Different Metal–Air Batteries as Range Extenders for the Electric Vehicle Market: A Comparative Study
Abstract
:1. Introduction
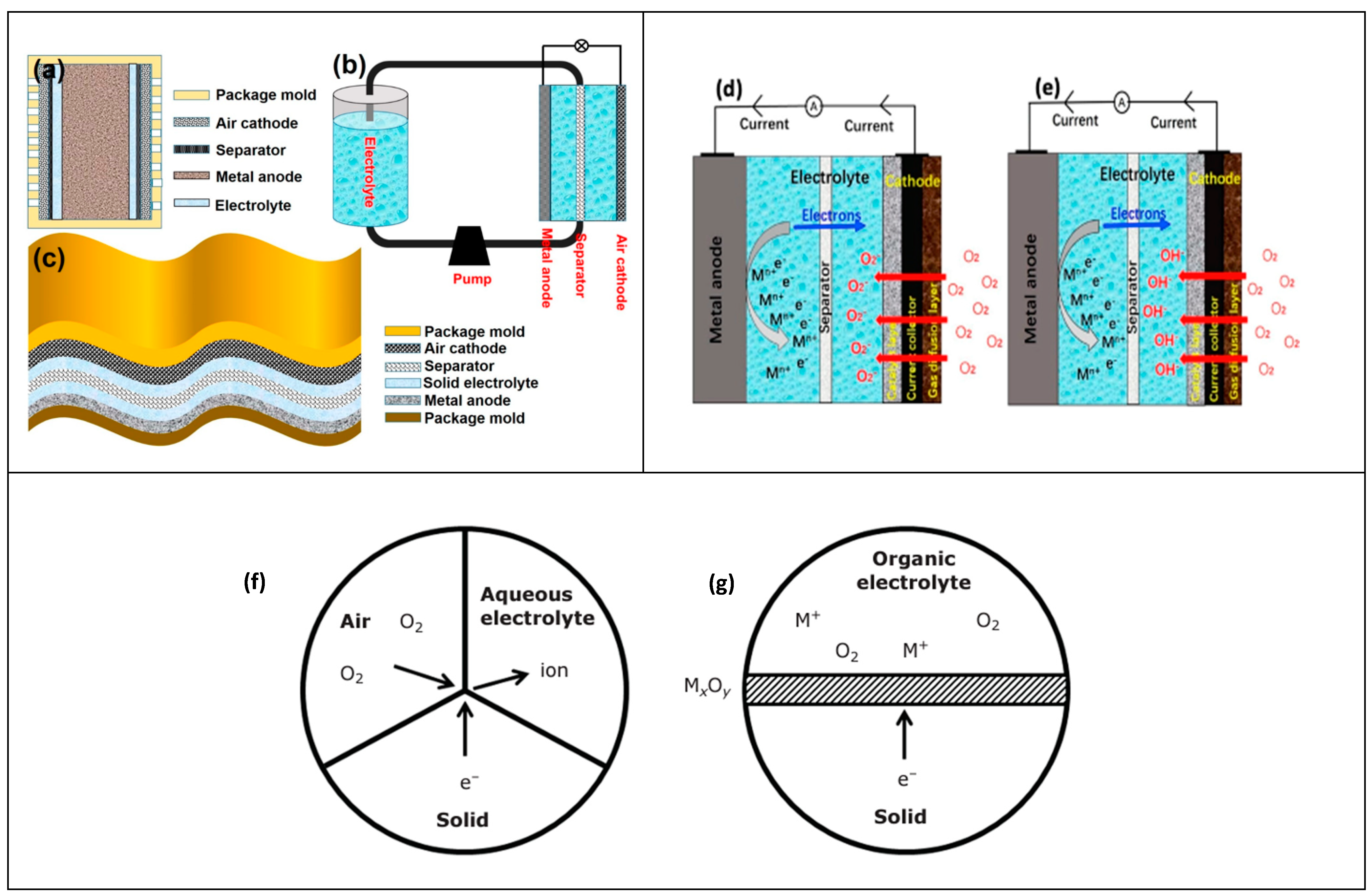
2. Metal–Air Batteries
| Parameter | Formula | Units | Explanation | Ref. |
|---|---|---|---|---|
| Specific capacity | mAh g−1 | I is the discharge current, t is the discharge time, and m is the mass of the electrode | [78,79] | |
| Capacity density | mA cm−2 | I is the current in mA, t is the discharge time in hours, and Δm is the weight loss in g | [38] | |
| Power density | mW cm−2 | E is the average discharge voltage (V), I is the current in mA, and S is the active surface area in cm2 | [38] | |
| Specific energy | Wh kg−1 | SE is the specific energy, V is the nominal battery voltage in V, C is the rated battery capacity in Ah, and MB is the battery mass | [80] | |
| Energy density | mWh g−1 | E is the average discharge voltage (V), I is the current in mA, t is the discharge time in hours, and Δm is the weight loss in g | [38] | |
| Instantaneous specific energy (including oxygen) | Wh kg−1 | F is Faraday’s constant, n is the number of electrons transferred per metal ion, OCV is the nominal voltage, MMetal is the molar mass of metal anode, and MM+O is the combined molar mass of metal anode and stoichiometric amount of oxygen that enters the battery | [50] | |
| Energy density (including oxygen) | Wh L−1 | SE is the specific energy in Whkg−1 and OAD is the oxidizing anode density in kgL−1 | [50] | |
| Nernst equation | V | R is the gas constant, T is the temperature in K, F is Faraday’s constant, n is the number of moles of electrons transferred, and Q is the reaction quotient | [81] | |
| Gibbs free energy | J | T is the temperature in K, F is Faraday’s constant, n is the number of moles of electrons transferred, S is the entropy, and H is the enthalpy | [81] | |
| Current efficiency | % | Q is the actual capacity density in mAcm−2 and Q0 is the theoretical capacity density in mAhg−1 | [38] | |
| Energy efficiency | % | W is the actual energy density in mWHg−1 and W0 is the theoretical energy density in mWhg−1 | [38] | |
| Charging energy efficiency | % | Numerator = net energy; denominator = charge energy; SOC = state of charge; U = battery voltage; Cn = battery standard capacity | [81] | |
| Discharging Energy efficiency | % | Numerator = discharge energy; denominator = net energy; SOC = state of charge; U = battery voltage; Cn = battery standard capacity | [81] | |
| Charge-discharge Energy Efficiency | % | Numerator = discharge energy; denominator = charge energy; SOC = state of charge; U = battery voltage; Cn = battery standard capacity | [81] | |
| Vehicle Range | km | EB is the battery pack energy in Wh, ECEV is the energy consumption efficiency of the vehicle in Wh km−1 kg−1, MV is the vehicle mass without a battery pack in kg, km, B is the battery mass overhead, and SEBC is the specific energy of the battery cell in Whkg−1 | [20] | |
| Total Vehicle Cost | USD | CV is the vehicle cost without battery pack in USD and CB is the battery pack cost in USD kWh−1 | [19] | |
| Battery Pack Volume | L | kvol,B is the battery volume overhead and EDBC is the energy density of the battery cell in WhL−1 | [19] |
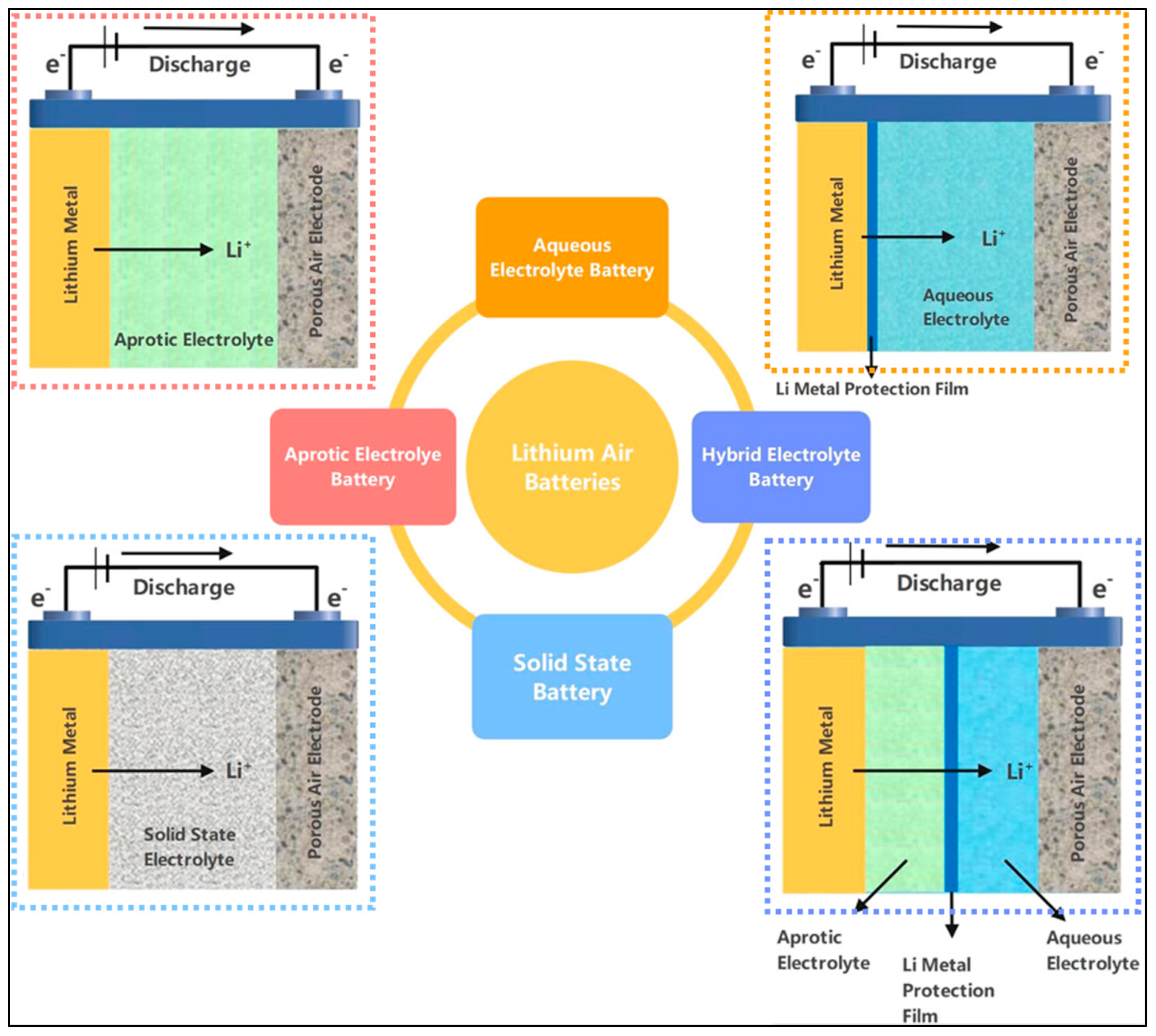
2.1. Lithium–Air Batteries
| Metal | Electrolyte | Cell Description | Reaction | Potential | Cell Reaction (Overall) | Refs. |
|---|---|---|---|---|---|---|
| Aluminum (Al) | Aqueous—Neutral | Anode: Aluminum Cathode: Air-breathing | Anode: Cathode: | Anode: −1.47 V Cathode: 0.81 V | (2.28 V) | [2,127,128] |
| Aqueous—Alkaline | Anode: Aluminum Cathode: Air-breathing | Anode: Cathode: Side: | Anode: −2.35 V Cathode: 0.39 V | (2.75 V) | [2,51,129] | |
| Aqueous—Acidic | Anode: Aluminum Cathode: Air-breathing | Anode: Cathode: Side: | Anode: −1.66 V Cathode: 1.23 V | (2.89 V) | [2] | |
| Organic Solvent Based: Solid-State Gel Polymer Electrolyte | Anode: Aluminum Cathode: Air-breathing (carbon and polymer-based) Electrolyte: PVA/LiCl polymer | Anode: Cathode: | Anode: −2.3 V Cathode: 0.81 V | (3.31 V) | [2,130,131] | |
| Room Temperature Ionic Liquid (RTIL)-Based | Anode: Aluminum Cathode: Air-breathing Electrolyte: Aprotic room temperature ionic liquids | Anode: Cathode: | Anode: −1.3 V Cathode: 0.695 V | (1.995 V) | [129,131,132,133,134] | |
| Germanium (Ge) | Aqueous—Alkaline: 1–6 M KOH | Anode: Germanium wafers Cathode: Air-breathing Electrolyte: Aqueous KOH | Anode: Cathode: Passivation: | Anode: 1.0 V Cathode: 0.81 V | (1.81 V) | [135] |
| Calcium (Ca) | Non-Aqueous—(Aprotic) Organic Electrolyte | Anode: Calcium Cathode: Air-breathing Electrolyte: Aprotic liquid | Anode: Cathode 1: Cathode 2: Cathode 3: | Anode: −2.368 V Cathode: varies | (3.25 V) (3.28 V) (3.13 V) | [136,137,138] |
| Non-Aqueous— Ionic Liquid | Anode: Calcium Cathode: Carbon nanotube sheet Electrolyte: Ca salt in 1:1 EMIM-BF4/DMSO solution | Anode: Cathode 1: Cathode 2: | Anode: −2.868 V Cathode: 0.715 V Cathode: 0.265 V | (3.583 V) (3.133 V) | [131,139] | |
| Iron (Fe) | Aqueous—Alkaline | Anode: Iron Cathode: Air-breathing Electrolyte: Basic media | Anode: Cathode: Side: | Anode: −0.88 V Cathode: 0.401 V | (1.28 V) | [140,141] |
| Solid Oxide | Anode: Ni-Fe alloy Cathode: Ba0.6La0.4CoO3 Electrolyte: LSGM Fuel: Iron powder | Anode: Cathode: Iron Powder: | Anode: - Cathode: - | (0.97 V) | [142,143,144,145,146] | |
| Lithium (Li) | Aprotic Solvent | Anode: Lithium Cathode: Porous carbon Electrolyte: Aprotic liquid | Anode: Cathode: Cathode: Cathode: Cathode: Cathode: | Anode: −3.0401 V Cathode: varies | (3.0 V) (2.96 V) (3.10 V) (2.91 V) (2.91 V) | [147,148,149,150] |
| Aqueous—Acidic | Anode: Lithium Cathode: Air-breathing Electrolyte: Acidic media |
Anode: Cathode: | Anode: −3.0401 V Cathode: 1.229 V | (4.274 V) | [131,147,151] | |
| Aqueous—Alkaline | Anode: Lithium Cathode: Air-breathing Electrolyte: Basic media |
Anode: Cathode: | Anode: −3.0401 V Cathode: 0.401 V | (3.44 V) | [131,151] | |
| Magnesium (Mg) | Aqueous—Neutral | Anode: Magnesium (or alloy) Cathode: Air-breathing Electrolyte: Saline | Anode: Cathode: Side: | Anode: −2.37 V Cathode: 0.81 V | (3.1 V) | [53,131] |
| Potassium (K) | K Salt Ether Solvent, Potassium Superoxide | Anode: Potassium Cathode: Air-breathing Electrolyte: Organic |
Anode: Cathode: | Anode: −2.931 V Cathode: −0.451 V | (2.48 V) | [131,152] |
| Silicon (Si) | Non-Aqueous—RTIL: EMIm (HF) 2.3 F | Anode: Silicon Cathode: Air-breathing Electrolyte: Room temperature ionic liquids | Anode: Cathode: SiO2 formation: | Anode: −2.71 V Cathode: 0.40 V | (2.21 V) | [153] |
| Aqueous—Alkaline: KOH | Anode: Silicon Cathode: Air-breathing |
Anode: Cathode: | Anode: −2.71 V Cathode: −0.44 V Alternate: −0.38 V | (2.09 V) | [153] | |
| Sodium (Na) | Aqueous—Neutral | Anode: Sodium Cathode: Air-breathing Electrolyte: Saline |
Anode: Cathode: | Anode: −0.96 V Cathode: 0.33 V | (3.11 V) | [154] |
| Aprotic Solvent | Anode: Sodium Cathode: Air-breathing | Anode: Cathode: Alternate Cathode: | Anode: −2.71 V Cathode: −0.44 V Alternate: −0.38 V | (2.27 V) (2.33 V) | [154,155] | |
| Tin (Sn) | Solid Oxide | Anode: Tin alloy Cathode: LSM/LSM-GDC Electrolyte: Ethylene glycol with tin powder (high temperature) |
Anode: Cathode: | Anode: −0.96 V Cathode: 0.33 V | (1.29 V) | [156,157] |
| Vanadium (Va) | Aqueous—Acidic | Anode: Zinc Cathode: Air-breathing Electrolyte: Acidic media |
Anode: Cathode: | Anode: −0.26 V Cathode: 1.0 V | (1.26 V) | [158,159] |
| Vanadium–Oxygen Fuel Cell | Anode: Vanadium Cathode: Oxygen diffusion electrode |
Anode: Cathode: | Anode: −0.26 V Cathode: 1.23 V | (1.49 V) | [143] | |
| Zinc (Zn) | Aqueous—Neutral | Anode: Zinc Cathode: Air-breathing Electrolyte: Any neutral media |
Anode: Cathode: | Anode: −0.762 V Cathode: 0.817 V | (1.579 V) | [160] |
| Aqueous—Alkaline | Anode: Zinc Cathode: Air-breathing Electrolyte: Any basic media |
Anode: Cathode: | Anode: −1.26 V Cathode: 0.40 V | (1.66 V) | [50,161] | |
| Non-Aqueous: Protic Room Temperature Ionic Liquids | Anode: Zinc Cathode: Air-breathing Electrolyte: Protic room temperature ionic liquid |
Anode: Cathode: | Anode: −0.7618 V Cathode: 0.695 V | (1.457 V) | [131,162] |
2.2. Zinc–Air Batteries
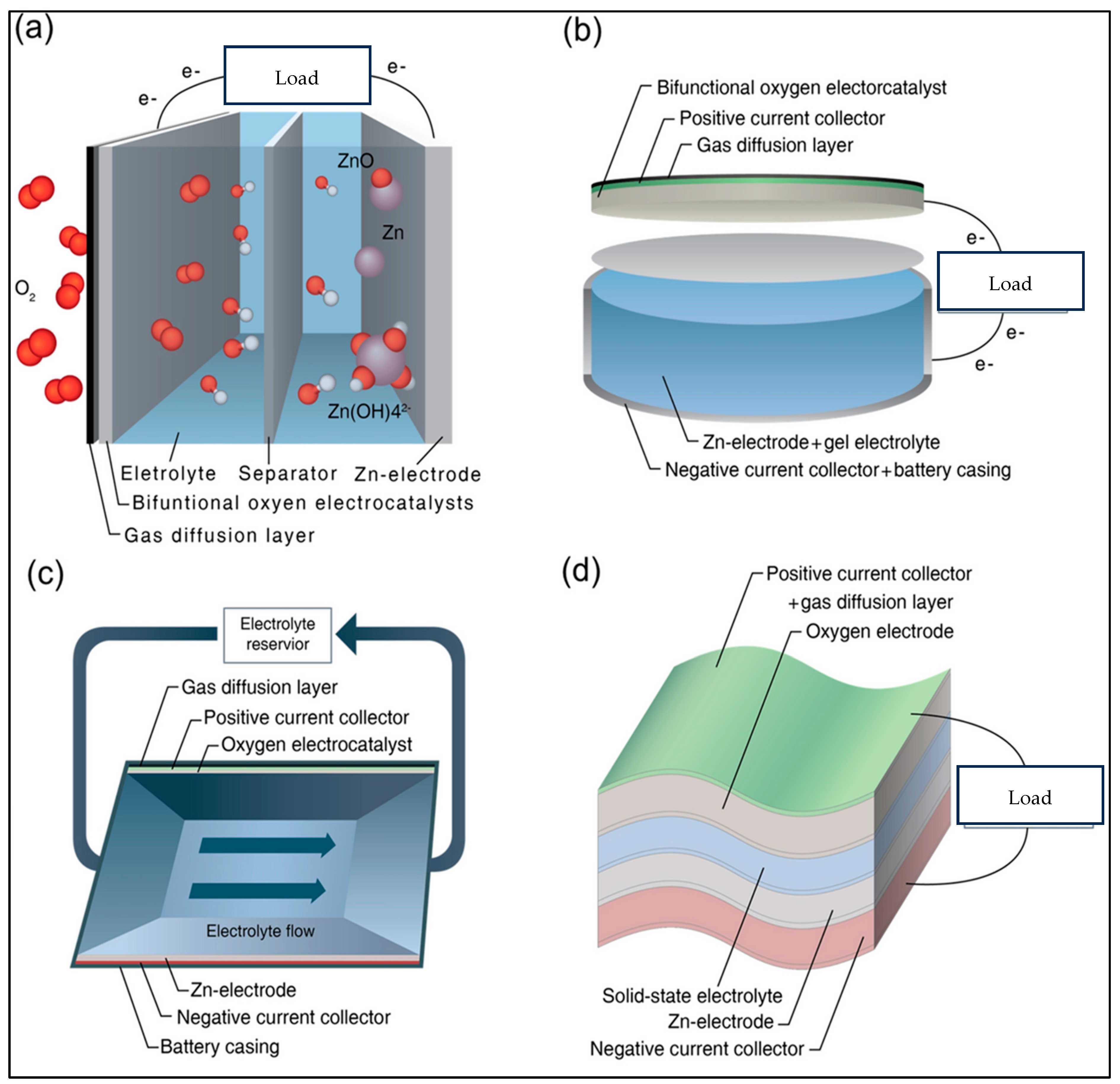
2.3. Aluminum–Air Batteries
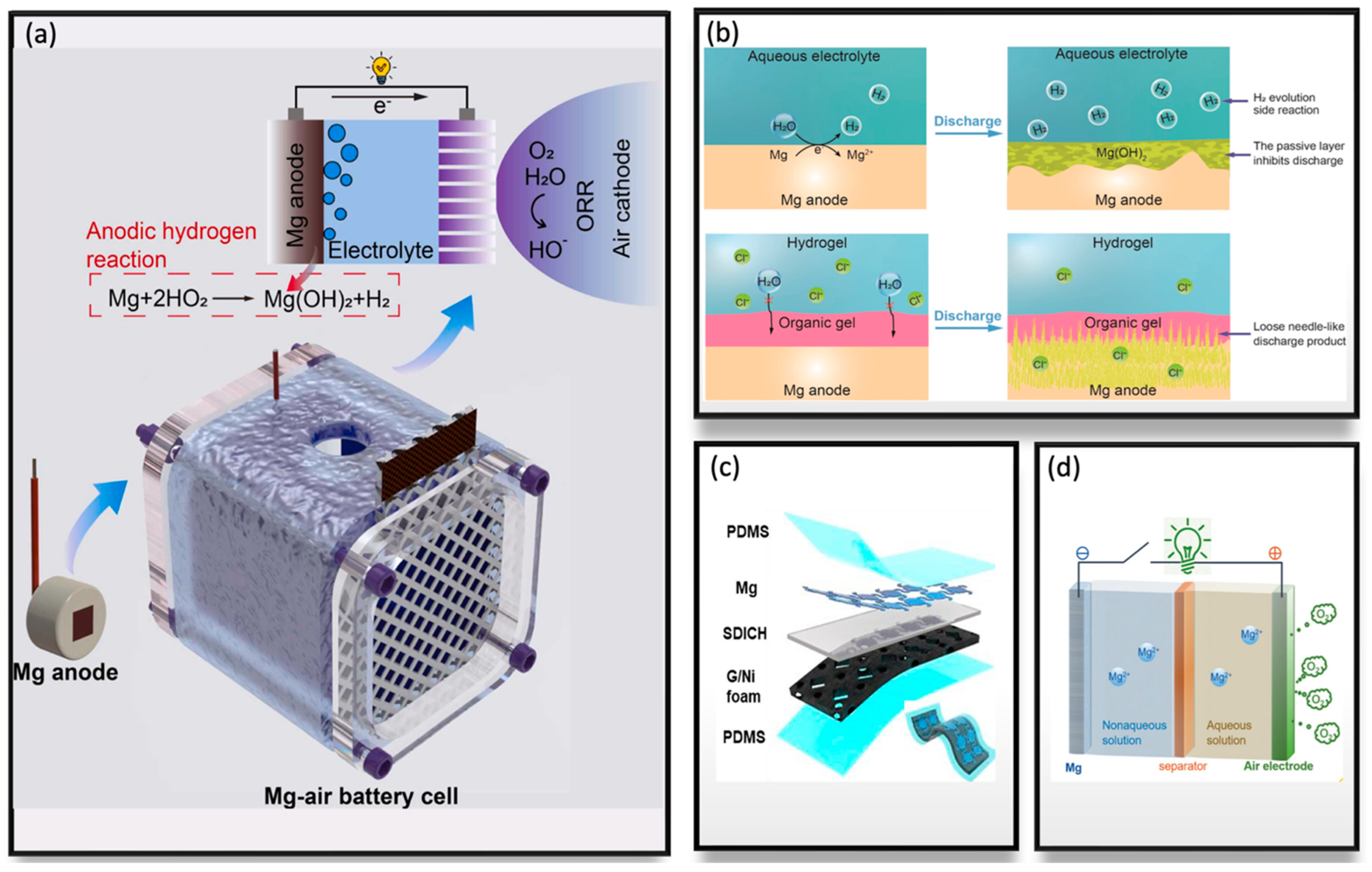
2.4. Magnesium–Air Batteries
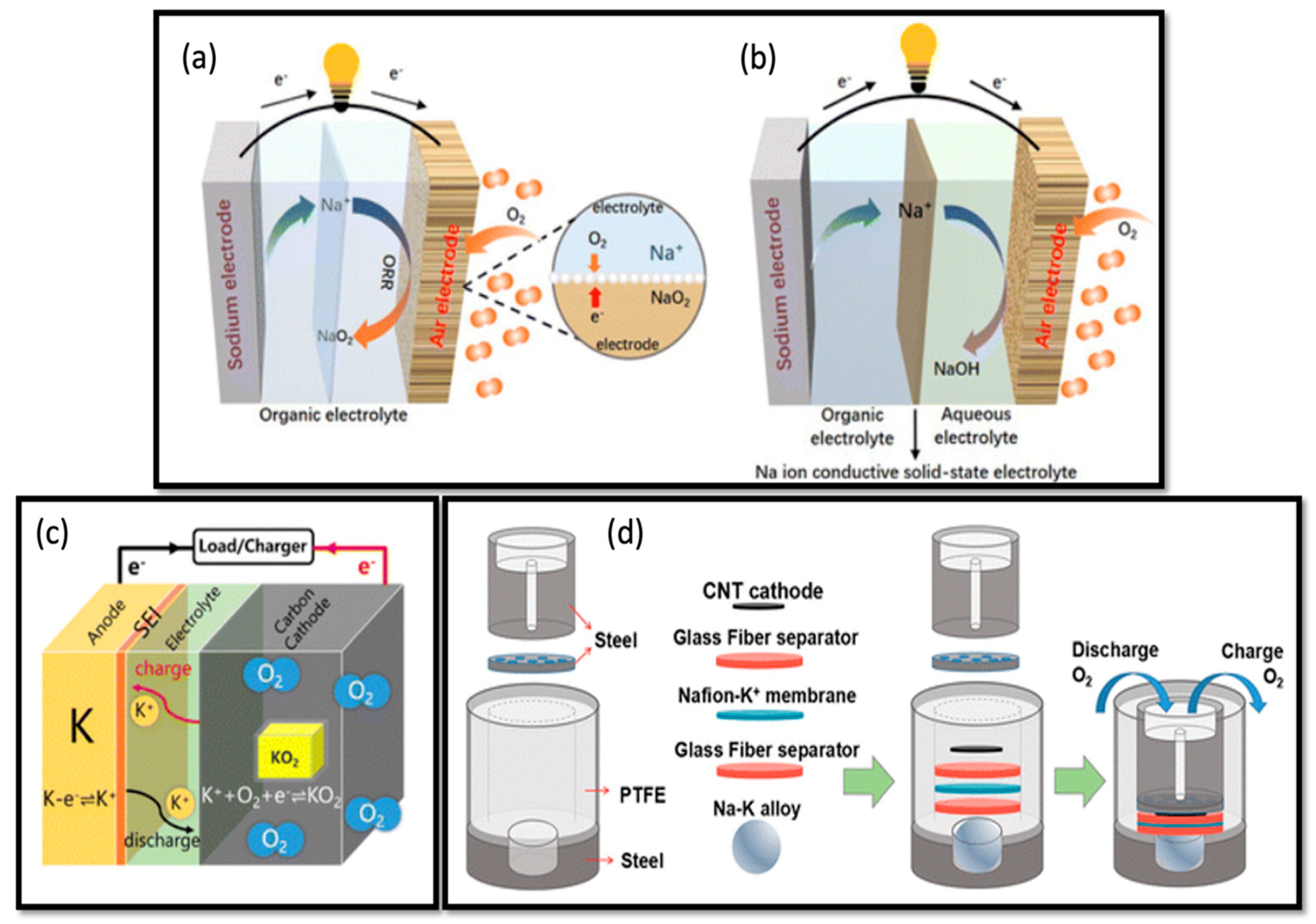
2.5. Sodium–Air and Potassium–Air Batteries
2.6. Other Metal–Air Batteries
3. Comparison of Performance Metrics
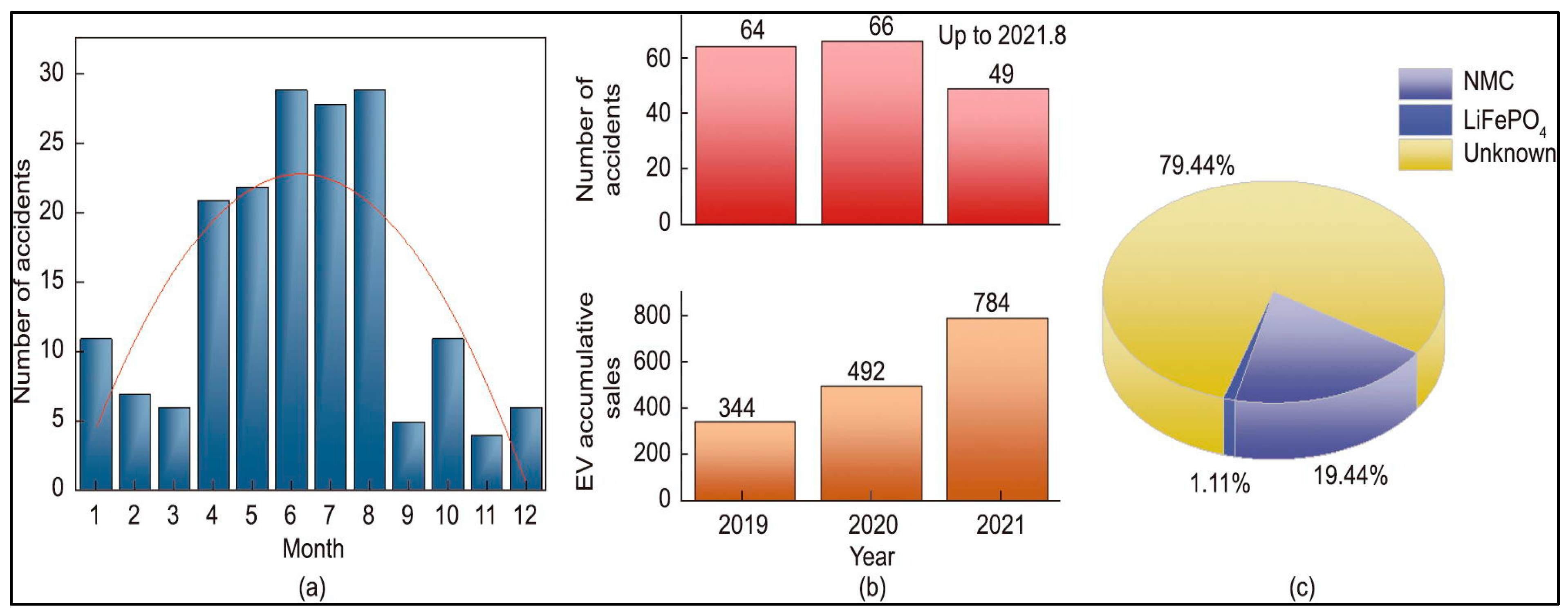
3.1. Cycle Life, Efficiency, and Safety Evaluation
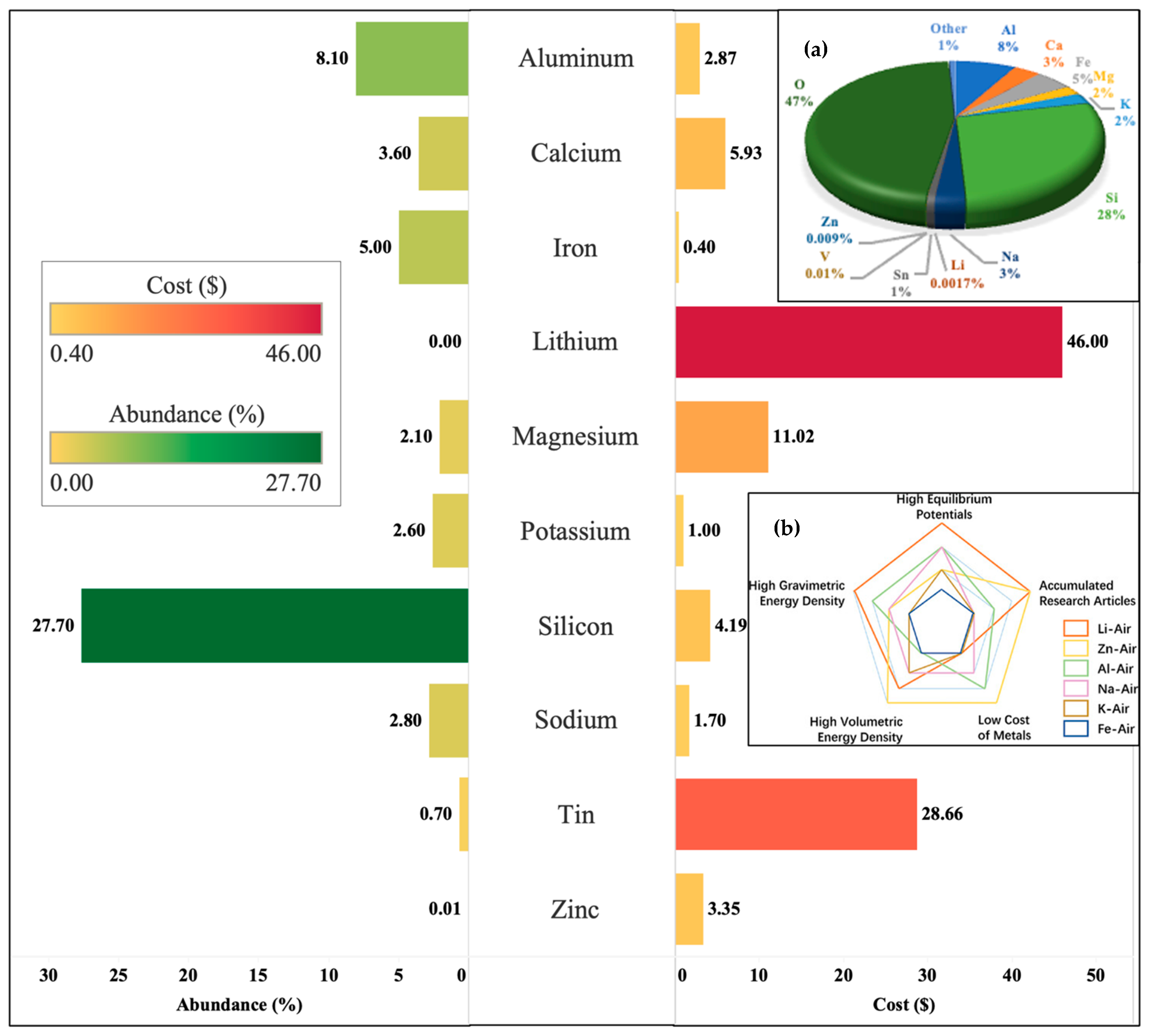
3.2. Cost Considerations
4. Conclusions
5. Future Outlook
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Al–air | Aluminum–air batteries |
| BEVs | Battery electric vehicles |
| Ca-O2 | Calcium–oxygen battery |
| CNT | Carbon nanotubes |
| CO2 | Carbon dioxide |
| EVs | Electric vehicles |
| Fe–air | Iron–air battery |
| GDL | Gas diffusion layer |
| Ge–air | Germanium–air batteries |
| GPEs | Gel polymer electrolytes |
| HER | Hydrogen evolution reaction |
| ICEVs | Internal combustion engine vehicles |
| IEA | International Energy Agency |
| LEDs | Light-emitting diodes |
| Li–air | Lithium–air |
| Li-ion | Lithium-ion |
| LISICON | Lithium superionic conductor |
| MABs | Metal–air batteries |
| Mg–air | Magnesium–air |
| Na–air | Sodium–air |
| OCV | Open circuit voltage |
| OER | Oxygen-evolution reaction |
| ORR | Oxygen-reduction reaction |
| SDICH | Dual-ion-conducting hydrogels |
| Si–air | Silicon–air batteries |
| SSE | Solid-state electrolytes |
| UHP | Ultra-high-purity |
| Zn–air | Zinc–air |
References
- Østergaard, P.A.; Duic, N.; Noorollahi, Y.; Kalogirou, S. Latest progress in Sustainable Development using renewable energy technology. Renew. Energy 2020, 162, 1554–1562. [Google Scholar] [CrossRef]
- Gaele, M.F.; Di Palma, T.M. Polymer electrolytes for Al-air batteries: Current state and future perspectives. Energy Fuels 2022, 36, 12875–12895. [Google Scholar] [CrossRef]
- Gouveia, J.; Mendes, A.; Monteiro, R.; Mata, T.; Caetano, N.; Martins, A. Life cycle assessment of a vanadium flow battery. Energy Rep. 2020, 6, 95–101. [Google Scholar] [CrossRef]
- Aldhafeeri, T.; Tran, M.-K.; Vrolyk, R.; Pope, M.; Fowler, M. A review of methane gas detection sensors: Recent developments and future perspectives. Inventions 2020, 5, 28. [Google Scholar] [CrossRef]
- Shittu, E.; Suman, R.; Ravikumar, M.K.; Shukla, A.K.; Zhao, G.; Patil, S.; Baker, J. Life cycle assessment of soluble lead redox flow battery. J. Clean. Prod. 2022, 337, 130503. [Google Scholar] [CrossRef]
- Branco, H.; Castro, R.; Lopes, A.S. Battery energy storage systems as a way to integrate renewable energy in small isolated power systems. Energy Sustain. Dev. 2018, 43, 90–99. [Google Scholar] [CrossRef]
- Network, R.E.P. Renewables Global Status Report. Paris: REN21 Secretariat, 272p. Available online: https://www.ren21.net/gsr-2023/ (accessed on 24 June 2017).
- Tooryan, F.; HassanzadehFard, H.; Collins, E.R.; Jin, S.; Ramezani, B. Smart integration of renewable energy resources, electrical, and thermal energy storage in microgrid applications. Energy 2020, 212, 118716. [Google Scholar] [CrossRef]
- Madhavan, P.V.; Shahgaldi, S.; Li, X. Ex-situ Characterization of Nb-Ti Alloy/Pt Coated Stainless Steel Bipolar Plates for Proton Exchange Membrane Fuel Cells. Energy Convers. Manag. 2024, 311, 118536. [Google Scholar] [CrossRef]
- Madhavan, P.V.; Zeng, X.; Shahgaldi, S.; Li, X. Investigation of Cr2SiC Ceramic MAX Phase Coated Metallic Bipolar Plates in Ex-situ Conditions for Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2024, 96, 1232–1242. [Google Scholar] [CrossRef]
- Madhavan, P.V.; Shahgaldi, S.; Li, X. Modelling Anti-Corrosion Coating Performance of Metallic Bipolar Plates for PEM Fuel Cells: A Machine Learning Approach. Energy AI 2024, 17, 100391. [Google Scholar] [CrossRef]
- Moradizadeh, L.; Madhavan, P.V.; Chellehbari, Y.M.; Gupta, A.; Li, X.; Shahgaldi, S. Porous transport layers with low Pt loading having Nb–Ta alloy as interlayer for proton exchange membrane water electrolyzers. Int. J. Hydrogen Energy 2024, 94, 1114–1129. [Google Scholar] [CrossRef]
- Gröger, O.; Gasteiger, H.A.; Suchsland, J.-P. Electromobility: Batteries or fuel cells? J. Electrochem. Soc. 2015, 162, A2605. [Google Scholar] [CrossRef]
- Legala, A.; Kubesh, M.; Chundru, V.R.; Conway, G.; Li, X. Machine Learning Modelling for Fuel Cell-Battery Hybrid Power System Dynamics in a Toyota Mirai 2 Vehicle Under Various Drive Cycles. Energy AI 2024, 17, 100415. [Google Scholar] [CrossRef]
- Li, W.; Xie, Y.; Hu, X.; Zhang, B.; Fowler, M.; Panchal, S.; Fraser, R.; Zhang, Y. An efficient two-stage heating strategy for embedded heat pipe system considering power and energy requirements from battery. Appl. Therm. Eng. 2024, 257, 124499. [Google Scholar] [CrossRef]
- Neubauer, J.; Wood, E. The impact of range anxiety and home, workplace, and public charging infrastructure on simulated battery electric vehicle lifetime utility. J. Power Sources 2014, 257, 12–20. [Google Scholar] [CrossRef]
- Singer, M. Consumer Views on Plug-In Electric Vehicles—National Benchmark Report; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2016. [Google Scholar]
- Li, W.; Long, R.; Chen, H.; Geng, J. A review of factors influencing consumer intentions to adopt battery electric vehicles. Renew. Sustain. Energy Rev. 2017, 78, 318–328. [Google Scholar] [CrossRef]
- Tran, M.-K.; Bhatti, A.; Vrolyk, R.; Wong, D.; Panchal, S.; Fowler, M.; Fraser, R. A review of range extenders in battery electric vehicles: Current progress and future perspectives. World Electr. Veh. J. 2021, 12, 54. [Google Scholar] [CrossRef]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Zackrisson, M.; Fransson, K.; Hildenbrand, J.; Lampic, G.; O’Dwyer, C. Life cycle assessment of lithium-air battery cells. J. Clean. Prod. 2016, 135, 299–311. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2022, 238, 121652. [Google Scholar] [CrossRef]
- Pu, J.H.; Li, Y.; Li, R.C.; Hua, N.; Zhang, H.; Lu, Y.; Panchal, S.; Fraser, R.; Fowler, M.; Zhang, X.-K. Design and performance of a compact lightweight hybrid thermal management system using phase change material and liquid cooling with a honeycomb-like structure for prismatic lithium-ion batteries. J. Power Sources 2024, 624, 235632. [Google Scholar] [CrossRef]
- Ma, W.; Xie, Y.; Guo, S.; Li, W.; Yang, R.; Panchal, S.; Zhang, Y. A mechanism-data driven resistance transfer algorithm for lithium-ion batteries and its application to thermal modeling. J. Energy Storage 2024, 102, 114066. [Google Scholar] [CrossRef]
- O’hayre, R.; Cha, S.-W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Alaswad, A.; Baroutaji, A.; Achour, H.; Carton, J.; Al Makky, A.; Olabi, A.-G. Developments in fuel cell technologies in the transport sector. Int. J. Hydrogen Energy 2016, 41, 16499–16508. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Rao, R.P.; Chellappan, V.; Ramakrishna, S. Towards sustainable fuel cells and batteries with an AI perspective. Sustainability 2022, 14, 16001. [Google Scholar] [CrossRef]
- Bank, D. Welcome to the Lithium-Ion Age; Deutsche Bank AG: Sydney, Australia, 2016. [Google Scholar]
- Van Noorden, R. A better battery. Nature 2014, 507, 26. [Google Scholar] [CrossRef] [PubMed]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From lithium-ion to sodium-ion batteries: Advantages, challenges, and surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef]
- Popp, D. Fit for 55: MEPs Back Objective of Zero Emissions for Cars and Vans in 2035. 2022. Available online: https://www.europarl.europa.eu/news (accessed on 5 October 2024).
- Yu, X.; Chen, R.; Gan, L.; Li, H.; Chen, L. Battery safety: From lithium-ion to solid-state batteries. Engineering 2023, 21, 9–14. [Google Scholar] [CrossRef]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating thermal runaway of lithium-ion batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Mokhtar, M.; Talib, M.Z.M.; Majlan, E.H.; Tasirin, S.M.; Ramli, W.M.F.W.; Daud, W.R.W.; Sahari, J. Recent developments in materials for aluminum–air batteries: A review. J. Ind. Eng. Chem. 2015, 32, 1–20. [Google Scholar] [CrossRef]
- Wen, H.; Liu, Z.; Qiao, J.; Chen, R.; Zhao, R.; Wu, J.; Qiao, G.; Yang, J. High energy efficiency and high power density aluminum-air flow battery. Int. J. Energy Res. 2020, 44, 7568–7579. [Google Scholar] [CrossRef]
- Hou, J.; Yang, M.; Wang, D.; Zhang, J. Fundamentals and challenges of lithium ion batteries at temperatures between −40 and 60° C. Adv. Energy Mater. 2020, 10, 1904152. [Google Scholar] [CrossRef]
- Bandhauer, T.M.; Garimella, S.; Fuller, T.F. A critical review of thermal issues in lithium-ion batteries. J. Electrochem. Soc. 2011, 158, R1. [Google Scholar] [CrossRef]
- Costa, C.M.; Barbosa, J.C.; Gonçalves, R.; Castro, H.; Del Campo, F.; Lanceros-Méndez, S. Recycling and environmental issues of lithium-ion batteries: Advances, challenges and opportunities. Energy Storage Materials 2021, 37, 433–465. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Grundish, N.S.; Goodenough, J.B.; Chen, Y.; Guo, L.; Peng, Z.; Qi, X.; Yang, F.; Qie, L. The 2021 battery technology roadmap. J. Phys. D Appl. Phys. 2021, 54, 183001. [Google Scholar] [CrossRef]
- Chen, J.; Kollmeyer, P.; Panchal, S.; Masoudi, Y.; Gross, O.; Emadi, A. Experimental results of battery power capability measurement on cells with different state of health levels. In Proceedings of the 2024 IEEE Transportation Electrification Conference and Expo (ITEC), Chicago, IL, USA, 19–21 June 2024; pp. 1–6. [Google Scholar]
- Liu, Q.; Pan, Z.; Wang, E.; An, L.; Sun, G. Aqueous metal-air batteries: Fundamentals and applications. Energy Storage Mater. 2020, 27, 478–505. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal–air batteries: Will they be the future electrochemical energy storage device of choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Li, T.; Huang, M.; Bai, X.; Wang, Y.-X. Metal–air batteries: A review on current status and future applications. Prog. Nat. Sci. Mater. Int. 2023, 33, 151–171. [Google Scholar] [CrossRef]
- Rani, B.; Yadav, J.K.; Saini, P.; Pandey, A.P.; Dixit, A. Aluminum–air batteries: Current advances and promises with future directions. RSC Adv. 2024, 14, 17628–17663. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, L.J.; De León, C.P. Rechargeable multi-valent metal-air batteries. Johns. Matthey Technol. Rev. 2018, 62, 134–149. [Google Scholar] [CrossRef]
- Alemu, M.A.; Getie, M.Z.; Worku, A.K. Advancement of electrically rechargeable multivalent metal-air batteries for future mobility. Ionics 2023, 29, 3421–3435. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically rechargeable zinc–air batteries: Progress, challenges, and perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef]
- Buckingham, R.; Asset, T.; Atanassov, P. Aluminum-air batteries: A review of alloys, electrolytes and design. J. Power Sources 2021, 498, 229762. [Google Scholar] [CrossRef]
- Feng, Y.; Xiong, W.; Zhang, J.; Wang, R.; Wang, N. Electrochemical discharge performance of the Mg–Al–Pb–Ce–Y alloy as the anode for Mg–air batteries. J. Mater. Chem. A 2016, 4, 8658–8668. [Google Scholar] [CrossRef]
- Zhang, T.; Tao, Z.; Chen, J. Magnesium–air batteries: From principle to application. Mater. Horiz. 2014, 1, 196–206. [Google Scholar] [CrossRef]
- Arafat, Y.; Azhar, M.R.; Zhong, Y.; Tadé, M.O.; Shao, Z. Metal-free carbon based air electrodes for Zn-air batteries: Recent advances and perspective. Mater. Res. Bull. 2021, 140, 111315. [Google Scholar] [CrossRef]
- Bi, X.; Jiang, Y.; Chen, R.; Du, Y.; Zheng, Y.; Yang, R.; Wang, R.; Wang, J.; Wang, X.; Chen, Z. Rechargeable zinc–air versus lithium–air battery: From fundamental promises toward technological potentials. Adv. Energy Mater. 2024, 14, 2302388. [Google Scholar] [CrossRef]
- Li, L.; Chang, Z.w.; Zhang, X.B. Recent progress on the development of metal-air batteries. Adv. Sustain. Syst. 2017, 1, 1700036. [Google Scholar] [CrossRef]
- Li, T.; Peng, X.; Cui, P.; Shi, G.; Yang, W.; Chen, Z.; Huang, Y.; Chen, Y.; Peng, J.; Zou, R. Recent progress and future perspectives of flexible metal-air batteries. SmartMat 2021, 2, 519–553. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2024; U.S. Geological Survey: Reston, VA, USA, 2024. Available online: https://www.usgs.gov/publications/mineral-commodity-summaries-2024 (accessed on 5 October 2024).
- Leimer, D.H.W. Abundances of Chemical Elements in the Earth’s Crust. Available online: https://sites.tntech.edu/hwleimer/geol-1040/geol-1040-lecture/abundances-of-chemical-elements-in-the-earths-crust/ (accessed on 6 June 2024).
- Wang, C.; Yu, Y.; Niu, J.; Liu, Y.; Bridges, D.; Liu, X.; Pooran, J.; Zhang, Y.; Hu, A. Recent progress of metal–air batteries—A mini review. Appl. Sci. 2019, 9, 2787. [Google Scholar] [CrossRef]
- Han, X.; Li, X.; White, J.; Zhong, C.; Deng, Y.; Hu, W.; Ma, T. Metal–air batteries: From static to flow system. Adv. Energy Mater. 2018, 8, 1801396. [Google Scholar] [CrossRef]
- Cheng, F.; Chen, J. Metal–air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, L.; Yang, Y.-S.; Wen, Y.-H.; Cao, G.-P.; Wang, X.-D. Preliminary study of single flow zinc–nickel battery. Electrochem. Commun. 2007, 9, 2639–2642. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, J.; Song, X.; Zarrin, H.; Tian, X.; Qiao, J.; Rasen, L.; Li, K.; Chen, Z. A flexible solid-state electrolyte for wide-scale integration of rechargeable zinc–air batteries. Energy Environ. Sci. 2016, 9, 663–670. [Google Scholar] [CrossRef]
- Zhou, G.; Li, F.; Cheng, H.-M. Progress in flexible lithium batteries and future prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Sumboja, A.; Ge, X.; Zong, Y.; Liu, Z. Progress in development of flexible metal–air batteries. Funct. Mater. Lett. 2016, 9, 1630001. [Google Scholar] [CrossRef]
- Wang, Y.; Kwok, H.; Pan, W.; Zhang, H.; Leung, D.Y. Innovative paper-based Al-air batteries as a low-cost and green energy technology for the miniwatt market. J. Power Sources 2019, 414, 278–282. [Google Scholar] [CrossRef]
- Li, F.; Zhang, T.; Zhou, H. Challenges of non-aqueous Li–O2 batteries: Electrolytes, catalysts, and anodes. Energy Environ. Sci. 2013, 6, 1125–1141. [Google Scholar] [CrossRef]
- Yadegari, H.; Sun, Q.; Sun, X. Sodium-oxygen batteries: A comparative review from chemical and electrochemical fundamentals to future perspective. Adv. Mater. 2016, 28, 7065–7093. [Google Scholar] [CrossRef]
- Ren, X.; Wu, Y. A low-overpotential potassium–oxygen battery based on potassium superoxide. J. Am. Chem. Soc. 2013, 135, 2923–2926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-B. Metal-Air Batteries: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Liu, Q.; Chang, Z.; Li, Z.; Zhang, X. Flexible metal–air batteries: Progress, challenges, and perspectives. Small Methods 2018, 2, 1700231. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Scheffler, R.; Speidel, A.; Bethune, D.S.; Shelby, R.M.; Luntz, A.C. On the efficacy of electrocatalysis in nonaqueous Li–O2 batteries. J. Am. Chem. Soc. 2011, 133, 18038–18041. [Google Scholar] [CrossRef]
- Peng, Z.; Freunberger, S.A.; Chen, Y.; Bruce, P.G. A reversible and higher-rate Li-O2 battery. Science 2012, 337, 563–566. [Google Scholar] [CrossRef]
- Chen, Y.; Freunberger, S.A.; Peng, Z.; Fontaine, O.; Bruce, P.G. Charging a Li–O2 battery using a redox mediator. Nat. Chem. 2013, 5, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Wang, Z.-L.; Xu, D.; Zhang, L.-L.; Zhang, X.-B. Tailoring deposition and morphology of discharge products towards high-rate and long-life lithium-oxygen batteries. Nat. Commun. 2013, 4, 2438. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, H. A reversible long-life lithium–air battery in ambient air. Nat. Commun. 2013, 4, 1817. [Google Scholar] [CrossRef]
- Tu, K.-N.; Gusak, A. Mean-time-to-failure equations for electromigration, thermomigration, and stress migration. IEEE Trans. Compon. Packag. Manuf. Technol. 2020, 10, 1427–1431. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Ye, L.; Liao, M.; Zhao, T.; Sun, H.; Zhao, Y.; Sun, X.; Wang, B.; Peng, H. A sodiophilic interphase-mediated, dendrite-free anode with ultrahigh specific capacity for sodium-metal batteries. Angew. Chem. 2019, 131, 17210–17216. [Google Scholar] [CrossRef]
- Olabi, A.G.; Sayed, E.T.; Wilberforce, T.; Jamal, A.; Alami, A.H.; Elsaid, K.; Rahman, S.M.A.; Shah, S.K.; Abdelkareem, M.A. Metal-air batteries—A review. Energies 2021, 14, 7373. [Google Scholar] [CrossRef]
- Arai, H. Introduction—General features of metal-air batteries. In Electrochemical Power Sources: Fundamentals, Systems, and Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–10. [Google Scholar]
- Arai, H.; Ikezawa, A. Electrochemical Devices| Electrochemical Power Sources: Fundamentals, Systems, and Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Arai, H. Metal Storage/Metal Air (Zn, Fe, Al, Mg). In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 337–344. [Google Scholar]
- Arai, H.; Hayashi, M. Secondary batteries–metal-air Systems| Overview (secondary and primary). Encycl. Electrochem. Power Sources 2009, 347–355. [Google Scholar] [CrossRef]
- Xu, S.; Lau, S.; Archer, L.A. CO2 and ambient air in metal–oxygen batteries: Steps towards reality. Inorg. Chem. Front. 2015, 2, 1070–1079. [Google Scholar] [CrossRef]
- Salado, M.; Lizundia, E. Advances, challenges, and environmental impacts in metal–air battery electrolytes. Mater. Today Energy 2022, 28, 101064. [Google Scholar] [CrossRef]
- Park, M.; Kim, K.Y.; Seo, H.; Cheon, Y.E.; Koh, J.H.; Sun, H.; Kim, T.J. Practical challenges associated with catalyst development for the commercialization of Li-air batteries. J. Electrochem. Sci. Technol. 2014, 5, 1–18. [Google Scholar] [CrossRef]
- Suryatna, A.; Raya, I.; Thangavelu, L.; Alhachami, F.R.; Kadhim, M.M.; Altimari, U.S.; Mahmoud, Z.H.; Mustafa, Y.F.; Kianfar, E. A Review of High-Energy Density Lithium-Air Battery Technology: Investigating the Effect of Oxides and Nanocatalysts. J. Chem. 2022, 2022, 2762647. [Google Scholar] [CrossRef]
- Li, J.; Hou, L.; Yu, M.; Li, Q.; Zhang, T.; Sun, H. Review and Recent Advances of Oxygen Transfer in Li-air Batteries. ChemElectroChem 2021, 8, 3588–3603. [Google Scholar] [CrossRef]
- Jilani, A.; Awan, Z.; Taqvi, S.A.A.; Khan, F.; Alshahrani, T. Recent Advances in the Development of Li-Air Batteries, Experimental and Predictive Approaches–Prospective, Challenges, and Opportunities. ChemBioEng Rev. 2024, 11, 95–114. [Google Scholar] [CrossRef]
- Manthiram, A.; Li, L. Hybrid and Aqueous Lithium-Air Batteries. Adv. Energy Mater. 2015, 5, 1401302. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, X.; Zhu, X.; Tan, P. Addressing transport issues in non-aqueous Li–air batteries to achieving high electrochemical performance. Electrochem. Energy Rev. 2023, 6, 18. [Google Scholar] [CrossRef]
- Luntz, A.C.; McCloskey, B.D. Nonaqueous Li–air batteries: A status report. Chem. Rev. 2014, 114, 11721–11750. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; He, P.; Zhou, H. Critical challenges in rechargeable aprotic Li–O2 batteries. Adv. Energy Mater. 2016, 6, 1502303. [Google Scholar] [CrossRef]
- Shu, C.; Wang, J.; Long, J.; Liu, H.K.; Dou, S.X. Understanding the reaction chemistry during charging in aprotic lithium–oxygen batteries: Existing problems and solutions. Adv. Mater. 2019, 31, 1804587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Imanishi, N.; Takeda, Y.; Yamamoto, O. Aqueous lithium/air rechargeable batteries. Chem. Lett. 2011, 40, 668–673. [Google Scholar] [CrossRef]
- Imanishi, N.; Yamamoto, O. Rechargeable lithium–air batteries: Characteristics and prospects. Mater. Today 2014, 17, 24–30. [Google Scholar] [CrossRef]
- Imanishi, N.; Yamamoto, O. Perspectives and challenges of rechargeable lithium–air batteries. Mater. Today Adv. 2019, 4, 100031. [Google Scholar] [CrossRef]
- Chen, P.; Bai, F.; Deng, J.W.; Liu, B.; Zhang, T. Recent progresses and challenges in aqueous lithium–air batteries relating to the solid electrolyte separator: A mini-review. Front. Chem. 2022, 10, 1035691. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, N.; Hasegawa, S.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Lithium anode for lithium-air secondary batteries. J. Power Sources 2008, 185, 1392–1397. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H. A lithium-air battery with a potential to continuously reduce O2 from air for delivering energy. J. Power Sources 2010, 195, 358–361. [Google Scholar] [CrossRef]
- Zhang, T.; Imanishi, N.; Hasegawa, S.; Hirano, A.; Xie, J.; Takeda, Y.; Yamamoto, O.; Sammes, N. Li/Polymer Electrolyte/Water Stable Lithium-Conducting Glass Ceramics Composite for Lithium–Air Secondary Batteries with an Aqueous Electrolyte. J. Electrochem. Soc. 2008, 155, A965. [Google Scholar] [CrossRef]
- He, P.; Wang, Y.; Zhou, H. A Li-air fuel cell with recycle aqueous electrolyte for improved stability. Electrochem. Commun. 2010, 12, 1686–1689. [Google Scholar] [CrossRef]
- Hasegawa, S.; Imanishi, N.; Zhang, T.; Xie, J.; Hirano, A.; Takeda, Y.; Yamamoto, O. Study on lithium/air secondary batteries—Stability of NASICON-type lithium ion conducting glass–ceramics with water. J. Power Sources 2009, 189, 371–377. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X.; Manthiram, A. A dual-electrolyte rechargeable Li-air battery with phosphate buffer catholyte. Electrochem. Commun. 2012, 14, 78–81. [Google Scholar] [CrossRef]
- Shimonishi, Y.; Zhang, T.; Johnson, P.; Imanishi, N.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. A study on lithium/air secondary batteries—Stability of NASICON-type glass ceramics in acid solutions. J. Power Sources 2010, 195, 6187–6191. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Shao, Y.; Chen, X.; Wang, Z.; Gao, F.; Liu, X.; Zhang, J.-G. H+ diffusion and electrochemical stability of Li1+ x+ yAlxTi2−xSiyP3−yO12 glass in aqueous Li/air battery electrolytes. J. Power Sources 2012, 214, 292–297. [Google Scholar] [CrossRef]
- Bidault, F.; Brett, D.; Middleton, P.; Brandon, N. Review of gas diffusion cathodes for alkaline fuel cells. J. Power Sources 2009, 187, 39–48. [Google Scholar] [CrossRef]
- He, P.; Zhang, T.; Jiang, J.; Zhou, H. Lithium–air batteries with hybrid electrolytes. J. Phys. Chem. Lett. 2016, 7, 1267–1280. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Choudhury, S.; Stalin, S.; Deng, Y.; Archer, L.A. Soft colloidal glasses as solid-state electrolytes. Chem. Mater. 2018, 30, 5996–6004. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Wang, Y.; Liu, Q.; Chen, Q.; Chen, M. Advances and prospects of PVDF based polymer electrolytes. J. Energy Chem. 2022, 64, 62–84. [Google Scholar] [CrossRef]
- Wang, H.-F.; Wang, X.-X.; Li, F.; Xu, J.-J. Fundamental Understanding and Construction of Solid-State Li−Air Batteries. Small Sci. 2022, 2, 2200005. [Google Scholar] [CrossRef]
- Vadhva, P.; Gill, T.E.; Cruddos, J.H.; Said, S.; Siniscalchi, M.; Narayanan, S.; Pasta, M.; Miller, T.S.; Rettie, A.J. Engineering solution-processed non-crystalline solid electrolytes for Li metal batteries. Chem. Mater. 2023, 35, 1168–1176. [Google Scholar] [CrossRef]
- Raj, H.; Fabre, T.; Lachal, M.; Neveu, A.; Jean, J.; Steil, M.C.; Bouchet, R.; Pralong, V. Stabilizing the NASICON Solid Electrolyte in an Inert Atmosphere as a Function of Physical Properties and Sintering Conditions for Solid-State Battery Fabrication. ACS Appl. Energy Mater. 2023, 6, 1197–1207. [Google Scholar] [CrossRef]
- Xu, A.; Wang, R.; Yao, M.; Cao, J.; Li, M.; Yang, C.; Liu, F.; Ma, J. Electrochemical properties of an Sn-doped LATP ceramic electrolyte and its derived sandwich-structured composite solid electrolyte. Nanomaterials 2022, 12, 2082. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Z.; Cheng, J.; Chen, H.; Huang, X.; Tian, B. Halide/sulfide composite solid-state electrolyte for Li-anode based all-solid-state batteries. Chin. Chem. Lett. 2023, 34, 108228. [Google Scholar] [CrossRef]
- Hamao, N.; Hamamoto, K. Fabrication of single-grain-layered garnet-type electrolyte sheets by a precursor method. J. Asian Ceram. Soc. 2022, 10, 1–8. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, N.; Li, Y.; Guo, X.; Feng, X.; Liu, X.; Liu, Z.; Cui, G.; Zheng, H.; Gu, L. A rechargeable Li-air fuel cell battery based on garnet solid electrolytes. Sci. Rep. 2017, 7, 41217. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Y. Perovskite-type Li-ion solid electrolytes: A review. J. Mater. Sci. Mater. Electron. 2021, 32, 9736–9754. [Google Scholar] [CrossRef]
- Hashishin, T.; Shimomura, H.; Kamiyama, R.; Matsuda, M. Analcime with high sodium ion conduction as a solid electrolyte. J. Phys. Chem. C 2022, 126, 19480–19486. [Google Scholar] [CrossRef]
- Cao, W.; Yang, Y.; Deng, J.; Li, Y.; Cui, C.; Zhang, T. Localization of electrons within interlayer stabilizes NASICON-type solid-state electrolyte. Mater. Today Energy 2021, 22, 100875. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Z.; Gu, Z.; Zhao, X.; Guo, D.; Yao, X. Polyimide-based solid-state gel polymer electrolyte for lithium–oxygen batteries with a long-cycling life. ACS Appl. Mater. Interfaces 2023, 15, 7014–7022. [Google Scholar] [CrossRef] [PubMed]
- Hamao, N.; Yamaguchi, Y.; Hamamoto, K. Densification of a NASICON-type LATP electrolyte sheet by a cold-sintering process. Materials 2021, 14, 4737. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Ma, J.; Li, S.; Mao, D. Solid-state electrolyte for lithium-air batteries: A review. Polymers 2023, 15, 2469. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Lu, H.; Fan, L.; Hong, Q.; Leng, J.; Chen, C. Performance of Al-air batteries based on Al–Ga, Al–In and Al–Sn alloy electrodes. J. Electrochem. Soc. 2015, 162, A2116. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, L.; Wang, S.; Xie, Y.; You, W.; Du, X. A review of the Al-gas batteries and perspectives for a “Real” Al-air battery. J. Power Sources 2023, 580, 233375. [Google Scholar] [CrossRef]
- Elia, G.A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R.; Knipping, E.; Peters, W.; Drillet, J.F.; Passerini, S.; Hahn, R. An overview and future perspectives of aluminum batteries. Adv. Mater. 2016, 28, 7564–7579. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, B.; Zhu, L.; Deng, X.; Sun, X.; Liu, Y.; Zhang, C.; Zhao, W.; Chen, X. A PVA/LiCl/PEO interpenetrating composite electrolyte with a three-dimensional dual-network for all-solid-state flexible aluminum–air batteries. RSC Adv. 2021, 11, 39476–39483. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 95th ed.; Taylor & Francis Group, LLC: Abingdon, UK, 2014. [Google Scholar]
- Hu, Y.; Sun, D.; Luo, B.; Wang, L. Recent progress and future trends of aluminum batteries. Energy Technol. 2019, 7, 86–106. [Google Scholar] [CrossRef]
- Bogolowski, N.; Drillet, J.-F. An electrically rechargeable Al-air battery with aprotic ionic liquid electrolyte. ECS Trans. 2017, 75, 85. [Google Scholar] [CrossRef]
- Carlin, R.T.; Osteryoung, R.A. Aluminum anodization in a basic ambient temperature molten salt. J. Electrochem. Soc. 1989, 136, 1409. [Google Scholar] [CrossRef]
- Ocon, J.D.; Kim, J.W.; Abrenica, G.H.A.; Lee, J.K.; Lee, J. Quasi-perpetual discharge behaviour in p-type Ge–air batteries. Phys. Chem. Chem. Phys. 2014, 16, 22487–22494. [Google Scholar] [CrossRef]
- Revel, R.; Audichon, T.; Gonzalez, S. Non-aqueous aluminium–air battery based on ionic liquid electrolyte. J. Power Sources 2014, 272, 415–421. [Google Scholar] [CrossRef]
- Wang, D.; Gao, X.; Chen, Y.; Jin, L.; Kuss, C.; Bruce, P.G. Plating and stripping calcium in an organic electrolyte. Nat. Mater. 2018, 17, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Schröder, D.; Janek, J.; Adelhelm, P. Nonlithium Aprotic Metal/Oxygen Batteries Using Na, K, Mg, or Ca as Metal Anode. Encycl. Electrochem. Online 2007, 1–29. [Google Scholar] [CrossRef]
- Xiao, N.; Ren, X.; McCulloch, W.D.; Gourdin, G.; Wu, Y. Potassium superoxide: A unique alternative for metal–air batteries. Acc. Chem. Res. 2018, 51, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Deyab, M.; Mohsen, Q. Improved battery capacity and cycle life in iron-air batteries with ionic liquid. Renew. Sustain. Energy Rev. 2021, 139, 110729. [Google Scholar] [CrossRef]
- McKerracher, R.; Ponce de Leon, C.; Wills, R.; Shah, A.A.; Walsh, F.C. A review of the iron–air secondary battery for energy storage. ChemPlusChem 2015, 80, 323–335. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, N.; Li, X.; Gong, Y.; Huang, K. Energy storage characteristics of a new rechargeable solid oxide iron–air battery. RSC Adv. 2012, 2, 10163–10166. [Google Scholar] [CrossRef]
- Arai, H.; Garche, J.; Colmenares, L.C. Electrochemical Power Sources: Fundamentals, Systems, and Applications: Metal–Air Batteries: Present and Perspectives; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sun, Q.; Dai, L.; Luo, T.; Wang, L.; Liang, F.; Liu, S. Recent advances in solid-state metal–air batteries. Carbon Energy 2023, 5, e276. [Google Scholar] [CrossRef]
- Zhao, X.; Gong, Y.; Li, X.; Xu, N.; Huang, K. Performance of solid oxide iron-air battery operated at 550 C. J. Electrochem. Soc. 2013, 160, A1241. [Google Scholar] [CrossRef]
- Jin, X.; Uddin, A.M.; Zhao, X.; White, R.; Huang, K. Understanding the High-Temperature Solid-Oxide Iron-Air Redox Battery Operated with an Oxygen Shuttle Mechanism: A Computational Study. J. Electrochem. Soc. 2015, 162, A1476. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium-air battery: Promise and challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Park, J.-B.; Sun, Y.-K.; Wu, F.; Amine, K. Aprotic and aqueous Li–O2 batteries. Chem. Rev. 2014, 114, 5611–5640. [Google Scholar] [CrossRef]
- Kowalczk, I.; Read, J.; Salomon, M. Li-air batteries: A classic example of limitations owing to solubilities. Pure Appl. Chem. 2007, 79, 851–860. [Google Scholar] [CrossRef]
- Geng, D.; Ding, N.; Hor, T.A.; Chien, S.W.; Liu, Z.; Wuu, D.; Sun, X.; Zong, Y. From lithium-oxygen to lithium-air batteries: Challenges and opportunities. Adv. Energy Mater. 2016, 6, 1502164. [Google Scholar] [CrossRef]
- Grande, L.; Paillard, E.; Hassoun, J.; Park, J.B.; Lee, Y.J.; Sun, Y.K.; Passerini, S.; Scrosati, B. The lithium/air battery: Still an emerging system or a practical reality? Adv. Mater. 2015, 27, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Staniewicz, R.J. A study of the calcium-thionyl chloride electrochemical system. J. Electrochem. Soc. 1980, 127, 782. [Google Scholar] [CrossRef]
- Weinrich, H.; Durmus, Y.E.; Tempel, H.; Kungl, H.; Eichel, R.-A. Silicon and iron as resource-efficient anode materials for ambient-temperature metal-air batteries: A review. Materials 2019, 12, 2134. [Google Scholar] [CrossRef]
- Xu, X.; San Hui, K.; Dinh, D.A.; Hui, K.N.; Wang, H. Recent advances in hybrid sodium–air batteries. Mater. Horiz. 2019, 6, 1306–1335. [Google Scholar] [CrossRef]
- Faktorovich-Simon, E.; Natan, A.; Peled, E.; Golodnitsky, D. Oxygen redox processes in PEGDME-based electrolytes for the Na-air battery. J. Solid State Electrochem. 2018, 22, 1015–1022. [Google Scholar] [CrossRef]
- Ju, H.; Lee, J. High-temperature liquid Sn-air energy storage cell. J. Energy Chem. 2015, 24, 614–619. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef] [PubMed]
- Skyllas-Kazacos, M.; Cao, L.; Kazacos, M.; Kausar, N.; Mousa, A. Vanadium electrolyte studies for the vanadium redox battery—A review. ChemSusChem 2016, 9, 1521–1543. [Google Scholar] [CrossRef]
- Skyllas-Kazacos, M.; Grossmith, F. Efficient vanadium redox flow cell. J. Electrochem. Soc. 1987, 134, 2950. [Google Scholar] [CrossRef]
- Wu, W.-F.; Yan, X.; Zhan, Y. Recent progress of electrolytes and electrocatalysts in neutral aqueous zinc-air batteries. Chem. Eng. J. 2023, 451, 138608. [Google Scholar] [CrossRef]
- Li, Y.; Dai, H. Recent advances in zinc–air batteries. Chem. Soc. Rev. 2014, 43, 5257–5275. [Google Scholar] [CrossRef]
- Xu, M.; Ivey, D.; Xie, Z.; Qu, W. Rechargeable Zn–air batteries: Progress in electrolyte development and cell configuration advancement. J. Power Sources 2015, 283, 358–371. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.Y.; Yang, H.; Dong, Z.; Liu, H.; Xia, B.Y. Advanced architectures and relatives of air electrodes in Zn–air batteries. Adv. Sci. 2018, 5, 1700691. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.W.; Wang, Z.; Lai, Z.; Liu, Y.; Ma, T.; Geng, J.; Yuan, Z.Y. Rechargeable zinc–air batteries: Advances, challenges, and prospects. Small 2024, 20, 2306396. [Google Scholar] [CrossRef]
- Li, Y.; Gong, M.; Liang, Y.; Feng, J.; Kim, J.-E.; Wang, H.; Hong, G.; Zhang, B.; Dai, H. Advanced zinc-air batteries based on high-performance hybrid electrocatalysts. Nat. Commun. 2013, 4, 1805. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.F.; Tang, C.; Zhang, Q. A review of precious-metal-free bifunctional oxygen electrocatalysts: Rational design and applications in Zn–air batteries. Adv. Funct. Mater. 2018, 28, 1803329. [Google Scholar] [CrossRef]
- Yu, P.; Wang, L.; Sun, F.; Xie, Y.; Liu, X.; Ma, J.; Wang, X.; Tian, C.; Li, J.; Fu, H. Co Nanoislands rooted on Co–N–C nanosheets as efficient oxygen electrocatalyst for Zn–air batteries. Adv. Mater. 2019, 31, 1901666. [Google Scholar] [CrossRef]
- Han, X.; Ling, X.; Wang, Y.; Ma, T.; Zhong, C.; Hu, W.; Deng, Y. Spatial isolation of zeolitic imidazole frameworks-derived cobalt catalysts: From nanoparticle, atomic cluster to single atom. Angew. Chem. Int. Ed. 2019, 58, 5359–5364. [Google Scholar] [CrossRef]
- Shang, H.; Zhou, X.; Dong, J.; Li, A.; Zhao, X.; Liu, Q.; Lin, Y.; Pei, J.; Li, Z.; Jiang, Z. Engineering unsymmetrically coordinated Cu-S1N3 single atom sites with enhanced oxygen reduction activity. Nat. Commun. 2020, 11, 3049. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Q.; Tang, Y.; Zhang, L.; Li, Y. Zinc–air batteries: Are they ready for prime time? Chem. Sci. 2019, 10, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Deiss, E.; Holzer, F.; Haas, O. Modeling of an electrically rechargeable alkaline Zn–air battery. Electrochim. Acta 2002, 47, 3995–4010. [Google Scholar] [CrossRef]
- Ma, H.; Wang, B.; Fan, Y.; Hong, W. Development and characterization of an electrically rechargeable zinc-air battery stack. Energies 2014, 7, 6549–6557. [Google Scholar] [CrossRef]
- Org, W.; Hong, W.; Li, H.; Wang, B. ELECTROCHEMICAL SCIENCE A Horizontal Three-Electrode Structure for Zinc-Air Batteries with Long-Term Cycle Life and High Performance. Int. J. Electrochem. Sci. 2016, 11, 3843–3851. [Google Scholar]
- Li, P.-C.; Chien, Y.-J.; Hu, C.-C. Novel configuration of bifunctional air electrodes for rechargeable zinc–air batteries. J. Power Sources 2016, 313, 37–45. [Google Scholar] [CrossRef]
- Park, M.G.; Lee, D.U.; Seo, M.H.; Cano, Z.P.; Chen, Z. 3D ordered mesoporous bifunctional oxygen catalyst for electrically rechargeable zinc–air batteries. Small 2016, 12, 2707–2714. [Google Scholar] [CrossRef]
- Nazir, G.; Rehman, A.; Lee, J.-H.; Kim, C.-H.; Gautam, J.; Heo, K.; Hussain, S.; Ikram, M.; AlObaid, A.A.; Lee, S.-Y. A Review of Rechargeable Zinc–Air Batteries: Recent Progress and Future Perspectives. Nano-Micro Lett. 2024, 16, 138. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, R.; He, C.; Xia, C.; Guo, W.; Xu, Z.-L.; Xia, B.Y. Advanced polymer-based electrolytes in zinc–air batteries. eScience 2022, 2, 453–466. [Google Scholar] [CrossRef]
- Belete, A.S.; Worku, A.K.; Ayele, D.W.; Assegie, A.A.; Teshager, M.A. The Recent Advancement of Graphene-Based Cathode Material for Rechargeable Zinc–Air Batteries. Processes 2024, 12, 1684. [Google Scholar] [CrossRef]
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y. Rechargeable Zn-air batteries: Recent trends and future perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111771. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, G.; Tong, H.; Liu, X.; Du, L.; Chen, N.; Wang, J.; Sun, T.; Regier, T.; Sun, S. Cobalt (II) oxide nanosheets with rich oxygen vacancies as highly efficient bifunctional catalysts for ultra-stable rechargeable Zn-air flow battery. Nano Energy 2021, 79, 105409. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, H.; Zhuang, J.; Zhou, M.; Gao, M.; Zhang, F.; Wang, Q. Redox-Mediated Two-Electron Oxygen Reduction Reaction with Ultrafast Kinetics for Zn–Air Flow Battery. Adv. Energy Mater. 2022, 12, 2103622. [Google Scholar] [CrossRef]
- Papanikolaou, M.; Hadjithoma, S.; Keramidas, O.; Drouza, C.; Amoiridis, A.; Themistokleous, A.; Hayes, S.C.; Miras, H.N.; Lianos, P.; Tsipis, A.C. Experimental and Theoretical Investigation of the Mechanism of the Reduction of O2 from Air to O22–by VIVO2+–N, N, N-Amidate Compounds and Their Potential Use in Fuel Cells. Inorg. Chem. 2024, 63, 3229–3249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, Y.-P.; Wang, J.; Jiang, Y.; Cui, G.; Shui, L.; Yu, A.; Wang, X.; Chen, Z. Recent progress on flexible Zn-air batteries. Energy Storage Mater. 2021, 35, 538–549. [Google Scholar] [CrossRef]
- Fang, W.; Zhao, J.; Zhang, W.; Chen, P.; Bai, Z.; Wu, M. Recent progress and future perspectives of flexible Zn-Air batteries. J. Alloys Compd. 2021, 869, 158918. [Google Scholar] [CrossRef]
- Liu, H.; Xie, W.; Huang, Z.; Yao, C.; Han, Y.; Huang, W. Recent advances in flexible Zn–Air batteries: Materials for electrodes and electrolytes. Small Methods 2022, 6, 2101116. [Google Scholar] [CrossRef]
- Jiang, L.; Luo, X.; Wang, D.W. A review on system and materials for aqueous flexible metal–air batteries. Carbon Energy 2023, 5, e284. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, H.; Wang, M.; Song, W.-l.; Xue, J.; Jiao, S. Current progresses and future prospects on aluminium–air batteries. Int. Mater. Rev. 2022, 67, 734–764. [Google Scholar] [CrossRef]
- Goel, P.; Dobhal, D.; Sharma, R. Aluminum–air batteries: A viability review. J. Energy Storage 2020, 28, 101287. [Google Scholar] [CrossRef]
- Mori, R. Recent developments for aluminum–air batteries. Electrochem. Energy Rev. 2020, 3, 344–369. [Google Scholar] [CrossRef]
- Mori, R. Rechargeable aluminum–air battery using various air-cathode materials and suppression of byproducts formation on both anode and air cathode. ECS Trans. 2017, 80, 377. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, H.; Fu, L.; Wu, P.; Li, Y.; Li, Y.; Sun, D.; Wang, H.; Tang, Y. Electrolytes for aluminum–air batteries: Advances, challenges, and applications. Sustain. Energy Fuels 2023, 7, 1353–1370. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Ren, J.; Zhang, Y.; Peng, H. An all-solid-state fiber-shaped aluminum–air battery with flexibility, stretchability, and high electrochemical performance. Angew. Chem. 2016, 128, 8111–8114. [Google Scholar] [CrossRef]
- Yang, S.; Knickle, H. Design and analysis of aluminum/air battery system for electric vehicles. J. Power Sources 2002, 112, 162–173. [Google Scholar] [CrossRef]
- Tang, Y.; Lu, L.; Roesky, H.W.; Wang, L.; Huang, B. The effect of zinc on the aluminum anode of the aluminum–air battery. J. Power Sources 2004, 138, 313–318. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.; Skeldon, P.; Thompson, G.; Smith, G. The effect of nickel on alloy microstructure and electrochemical behaviour of AA1050 aluminium alloy in acid and alkaline solutions. Electrochim. Acta 2012, 75, 229–238. [Google Scholar] [CrossRef]
- Ryu, J.; Park, M.; Cho, J. Advanced technologies for high-energy aluminum–air batteries. Adv. Mater. 2019, 31, 1804784. [Google Scholar] [CrossRef] [PubMed]
- Yirka, B. Phinergy Demonstrates Aluminum–air Battery Capable of Fueling an Electric Vehicle for 1000 Miles. PHYS ORG. 27 March 2013. Available online: https://phys.org/news/2013-03-phinergy-aluminum-air-battery-capable-fueling.html (accessed on 16 August 2024).
- Vegh, J.M.; Alonso, J.J. Design and optimization of short-range aluminum-air powered aircraft. In Proceedings of the 54th AIAA Aerospace Sciences Meeting, San Diego, CA, USA, 4–8 January 2016; p. 1026. [Google Scholar]
- Rewatkar, P.; Goel, S. Catalyst-mitigated arrayed aluminum-air origami fuel cell with ink-jet printed custom-porosity cathode. Energy 2021, 224, 120017. [Google Scholar] [CrossRef]
- Huang, H.; Liu, P.; Ma, Q.; Tang, Z.; Wang, M.; Hu, J. Enabling a high-performance saltwater Al-air battery via ultrasonically driven electrolyte flow. Ultrason. Sonochem. 2022, 88, 106104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shao, Q.; Zhang, J. An overview of non-noble metal electrocatalysts and their associated air cathodes for Mg-air batteries. Mater. Rep. Energy 2021, 1, 100002. [Google Scholar] [CrossRef]
- Raghavan, P.; Das, A.; Jabeen Fatima, M.J. Advanced Technologies for Rechargeable Batteries: Metal Ion, Hybrid, and Metal-Air Batteries; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Li, C.S.; Sun, Y.; Gebert, F.; Chou, S.L. Current progress on rechargeable magnesium–air battery. Adv. Energy Mater. 2017, 7, 1700869. [Google Scholar] [CrossRef]
- Chen, X.; Venezuela, J.; Shi, Z.; Wang, L.; Dargusch, M. Ultra-high-purity Mg-Ge anodes enable a long-lasting, high energy-density Mg-air battery. Nano Energy 2024, 122, 109269. [Google Scholar] [CrossRef]
- Li, L.; Chen, H.; He, E.; Wang, L.; Ye, T.; Lu, J.; Jiao, Y.; Wang, J.; Gao, R.; Peng, H. High-energy-density magnesium-air battery based on dual-layer gel electrolyte. Angew. Chem. 2021, 133, 15445–15450. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Park, W.; Zhao, Z.; Li, J.; Lim, C.K.; Wong, T.H.; Yiu, C.K.; Gao, Y.; Zhou, J. Stretchable magnesium–air battery based on dual ions conducting hydrogel for intelligent biomedical applications. InfoMat 2023, 5, e12388. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, S.; Li, T.; Su, D.; Guo, S.; Wang, G. Recent advances in rechargeable magnesium-based batteries for high-efficiency energy storage. Adv. Energy Mater. 2020, 10, 1903591. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Mazor, H.; Goor, M.; Avshalomov, S. Parameter analysis of a practical lithium-and sodium-air electric vehicle battery. J. Power Sources 2011, 196, 6835–6840. [Google Scholar] [CrossRef]
- Qin, L.; Xiao, N.; Zhang, S.; Chen, X.; Wu, Y. From K-O2 to K-Air batteries: Realizing superoxide batteries on the basis of dry ambient air. Angew. Chem. Int. Ed. 2020, 59, 10498–10501. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Arandiyan, H.; Mofarah, S.S.; Koshy, P.; Pozo-Gonzalo, C.; Zheng, R.; Wang, Z.; Wang, Y.; Bhargava, S.K.; Sun, H. A comprehensive review of cathode materials for Na–air batteries. Energy Adv. 2023, 2, 465–502. [Google Scholar] [CrossRef]
- Qin, L.; Ao, H.; Wu, Y. Feasibility of achieving two-electron K–O2 batteries. Faraday Discuss. 2024, 248, 60–74. [Google Scholar] [CrossRef]
- Yu, W.; Lau, K.C.; Lei, Y.; Liu, R.; Qin, L.; Yang, W.; Li, B.; Curtiss, L.A.; Zhai, D.; Kang, F. Dendrite-free potassium–oxygen battery based on a liquid alloy anode. ACS Appl. Mater. Interfaces 2017, 9, 31871–31878. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Han, Y.; Xu, P.; Feng, X.; Ji, W.; Arandiyan, H. In-situ generating highly efficient sites for multifunctional water splitting/Zn-air battery over hierarchically architectured Fe2Ni3 nanoalloy/N-incorporated carbon matrix. Chem. Eng. J. 2022, 450, 138245. [Google Scholar] [CrossRef]
- Lutz, L.; Alves Dalla Corte, D.; Tang, M.; Salager, E.; Deschamps, M.; Grimaud, A.; Johnson, L.; Bruce, P.G.; Tarascon, J.-M. Role of electrolyte anions in the Na–O2 battery: Implications for NaO2 solvation and the stability of the sodium solid electrolyte interphase in glyme ethers. Chem. Mater. 2017, 29, 6066–6075. [Google Scholar] [CrossRef]
- Yadegari, H.; Sun, X. Sodium–oxygen batteries: Recent developments and remaining challenges. Trends Chem. 2020, 2, 241–253. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, S.; Zhu, S.; Gao, H.; San Hui, K.; Yuan, C.-Z.; Yin, H.; Bin, F.; Wu, X.-L.; Mai, W. Iron-modulated nickel cobalt phosphide embedded in carbon to boost power density of hybrid sodium–air battery. Appl. Catal. B Environ. 2021, 285, 119786. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, K.; Zhang, D.; Chang, S.; Liang, F.; Yan, P.; Yao, Y.; Qu, T.; Zhan, J.; Ma, W. Reversible hybrid sodium-CO2 batteries with low charging voltage and long-life. Nano Energy 2020, 68, 104318. [Google Scholar] [CrossRef]
- Enterría, M.; Reynaud, M.; Paredes, J.I.; Medinilla, L.; Younesi, R.; Ortiz-Vitoriano, N. Driving the sodium-oxygen battery chemistry towards the efficient formation of discharge products: The importance of sodium superoxide quantification. J. Energy Chem. 2022, 68, 709–720. [Google Scholar] [CrossRef]
- Duan, C.; Li, X.; Wang, D.; Wang, Z.; Sun, H.; Zheng, R.; Liu, Y. Nanosized high entropy spinel oxide (FeCoNiCrMn)3O4 as a highly active and ultra-stable electrocatalyst for the oxygen evolution reaction. Sustain. Energy Fuels 2022, 6, 1479–1488. [Google Scholar] [CrossRef]
- Cohn, G.; Starosvetsky, D.; Hagiwara, R.; Macdonald, D.D.; Ein-Eli, Y. Silicon–air batteries. Electrochem. Commun. 2009, 11, 1916–1918. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, H.; Liu, Y.; Bai, J.; Liao, L.; Huang, Y.; Duan, X. High-Capacity Silicon–Air Battery in Alkaline Solution. ChemSusChem 2012, 5, 177–180. [Google Scholar] [CrossRef]
- Park, D.-W.; Kim, S.; Ocon, J.D.; Abrenica, G.H.A.; Lee, J.K.; Lee, J. Controlled electrochemical etching of nanoporous Si anodes and its discharge behavior in alkaline Si–air batteries. ACS Appl. Mater. Interfaces 2015, 7, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Durmus, Y.E.; Aslanbas, Ö.; Kayser, S.; Tempel, H.; Hausen, F.; De Haart, L.; Granwehr, J.; Ein-Eli, Y.; Eichel, R.-A.; Kungl, H. Long run discharge, performance and efficiency of primary Silicon–air cells with alkaline electrolyte. Electrochim. Acta 2017, 225, 215–224. [Google Scholar] [CrossRef]
- Schalinski, R.; Schweizer, S.L.; Wehrspohn, R.B. The Role of Silicate Enrichment on the Discharge Duration of Silicon-Air Batteries. ChemSusChem 2023, 16, e202300077. [Google Scholar] [CrossRef]
- Montiel Guerrero, S.S.; Durmus, Y.E.; Tempel, H.; Roth, C.; Kungl, H.; van Waasen, S.; Ein-Eli, Y.; Eichel, R.A. Unveiling the Potential of Silicon-Air Batteries for Low-Power Transient Electronics: Electrochemical Insights and Practical Application. Batter. Supercaps 2024, 7, e202300573. [Google Scholar] [CrossRef]
- Yang, W.-T.; Yu, J.; Li, D.-X.; Hu, S.-Q.; Ding, X.; Chen, F.-Y.; Qiao, C. Performance of silicon-air batteries using industrial silicon as anode. J. Mater. Sci. Mater. Electron. 2023, 34, 800. [Google Scholar] [CrossRef]
- Lu, Y.-T.; Neale, A.R.; Hu, C.-C.; Hardwick, L.J. Divalent nonaqueous metal-air batteries. Front. Energy Res. 2021, 8, 602918. [Google Scholar] [CrossRef]
- Ocon, J.D.; Kim, J.W.; Uhm, S.; Mun, B.S.; Lee, J. An etched nanoporous Ge anode in a novel metal–air energy conversion cell. Phys. Chem. Chem. Phys. 2013, 15, 6333–6338. [Google Scholar] [CrossRef]
- Ye, L.; Liao, M.; Zhang, K.; Zheng, M.; Jiang, Y.; Cheng, X.; Wang, C.; Xu, Q.; Tang, C.; Li, P. A rechargeable calcium–oxygen battery that operates at room temperature. Nature 2024, 626, 313–318. [Google Scholar] [CrossRef]
- Fang, C.; Tang, X.; Wang, J.; Yi, Q. Performance of iron-air battery with iron nanoparticle-encapsulated CN composite electrode. Front. Energy 2024, 18, 42–53. [Google Scholar] [CrossRef]
- Global EV Outlook 2024, Paris. 2024. Available online: https://www.iea.org/reports/global-ev-outlook-2024 (accessed on 15 October 2024).
- Gallagher, K.G.; Goebel, S.; Greszler, T.; Mathias, M.; Oelerich, W.; Eroglu, D.; Srinivasan, V. Quantifying the promise of lithium–air batteries for electric vehicles. Energy Environ. Sci. 2014, 7, 1555–1563. [Google Scholar] [CrossRef]
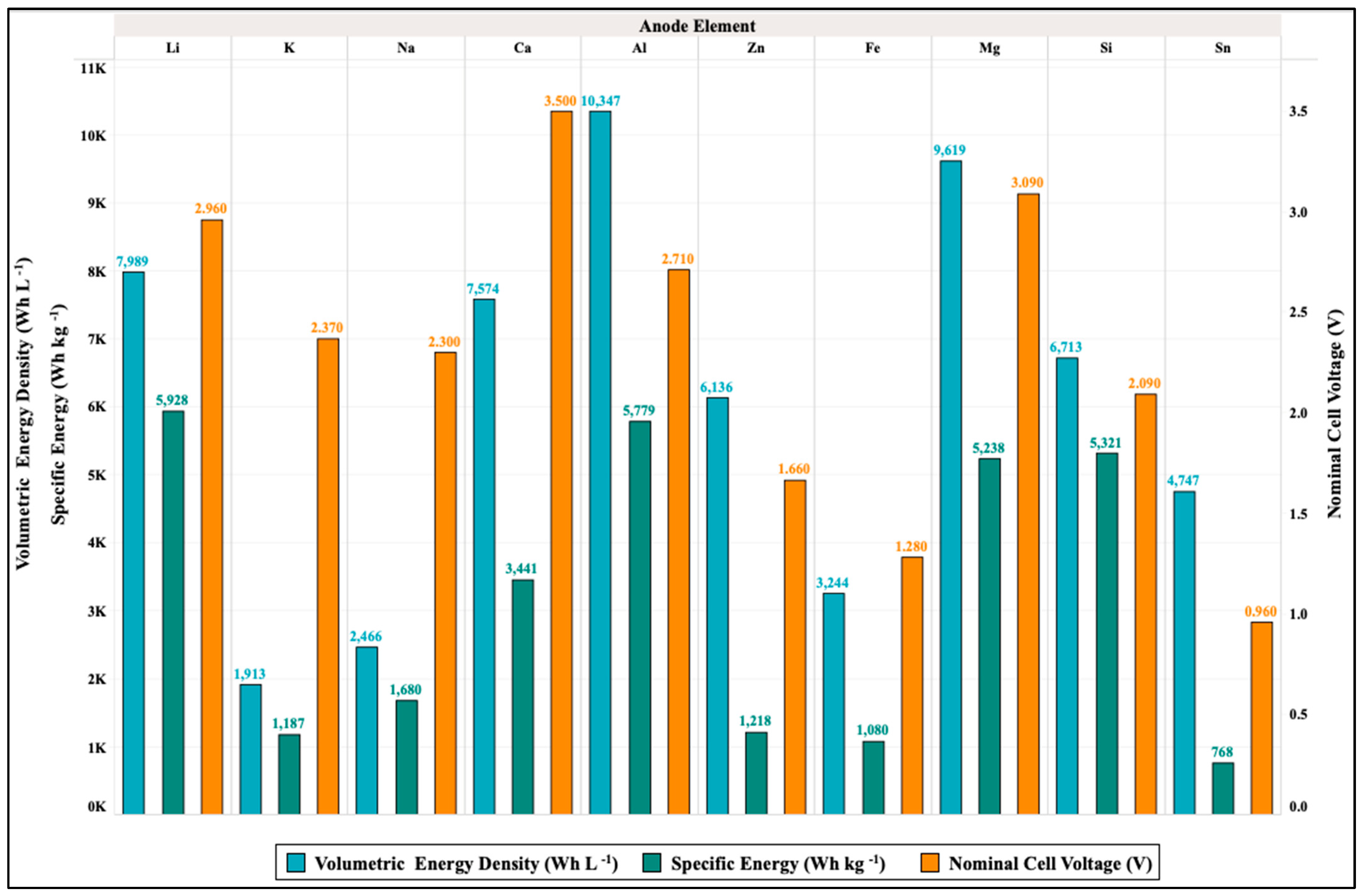


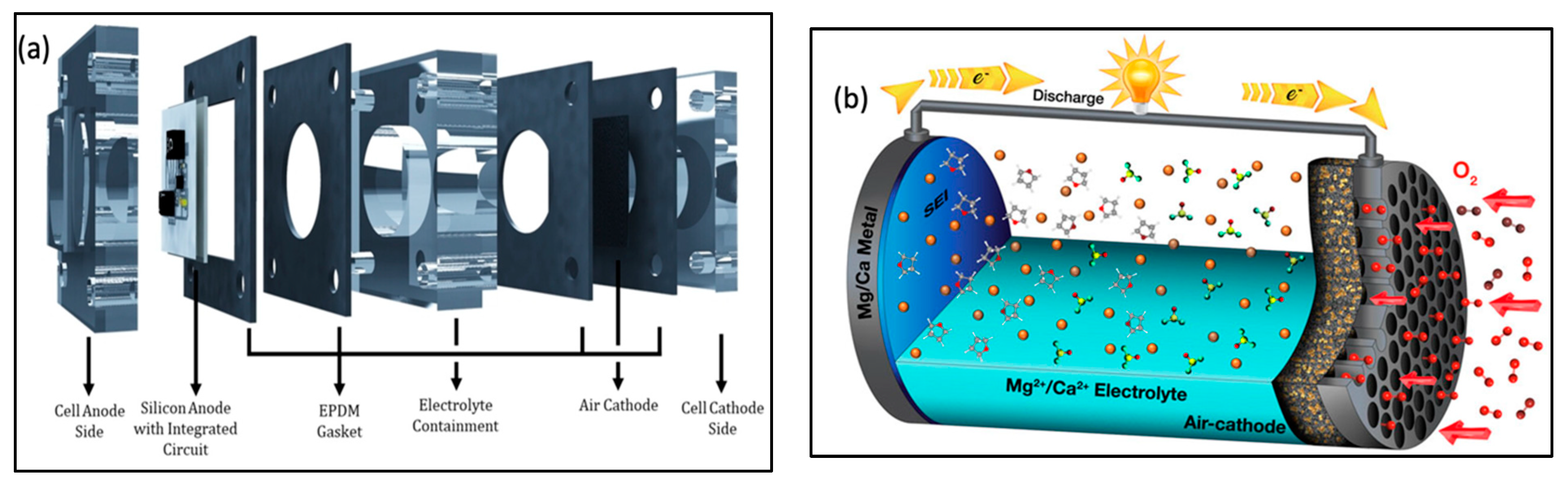
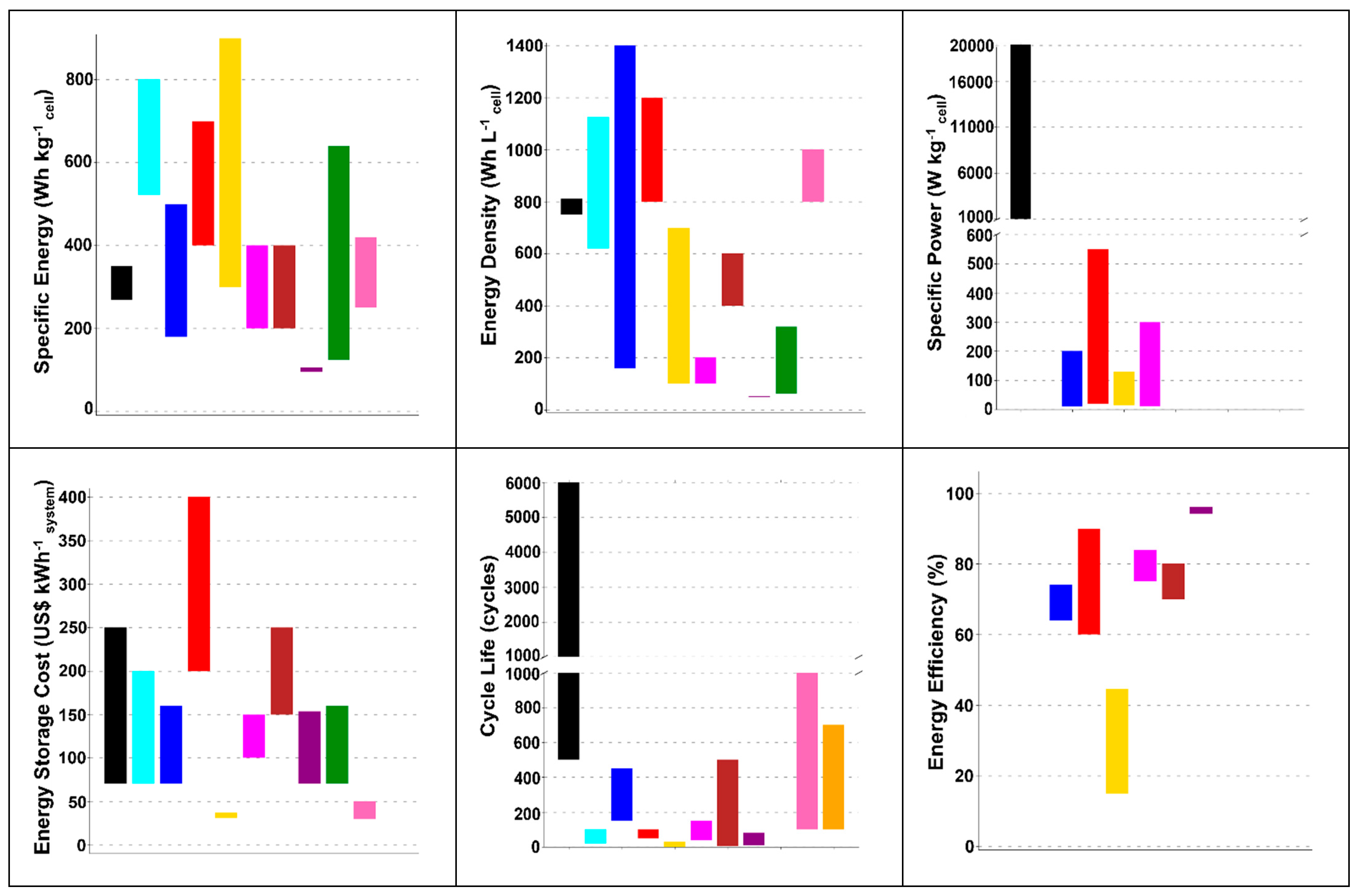
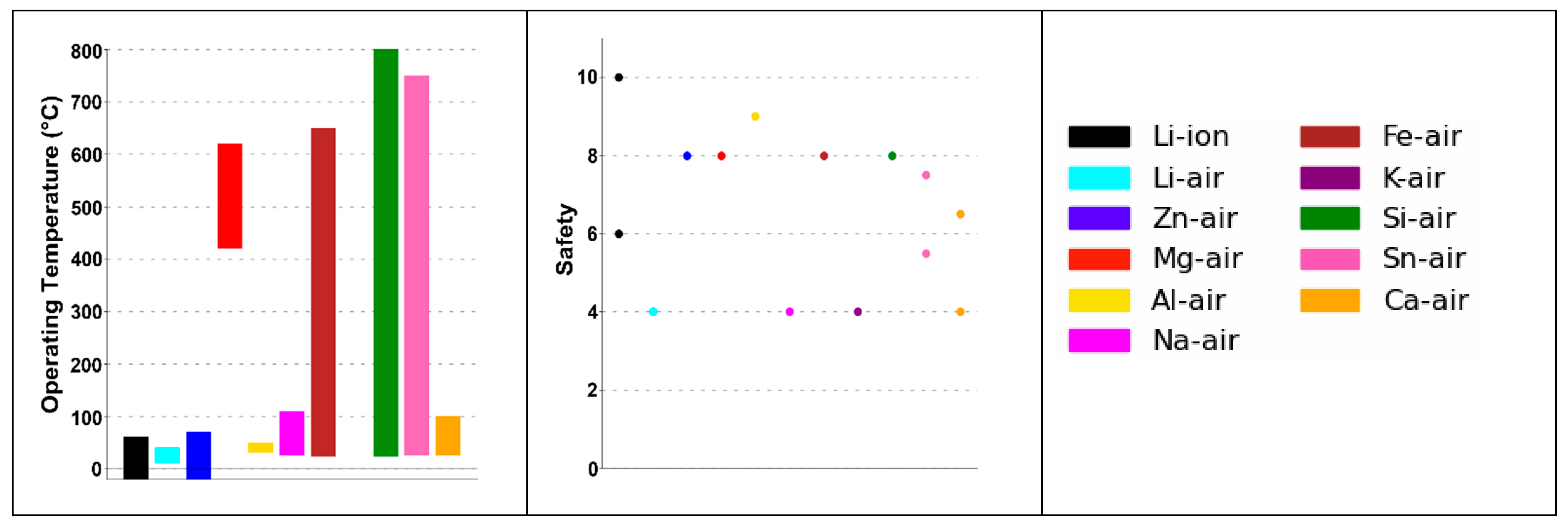
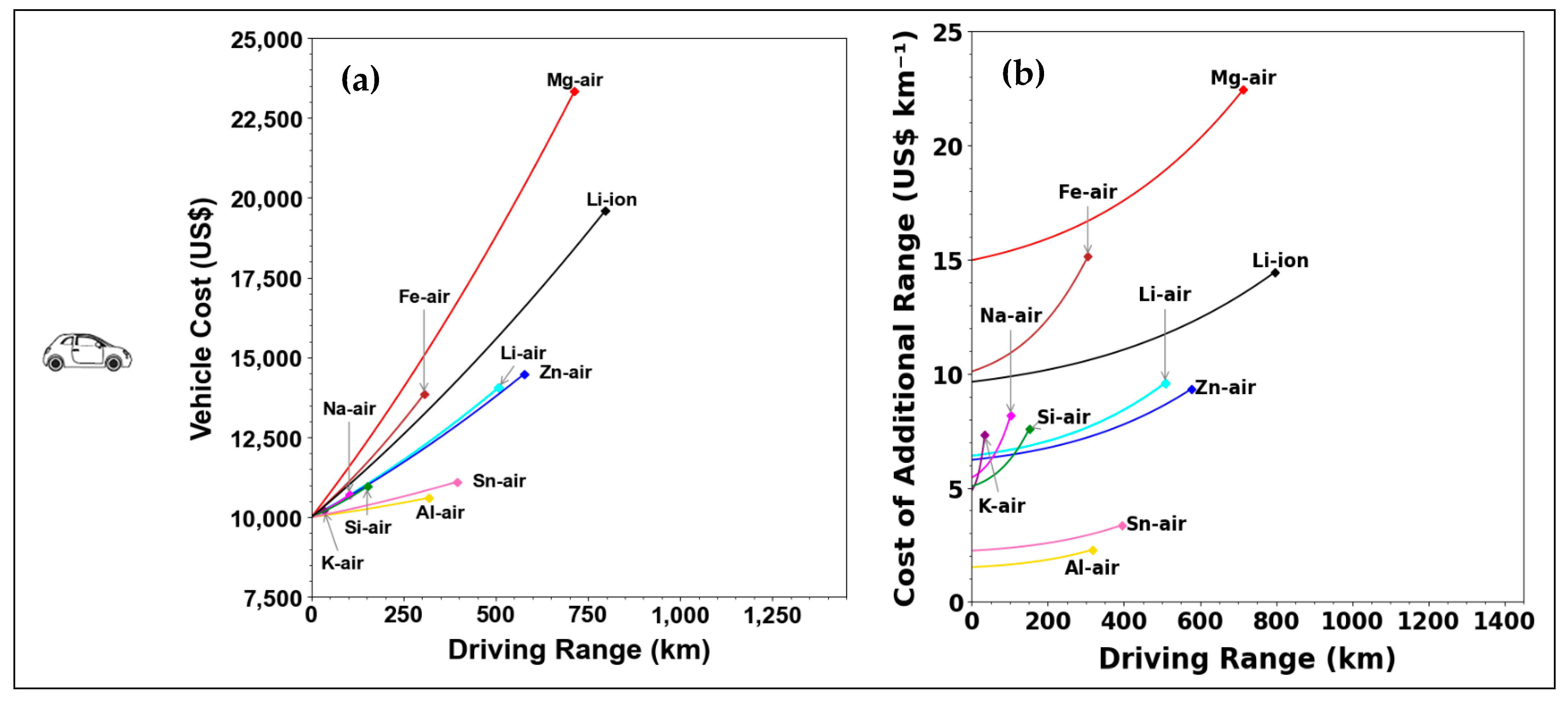
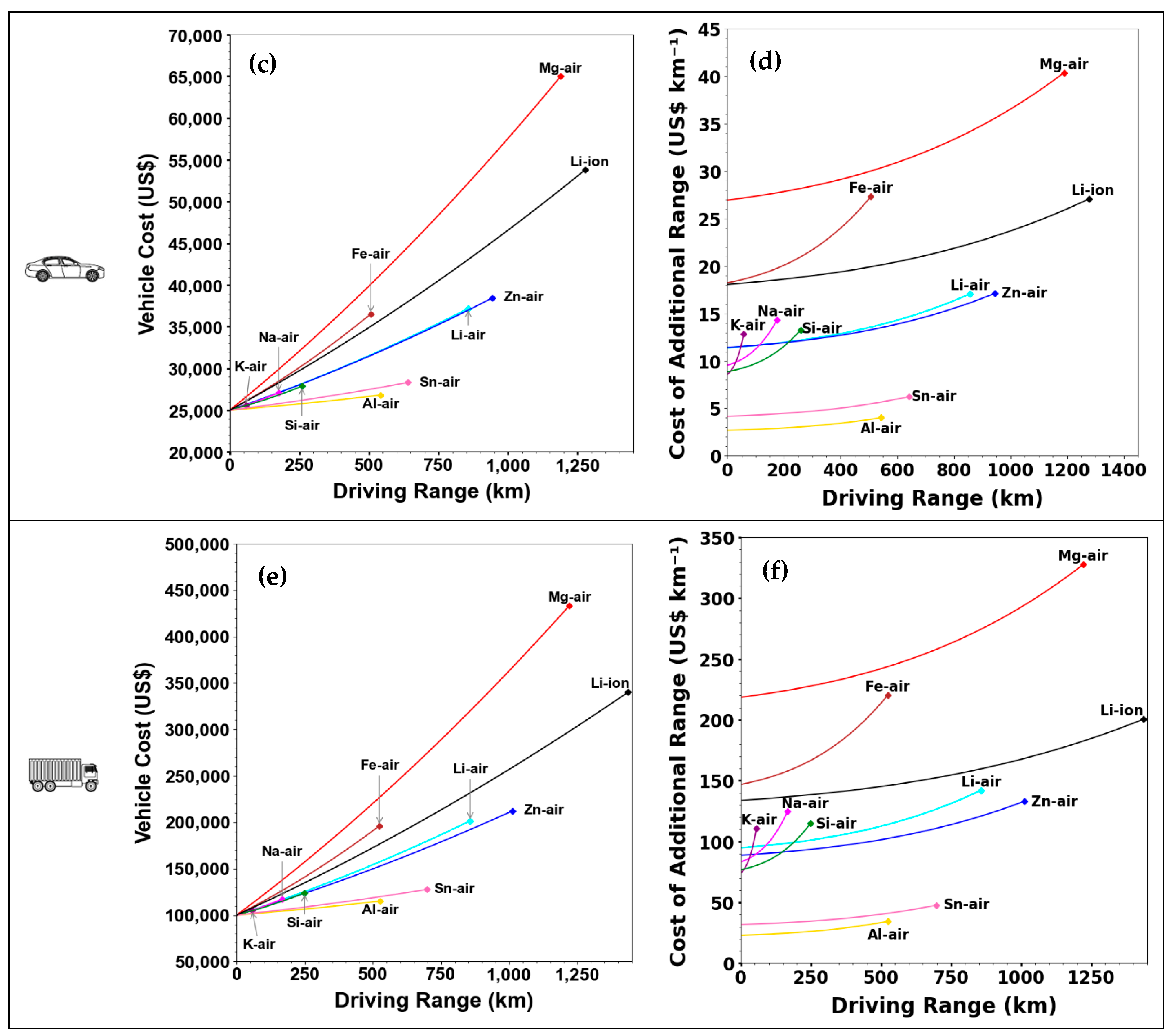
| Parameter | Li–Air | K–Air | Na–Air | Mg–Air | Al–Air | Zn–Air | Ca–Air | Fe–Air | Sn–Air | Si–Air | Ge–Air |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Specific Energy (Wh kg−1) | 5928 | 187 | 680 | 5238 | 5779 | 1218 | 3441 | 1080 | 768 | 5321 | 2214 |
| Specific Power (W kg−1) | - | - | 12–300 | 20–550 | 15–130 | 10–200 | - | - | - | - | - |
| Specific Capacity (Ah kg−1) | 2003 | 501 | 730 | 1695 | 2132 | 733 | 983.1 | 844 | 800 | 2548 | 1223 |
| Volumetric Capacity density (Ah L−1) | 2699 | 807 | 1072 | 3112 | 3817 | 3694 | 2164 | 2537 | 4944 | 3212 | 2633 |
| Volumetric Energy Density (Wh L−1) | 7989 | 1913 | 2466 | 9619 | 10347 | 6136 | 7574 | 3244 | 4747 | 6713 | 4766 |
| Theoretical Open Circuit Voltage (V cell−1) | 2.96 | 2.37 | 2.3 | 3.09 | 2.71 | 1.66 | 3.5 | 1.28 | 0.96 | 2.09 | 1.81 |
| Energy Efficiency (%) | 68–94 | - | - | - | 70 | - | - | 96 | 70–90 | - | - |
| Operational Temperature Range (°C) | 10–40 | - | 105–110 | 420–620 | 30–50 | −20–70 | - | - | - | - | - |
| Cost (USDkg−1) | 46 | 13.02 | 1.7 | 11.02 | 2.866 | 3.351 | 5.93 | 0.4 | 28.66 | 4.19 | 1400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabeer, Y.; Madani, S.S.; Panchal, S.; Mousavi, M.; Fowler, M. Different Metal–Air Batteries as Range Extenders for the Electric Vehicle Market: A Comparative Study. Batteries 2025, 11, 35. https://doi.org/10.3390/batteries11010035
Shabeer Y, Madani SS, Panchal S, Mousavi M, Fowler M. Different Metal–Air Batteries as Range Extenders for the Electric Vehicle Market: A Comparative Study. Batteries. 2025; 11(1):35. https://doi.org/10.3390/batteries11010035
Chicago/Turabian StyleShabeer, Yasmin, Seyed Saeed Madani, Satyam Panchal, Mahboubeh Mousavi, and Michael Fowler. 2025. "Different Metal–Air Batteries as Range Extenders for the Electric Vehicle Market: A Comparative Study" Batteries 11, no. 1: 35. https://doi.org/10.3390/batteries11010035
APA StyleShabeer, Y., Madani, S. S., Panchal, S., Mousavi, M., & Fowler, M. (2025). Different Metal–Air Batteries as Range Extenders for the Electric Vehicle Market: A Comparative Study. Batteries, 11(1), 35. https://doi.org/10.3390/batteries11010035









