Fluorinated Hollow Porous Carbon Spheres as High-Performance Cathode Material for Primary Battery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterization
2.3. Electrochemical Measurements
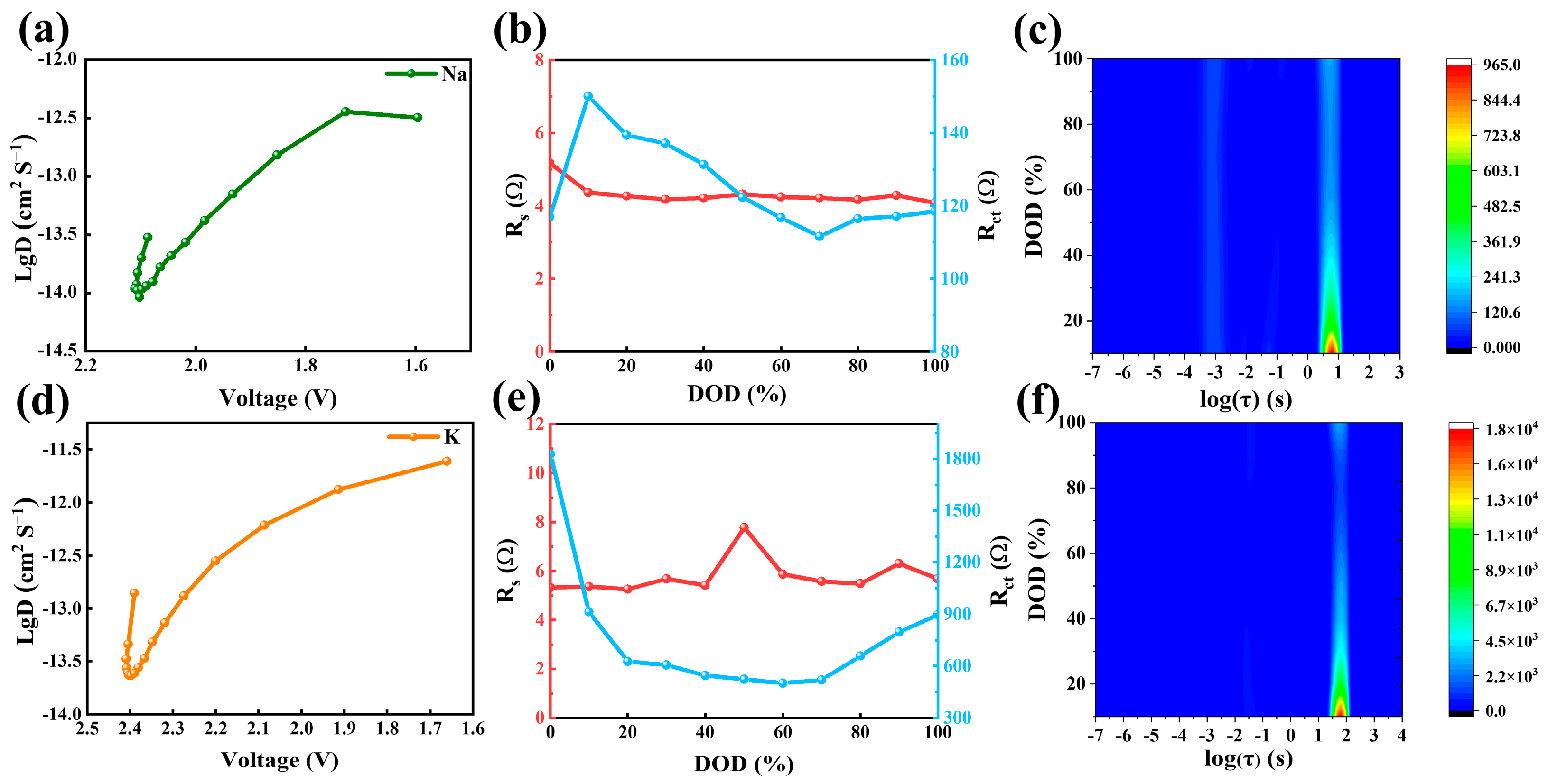
3. Results and Discussion
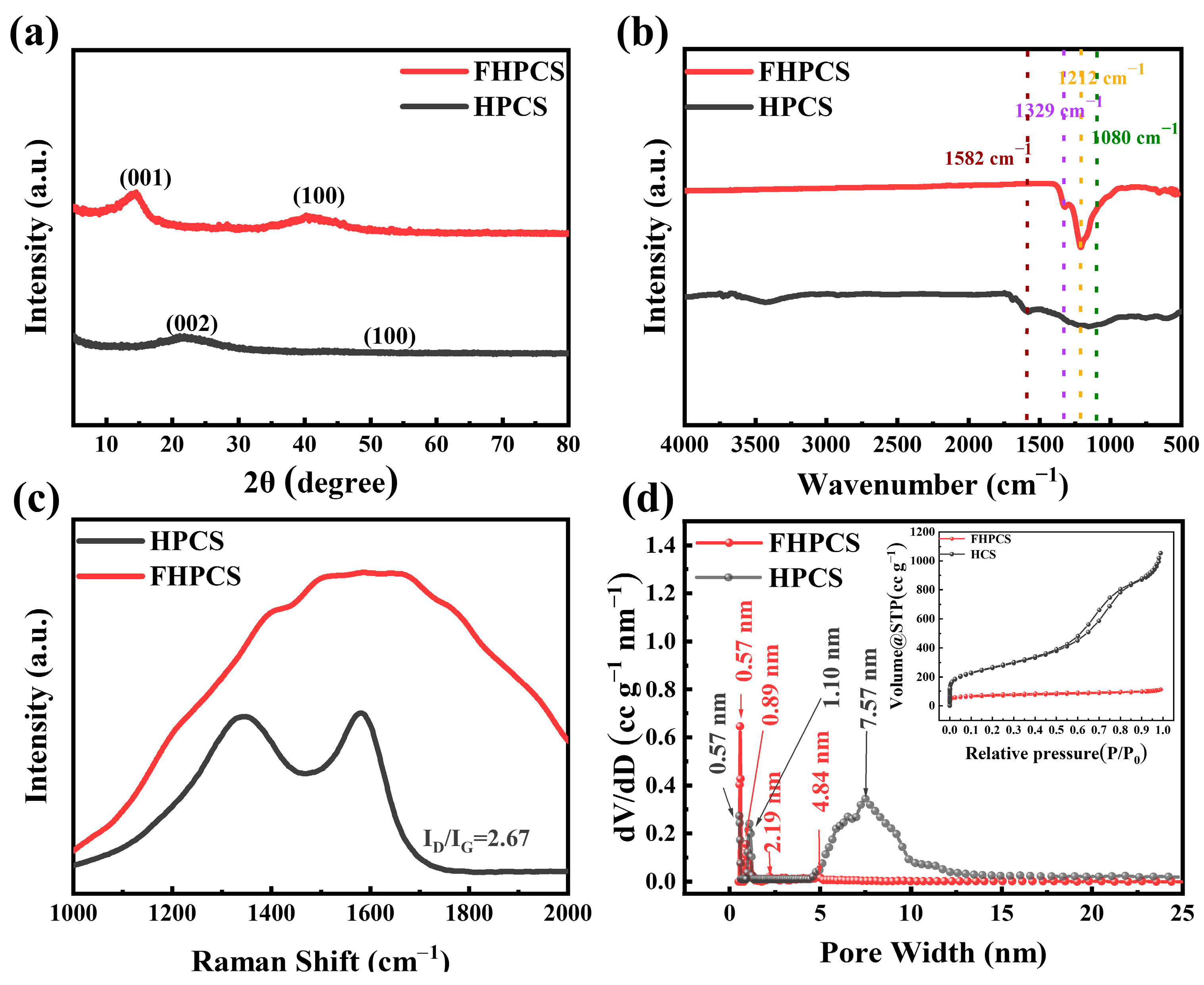
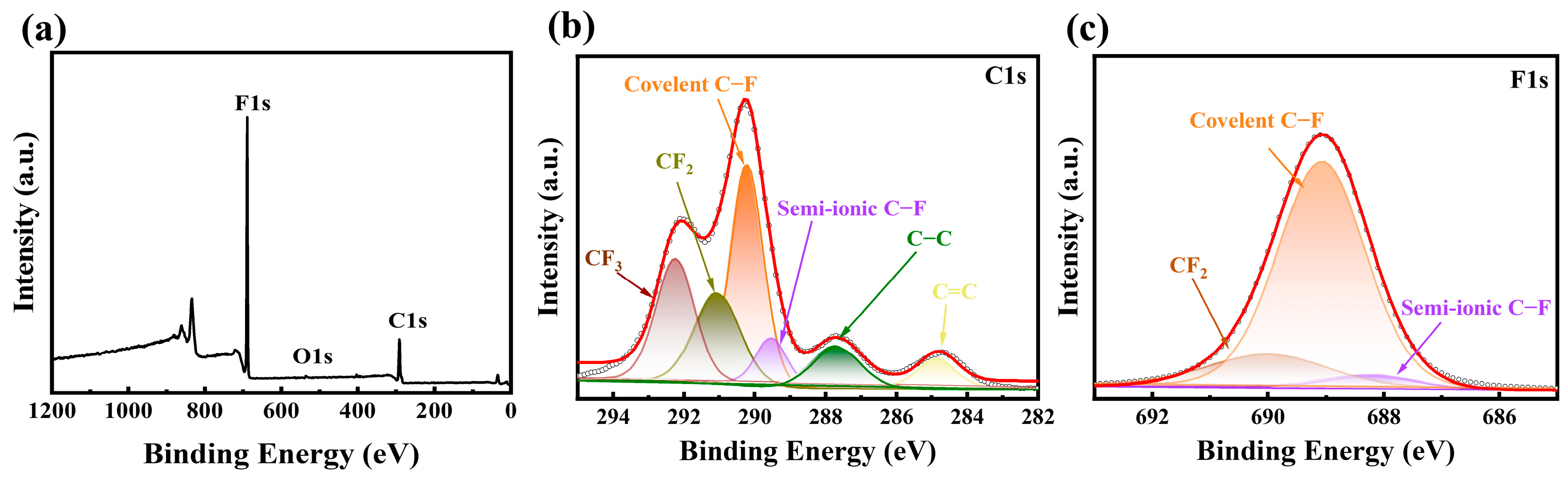
3.1. Structural and Physicochemical Properties of FHPCS
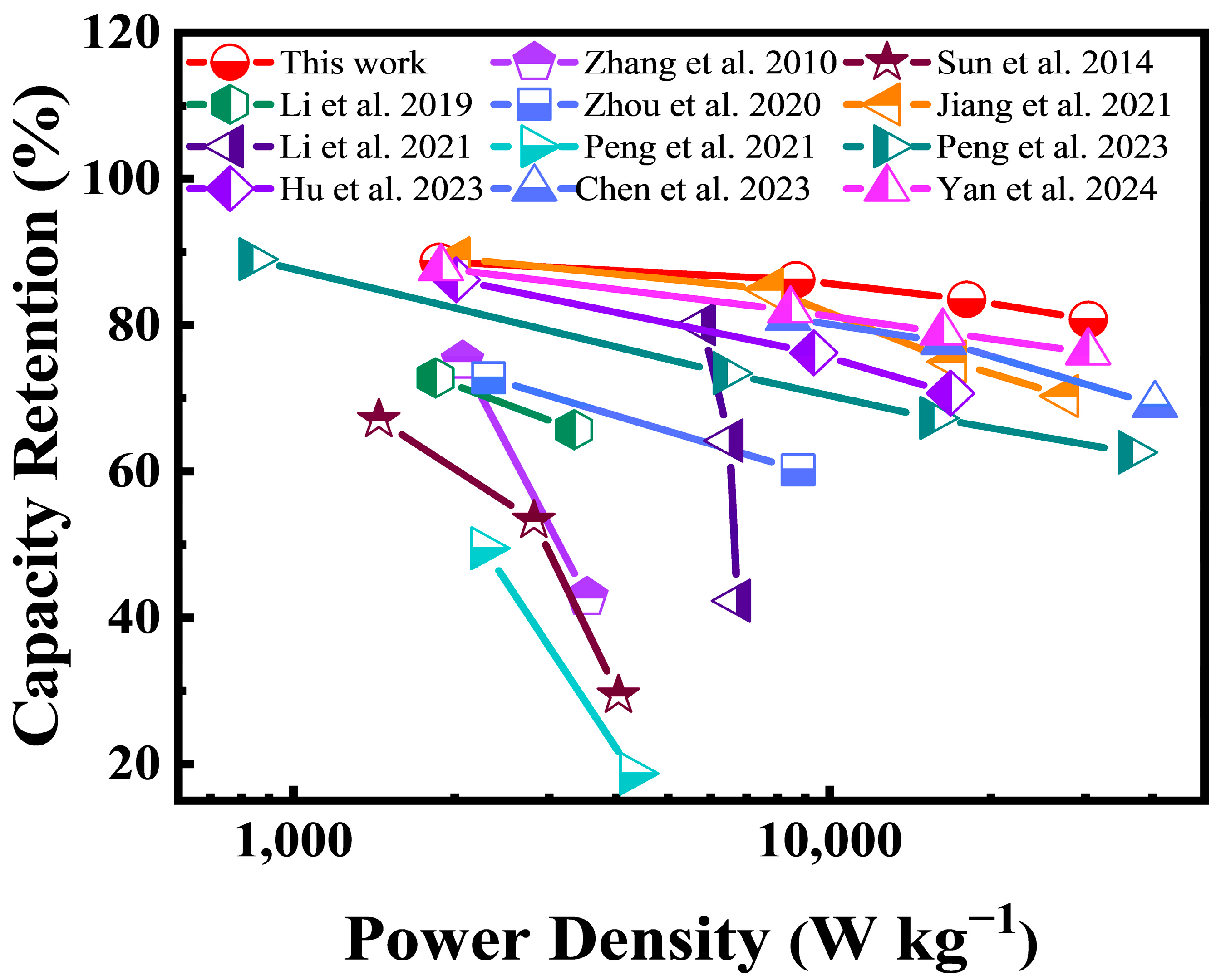
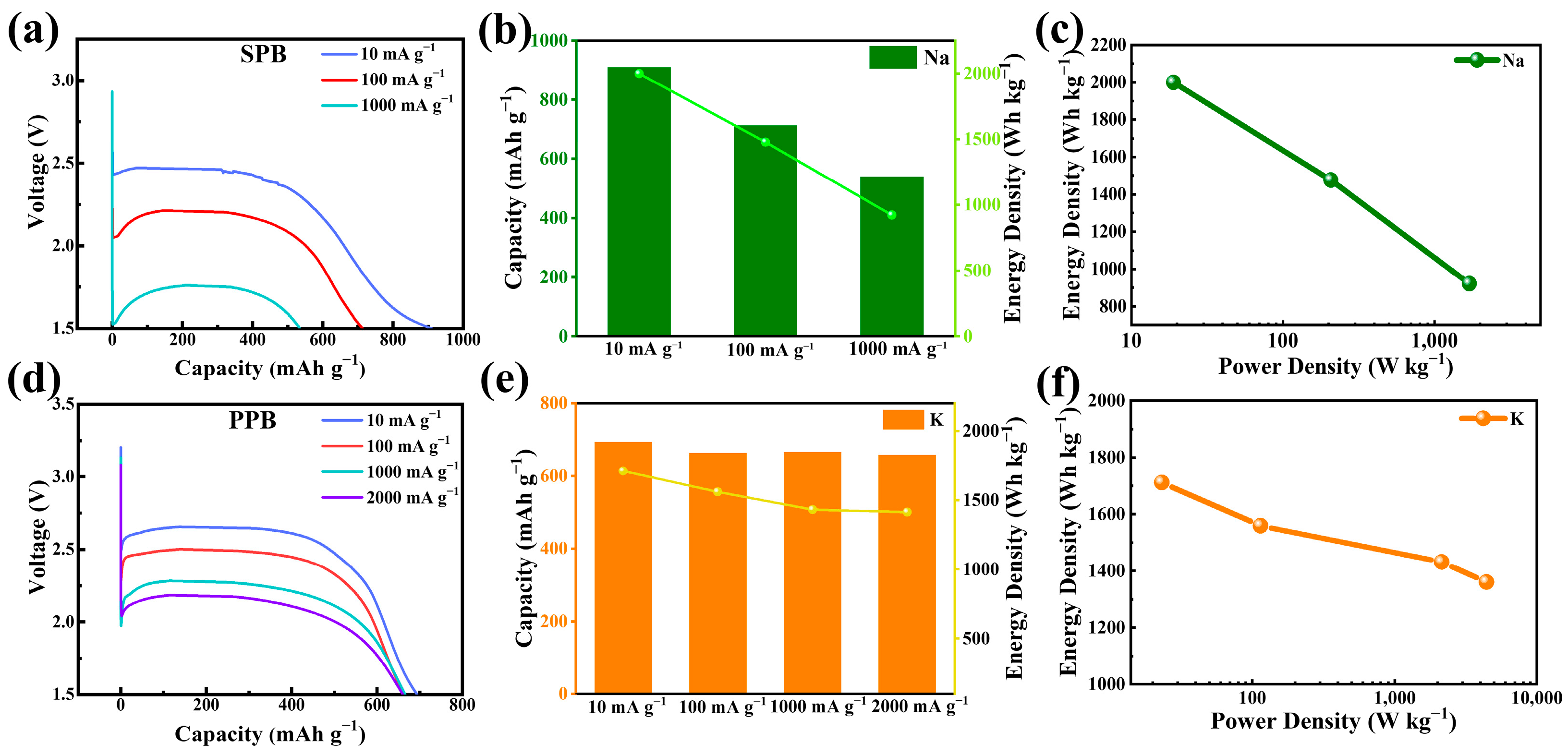
3.2. Electrochemical Properties of FHPCS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Fan, K.; Liu, Y.; Li, Y.; Liu, X.; Feng, W.; Wang, X. Recent Advances in Fluorinated Graphene from Synthesis to Applications: Critical Review on Functional Chemistry and Structure Engineering. Adv. Mater. 2022, 34, 2101665. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zang, X.; Wang, X.; Gu, X.; Shao, Q.; Cao, N. Recent advances in fluorine-doped/fluorinated carbon-based materials for supercapacitors. Energy Storage Mater. 2020, 30, 367–384. [Google Scholar] [CrossRef]
- Feng, W.; Long, P.; Feng, Y.; Li, Y. Two-Dimensional Fluorinated Graphene: Synthesis, Structures, Properties and Applications. Adv. Sci. 2016, 3, 1500413. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Silva, S.R.P.; Ding, B.; Zhang, P.; Shao, G. Lithium-Sulfur Batteries Meet Electrospinning: Recent Advances and the Key Parameters for High Gravimetric and Volume Energy Density. Adv. Sci. 2022, 9, 2103879. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, M.; Li, R.; Li, C.; He, J.; Qiu, M.; Xiao, L.; Wen, Z.; Qian, Q.; Li, X.; et al. Functionalized N-doped hollow graphitic carbon-nanotube/carbon -nanosphere composite. Compos. Commun. 2021, 23, 100578. [Google Scholar] [CrossRef]
- Zhang, W.; Spinelle, L.; Dubois, M.; Guerin, K.; Kharbache, H.; Masin, F.; Kharitonov, A.P.; Hamwi, A.; Brunet, J.; Varenne, C.; et al. New synthesis methods for fluorinated carbon nanofibres and applications. J. Fluor. Chem. 2010, 131, 676–683. [Google Scholar] [CrossRef]
- Schonberg, W.P.; Hull, S.M. Current Design Criteria for MMOD Impact of Metallic Pressurized Tanks. J. Aerosp. Eng. 2016, 29, 06016004. [Google Scholar] [CrossRef]
- Guerin, K.; Dubois, M.; Houdayer, A.; Hamwi, A. Applicative performances of fluorinated carbons through fluorination routes: A review. J. Fluor. Chem. 2012, 134, 11–17. [Google Scholar] [CrossRef]
- Zhang, S.; Kong, L.; Li, Y.; Peng, C.; Feng, W. Fundamentals of Li/CFx battery design and application. Energy Environ. Sci. 2023, 16, 1907–1942. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, X.; Chen, D.; Chang, Q.; Xie, S.; Ma, Z.; Lei, W.; Pan, J.; Pan, Y.; Huang, J. Ultrafast Li/Fluorinated Graphene Primary Batteries with High Energy Density and Power Density. ACS Appl. Mater. Interfaces 2021, 13, 18809–18820. [Google Scholar] [CrossRef]
- Wang, K.; Feng, Y.; Kong, L.; Peng, C.; Hu, Y.; Li, W.; Li, Y.; Feng, W. The Fluorination of Boron-Doped Graphene for CFx Cathode with Ultrahigh Energy Density. Energy Environ. Mater. 2023, 6, e12437. [Google Scholar] [CrossRef]
- Fan, R.; Yang, B.; Li, Z.; Ma, D.; Yuan, W.; Ma, J.; Ren, H. First-principles study of the adsorption behaviors of Li atoms and LiF on the CFx (x=1.0, 0.9, 0.8, 0.5,∼0.0) surface. RSC Adv. 2020, 10, 31881–31888. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, L.; Wang, H.; Wang, H.; Jiao, W.; Chen, G.; Zhang, P.; Hui, D.; Jian, X. A brief review for fluorinated carbon: Synthesis, properties and applications. Nanotechnol. Rev. 2019, 8, 573–586. [Google Scholar] [CrossRef]
- Yazami, R.; Hamwi, A.; Guérin, K.; Ozawa, Y.; Dubois, M.; Giraudet, J.; Masin, F. Fluorinated carbon nanofibres for high energy and high power densities primary lithium batteries. Electrochem. Commun. 2007, 9, 1850–1855. [Google Scholar] [CrossRef]
- Ahmad, Y.; Guérin, K.; Dubois, M.; Zhang, W.; Hamwi, A. Enhanced performances in primary lithium batteries of fluorinated carbon nanofibers through static fluorination. Electrochim. Acta 2013, 114, 142–151. [Google Scholar] [CrossRef]
- Ahmad, Y.; Dubois, M.; Guerin, K.; Hamwi, A.; Flahaut, E. High energy density of primary lithium batteries working with sub-fluorinated few walled carbon nanotubes cathode. J. Alloys Compd. 2017, 726, 852–859. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Yao, F.; Fu, H.; Zhou, R.; Feng, Y.; Feng, W. Ultrahigh-energy-density fluorinated calcinated macadamia nut shell cathodes for lithium/fluorinated carbon batteries. Carbon 2019, 153, 783–791. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, S.; Kong, L.; Xu, H.; Li, Y.; Feng, W. Fluorinated Carbon Nanohorns as Cathode Materials for Ultra-High Power Li/CFx Batteries. Small Methods 2023, 8, 2301090. [Google Scholar] [CrossRef]
- Jiang, S.; Huang, P.; Lu, J.; Liu, Z. The electrochemical performance of fluorinated ketjenblack as a cathode for lithium/fluorinated carbon batteries. RSC Adv. 2021, 11, 25461–25470. [Google Scholar] [CrossRef]
- Hu, Y.; Kong, L.; Li, W.; Sun, L.; Peng, C.; Qin, M.; Zhao, Z.; Li, Y.; Feng, W. Fluorinated microporous carbon spheres for Li/CF batteries with high volumetric energy density. Compos. Commun. 2023, 40, 101607. [Google Scholar] [CrossRef]
- Matsuda, Y.; Nakashima, H.; Morita, M.; Takasu, Y. Behavior of Some Ions in Mixed Organic Electrolytes of High-Energy Density Batteries. J. Electrochem. Soc. 1981, 128, 2552–2556. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Liu, C.; Wang, S.; Zhou, P.; Zhou, T.; Miao, Z.; Xing, W.; Zhuo, S.; Zhou, J. Fluorinated multi-walled carbon nanotubes as cathode materials of lithium and sodium primary batteries: Effect of graphitization of carbon nanotubes. J. Mater. Chem. A 2019, 7, 7128–7137. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, B.; Wu, Z.; Qiu, J.; Ding, Z.; Zou, J.; Chen, S.; Gao, P.; Niu, X.; Wang, L.; et al. Electrolyte-assisted dissolution-recrystallization mechanism towards high energy density and power density CF cathodes in potassium cell. Nano Energy 2020, 70, 104552. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, D.; Wang, X.; Huang, J.; Pan, Y.; Lei, W.; Pan, J. Accordion-Like Fluorinated Graphite Nanosheets with High Power and Energy Densities for Wide-Temperature, Long Shelf-Life Sodium/Potassium Primary Batteries. Small 2021, 17, 2008163. [Google Scholar] [CrossRef]
- Ahmad, Y.; Dubois, M.; Guérin, K.; Hamwi, A.; Zhang, W. Pushing the theoretical limit of Li–CFx batteries using fluorinated nanostructured carbon nanodiscs. Carbon 2015, 94, 1061–1070. [Google Scholar] [CrossRef]
- Pei, F.; An, T.; Zang, J.; Zhao, X.; Fang, X.; Zheng, M.; Dong, Q.; Zheng, N. From Hollow Carbon Spheres to N-Doped Hollow Porous Carbon Bowls: Rational Design of Hollow Carbon Host for Li-S Batteries. Adv. Energy Mater. 2016, 6, 1502539. [Google Scholar] [CrossRef]
- Xia, Q.; Zou, Y.; Yan, K.; Bao, L.; Chen, H.; Yue, H. In-situ texturing hollow carbon host anchored with Fe single atoms accelerating solid-phase redox for Li-Se batteries. J. Colloid Interface Sci. 2024, 667, 282–290. [Google Scholar] [CrossRef]
- Wan, X.K.; Wu, H.B.; Guan, B.Y.; Luan, D.; Lou, X.W. Confining Sub-Nanometer Pt Clusters in Hollow Mesoporous Carbon Spheres for Boosting Hydrogen Evolution Activity. Adv. Mater. 2019, 32, 1901349. [Google Scholar] [CrossRef]
- Xiao, L.; Lu, H.; Fang, Y.; Sushko, M.L.; Cao, Y.; Ai, X.; Yang, H.; Liu, J. Low-Defect and Low-Porosity Hard Carbon with High Coulombic Efficiency and High Capacity for Practical Sodium Ion Battery Anode. Adv. Energy Mater. 2018, 8, 1703238. [Google Scholar] [CrossRef]
- Wei, Z.; Sarwar, S.; Azam, S.; Ahasan, M.R.; Voyda, M.; Zhang, X.; Wang, R. Ultrafast microwave synthesis of MoTe2@graphene composites accelerating polysulfide conversion and promoting Li2S nucleation for high-performance Li-S batteries. J. Colloid Interface Sci. 2023, 635, 391–405. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Peng, C.; Sun, L.; Wang, K.; Liu, Y.; Feng, W. Defective nano-structure regulating C-F bond for lithium/fluorinated carbon batteries with dual high-performance. Nano Energy 2022, 104, 107905. [Google Scholar] [CrossRef]
- He, Q.; Chen, H.; Chen, X.; Zheng, J.; Que, L.; Yu, F.; Zhao, J.; Xie, Y.; Huang, M.; Lu, C.; et al. Tea-Derived Sustainable Materials. Adv. Funct. Mater. 2024, 34, 2310226. [Google Scholar] [CrossRef]
- Giraudet, J.; Delabarre, C.; Guerin, K.; Dubois, M.; Masin, F.; Hamwi, A. Comparative performances for primary lithium batteries of some covalent and semi-covalent graphite fluorides. J. Power Sources 2006, 158, 1365–1372. [Google Scholar] [CrossRef]
- Azam, S.; Wei, Z.; Wang, R. Cerium oxide nanorods anchored on carbon nanofibers derived from cellulose paper as effective interlayer for lithium sulfur battery. J. Colloid Interface Sci. 2022, 615, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, H.F. Experimental and computational investigations of the properties of fluorinated single-walled carbon nanotubes. Chemphyschem 2003, 4, 1283–1289. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, G.; Chen, H.; Yue, H. Conductive Carbon-Wrapped Fluorinated Hard Carbon Composite as High-Performance Cathode for Primary Lithium Batteries. Coatings 2023, 13, 812. [Google Scholar] [CrossRef]
- Yan, K.; Zou, Y.; Bao, L.-X.; Xia, Q.; Meng, L.-Y.; Lin, H.-C.; Chen, H.-X.; Yue, H.-J. Fluorinated N,P co-doped biomass carbon with high-rate performance as cathode material for lithium/fluorinated carbon battery. Rare Met. 2024. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Liu, C.; Qiao, J.; Zhou, X. A MOF-derived multifunctional nano-porous fluorinated carbon for high performance lithium/fluorinated carbon primary batteries. Microporous Mesoporous Mater. 2021, 310, 110650. [Google Scholar] [CrossRef]
- Zhang, Q.; D’Astorg, S.; Xiao, P.; Zhang, X.; Lu, L. Carbon-coated fluorinated graphite for high energy and high power densities primary lithium batteries. J. Power Sources 2010, 195, 2914–2917. [Google Scholar] [CrossRef]
- Sun, C.; Feng, Y.; Li, Y.; Qin, C.; Zhang, Q.; Feng, W. Solvothermally exfoliated fluorographene for high-performance lithium primary batteries. Nanoscale 2014, 6, 2634–2641. [Google Scholar] [CrossRef]
- Zhou, R.; Li, Y.; Feng, Y.; Peng, C.; Feng, W. The electrochemical performances of fluorinated hard carbon as the cathode of lithium primary batteries. Compos. Commun. 2020, 21, 100396. [Google Scholar] [CrossRef]
- Peng, C.; Kong, L.; Li, Y.; Fu, H.; Sun, L.; Feng, Y.; Feng, W. Fluorinated graphene nanoribbons from unzipped single-walled carbon nanotubes for ultrahigh energy density lithium-fluorinated carbon batteries. Sci. China Mater. 2021, 64, 1367–1377. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, C.-Z.; Huang, J.-Q.; Zhang, Q. The timescale identification decoupling complicated kinetic processes in lithium batteries. Joule 2022, 6, 1172–1198. [Google Scholar] [CrossRef]
- Yue, H.; Chen, H.; Zhao, C.; Zheng, Z.; Zhou, K.; Zhang, Q.; Zhong, G.; Lu, C.-Z.; Yang, Y. Reversible potassium storage in ultrafine CF: A superior cathode material for potassium batteries and its mechanism. J. Energy Chem. 2021, 53, 347–353. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Y.; Yan, K.; Bao, L.; Xia, Q.; Chen, H.; Yue, H. Fluorinated Hollow Porous Carbon Spheres as High-Performance Cathode Material for Primary Battery. Batteries 2024, 10, 310. https://doi.org/10.3390/batteries10090310
Zou Y, Yan K, Bao L, Xia Q, Chen H, Yue H. Fluorinated Hollow Porous Carbon Spheres as High-Performance Cathode Material for Primary Battery. Batteries. 2024; 10(9):310. https://doi.org/10.3390/batteries10090310
Chicago/Turabian StyleZou, Yan, Ke Yan, Liangxue Bao, Qi Xia, Huixin Chen, and Hongjun Yue. 2024. "Fluorinated Hollow Porous Carbon Spheres as High-Performance Cathode Material for Primary Battery" Batteries 10, no. 9: 310. https://doi.org/10.3390/batteries10090310
APA StyleZou, Y., Yan, K., Bao, L., Xia, Q., Chen, H., & Yue, H. (2024). Fluorinated Hollow Porous Carbon Spheres as High-Performance Cathode Material for Primary Battery. Batteries, 10(9), 310. https://doi.org/10.3390/batteries10090310





