [Google Scholar] [CrossRef]
- Ji, Y.Q.; Yu, Z.H.; Yan, L.G.; Song, W. Research progress in preparation, modification and application of biomass-based single-atom catalysts. China Powder Sci. Technol. 2023, 29, 100. [Google Scholar]
- Zhu, X.; Meng, F.; Zhang, Q.; Xue, L.; Zhu, H.; Lan, S.; Liu, Q.; Zhao, J.; Zhuang, Y.; Guo, Q.; et al. LiMnO2cathode stabilised by interfacial orbital ordering for sustainable lithium-ion batteries. Nat. Sustain. 2021, 4, 392. [Google Scholar] [CrossRef]
- Hou, P.; Zhang, H.; Deng, X.; Xu, X.; Zhang, L. Stabilising the Electrode/Electrolyte Interface of LiNi0.8Co0.15Al0.05O2through Tailoring Aluminum Distribution in Microspheres as Long-Life, High-Rate, and Safe Cathode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 29643. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Namkoong, B.; Kim, J.-H.; Belharouak, I.; Yoon, C.S.; Sun, Y.-K. Capacity Fading Mechanisms in Ni-Rich Single-Crystal NCM Cathodes. ACS Energy Lett. 2021, 6, 2726. [Google Scholar] [CrossRef]
- Hu, D.; Su, Y.; Chen, L.; Li, N.; Bao, L.; Lu, Y.; Zhang, Q.; Wang, J.; Chen, S.; Wu, F. The mechanism of side reaction induced capacity fading of Ni-rich cathode materials for lithium ion batteries. J. Energy Chem. 2021, 58, 1. [Google Scholar] [CrossRef]
- Yang, J.P.; Zhang, F.Z.; Chen, J. Structural design and application of fiber-based electrocatalytic materials. China Powder Sci. Technol. 2024, 30, 161. [Google Scholar]
- Chen, S.; Qi, G.; Yin, R.; Liu, Q.; Feng, L.; Feng, X.; Hu, G.; Luo, J.; Liu, X.; Liu, W. Electrocatalytic nitrate-to-ammonia conversion on CoO/CuO nanoarrays using Zn–nitrate batteries. Nanoscale 2023, 15, 19577. [Google Scholar] [CrossRef]
- Lu, G.; Meng, G.; Liu, Q.; Feng, L.; Luo, J.; Liu, X.; Luo, Y.; Chu, P.K. Advanced strategies for solid electrolyte interface design with MOF materials. Adv. Powder Mater. 2024, 3, 100154. [Google Scholar] [CrossRef]
- Qin, D.D.; Ding, J.Y.; Linag, C.; Liu, Q.; Feng, L.G.; Luo, Y.; Hu, G.Z.; Luo, J.; Liu, X.J. Addressing Challenges and Enhancing Performance of Manganese-based Cathode Materials in Aqueous Zinc-Ion Batteries. Acta Phys.-Chim. Sin. 2024, 40, 2310034. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Hou, S.; Qin, N.; Huang, T.; Guo, J.; Hou, X.; Huang, N.; Liu, Y.; Liu, X. Optimization of the Operating Voltage of Cobalt-Free Nickel-in-Medium Cathodes for High-Performance Lithium-Ion Batteries. Batteries 2024, 10, 273. https://doi.org/10.3390/batteries10080273
Qi Y, Hou S, Qin N, Huang T, Guo J, Hou X, Huang N, Liu Y, Liu X. Optimization of the Operating Voltage of Cobalt-Free Nickel-in-Medium Cathodes for High-Performance Lithium-Ion Batteries. Batteries. 2024; 10(8):273. https://doi.org/10.3390/batteries10080273
Chicago/Turabian StyleQi, Yuchuan, Shuheng Hou, Ningbo Qin, Ting Huang, Jiawen Guo, Xianghua Hou, Ning Huang, Yifan Liu, and Xijun Liu. 2024. "Optimization of the Operating Voltage of Cobalt-Free Nickel-in-Medium Cathodes for High-Performance Lithium-Ion Batteries" Batteries 10, no. 8: 273. https://doi.org/10.3390/batteries10080273
APA StyleQi, Y., Hou, S., Qin, N., Huang, T., Guo, J., Hou, X., Huang, N., Liu, Y., & Liu, X. (2024). Optimization of the Operating Voltage of Cobalt-Free Nickel-in-Medium Cathodes for High-Performance Lithium-Ion Batteries. Batteries, 10(8), 273. https://doi.org/10.3390/batteries10080273






