Magnesium Alginate as an Electrolyte for Magnesium Batteries
Abstract
:1. Introduction
2. Results and Discussion
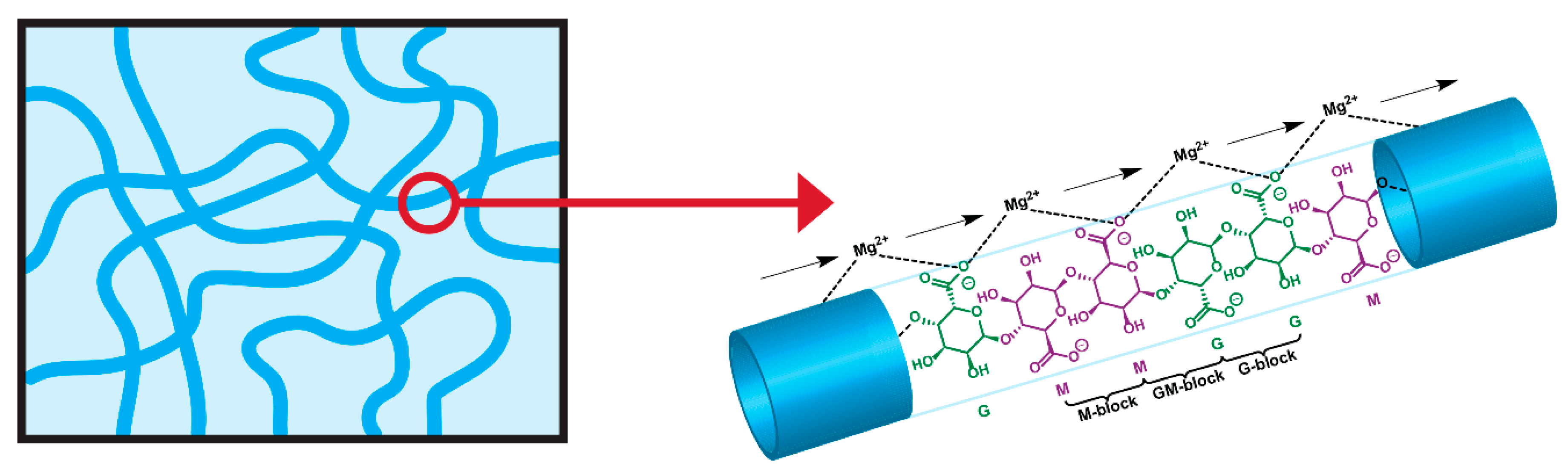
2.1. Synthesis and Characterization
2.1.1. Synthesis
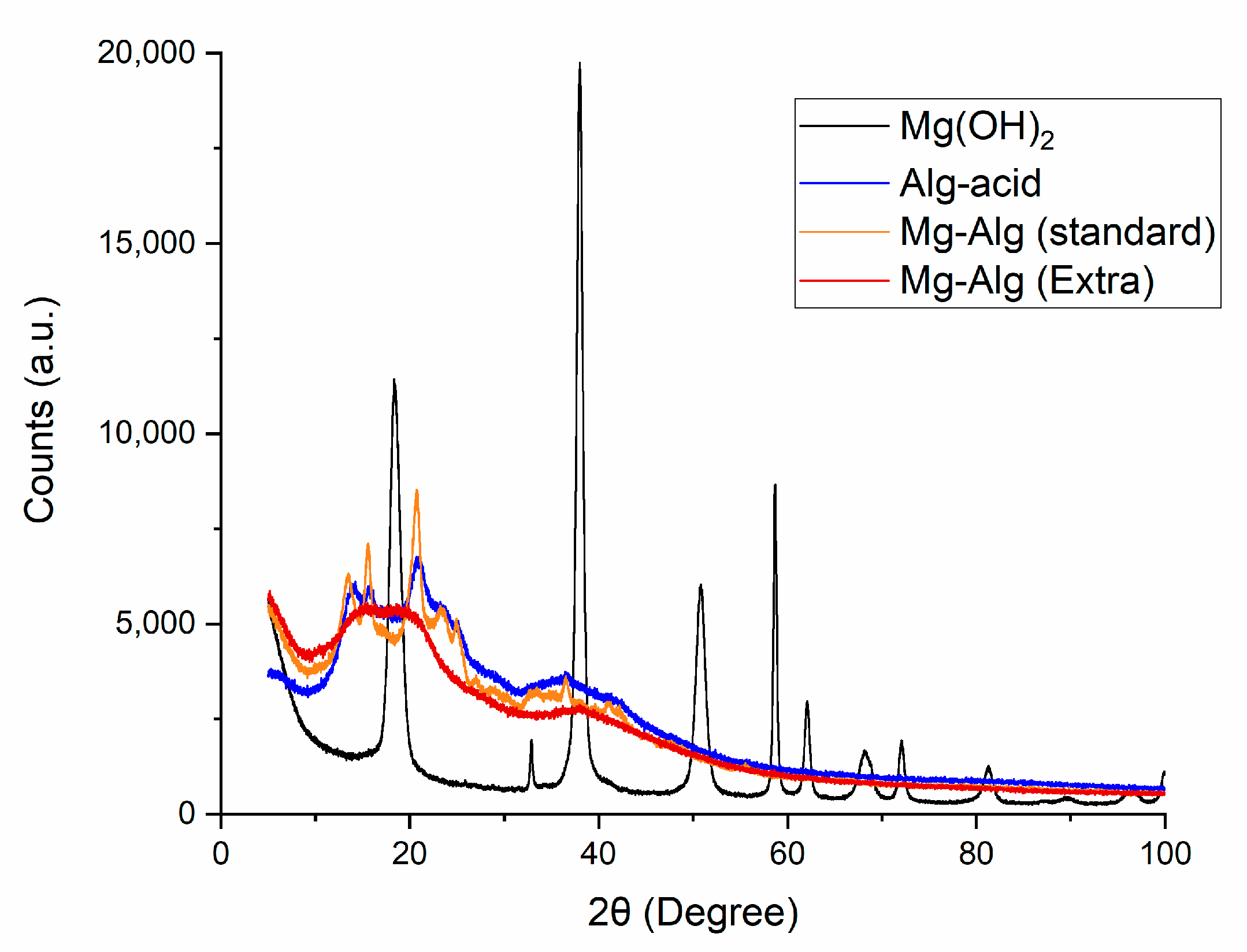
2.1.2. X-Ray Diffraction (XRD) Analysis
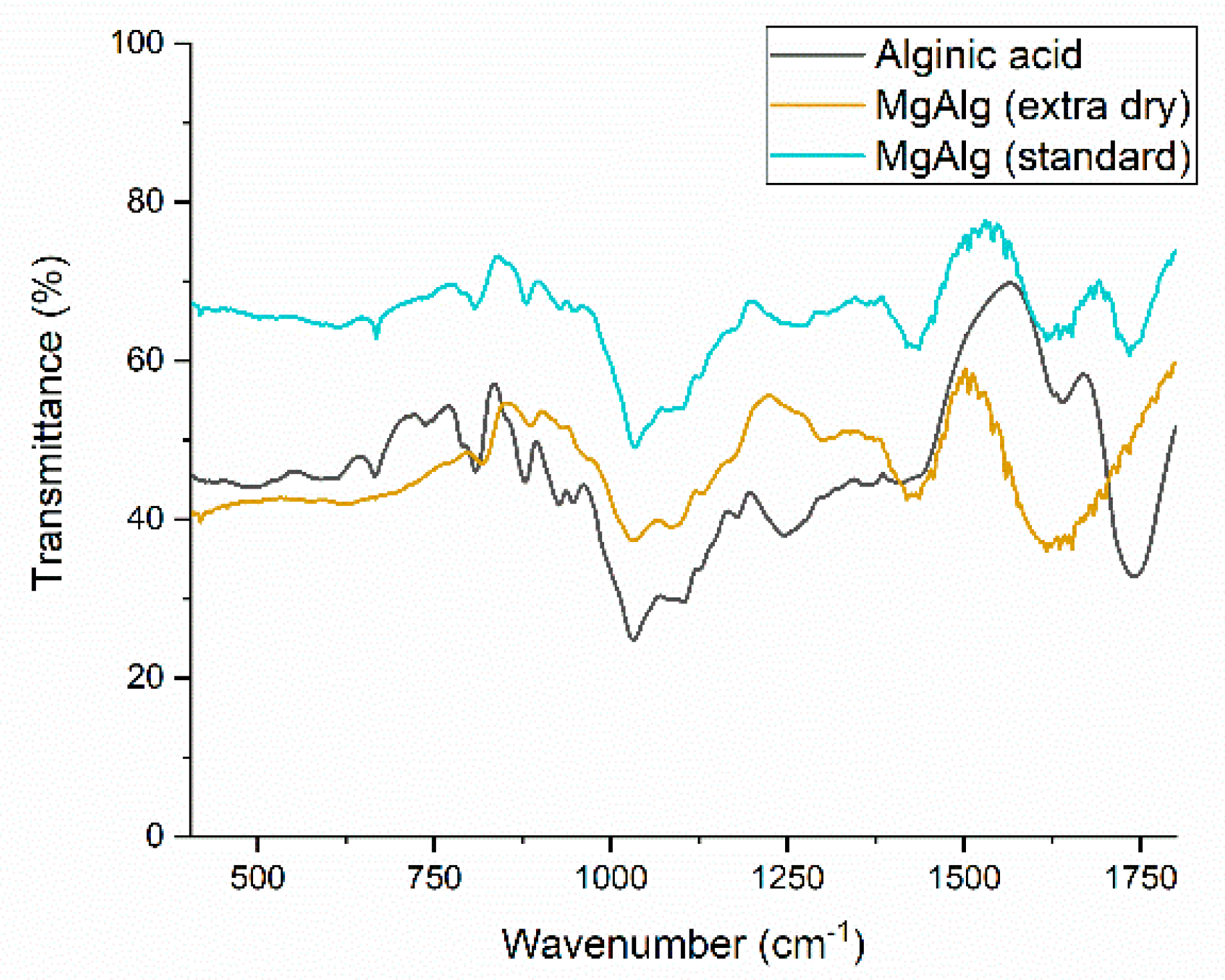
2.1.3. Characterization Using Fourier Transform Infrared Spectroscopy (FTIR)
2.1.4. Pair Distribution Function (PDF) Analysis
2.1.5. Modeling
2.2. Electrochemical Experiments
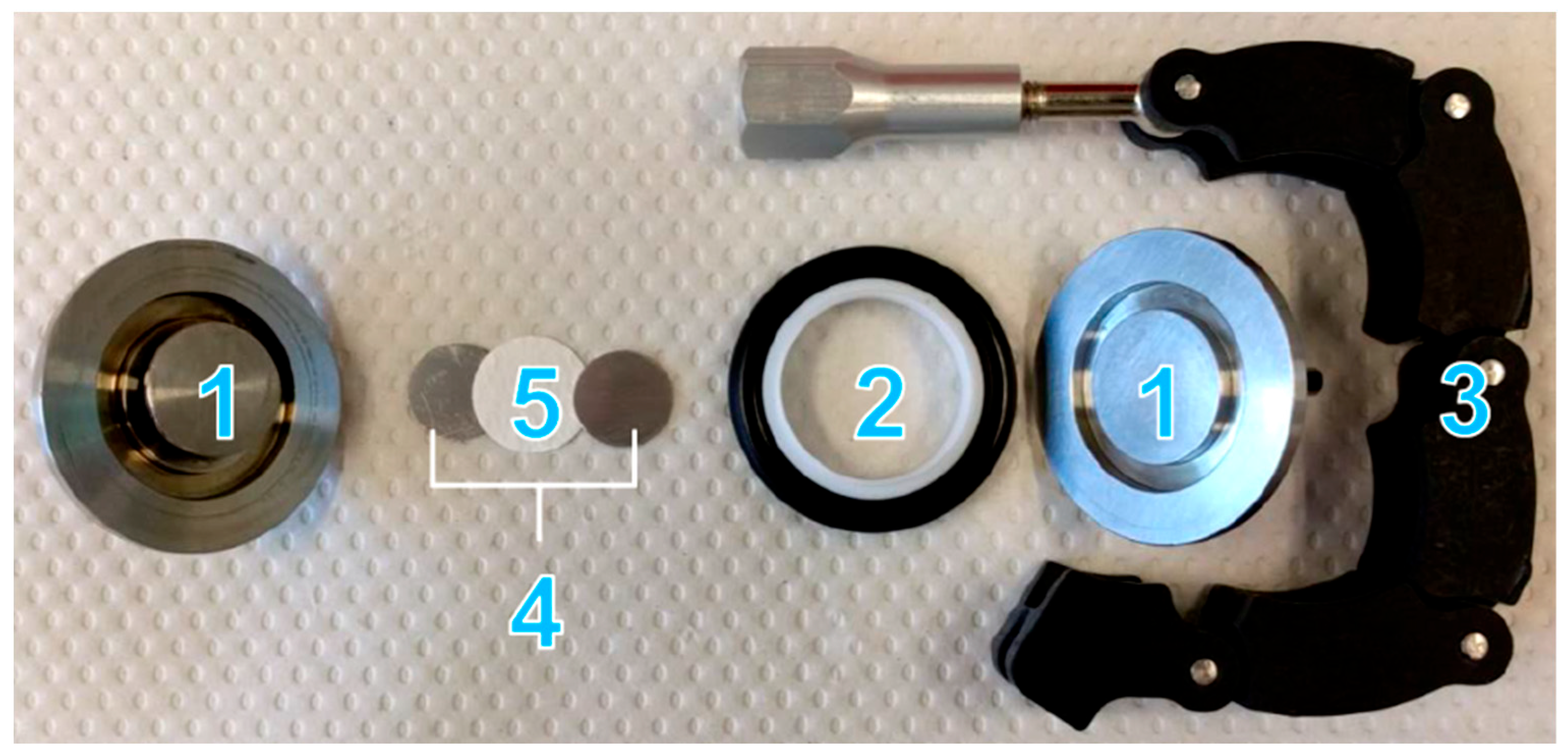
2.2.1. Electrolyte Preparation and Cell Assembly
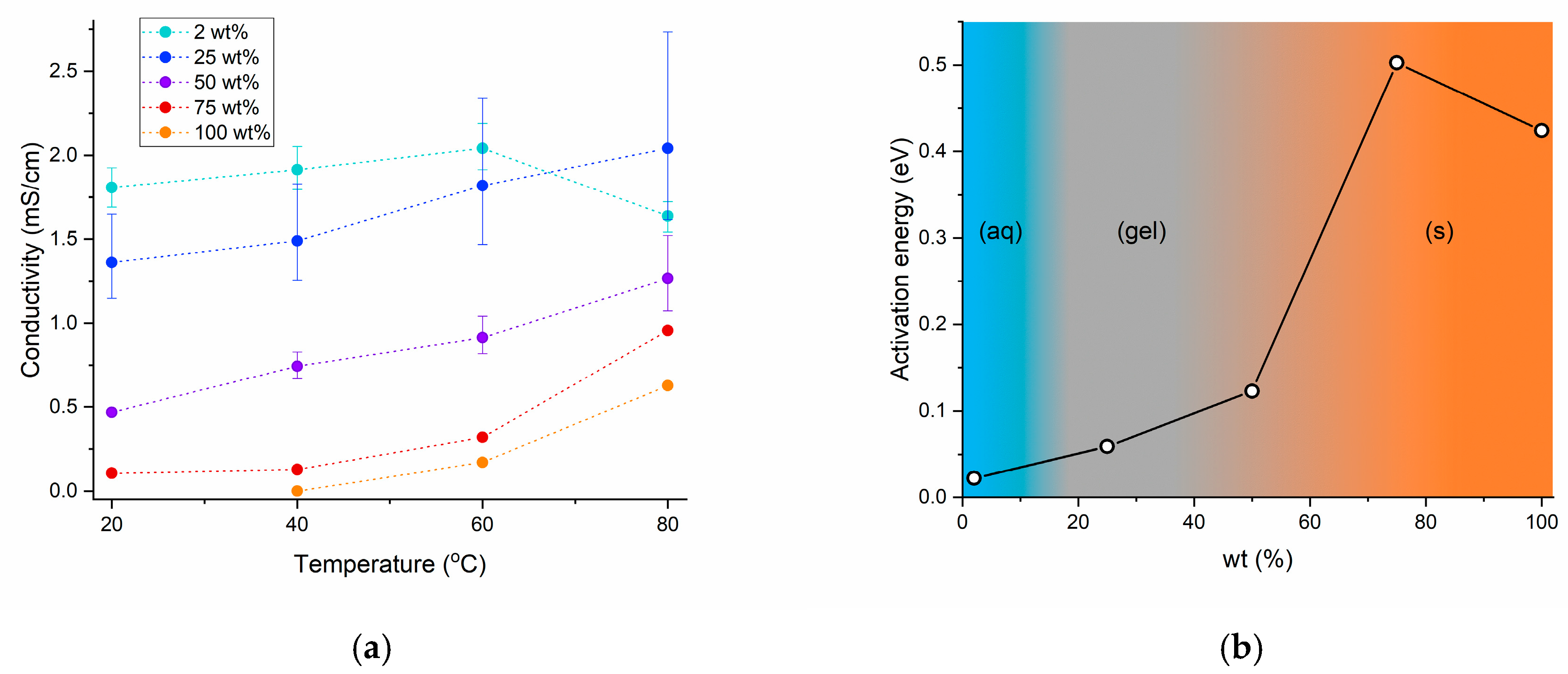
2.2.2. Electrolyte Conductivity
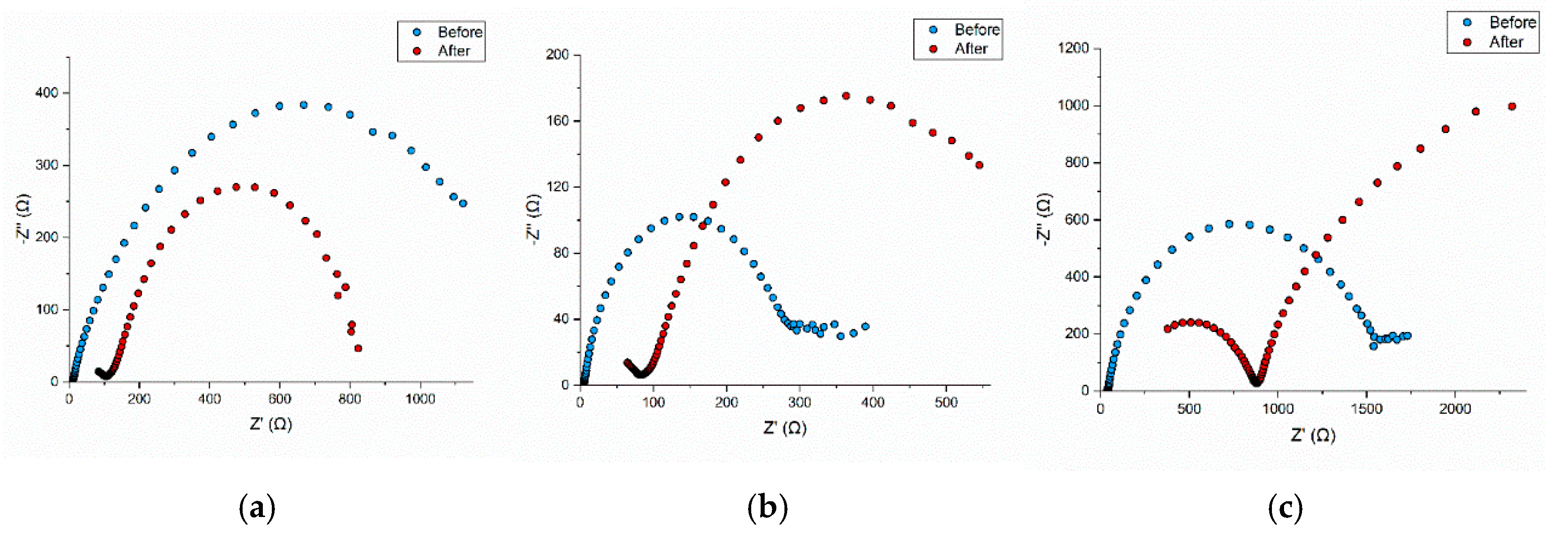
2.2.3. Electrochemical Impedance Spectroscopy (EIS) Profiles
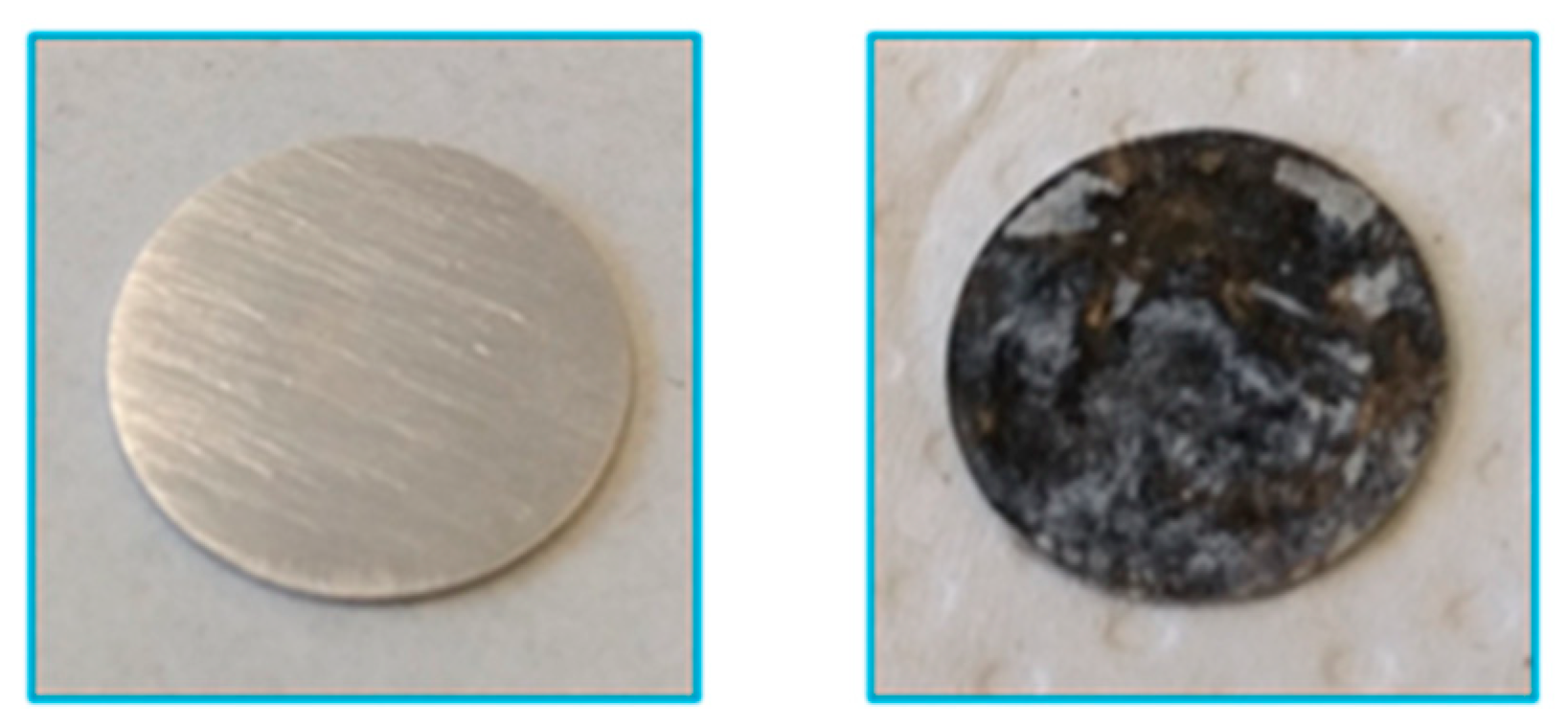
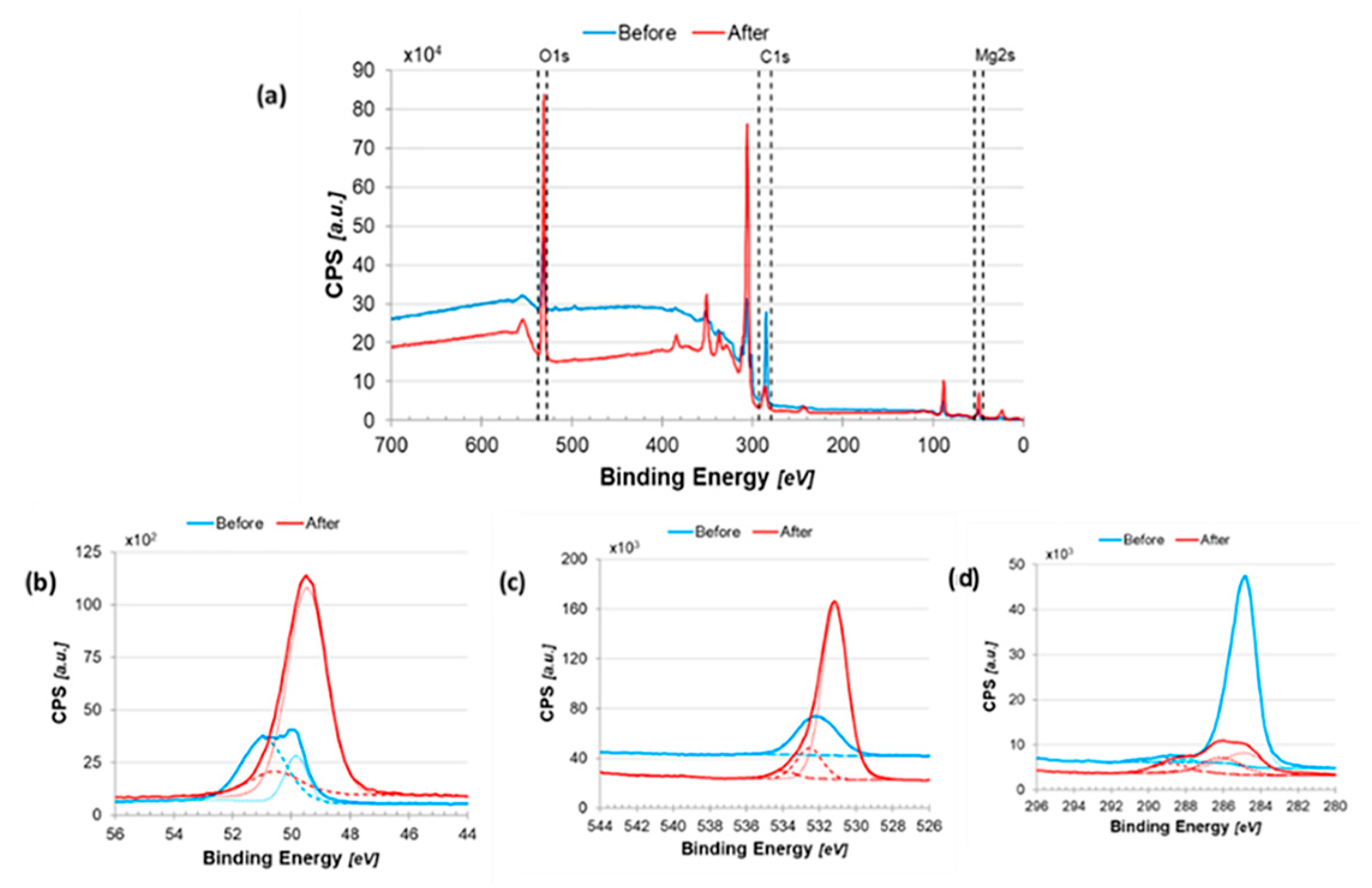
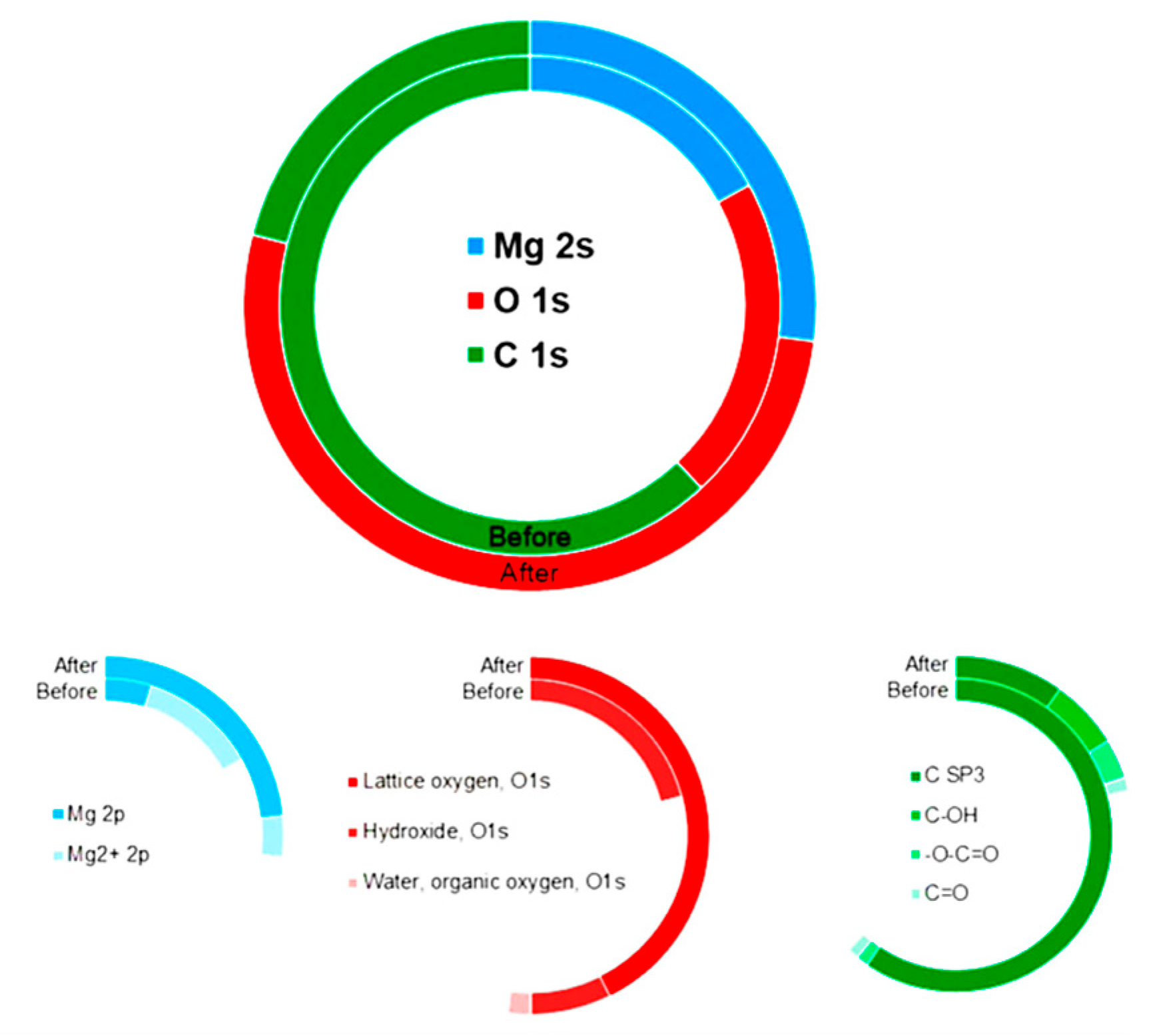
2.2.4. Surface Analysis Using X-Ray Photoelectron Spectroscopy (XPS)
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of Magnesium Alginate
3.3. Pair Distribution Function (PDF)
3.4. Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, C.; Wu, X.; Yuan, X.; Chen, Y.; Liu, L.; Zhu, Y.; Fu, L.; Wu, Y.; Guo, Y.-G.; Van Ree, T. Advances in Rechargeable Mg Batteries. J. Mater. Chem. A 2020, 8, 25601–25625. [Google Scholar] [CrossRef]
- Kotobuki, M.; Yan, B.; Lu, L. Recent Progress on Cathode Materials for Rechargeable Magnesium Batteries. Energy Storage Mater. 2023, 54, 227–253. [Google Scholar] [CrossRef]
- Dominko, R.; Bitenc, J.; Berthelot, R.; Gauthier, M.; Pagot, G.; Di Noto, V. Magnesium Batteries: Current Picture and Missing Pieces of the Puzzle. J. Power Sources 2020, 478, 229027. [Google Scholar] [CrossRef]
- Johnson, I.D.; Ingram, B.J.; Cabana, J. The Quest for Functional Oxide Cathodes for Magnesium Batteries: A Critical Perspective. ACS Energy Lett. 2021, 6, 1892–1900. [Google Scholar] [CrossRef]
- Yoo, H.D.; Shterenberg, I.; Gofer, Y.; Gershinsky, G.; Pour, N.; Aurbach, D. Mg Rechargeable Batteries: An on-Going Challenge. Energy Environ. Sci. 2013, 6, 2265. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Schechter, A.; Moshkovich, M.; Aurbach, D. On the Electrochemical Behavior of Magnesium Electrodes in Polar Aprotic Electrolyte Solutions. J. Electroanal. Chem. 1999, 466, 203–217. [Google Scholar] [CrossRef]
- Yagi, S.; Tanaka, A.; Ichikawa, Y.; Ichitsubo, T.; Matsubara, E. Effects of Water Content on Magnesium Deposition from a Grignard Reagent-Based Tetrahydrofuran Electrolyte. Res. Chem. Intermed. 2014, 40, 3–9. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, B.; Liu, X.; Liu, J.; Ding, J.; Zhong, C.; Hu, W. Water-in-Salt Electrolyte for Safe and High-Energy Aqueous Battery. Energy Storage Mater. 2021, 34, 461–474. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-Salt” Electrolyte Enables High-Voltage Aqueous Lithium-Ion Chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.W.; Pan, W.; Wang, Y.; Luo, S.; Zhao, X.; Leung, D.Y.C. Reversibility of a High-Voltage, Cl–-Regulated, Aqueous Mg Metal Battery Enabled by a Water-in-Salt Electrolyte. ACS Energy Lett. 2022, 7, 2657–2666. [Google Scholar] [CrossRef]
- Shapiro, Y.E. Structure and Dynamics of Hydrogels and Organogels: An NMR Spectroscopy Approach. Prog. Polym. Sci. 2011, 36, 1184–1253. [Google Scholar] [CrossRef]
- McConville, P.; Whittaker, M.K.; Pope, J.M. Water and Polymer Mobility in Hydrogel Biomaterials Quantified by 1H NMR: A Simple Model Describing Both T1 and T2 Relaxation. Macromolecules 2002, 35, 6961–6969. [Google Scholar] [CrossRef]
- El Hariri El Nokab, M.; Lasorsa, A.; Sebakhy, K.O.; Picchioni, F.; van der Wel, P.C.A. Solid-State NMR Spectroscopy Insights for Resolving Different Water Pools in Alginate Hydrogels. Food Hydrocoll. 2022, 127, 107500. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Kiriinya, L.K.; Kwakernaak, M.C.; Van den Akker, S.C.D.; Verbist, G.L.M.M.; Picken, S.J.; Kelder, E.M. Iron and Manganese Alginate for Rechargeable Battery Electrodes. Polymers 2023, 15, 639. [Google Scholar] [CrossRef]
- Sikorski, P.; Mo, F.; Skjåk-Bræk, G.; Stokke, B.T. Evidence for Egg-Box-Compatible Interactions in Calcium−Alginate Gels from Fiber X-Ray Diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium Ions and Alginate Do Form Hydrogels: A Rheological Study. Soft Matter 2012, 8, 4877. [Google Scholar] [CrossRef]
- Kurosawa, R.; Takeuchi, M.; Ryu, J. Fourier-Transform Infrared Analysis of the Dehydration Mechanism of Mg(OH)2 and Chemically Modified Mg(OH)2. J. Phys. Chem. C 2021, 125, 5559–5571. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–Carboxylate Interactions in Metal–Alginate Complexes Studied with FTIR Spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Agulhon, P.; Markova, V.; Robitzer, M.; Quignard, F.; Mineva, T. Structure of Alginate Gels: Interaction of Diuronate Units with Divalent Cations from Density Functional Calculations. Biomacromolecules 2012, 13, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Perić, L.; Pereira, C.S.; Pérez, S.; Hünenberger, P.H. Conformation, Dynamics and Ion-Binding Properties of Single-Chain Polyuronates: A Molecular Dynamics Study. Mol. Simul. 2008, 34, 421–446. [Google Scholar] [CrossRef]
- Rutkowska-Zbik, D.; Witko, M.; Fiedor, L. Ligation of Water to Magnesium Chelates of Biological Importance. J. Mol. Model 2013, 19, 4661–4667. [Google Scholar] [CrossRef] [PubMed]
- Perić-Hassler, L.; Hünenberger, P.H. Interaction of Alginate Single-Chain Polyguluronate Segments with Mono- and Divalent Metal Cations: A Comparative Molecular Dynamics Study. Mol. Simul. 2010, 36, 778–795. [Google Scholar] [CrossRef]
- du Poset, A.M.; Zitolo, A.; Cousin, F.; Assifaoui, A.; Lerbret, A. Evidence for an Egg-Box-like Structure in Iron(II)–Polygalacturonate Hydrogels: A Combined EXAFS and Molecular Dynamics Simulation Study. Phys. Chem. Chem. Phys. 2020, 22, 2963–2977. [Google Scholar] [CrossRef] [PubMed]
- Engelsen, S.B.; Hansen, P.I.; Pérez, S. POLYS 2.0: An Open Source Software Package for Building Three-Dimensional Structures of Polysaccharides. Biopolymers 2014, 101, 733–743. [Google Scholar] [CrossRef]

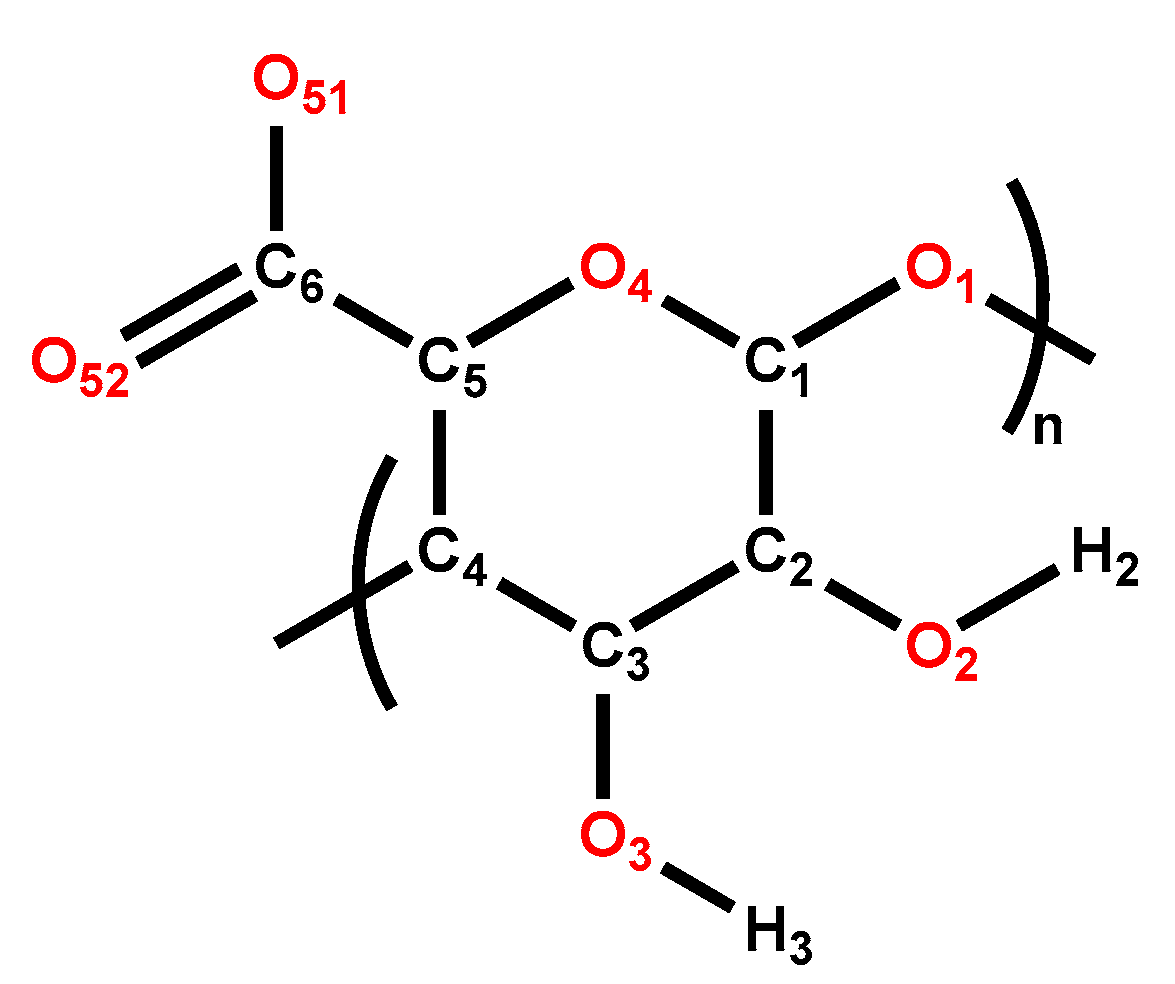
| H2O:Mg2+ Ratio | O51 (Carboxyl) | O52 (Carboxyl) | O3 (Hydroxyl) | O2 (Hydroxyl) | O4 (Ether) | O1 (Ether) | O (H2O) |
|---|---|---|---|---|---|---|---|
| 0 | 2.07 ± 0.05 | 2.09 ± 0.05 | 0.5 ± 0.03 | 0.95 ± 0.04 | 0.12 ± 0.02 | 0.05 ± 0.01 | - |
| 6 | 0.86 ± 0.04 | 1.00 ± 0.03 | 0.034 ± 0.007 | 0.103 ± 0.014 | 0.007 ± 0.003 | - | 4.01 ± 0.01 |
| 12 | 0.27 ± 0.02 | 0.42 ± 0.03 | 0.041 ± 0.009 | 0.146 ± 0.016 | - | - | 5.1 ± 0.08 |
| 24 | 0.062 ± 0.008 | 0.062 ± 0.008 | 0.027 ± 0.011 | 0.094 ± 0.013 | - | - | 5.74 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwakernaak, M.C.; Kiriinya, L.K.; Legerstee, W.J.; Berghmans, W.M.J.; Hofman, C.G.T.; Kelder, E.M. Magnesium Alginate as an Electrolyte for Magnesium Batteries. Batteries 2025, 11, 16. https://doi.org/10.3390/batteries11010016
Kwakernaak MC, Kiriinya LK, Legerstee WJ, Berghmans WMJ, Hofman CGT, Kelder EM. Magnesium Alginate as an Electrolyte for Magnesium Batteries. Batteries. 2025; 11(1):16. https://doi.org/10.3390/batteries11010016
Chicago/Turabian StyleKwakernaak, Markus C., Lindah K. Kiriinya, Walter J. Legerstee, Winok M. J. Berghmans, Caspar G. T. Hofman, and Erik M. Kelder. 2025. "Magnesium Alginate as an Electrolyte for Magnesium Batteries" Batteries 11, no. 1: 16. https://doi.org/10.3390/batteries11010016
APA StyleKwakernaak, M. C., Kiriinya, L. K., Legerstee, W. J., Berghmans, W. M. J., Hofman, C. G. T., & Kelder, E. M. (2025). Magnesium Alginate as an Electrolyte for Magnesium Batteries. Batteries, 11(1), 16. https://doi.org/10.3390/batteries11010016







