Enhancing Mass Transport in Organic Redox Flow Batteries Through Electrode Obstacle Design
Abstract
:1. Introduction
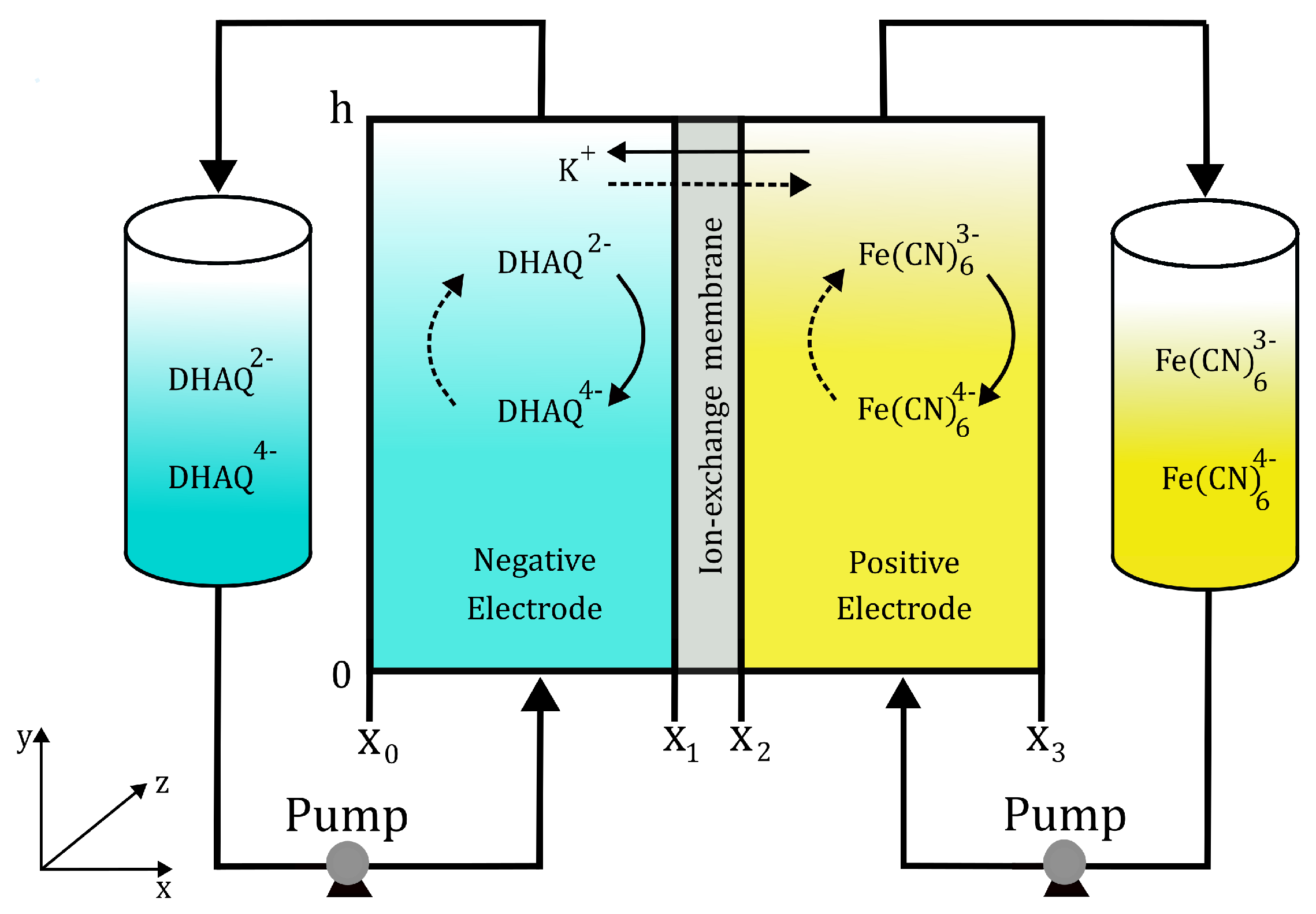
2. Model Description
- The model is stationary.
- Every property of the electrode and membrane is isotropic.
- It is assumed that the entire cell is isothermal.
- The electrolyte is considered incompressible.
- There is no modeling of parasitic reactions.
- Infinite dilute approximation is considered.
- The membrane only permits K+ ions to pass through. All other ion crossover is disregarded.
2.1. Governing Equations
2.1.1. Mass Transport
2.1.2. Reaction Kinetics
2.1.3. Charge Conservation
2.1.4. Boundary Conditions
2.2. Battery Performance Parameters
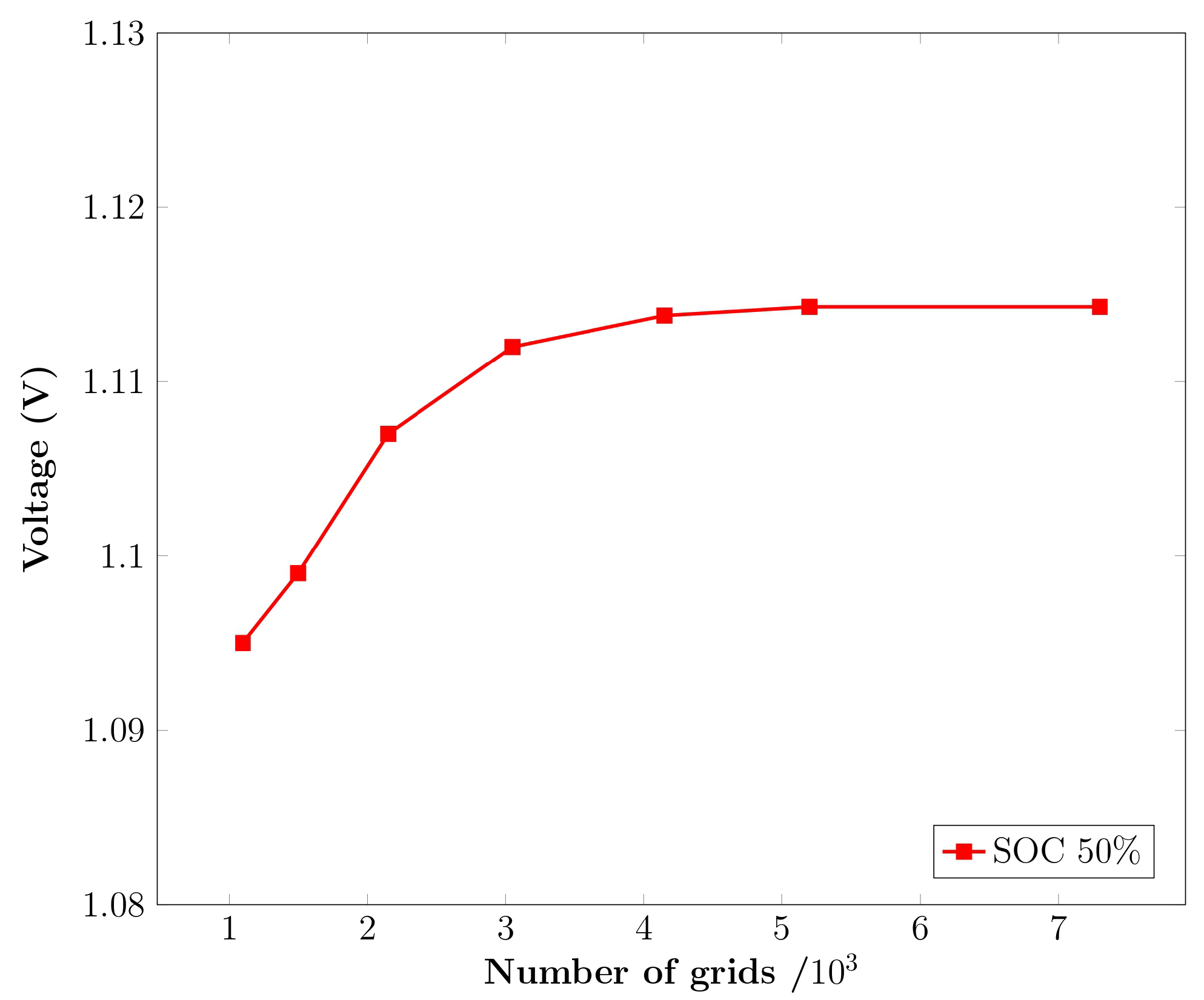
2.3. Numerical Model
3. Results
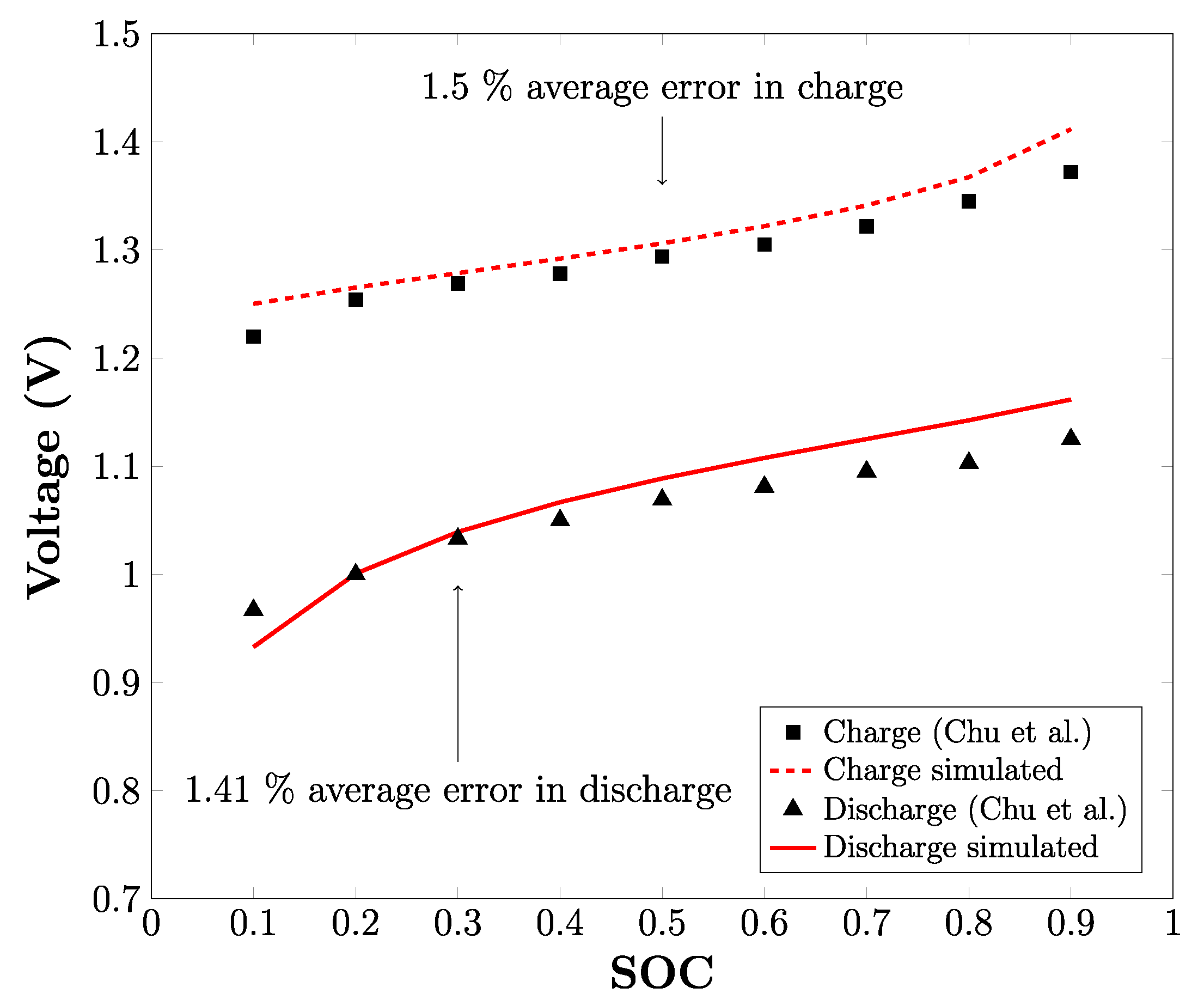
3.1. Experimental Validation
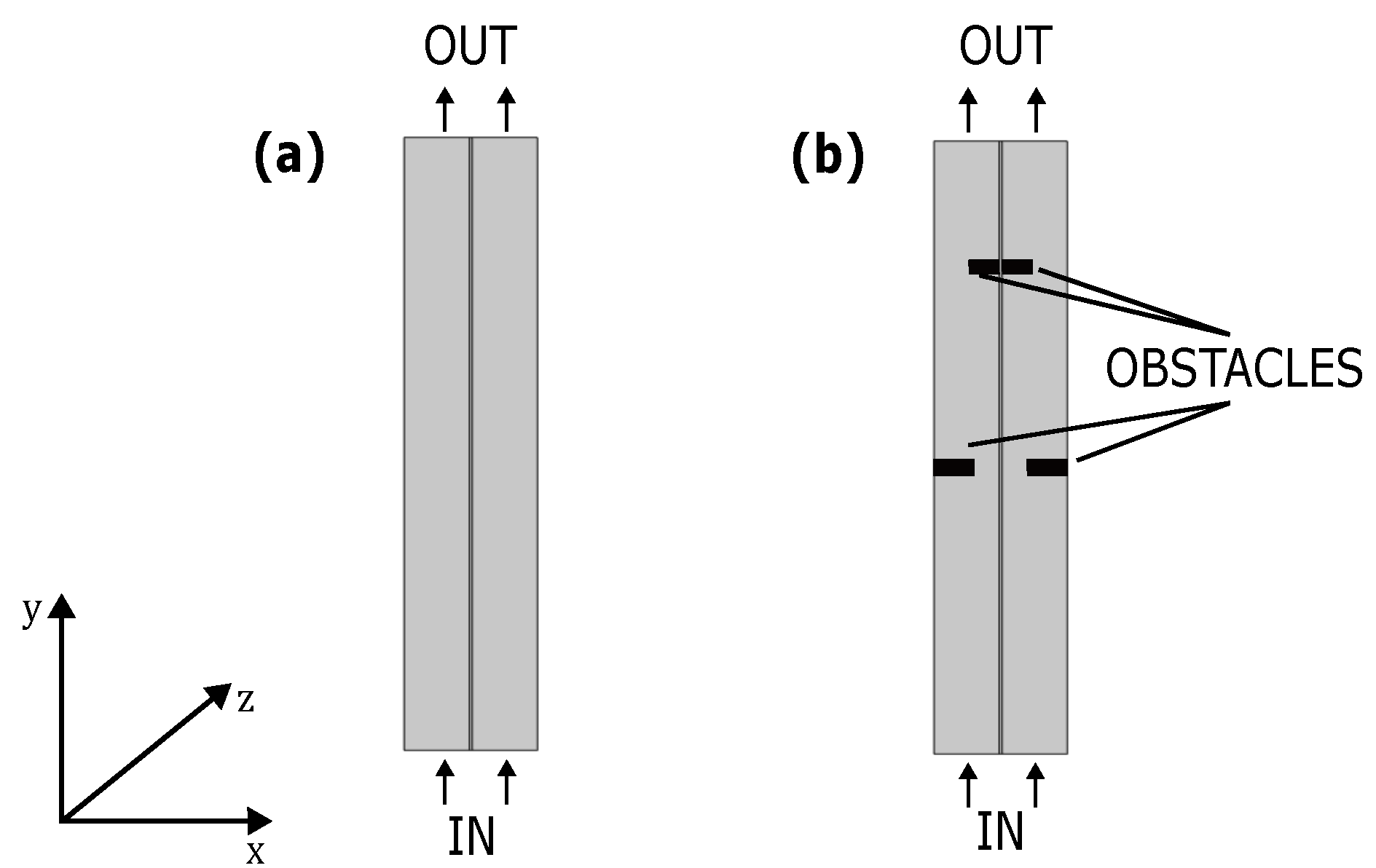
3.2. Case Studies
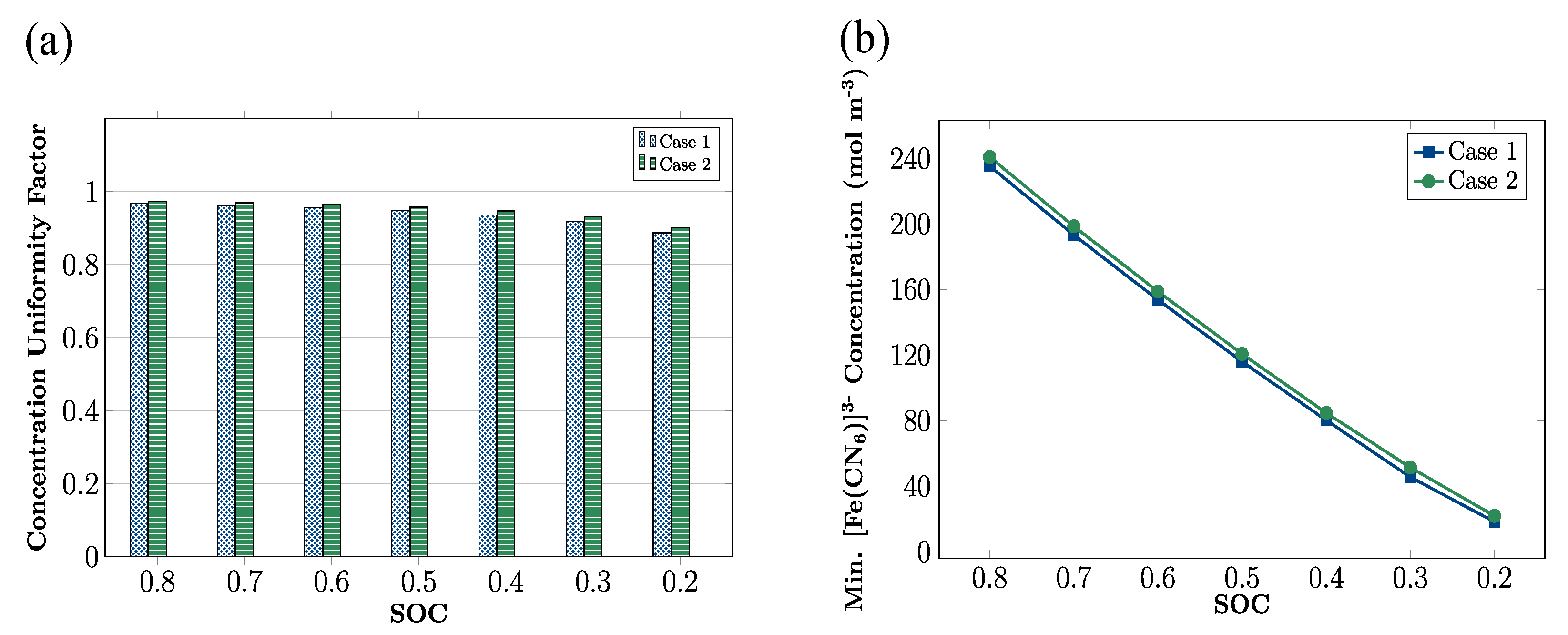
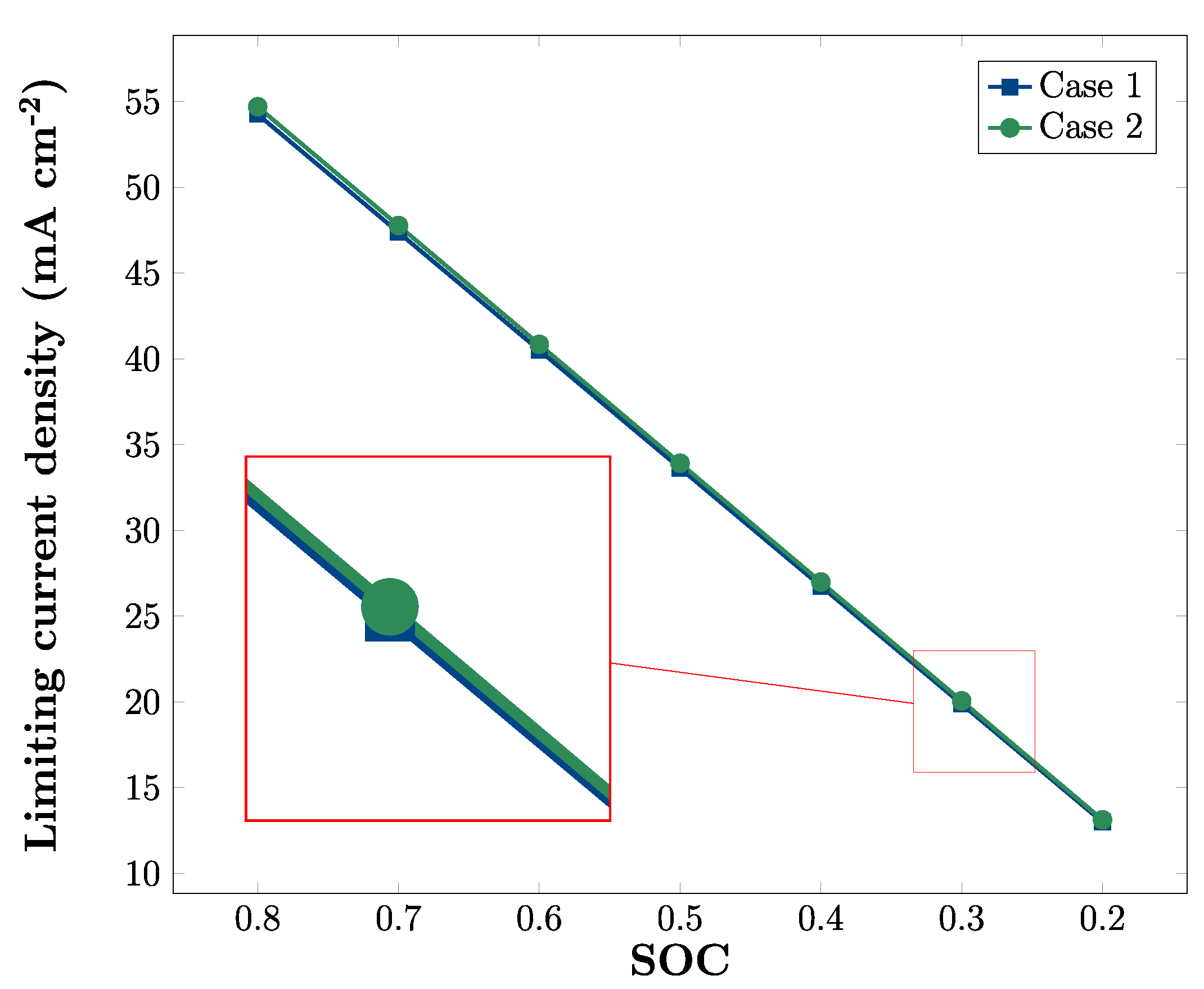
3.3. Effects of SOC
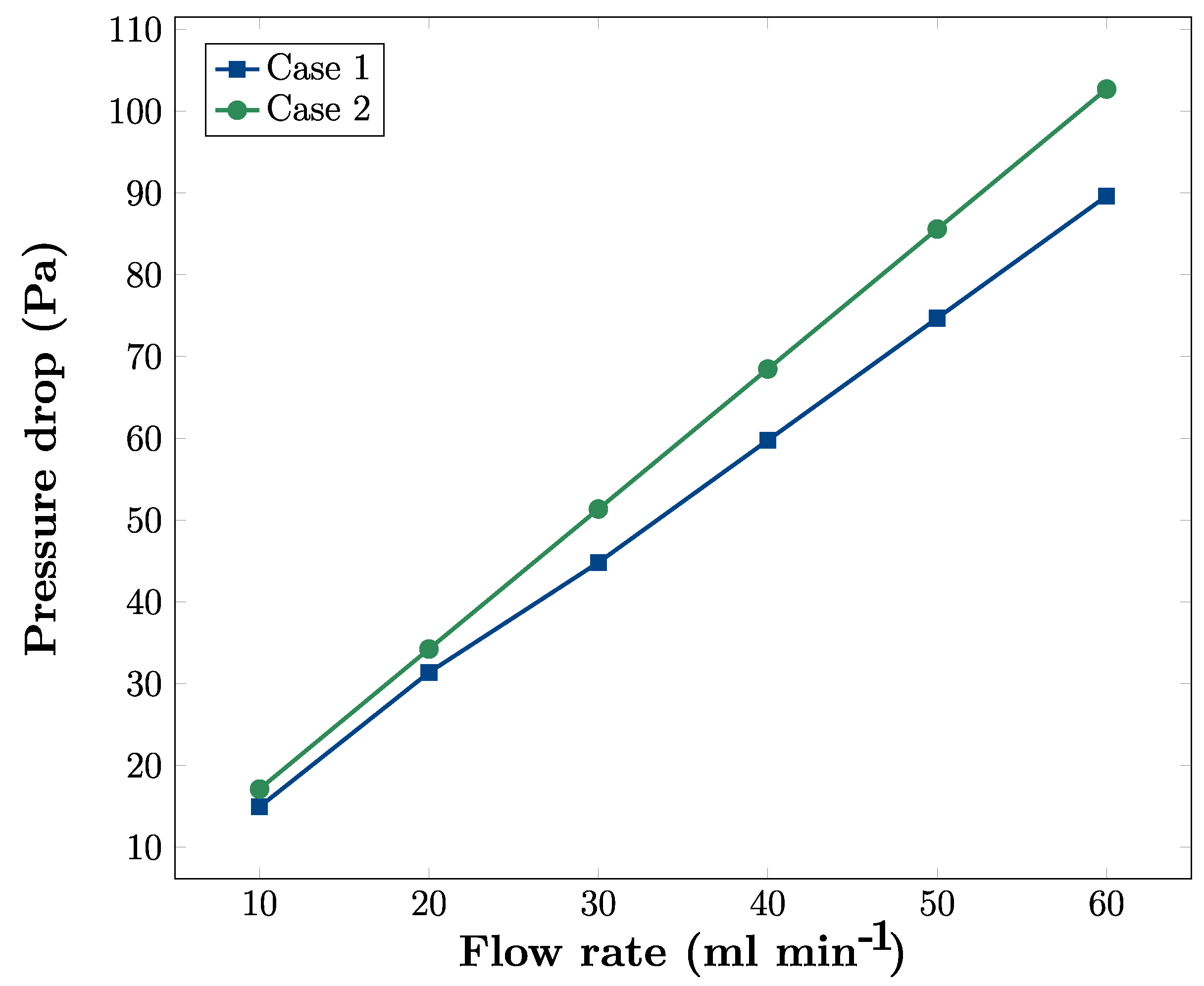
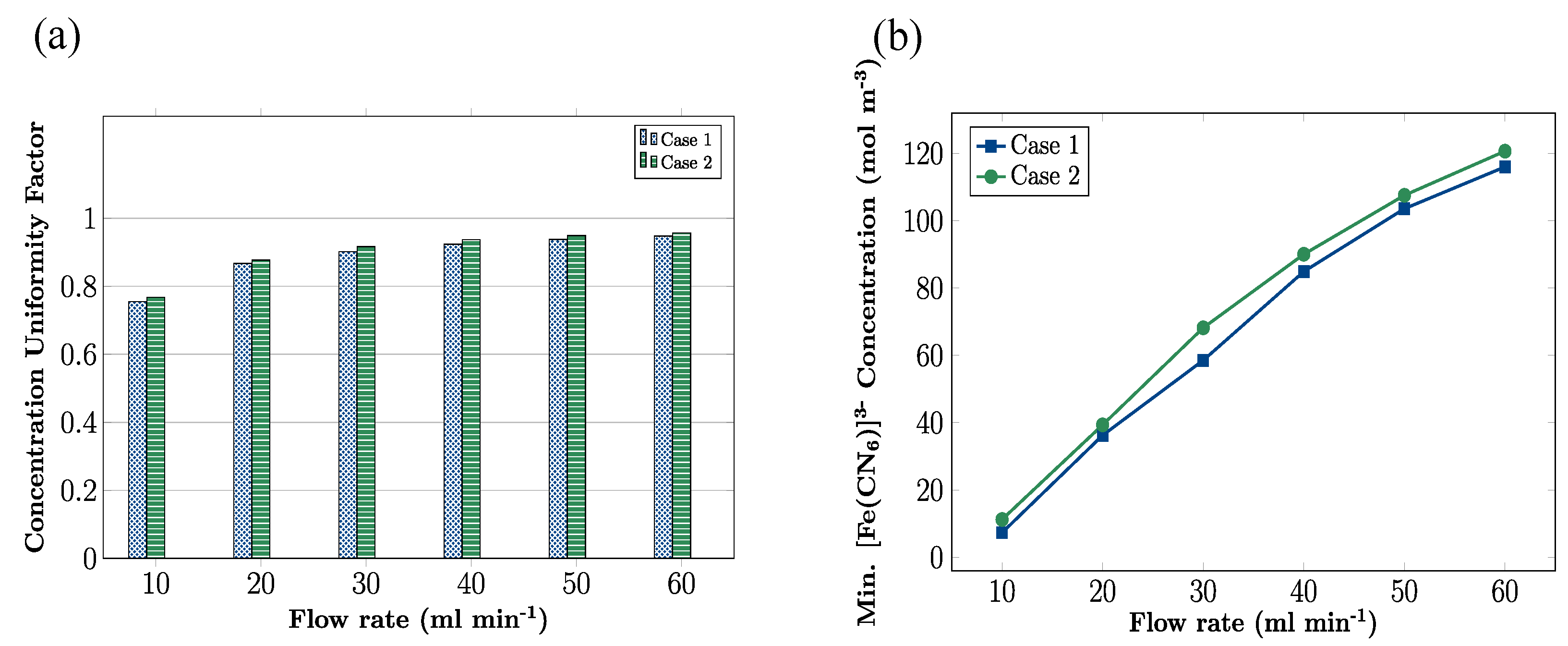
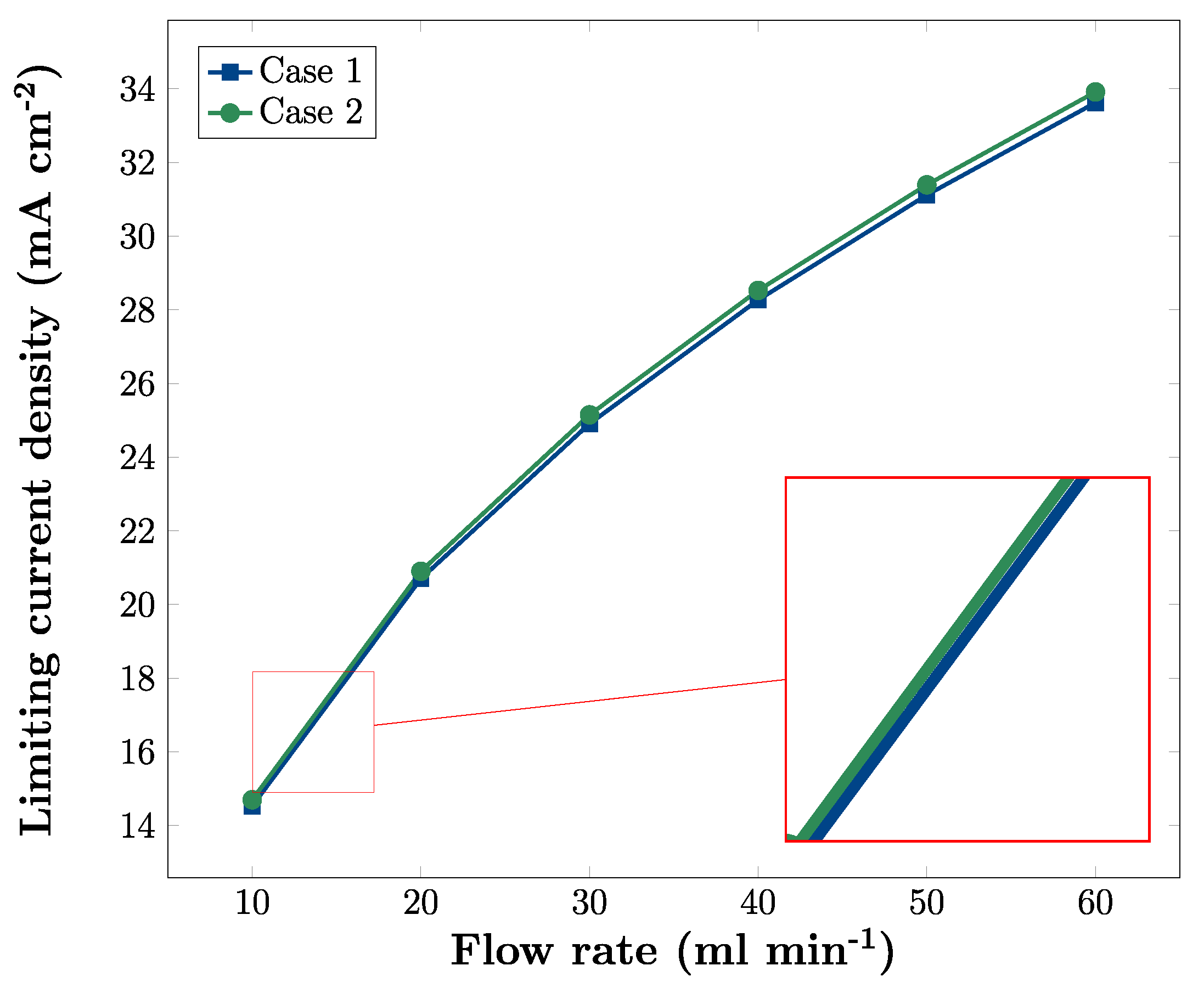
3.4. Effects of Flow Rate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| a | Specific surface area |
| c | Concentration |
| D | Diffusion coefficient |
| Fiber diameter | |
| E | Equilibrium potential |
| F | Faraday constant |
| h | Height |
| Exchange current density | |
| Electrochemical reaction rate | |
| K | Permeability |
| Kozeny–Carman constant | |
| k | Reaction rate constant |
| N | Flux of charged species |
| p | Pressure |
| Q | Volumetric flow rate |
| R | Constant of ideal gases |
| S | Source term |
| T | Temperature |
| t | Time |
| u | Mobility |
| u | Velocity |
| w | Width |
| z | Species charge |
| Greek | |
| Charge transfer coefficient | |
| Electrode porosity | |
| Overpotential | |
| Potential | |
| Conductivity | |
| Dynamic viscosity | |
| Superscripts and subscripts | |
| + | Positive side |
| − | Negative side |
| Standard | |
| Average | |
| e | Electrode |
| Effective | |
| i | Species |
| l | Liquid |
| Membrane | |
| Outlet | |
| s | Solid |
Abbreviations
| CFD | Computational fluid dynamics |
| RFB | Redox flow battery |
| VRFB | Vanadium redox flow battery |
| ORFB | Organic redox flow battery |
References
- Nguyen, T.; Savinell, R.F. Flow Batteries. Electrochem. Soc. Interface 2010, 19, 54. [Google Scholar] [CrossRef]
- Asiaban, S.; Kayedpour, N.; Ebneali Samani, A.; Bozalakov, D.; De Kooning, J.; Crevecoeur, G.; Vandevelde, L. Wind and solar intermittency and the associated integration challenges: A comprehensive review including the status in the Belgian power system. Energies 2021, 14, 630. [Google Scholar] [CrossRef]
- Mutz, M.; Perovic, M.; Gümbel, P.; Königer, V.; Taranovskyy, A.; Li, Y.; Beran, L.; Käfer, T.; Dröder, K.; Knoblauch, V.; et al. Toward a Li-Ion Battery Ontology Covering Production and Material Structure. Energy Technol. 2022, 11, 2200681. [Google Scholar] [CrossRef]
- Braff, W.A.; Mueller, J.M.; Trancik, J.E. Value of storage technologies for wind and solar energy. Nat. Clim. Chang. 2016, 6, 964–969. [Google Scholar] [CrossRef]
- Yu, J.; Fan, L.; Yan, R.; Zhou, M.; Wang, Q. Redox Targeting-Based Aqueous Redox Flow Lithium Battery. ACS Energy Lett. 2018, 3, 2314–2320. [Google Scholar] [CrossRef]
- Reed, D.; Thomsen, E.; Li, B.; Wang, W.; Nie, Z.; Koeppel, B.; Kizewski, J.; Sprenkle, V. Stack Developments in a kW Class All Vanadium Mixed Acid Redox Flow Battery at the Pacific Northwest National Laboratory. J. Electrochem. Soc. 2015, 163, A5211. [Google Scholar] [CrossRef]
- Arevalo-Cid, P.; Dias, P.; Mendes, A.; Azevedo, J. Redox flow batteries: A new frontier on energy storage. Sustain. Energy Fuels 2021, 5, 5366–5419. [Google Scholar] [CrossRef]
- Lourenssen, K.; Williams, J.; Ahmadpour, F.; Clemmer, R.; Tasnim, S. Vanadium redox flow batteries: A comprehensive review. J. Energy Storage 2019, 25, 100844. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.; Lu, S.; Xiang, Y. Titanium Nitride Nanorods Array-Decorated Graphite Felt as Highly Efficient Negative Electrode for Iron-Chromium Redox Flow Battery. Small 2023, 19, e2300943. [Google Scholar] [CrossRef]
- Cunha, A.; Martins, J.; Rodrigues, N.; Brito, F. Vanadium redox flow batteries: A technology review. Int. J. Energy Res. 2014, 39, 889–918. [Google Scholar] [CrossRef]
- Bhattarai, A.; Whitehead, A.; Schweiss, R.; Scherer, G.; Skyllas-Kazacos, M.; Wai, N.; Nguyen, T.; Ghimire, P.; Oo, M.; Hng, H. Anomalous Behavior of Anion Exchange Membrane during Operation of a Vanadium Redox Flow Battery. ACS Appl. Energy Mater. 2019, 2, 1712–1719. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Chakrabarti, B.; Hajimolana, Y.S.; Mjalli, F.; Saleem, M. Progress in Flow Battery Research and Development. J. Electrochem. Soc. 2011, 158, R55–R79. [Google Scholar] [CrossRef]
- Minke, C.; Kunz, U.; Turek, T. Techno-economic assessment of novel vanadium redox flow batteries with large-area cells. J. Power Sources 2017, 361, 105–114. [Google Scholar] [CrossRef]
- Leung, P.; Shah, A.A.; Sanz, L.; Flox, C.; Morante, J.R.; Xu, Q.; Mohamed, M.R.; Ponce de León, C.; Walsh, F.C. Recent developments in organic redox flow batteries: A critical review. J. Power Sources 2017, 360, 243–283. [Google Scholar] [CrossRef]
- Yu, L.C.; Luo, Y.C.; Feng, W.; Zhang, S.; Zhang, X. Fluorinated TEMPO: A new redox-active catholyte material for aqueous Zn-anode hybrid flow batteries. J. Mater. Chem. A 2023, 11, 18911–18921. [Google Scholar] [CrossRef]
- Sánchez-Díez, E.; Ventosa, E.; Guarnieri, M.; Trovò, A.; Flox, C.; Marcilla, R.; Soavi, F.; Mazur, P.; Aranzabe, E.; Ferret, R. Redox flow batteries: Status and perspective towards sustainable stationary energy storage. J. Power Sources 2021, 481, 228804. [Google Scholar] [CrossRef]
- Lin, K.; Chen, Q.; Gerhardt, M.R.; Tong, L.; Kim, S.B.; Eisenach, L.; Valle, A.W.; Hardee, D.; Gordon, R.G.; Aziz, M.J.; et al. Alkaline quinone flow battery. Science 2015, 349, 1529–1532. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, H.; Salla, M.; Zhuang, J.; Zhi, Y.; Wang, X.; Wang, Q. Molecular engineering of dihydroxyanthraquinone-based electrolytes for high-capacity aqueous organic redox flow batteries. Nat. Commun. 2022, 13, 4746. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Li, Y.; Tang, G.; Liu, Y.; Yang, Z.; Xu, T. Solvation regulation to mitigate the decomposition of 2,6-dihydroxyanthraquinone in aqueous organic redox flow batteries. Energy Environ. Sci. 2023, 16, 430–437. [Google Scholar] [CrossRef]
- Jin, S.; Fell, E.M.; Vina-Lopez, L.; Jing, Y.; Michalak, P.W.; Gordon, R.G.; Aziz, M.J. Near Neutral pH Redox Flow Battery with Low Permeability and Long-Lifetime Phosphonated Viologen Active Species. Adv. Energy Mater. 2020, 10, 2000100. [Google Scholar] [CrossRef]
- Zou, H.; Xu, Z.; Xiong, L.; Wang, J.; Fu, H.; Cao, J.; Ding, M.; Wang, X.; Jia, C. An alkaline S/Fe redox flow battery endowed with high volumetric-capacity and long cycle-life. J. Power Sources 2024, 591, 233856. [Google Scholar] [CrossRef]
- Shen, X.; Kellamis, C.; Tam, V.; Sinclair, N.; Wainright, J.; Savinell, R. An All-Soluble Fe/Mn-Based Alkaline Redox Flow Battery System. ACS Appl. Mater. Interfaces 2024, 16, 18686–18692. [Google Scholar] [CrossRef]
- Aramendia, I.; Fernandez-Gamiz, U.; Martinez-San-Vicente, A.; Zulueta, E.; Lopez-Guede, J.M. Vanadium Redox Flow Batteries: A Review Oriented to Fluid-Dynamic Optimization. Energies 2021, 14, 176. [Google Scholar] [CrossRef]
- Esan, O.C.; Shi, X.; Pan, Z.; Huo, X.; An, L.; Zhao, T. Modeling and Simulation of Flow Batteries. Adv. Energy Mater. 2020, 10, 2000758. [Google Scholar] [CrossRef]
- Sun, J.; Guo, Z.; Pan, L.; Fan, X.; Wei, L.; Zhao, T. Redox flow batteries and their stack-scale flow fields. Carbon Neutrality 2023, 2, 30. [Google Scholar] [CrossRef]
- Pan, L.; Xie, J.; Guo, J.; Wei, D.; Qi, H.; Rao, H.; Leung, P.; Zeng, L.; Zhao, T.; Wei, L. In-plane gradient design of flow fields enables enhanced convections for redox flow batteries. Energy Adv. 2023, 2, 2006–2017. [Google Scholar] [CrossRef]
- Martinez Lopez, J.; Aramendia, I.; Fernandez-Gamiz, U.; Sanchez-Diez, E.; Beloki, A.; Kurt, E.; Lopez-Guede, J.M. Computational Modeling of a 2D Vanadium Redox Flow Battery Cell. JOM 2024, 76, 130–140. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, T.S.; Leung, P.K. Numerical investigations of flow field designs for vanadium redox flow batteries. Appl. Energy 2013, 105, 47–56. [Google Scholar] [CrossRef]
- Chu, F.; Su, M.; Xiao, G.; Tan, Z.; Yang, G. Analysis of Electrode Configuration Effects on Mass Transfer and Organic Redox Flow Battery Performance. Ind. Eng. Chem. Res. 2022, 61, 2915–2925. [Google Scholar] [CrossRef]
- Aparicio-Mauricio, G.; Jiménez-Lima, L.; Rivero, E.P.; Cruz-Díaz, M.R. 3D modeling and simulation of an alkaline flow battery considering the tertiary current distribution on the electrodes: Anthraquinone-ferro/ferricyanide as organic electroactive species. J. Power Sources 2023, 582, 233533. [Google Scholar] [CrossRef]
- Akuzum, B.; Alparslan, Y.C.; Robinson, N.C.; Agar, E.; Kumbur, E.C. Obstructed flow field designs for improved performance in vanadium redox flow batteries. J. Appl. Electrochem. 2019, 49, 551–561. [Google Scholar] [CrossRef]
- Messaggi, M.; Gambaro, C.; Casalegno, A.; Zago, M. Development of innovative flow fields in a vanadium redox flow battery: Design of channel obstructions with the aid of 3D computational fluid dynamic model and experimental validation through locally-resolved polarization curves. J. Power Sources 2022, 526, 231155. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Z.; Xie, X.; Liu, Y.; Wu, J.; Guo, Z.; Huang, Q. Redox flow battery:Flow field design based on bionic mechanism with different obstructions. Chem. Eng. J. 2024, 498, 155663. [Google Scholar] [CrossRef]
- Gurieff, N.; Keogh, D.F.; Timchenko, V.; Menictas, C. Enhanced Reactant Distribution in Redox Flow Cells. Molecules 2019, 24, 3877. [Google Scholar] [CrossRef] [PubMed]
- Gurieff, N.; Keogh, D.F.; Baldry, M.; Timchenko, V.; Green, D.; Koskinen, I.; Menictas, C. Mass Transport Optimization for Redox Flow Battery Design. Appl. Sci. 2020, 10, 2801. [Google Scholar] [CrossRef]
- Dhillon, A. Multiphysics Modelling of an Alkaline All-Iron, All-Soluble Aqueous Redox Flow Battery. Master’s Thesis, University of Waterloo, Waterloo, ON, Canada, 2021. [Google Scholar]
- Chen, Z.; Yu, W.; Liu, Y.; Zeng, Y.; He, Q.; Tan, P.; Ni, M. Mathematical modeling and numerical analysis of alkaline zinc-iron flow batteries for energy storage applications. Chem. Eng. J. 2021, 405, 126684. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Z.G.; Jiang, Z.Y.; Yang, W.W. Experimental study on the performance of a vanadium redox flow battery with non-uniformly compressed carbon felt electrode. Appl. Energy 2018, 213, 293–305. [Google Scholar] [CrossRef]
- Knehr, K.W.; Agar, E.; Dennison, C.R.; Kalidindi, A.R.; Kumbur, E.C. A Transient Vanadium Flow Battery Model Incorporating Vanadium Crossover and Water Transport through the Membrane. J. Electrochem. Soc. 2012, 159, A1446. [Google Scholar] [CrossRef]
- You, D.; Zhang, H.; Chen, J. A simple model for the vanadium redox battery. Electrochim. Acta 2009, 54, 6827–6836. [Google Scholar] [CrossRef]

| Species | Positive Electrode | Negative Electrode |
|---|---|---|
| [Fe(CN6)]4− | - | |
| [Fe(CN6)]3− | - | |
| DHAQ2− | - | |
| DHAQ4− | - |
| Quantity | Symbol | Value | References |
|---|---|---|---|
| Diffusivity of [Fe(CN6)]4− | m2 s−1 | [36] | |
| Diffusivity of [Fe(CN6)]3− | m2 s−1 | [36] | |
| Diffusivity of DHAQ2− | m2 s−1 | [17] | |
| Diffusivity of DHAQ4− | m2 s−1 | [17] | |
| Diffusivity of K+ | m2 s−1 | [37] | |
| Diffusivity of OH− | m2 s−1 | [37] |
| Quantity | Symbol | Value | References |
|---|---|---|---|
| Non-compressed electrode porosity | 0.895 | [38] | |
| Non-compressed electrode specific surface area | a | m2 m−3 | Fitted |
| Non-compressed electrode conductivity | 66.7 S m−1 | [39] | |
| Kozeny–Carman constant | 4.28 | [40] |
| Quantity | Symbol | Value | References |
|---|---|---|---|
| Standard equilibrium potential for positive reaction | 0.33 V | [29] | |
| Standard equilibrium potential for negative reaction | −0.71 V | [29] | |
| Cathodic transfer coefficient | 0.5 | [17] | |
| Anodic transfer coefficient | 0.5 | [17] | |
| Rate constant for positive reaction | m s−1 | [29] | |
| Rate constant for negative reaction | m s−1 | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-López, J.; Fernández-Gamiz, U.; Sánchez-Díez, E.; Beloki-Arrondo, A.; Ortega-Fernández, Í. Enhancing Mass Transport in Organic Redox Flow Batteries Through Electrode Obstacle Design. Batteries 2025, 11, 29. https://doi.org/10.3390/batteries11010029
Martínez-López J, Fernández-Gamiz U, Sánchez-Díez E, Beloki-Arrondo A, Ortega-Fernández Í. Enhancing Mass Transport in Organic Redox Flow Batteries Through Electrode Obstacle Design. Batteries. 2025; 11(1):29. https://doi.org/10.3390/batteries11010029
Chicago/Turabian StyleMartínez-López, Joseba, Unai Fernández-Gamiz, Eduardo Sánchez-Díez, Aitor Beloki-Arrondo, and Íñigo Ortega-Fernández. 2025. "Enhancing Mass Transport in Organic Redox Flow Batteries Through Electrode Obstacle Design" Batteries 11, no. 1: 29. https://doi.org/10.3390/batteries11010029
APA StyleMartínez-López, J., Fernández-Gamiz, U., Sánchez-Díez, E., Beloki-Arrondo, A., & Ortega-Fernández, Í. (2025). Enhancing Mass Transport in Organic Redox Flow Batteries Through Electrode Obstacle Design. Batteries, 11(1), 29. https://doi.org/10.3390/batteries11010029









