Carbon-Coated CF-Si/Al Anodes for Improved Lithium-Ion Battery Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
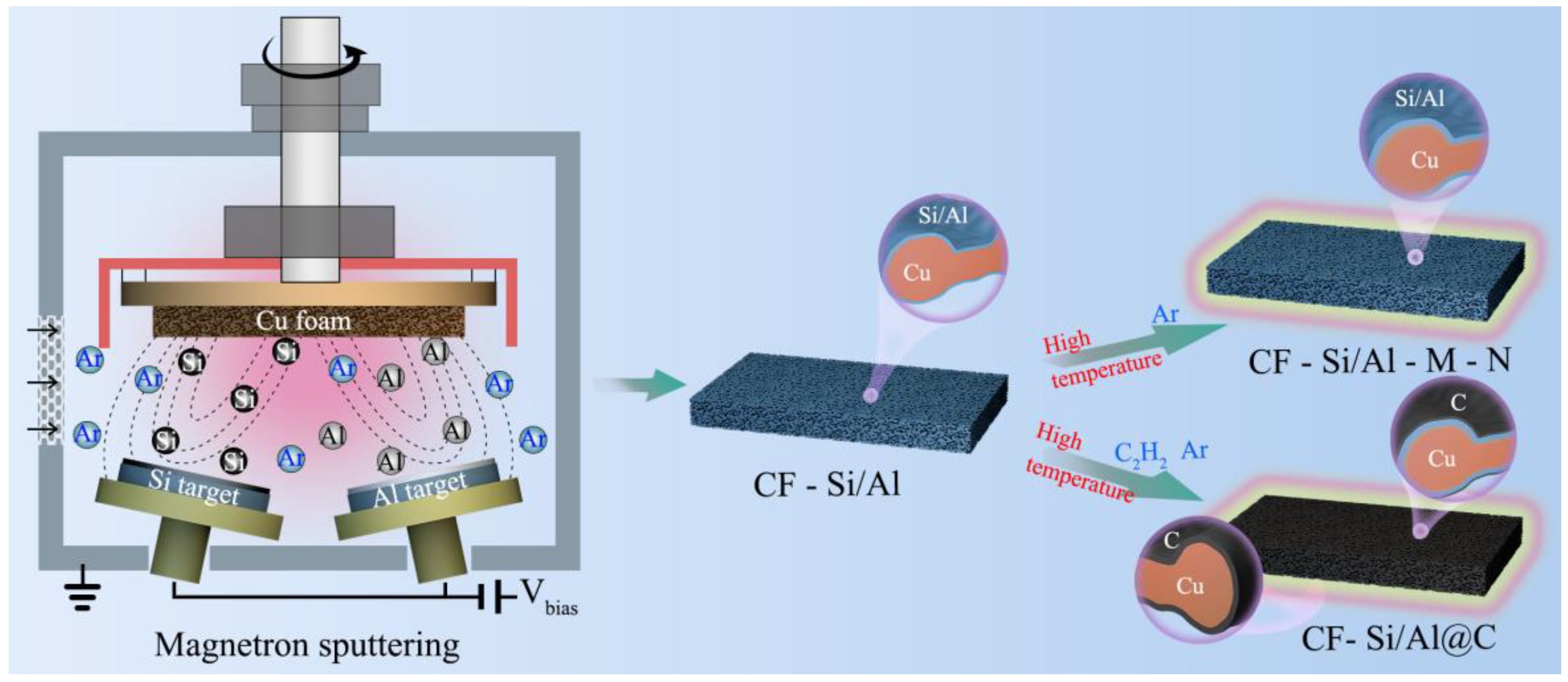
2.2. Preparation of CF-Si/Al Materials
2.3. Preparation of CF-Si/Al-M-N and CF-Si/Al@C-M-N Materials
2.4. Material Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
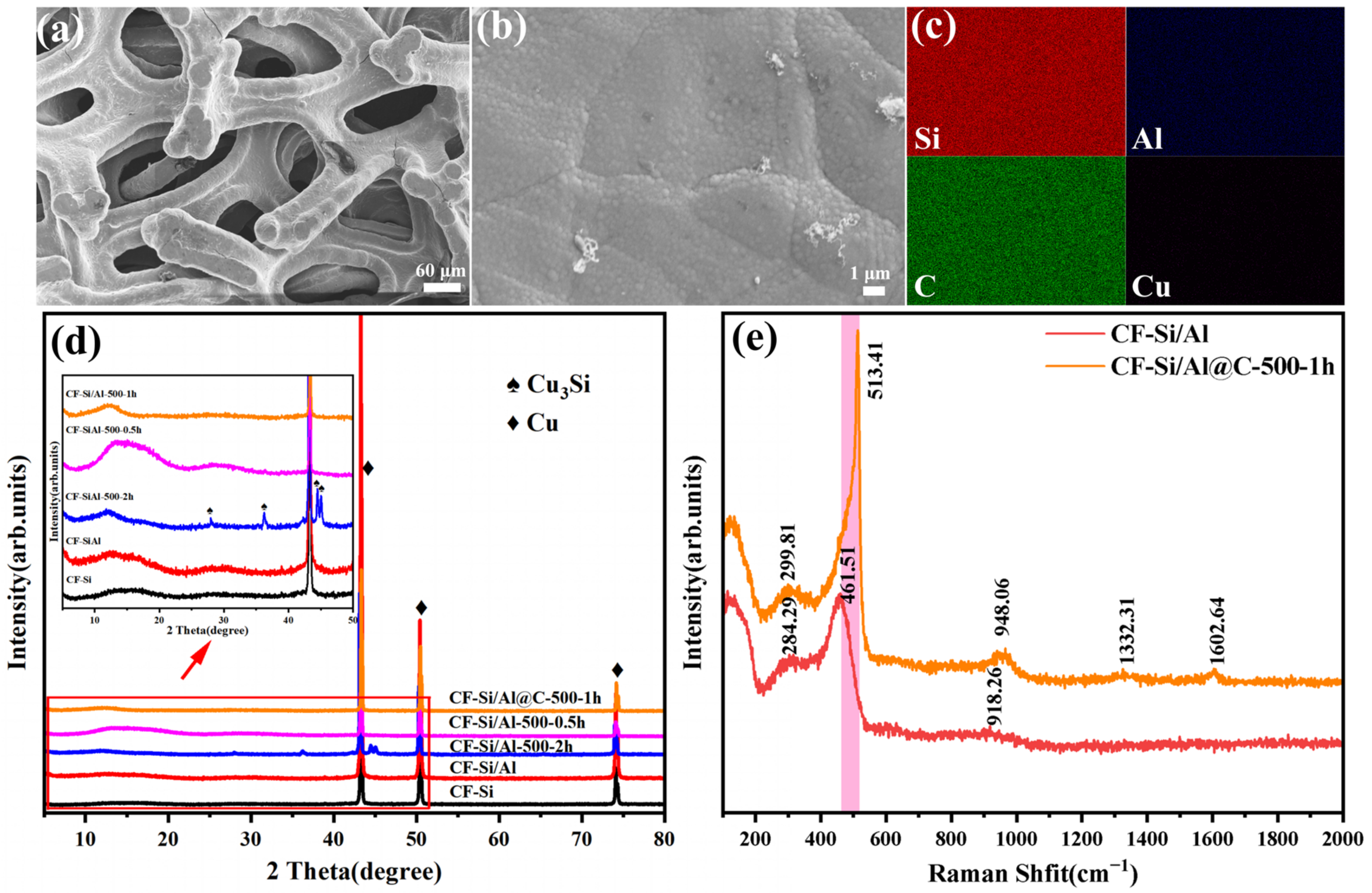
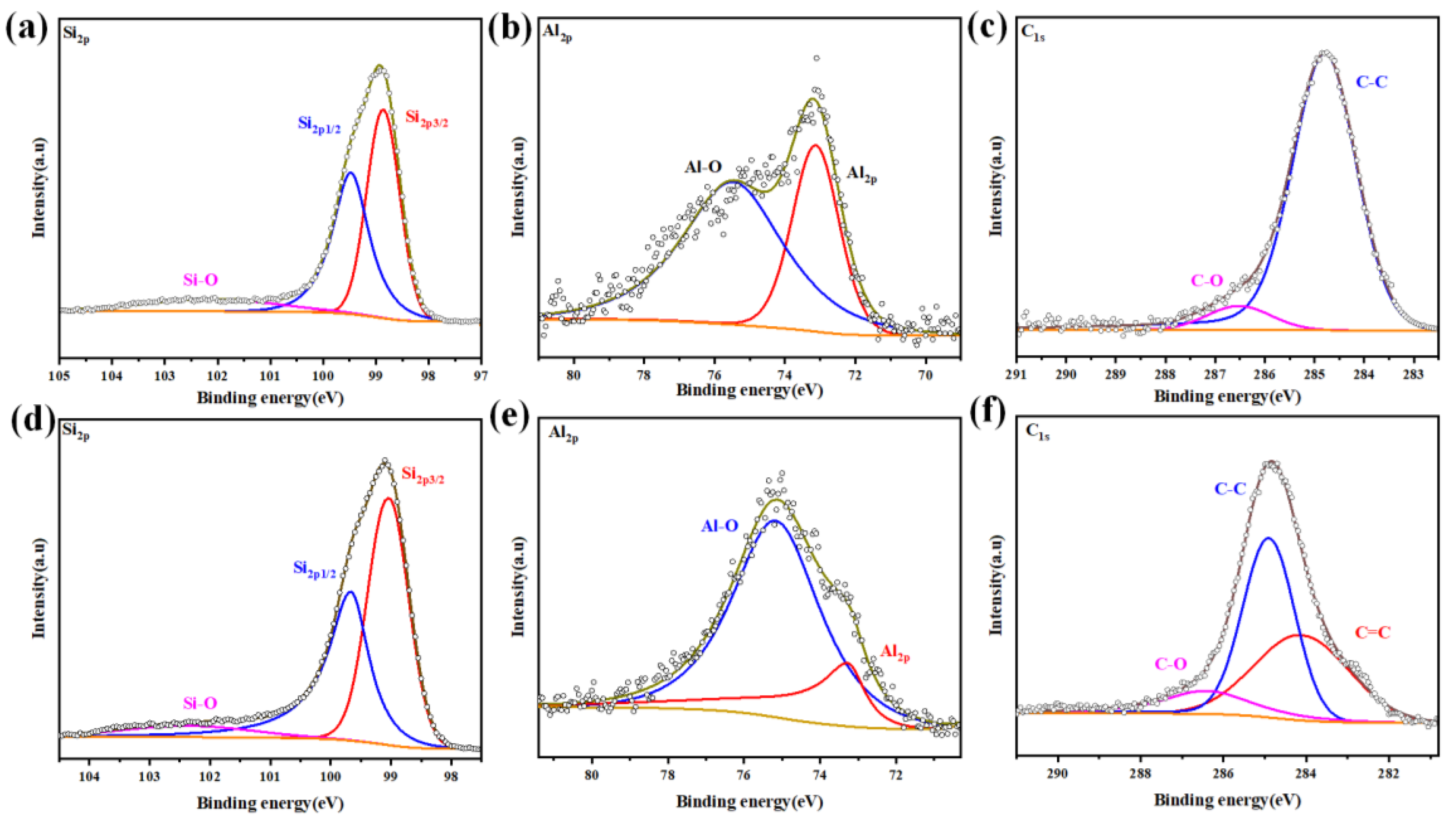
3.1. Material Morphology and Composition Analysis
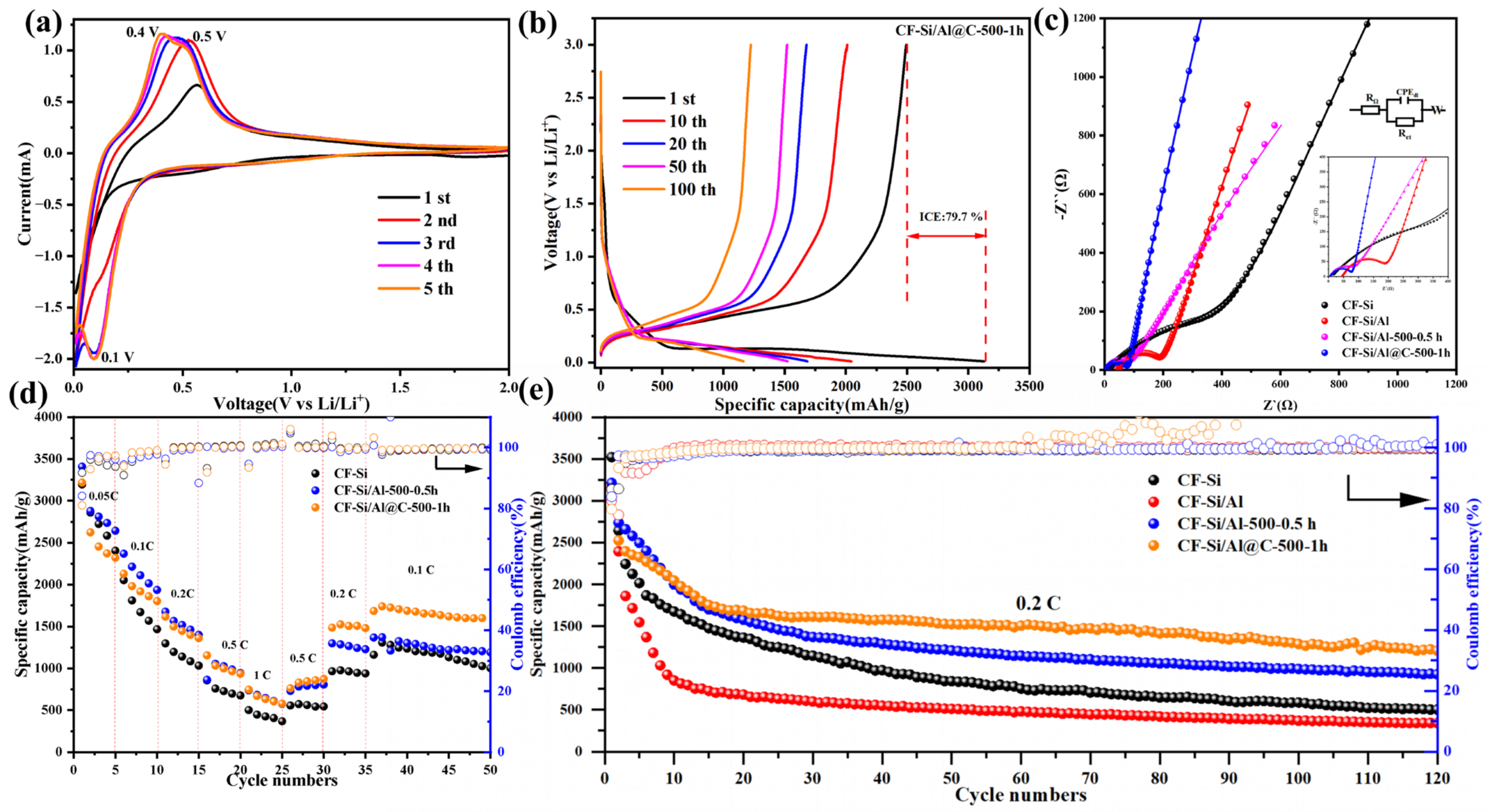
3.2. Electrochemical Performance Analysis
3.3. Electrochemical Kinetics
3.4. Changes in Film Surface Morphology Before and After Cycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H.; Li, H.; Lai, Y.; Yang, Z.; Yang, Q.; Liu, Y.; Zheng, Z.; Liu, Y.; Sun, Y.; Zhong, B.; et al. Revisiting the Preparation Progress of Nano-structured Si Anodes toward Industrial Application from the Perspective of Cost and Scalability. Adv. Energy Mater. 2022, 12, 2102181. [Google Scholar] [CrossRef]
- Yang, X.; Kong, W.; Du, G.; Li, S.; Tang, Y.; Cao, J.; Lu, X.; Tan, R.; Qian, G. Synthesis of a Yolk-Shell Nanostructured Silicon-Based Anode for High-Performance Li-Ion Batteries. Batteries 2023, 9, 446. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Chai, J.; Wang, Y.; Du, J.; Chen, Z.; Rui, Y.; Jiang, L.; Tang, B. Si-Based Anode Lithium-Ion Batteries: A Comprehensive Review of Recent Progress. ACS Mater. Lett. 2023, 5, 2948–2970. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, Z.; Xia, Y.; Wu, G.; Chen, C.; Wang, J.; Rao, P.; Dong, A. Facile Electrostatic Assembly of Si@MXene Superstructures for Enhanced Lithium-Ion Storage. J. Colloid Interface Sci. 2020, 580, 68–76. [Google Scholar] [CrossRef]
- Fang, Q.; Xu, S.; Sha, X.; Liu, D.; Zhang, X.; Li, W.; Weng, S.; Li, X.; Chen, L.; Li, H.; et al. Interfacial Degradation of Silicon Anodes in Pouch Cells. Energy Environ. Sci. 2024, 17, 6368–6376. [Google Scholar] [CrossRef]
- Kulova, T.L.; Mironenko, A.A.; Skundin, A.M.; Rudy, A.S.; Naumov, V.V.; Pukhov, D.E. Study of Silicon Composite for Negative Electrode of Lithium-Ion Battery. Int. J. Electrochem. Sci. 2016, 11, 1370–1381. [Google Scholar] [CrossRef]
- Cheng, Y.; Wei, K.; Yu, Z.; Fan, D.; Yan, D.L.; Pan, Z.; Tian, B. Ternary Si-SiO-Al Composite Films as High-Performance Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 34447–34456. [Google Scholar] [CrossRef]
- Han, M.; Mu, Y.; Wei, L.; Zeng, L.; Zhao, T. Multilevel Carbon Architecture of Subnanoscopic Silicon for Fast-charging High-energy-density Lithiumion Batteries. Carbon Energy 2024, 6, e377. [Google Scholar] [CrossRef]
- Huo, H.; Janek, J. Silicon as Emerging Anode in Solid-State Batteries. ACS Energy Lett. 2022, 7, 4005–4016. [Google Scholar] [CrossRef]
- Keles, O.; Karahan, B.D.; Eryilmaz, L.; Amine, R.; Abouimrane, A.; Chen, Z.; Zuo, X.; Zhu, Z.; Al-Hallaj, S.; Amine, K. Superlattice-Structured Films by Magnetron Sputtering as New Era Electrodes for Advanced Lithium-Ion Batteries. Nano Energy 2020, 76, 105094. [Google Scholar] [CrossRef]
- Salah, M.; Hall, C.; Francis, C.; Rollo-Walker, G.; Fabretto, M. Binary Silicon-Based Thin-Film Anodes for Lithium-Ion Batteries: A Review. J. Power Sources 2022, 520, 230871. [Google Scholar] [CrossRef]
- Wang, H.; Man, H.; Yang, J.; Zang, J.; Che, R.; Wang, F.; Sun, D.; Fang, F. Self-adapting Electrochemical Grinding Strategy for Stable Silicon Anode. Adv. Funct. Mater. 2022, 32, 2109887. [Google Scholar] [CrossRef]
- Manthiram, A. An Outlook on Lithium-Ion Battery Technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, Y.; Liu, L.; Zhao, D.; Zhang, Z.; Wu, Y.; Zhang, Y.; Liu, X.; Wang, Z. A Hollow Silicon Nanosphere/Carbon Nanotube Composite as an Anode Material for Lithium-Ion Batteries. Coatings 2022, 12, 1515. [Google Scholar] [CrossRef]
- Collins, G.A.; Kilian, S.; Geaney, H.; Ryan, K.M. A Nanowire Nest Structure Comprising Copper Silicide and Silicon Nanowires for Lithium-ion Battery Anodes with High Areal Loading. Small 2021, 17, 2102333. [Google Scholar] [CrossRef]
- Tang, W.; Guo, X.; Liu, X.; Chen, G.; Wang, H.; Zhang, N.; Wang, J.; Qiu, G.; Ma, R. Interconnected Silicon Nanoparticles Originated from Halloysite Nanotubes through the Magnesiothermic Reduction: A High-Performance Anode Material for Lithium-Ion Batteries. Appl. Clay Sci. 2018, 162, 499–506. [Google Scholar] [CrossRef]
- Wu, H.; Cui, Y. Designing Nanostructured Si Anodes for High Energy Lithium-Ion Batteries. Nano Today 2012, 7, 414–429. [Google Scholar] [CrossRef]
- Leung, O.M.; Gordon, L.W.; Messinger, R.J.; Prodromakis, T.; Wharton, J.A.; Ponce De León, C.; Schoetz, T. Solid Polymer Electrolytes with Enhanced Electrochemical Stability for High-capacity Aluminum Batteries. Adv. Energy Mater. 2024, 14, 2303285. [Google Scholar] [CrossRef]
- Aslanbas, Ö.; Durmus, Y.E.; Tempel, H.; Hausen, F.; Ein-Eli, Y.; Eichel, R.-A.; Kungl, H. Electrochemical Analysis and Mixed Potentials Theory of Ionic Liquid Based Metal–Air Batteries with Al/Si Alloy Anodes. Electrochim. Acta 2018, 276, 399–411. [Google Scholar] [CrossRef]
- Sun, B.; Xu, Y.; Yang, S.; Zhang, D.; Pei, C.; Ni, S. Al-Based Materials for Advanced Lithium Rechargeable Batteries: Recent Progress and Prospects. Mater. Chem. Front. 2023, 7, 2554–2569. [Google Scholar] [CrossRef]
- Fleischauer, M.D.; Obrovac, M.N.; Dahn, J.R. Al-Si Thin-Film Negative Electrodes for Li-Ion Batteries. J. Electrochem. Soc. 2008, 155, A851. [Google Scholar] [CrossRef]
- Johnson, B.C.; Stuiber, M.; Creedon, D.L.; Bose, M.; Berhane, A.; Willems Van Beveren, L.H.; Rubanov, S.; Cole, J.H.; Mourik, V.; Hamilton, A.R.; et al. Silicon-Aluminum Phase-Transformation-Induced Superconducting Rings. Nano Lett. 2023, 23, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Reyes Jiménez, A.; Klöpsch, R.; Wagner, R.; Rodehorst, U.C.; Kolek, M.; Nölle, R.; Winter, M.; Placke, T. A Step toward High-Energy Silicon-Based Thin Film Lithium-Ion Batteries. ACS Nano 2017, 11, 4731–4744. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, S.; Zhao, Y.; Shi, J.; Lv, L.; Wang, H.; Zhang, Z.; Feng, W. The Influence of Different Si: C Ratios on the Electrochemical Performance of Silicon/Carbon Layered Film Anodes for Lithium-Ion Batteries. RSC Adv. 2018, 8, 6660–6666. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Wang, X.; Wang, T.; Zheng, J.; Li, X. Intercalated Si/C Films as the Anode for Li-Ion Batteries with near Theoretical Stable Capacity Prepared by Dual Plasma Deposition. J. Power Sources 2013, 221, 242–246. [Google Scholar] [CrossRef]
- Korzhenko, D.V.; Yurjev, Y.N.; Emlin, D.R.; Plotnikov, S.A.; Vladimirov, A.B.; Romanov, I.Y.; Loginov, B.A.; Loginov, A.B. Comparative Analysis of Properties of the Carbon-Based Coatings Obtained through Various PVD and CVD Deposition Methods. J. Phys. Conf. Ser. 2020, 1443, 12006. [Google Scholar] [CrossRef]
- Windischmann, H. Intrinsic Stress in Sputter-Deposited Thin Films. Crit. Rev. Solid State Mater. Sci. 1992, 17, 547–596. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, N.; Han, Y.; Zhou, J.; Zhu, Y.; Du, J.; Qian, Y. Cu3Si@Si Core-Shell Nanoparticles Synthesized Using a Solid-State Reaction and Their Performance as Anode Materials for Lithium-Ion Batteries. Nanoscale 2015, 7, 15075–15079. [Google Scholar] [CrossRef]
- Ruttert, M.; Siozios, V.; Winter, M.; Placke, T. Mechanochemical Synthesis of Fe-Si-Based Anode Materials for High-Energy Lithium Ion Full-Cells. ACS Appl. Energy Mater. 2020, 3, 743–758. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, J.; Liu, Q.; Zhou, Q.; Liu, H.; Xu, C. Graphene Quantum Dots Modified Nanoporous SiAl Composite as an Advanced Anode for Lithium Storage. Electrochim. Acta 2019, 318, 228–235. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Zhu, X.; Xie, M.; Su, Y.; Hu, N.; Yang, Z.; Zhang, Y. Synthesis of Carbon Nanotubes on Graphene Quantum Dot Surface by Catalyst Free Chemical Vapor Deposition. Carbon 2014, 68, 399–405. [Google Scholar] [CrossRef]
- Li, K.; Kang, Y.; Deng, C.; Wang, Y.; Ba, H.; An, Q.; Han, X.; Huang, S. S, Se-Codoped Dual Carbon Coating and Se Substitution in Co-Alkoxide-Derived CoS2 through SeS2 Triggered Selenization for High-Performance Sodium-Ion Batteries. Batteries 2025, 11, 28. [Google Scholar] [CrossRef]
- Brubaker, Z.E.; Langford, J.J.; Kapsimalis, R.J.; Niedziela, J.L. Quantitative Analysis of Raman Spectral Parameters for Carbon Fibers: Practical Considerations and Connection to Mechanical Properties. J. Mater. Sci. 2021, 56, 15087–15121. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Tian, L.; Xiang, W.; Wang, T.; Hu, W.; Hu, Y.; Chen, S.; Chen, J.; Dai, Z. Synthesis of Graphene Quantum Dots from Natural Polymer Starch for Cell Imaging. Green Chem. 2018, 20, 4438–4442. [Google Scholar] [CrossRef]
- Sharma, M.; Rani, S.; Pathak, D.K.; Bhatia, R.; Kumar, R.; Sameera, I. Temperature Dependent Raman Modes of Reduced Graphene Oxide: Effect of Anharmonicity, Crystallite Size and Defects. Carbon 2021, 184, 437–444. [Google Scholar] [CrossRef]
- Yogi, P.; Tanwar, M.; Saxena, S.K.; Mishra, S.; Pathak, D.K.; Chaudhary, A.; Sagdeo, P.R.; Kumar, R. Quantifying the Short-Range Order in Amorphous Silicon by Raman Scattering. Anal. Chem. 2018, 90, 8123–8129. [Google Scholar] [CrossRef]
- Cao, W.; Chen, M.; Liu, Y.; Han, K.; Chen, X.; Ye, H.; Sang, S. C2H2O4 Etching of AlSi Alloy Powder: An Efficient and Mild Preparation Approach for High Performance Micro Si Anode. Electrochim. Acta 2019, 320, 134615. [Google Scholar] [CrossRef]
- Ye, Z.; Dong, J.; Jin, J.; Chen, Y.; Yang, W. The Effect of Annealing and Multilayer Structure on Promoting the Electrochemical Performance of Al/Si Thin Film Anodes. J. Electroanal. Chem. 2024, 963, 118304. [Google Scholar] [CrossRef]
- Shi, H.; Yuan, A.; Xu, J. Tailored Synthesis of Monodispersed Nano/Submicron Porous Silicon Oxycarbide (SiOC) Spheres with Improved Li-Storage Performance as an Anode Material for Li-Ion Batteries. J. Power Sources 2017, 364, 288–298. [Google Scholar] [CrossRef]
- He, W.; Tian, H.; Xin, F.; Han, W. Scalable Fabrication of Micro-Sized Bulk Porous Si from Fe-Si Alloy as a High-Performance Anode for Lithium-Ion Batteries. J. Mater. Chem. A 2015, 3, 17956–17962. [Google Scholar] [CrossRef]
- Parekh, M.H.; Sediako, A.D.; Naseri, A.; Thomson, M.J.; Pol, V.G. In Situ Mechanistic Elucidation of Superior Si-C-Graphite Li-Ion Battery Anode Formation with Thermal Safety Aspects. Adv. Energy Mater. 2020, 10, 1902799. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Wu, Z.-Y.; Li, J.-T.; Huang, L.; Sun, S.-G. 3D Nanostructured Multilayer Si/Al Film with Excellent Cycle Performance as Anode Material for Lithium-Ion Battery. J. Alloys Compd. 2016, 657, 559–564. [Google Scholar] [CrossRef]
- Nogales, P.M.; Lee, S.; Yang, S.; Yang, I.; Choi, S.H.; Park, S.-M.; Lee, J.H.; Kim, C.J.; An, J.-C.; Jeong, S.-K. Stabilizing the Solid Electrolyte Interphase of SiOx Negative Electrodes: The Role of Fluoroethylene Carbonate in Enhancing Electrochemical Performance. Batteries 2024, 10, 385. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not to Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Yang, X.; Rogach, A.L. Electrochemical Techniques in Battery Research: A Tutorial for Nonelectrochemists. Adv. Energy Mater. 2019, 9, 1900747. [Google Scholar] [CrossRef]
- Yu, P.; Li, C.; Guo, X. Sodium Storage and Pseudocapacitive Charge in Textured Li4Ti5O12 Thin Films. J. Phys. Chem. C 2014, 118, 10616–10624. [Google Scholar] [CrossRef]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive Contributions to Electrochemical Energy Storage in TiO2 (Anatase) Nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Klingler, R.J.; Kochi, J.K. Electron-Transfer Kinetics from Cyclic Voltammetry. Quantitative Description of Electrochemical Reversibility. J. Phys. Chem. 1981, 85, 1731–1741. [Google Scholar]
- Sun, X.; Luo, Y.; Li, X.; Wang, Y.; Lin, S.; Ding, W.; Guo, K.; Zhang, K.; Qin, A. Pompon Mum-like SiO2/C Nanospheres with High Performance as Anodes for Lithium-Ion Batteries. Batteries 2024, 10, 149. [Google Scholar] [CrossRef]
- Pu, X.; Zhao, D.; Fu, C.; Chen, Z.; Cao, S.; Wang, C.; Cao, Y. Understanding and Calibration of Charge Storage Mechanism in Cyclic Voltammetry Curves. Angew. Chem. Int. Ed. 2021, 60, 21310–21318. [Google Scholar] [CrossRef]
- Gaberšček, M. Understanding Li-Based Battery Materials via Electrochemical Impedance Spectroscopy. Nat. Commun. 2021, 12, 6513. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Q.; Luo, M.; Guo, Q.; Yu, G.; Deng, S.; Xu, Z.; Yang, B.; Liang, X. Electrochemical Oxidation of Environmentally Persistent Perfluorooctane Sulfonate by a Novel Lead Dioxide Anode. Electrochim. Acta 2016, 213, 358–367. [Google Scholar] [CrossRef]
- Liu, H.; Ren, L.; Li, J.; Tuo, H. Iron-Assisted Carbon Coating Strategy for Improved Electrochemical LiMn0.8Fe0.2PO4 Cathodes. Electrochim. Acta 2016, 212, 800–807. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, J.; Qiao, Y.; Li, H.; Wang, J.; Wu, M. Fabrication and Characterization of Porous Si-al Films Anode with Different Macroporous Substrates for Lithium-Ion Batteries. J. Solid State Electrochem. 2014, 18, 1799–1806. [Google Scholar] [CrossRef]
- Li, W.; Ma, Q.; Liu, X.; Chen, A.; Wang, J.-H.; Min, D.H.; Xiong, P.; Liu, M.; Park, H.S. Enhanced Reaction Kinetics Enabled by a Bi-Element Co-Doping Strategy for High-Performance Ternary Si-Based Anodes of Li-Ion Batteries. Chem. Eng. J. 2023, 453, 139567. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, P.; Ma, R.; Gao, M.; Pan, H. Electrochemical Properties of the Ternary Alloy Li5AlSi2 Synthesized by Reacting LiH, al and Si as an Anodic Material for Lithium-Ion Batteries. J. Power Sources 2015, 283, 54–60. [Google Scholar] [CrossRef]
- Cen, Y.; Fan, Y.; Qin, Q.; Sisson, R.D.; Apelian, D.; Liang, J. Synthesis of Si Anode with a Microsized-Branched Structure from Recovered al Scrap for Use in Li-Ion Batteries. J. Power Sources 2019, 410–411, 31–37. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, J.; Lv, X.; Yang, W. Gyrification Structure of Si-Al Thin Film Anodes with High-Rate Performance. Mater. Sci. Semicond. Process. 2022, 151, 106981. [Google Scholar] [CrossRef]
- Hwang, G.; Park, H.; Bok, T.; Choi, S.; Lee, S.; Hwang, I.; Choi, N.-S.; Seo, K.; Park, S. A High-Performance Nanoporous Si/Al2O3 Foam Lithium-Ion Battery Anode Fabricated by Selective Chemical Etching of the Al-Si Alloy and Subsequent Thermal Oxidation. Chem. Commun. 2015, 51, 4429–4432. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Chen, Y.-L.; Chen, Y.-S.; Huang, S.-M.; Huang, H.-M.; Lin, S.-J.; Yang, C.-Y. Utilization of Si/SiOx/Al2O3 Materials from Recycled Solar Cells for a High-Performance Lithium-Ion Battery Anode. Green Chem. 2022, 24, 5151–5161. [Google Scholar] [CrossRef]



| Sample | C Atomic Ratio/% | Si Atomic Ratio/% | Al Atomic Ratio/% |
|---|---|---|---|
| CF-Si/Al | 25.13 | 69.71 | 5.16 |
| CF-Si/Al@C-500-1h | 43.62 | 52.00 | 4.38 |
| Sample | RΩ (Ω) | Rct (Ω) | σ | DLi+ (cm2/s) |
|---|---|---|---|---|
| CF-Si | 12.52 | 155.5 | 4296.67 | 8.52 × 10−13 |
| CF-Si/Al | 46.32 | 98.23 | 488.38 | 8.51 × 10−11 |
| CF-Si/Al@C-500-1h | 5.709 | 45.57 | 317.18 | 2.01 × 10−10 |
| CF-Si after cycling | 4.647 | 67.18 | 429.80 | 8.08 × 10−11 |
| CF-Si/Al after cycling | 45.04 | 136.5 | 667.77 | 3.35 × 10−12 |
| CF-Si/Al@C-500-1h after cycling | 5.109 | 28.62 | 34.40 | 1.26 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, L.; Li, P.; Ouyang, M.; Gao, S.; Liang, K. Carbon-Coated CF-Si/Al Anodes for Improved Lithium-Ion Battery Performance. Batteries 2025, 11, 114. https://doi.org/10.3390/batteries11030114
Zeng L, Li P, Ouyang M, Gao S, Liang K. Carbon-Coated CF-Si/Al Anodes for Improved Lithium-Ion Battery Performance. Batteries. 2025; 11(3):114. https://doi.org/10.3390/batteries11030114
Chicago/Turabian StyleZeng, Liangliang, Peng Li, Mi Ouyang, Shujuan Gao, and Kun Liang. 2025. "Carbon-Coated CF-Si/Al Anodes for Improved Lithium-Ion Battery Performance" Batteries 11, no. 3: 114. https://doi.org/10.3390/batteries11030114
APA StyleZeng, L., Li, P., Ouyang, M., Gao, S., & Liang, K. (2025). Carbon-Coated CF-Si/Al Anodes for Improved Lithium-Ion Battery Performance. Batteries, 11(3), 114. https://doi.org/10.3390/batteries11030114









