Tailoring Two-Dimensional NiFeCo-Layered Double Hydroxide onto One-Dimensional N-Doped CNTs for High-Performance Bifunctional Air Electrodes in Flexible Zinc–Air Batteries
Abstract
:1. Introduction
2. Materials and Methods
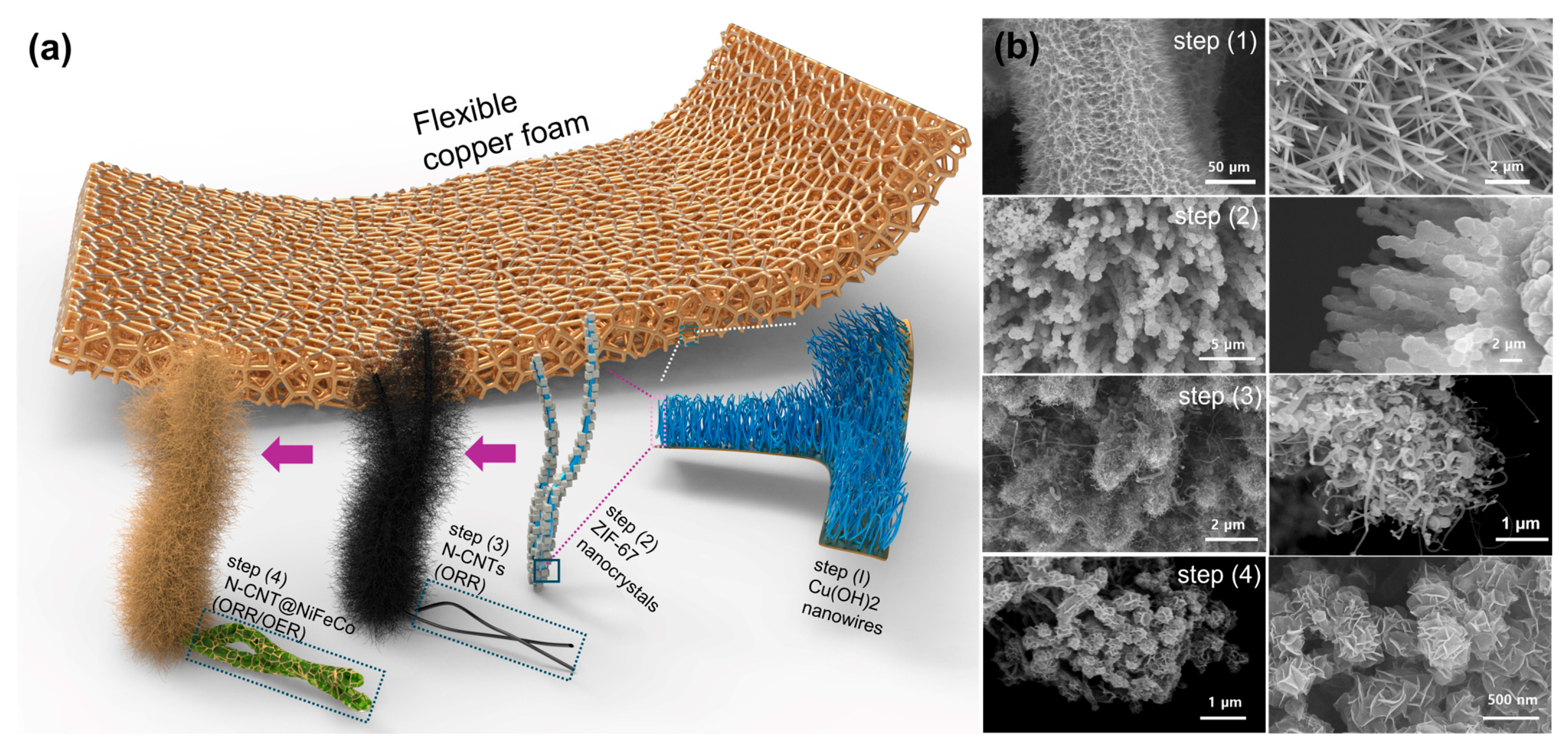
2.1. Synthesis of Cu(OH)2-Coated Copper Foam
2.2. Growth of N-Doped CNTs on Copper Foam
2.3. Electrodeposition of NiFeCo LDH Nanosheets
2.4. Electrochemical Analysis
2.5. Assembly of Quasi-Solid-State Zinc–Air Batteries
2.6. Characterization Techniques
3. Results
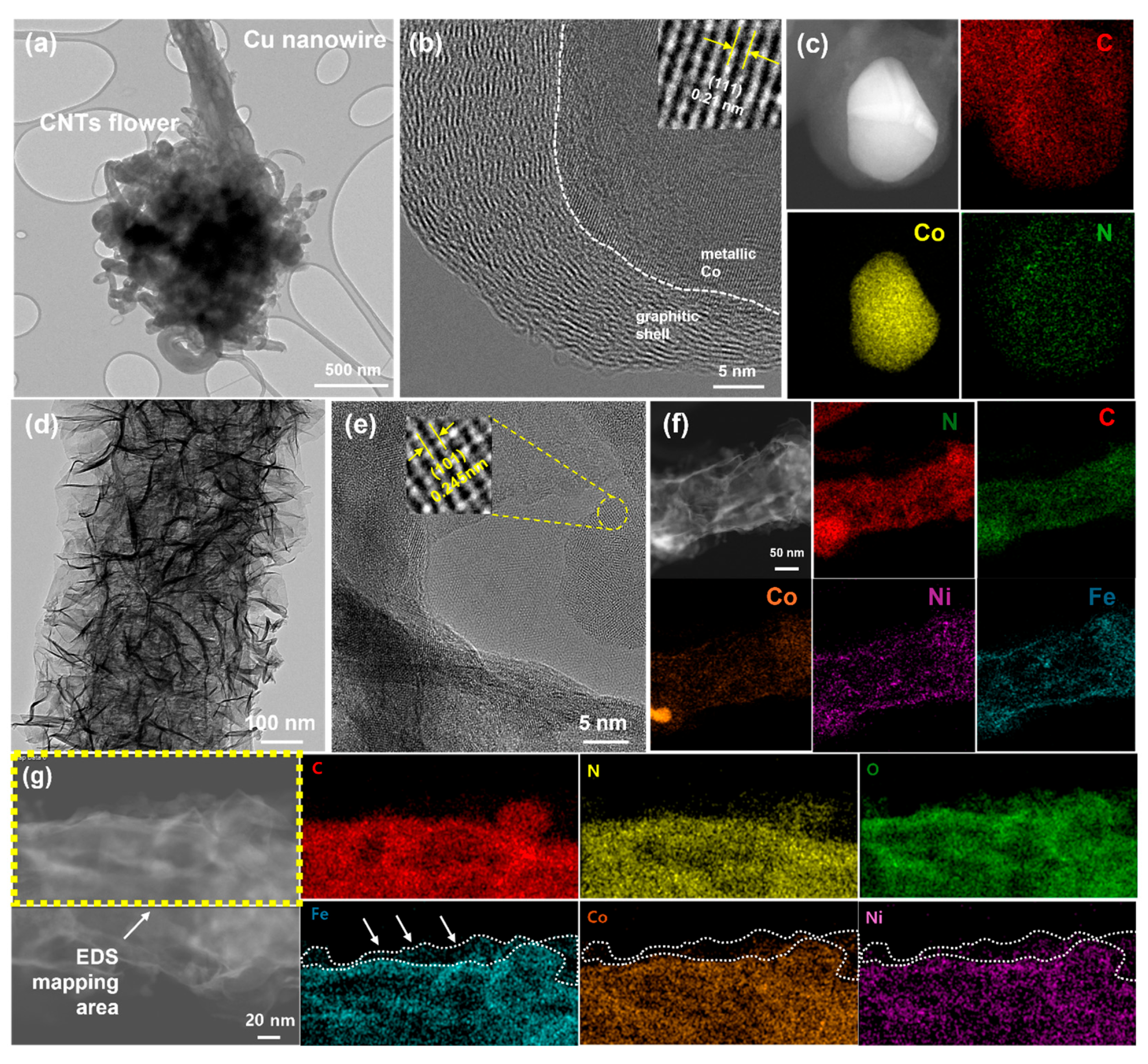
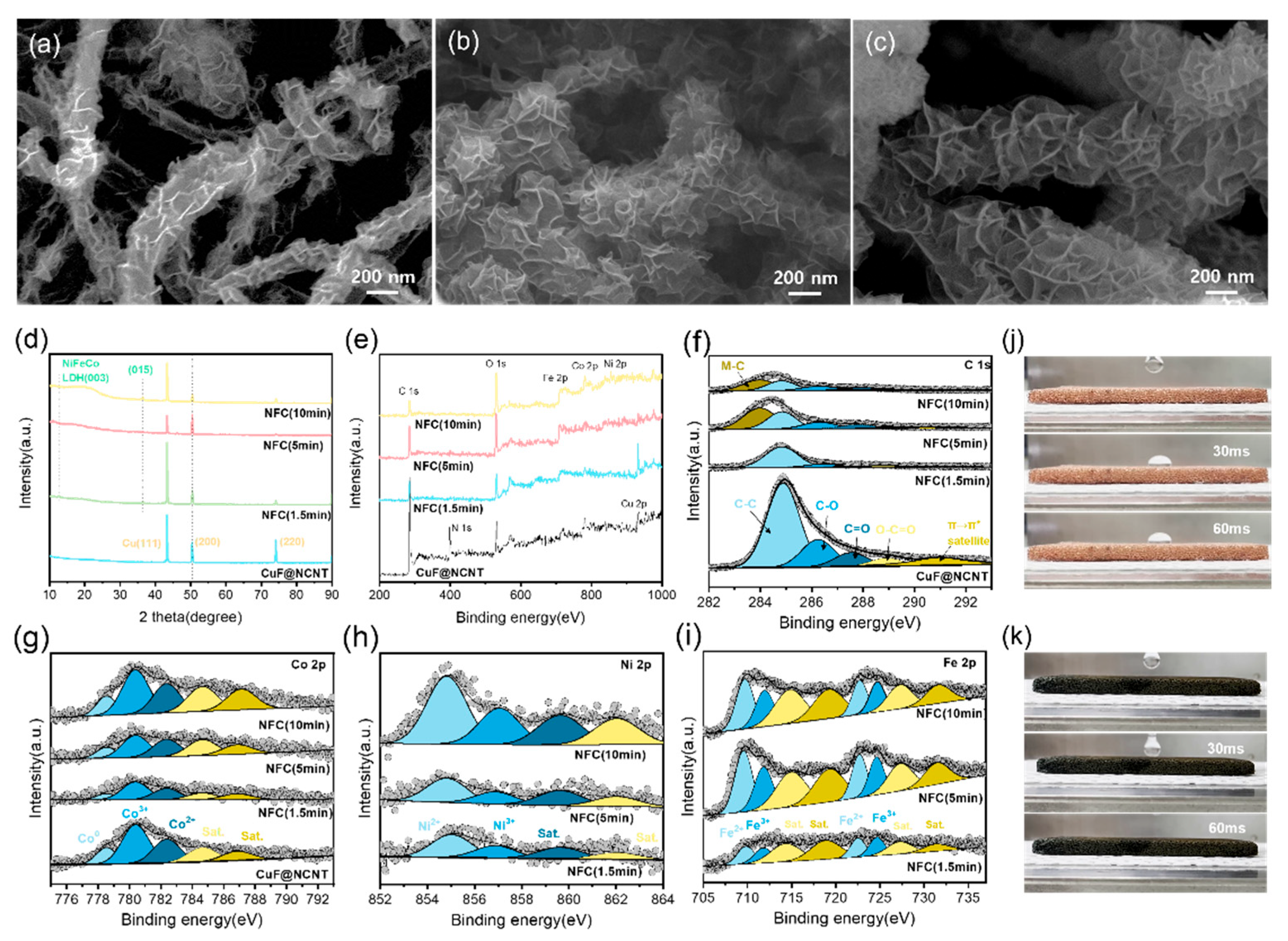
3.1. Structural and Morphological Characterization of Copper Foam-Based Electrodes
3.2. Chemical Composition and Surface Characteristics of CuF-Based Electrodes
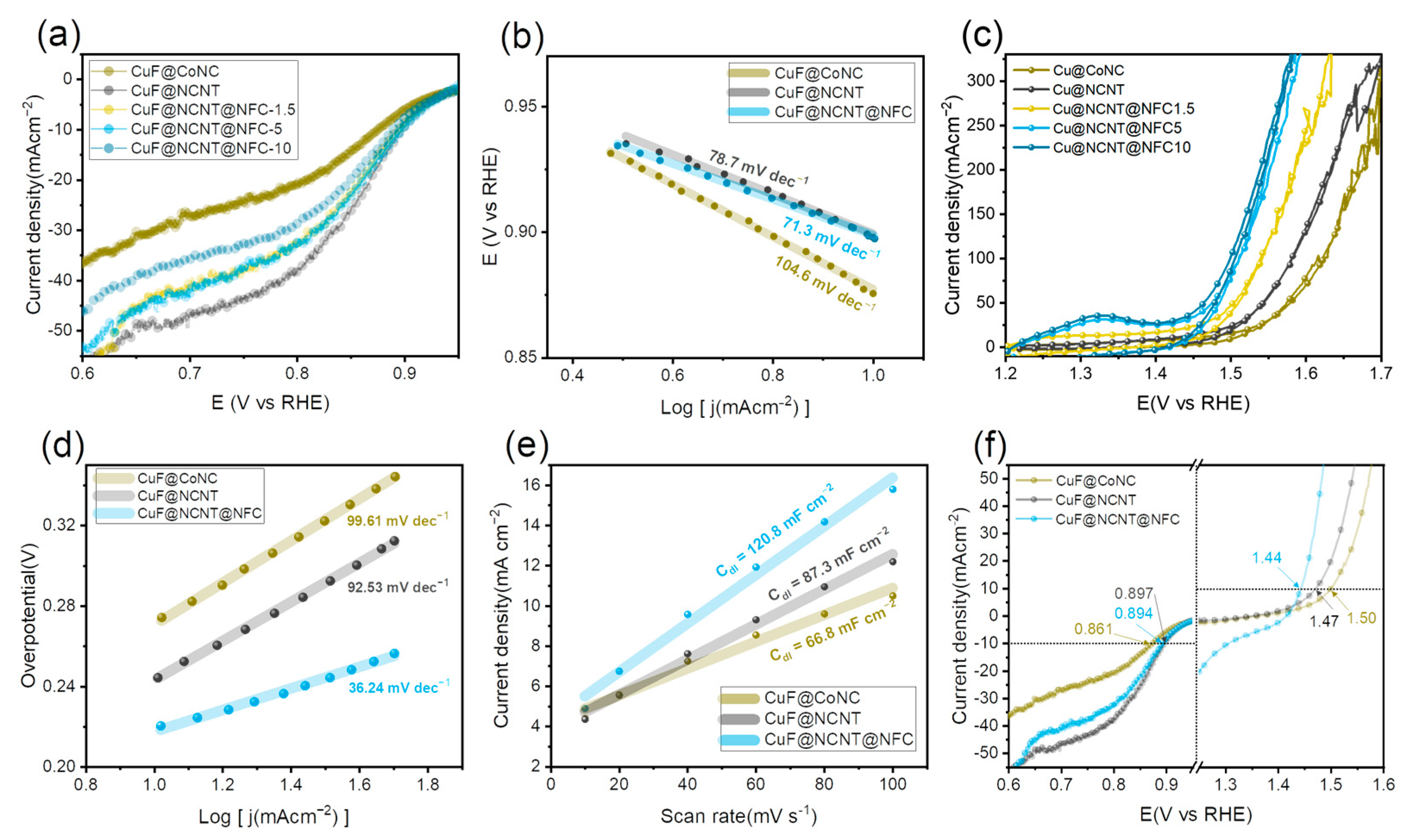
3.3. Electrochemical Performance Evaluation
3.4. Fabrication and Electrochemical Performance of Pouch-Type Solid-State Zinc–Air Batteries
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ZAB | Zinc–air battery |
| CuF | Copper foam |
| CNT | Carbon nanotube |
| N-CNT | Nitrogen-doped carbon nanotube |
| NFC | NiFeCo hydroxide |
| LDH | Layered double hydroxide |
| ORR | Oxygen reduction reaction |
| OER | Oxygen evolution reaction |
| PMMA | Poly(methyl methacrylate) |
| ECSA | Electrochemical surface area |
| LSV | Linear sweep voltammetry |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| FE-SEM | Field-emission scanning electron microscopy |
| HR-TEM | High-resolution transmission electron microscopy |
| STEM | Scanning transmission electron microscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
References
- Zhao, C.X.; Liu, J.N.; Wang, J.; Ren, D.; Li, B.Q.; Zhang, Q. Recent advances of noble-metal-free bifunctional oxygen reduction and evolution electrocatalysts. Chem. Soc. Rev. 2021, 50, 7745–7778. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Zheng, M.B.; Zhao, Q.X.; Xiao, X.; Xue, H.G.; Pang, H. Rechargeable zinc-air batteries: A promising way to green energy. J. Mater. Chem. A 2017, 5, 7651–7666. [Google Scholar] [CrossRef]
- Zhu, Z.X.; Jiang, T.L.; Ali, M.; Meng, Y.H.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef] [PubMed]
- Mainar, A.R.; Iruin, E.; Colmenares, L.C.; Kvasha, A.; de Meatza, I.; Bengoechea, M.; Leonet, O.; Boyano, I.; Zhang, Z.C.; Blazquez, J.A. An overview of progress in electrolytes for secondary zinc-air batteries and other storage systems based on zinc. J. Energy Storage 2018, 15, 304–328. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Yang, X.Y.; Liu, T.; Zhang, X.B. Flexible 1D Batteries: Recent Progress and Prospects. Adv. Mater. 2020, 32, 1901961. [Google Scholar] [CrossRef]
- Tan, P.; Chen, B.; Xu, H.R.; Zhang, H.C.; Cai, W.Z.; Ni, M.; Liu, M.L.; Shao, Z.P. Flexible Zn- and Li-air batteries: Recent advances, challenges, and future perspectives. Energy Environ. Sci. 2017, 10, 2056–2080. [Google Scholar] [CrossRef]
- Liu, Q.C.; Chang, Z.W.; Li, Z.J.; Zhang, X.B. Flexible Metal-Air Batteries: Progress, Challenges, and Perspectives. Small Methods 2018, 2, 1700231. [Google Scholar] [CrossRef]
- Wang, S.G.; Qin, J.W.; Meng, T.; Cao, M.H. Metal-organic framework-induced construction of actiniae-like carbon nanotube assembly as advanced multifunctional electrocatalysts for overall water splitting and Zn-air batteries. Nano Energy 2017, 39, 626–638. [Google Scholar] [CrossRef]
- Chang, H.; Guo, Y.F.; Liu, X.; Wang, P.F.; Xie, Y.; Yi, T.F. Dual MOF-derived Fe/N/P-tridoped carbon nanotube as high-performance oxygen reduction catalysts for zinc-air batteries. Appl. Catal. B-Environ. Energy 2023, 327, 122469. [Google Scholar] [CrossRef]
- Liu, X.; Liu, W.; Ko, M.; Park, M.; Kim, M.G.; Oh, P.; Chae, S.; Park, S.; Casimir, A.; Wu, G.; et al. Metal (Ni, Co)-Metal Oxides/Graphene Nanocomposites as Multifunctional Electrocatalysts. Adv. Funct. Mater. 2015, 25, 5799–5808. [Google Scholar] [CrossRef]
- Tang, C.; Wang, B.; Wang, H.F.; Zhang, Q. Defect Engineering toward Atomic Co-Nx-C in Hierarchical Graphene for Rechargeable Flexible Solid Zn-Air Batteries. Adv. Mater. 2017, 29, 1703185. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.B.; Miao, J.W.; Hung, S.F.; Chen, J.Z.; Tao, H.B.; Wang, X.Z.; Zhang, L.P.; Chen, R.; Gao, J.J.; Chen, H.M.; et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: Development of highly efficient metal-free bifunctional electrocatalyst. Sci. Adv. 2016, 2, 1501122. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Liu, Y.L.; Zhao, F.P.; Nie, K.Q.; Han, N.; Wang, X.X.; Huang, W.J.; Song, X.N.; Zhong, J.; Li, Y.G. Metallic Cobalt Nanoparticles Encapsulated in Nitrogen-Enriched Graphene Shells: Its Bifunctional Electrocatalysis and Application in Zinc-Air Batteries. Adv. Funct. Mater. 2016, 26, 4397–4404. [Google Scholar] [CrossRef]
- Wang, Z.; Ang, J.M.; Liu, J.; Ma, X.Y.D.; Kong, J.H.; Zhang, Y.F.; Yan, T.; Lu, X.H. FeNi alloys encapsulated in N-doped CNTs-tangled porous carbon fibers as highly efficient and durable bifunctional oxygen electrocatalyst for rechargeable zinc-air battery. Appl. Catal. B-Environ. 2020, 263, 118344. [Google Scholar] [CrossRef]
- Hao, X.Q.; Jiang, Z.Q.; Zhang, B.A.; Tian, X.N.; Song, C.S.; Wang, L.K.; Maiyalagan, T.; Hao, X.G.; Jiang, Z.J. N-Doped Carbon Nanotubes Derived from Graphene Oxide with Embedment of FeCo Nanoparticles as Bifunctional Air Electrode for Rechargeable Liquid and Flexible All-Solid-State Zinc-Air Batteries. Adv. Sci. 2021, 8, 2004572. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Y.-P.; Liang, R.; Fu, J.; Luo, D.; Liu, G.; Li, J.; Zhang, Z.; Hu, Y.; Chen, Z. Multidimensional Ordered Bifunctional Air Electrode Enables Flash Reactants Shuttling for High-Energy Flexible Zn-Air Batteries. Adv. Energy Mater. 2019, 9, 1900911. [Google Scholar] [CrossRef]
- Jin, Q.; Ren, B.; Cui, H.; Wang, C. Nitrogen and cobalt co-doped carbon nanotube films as binder-free trifunctional electrode for flexible zinc-air battery and self-powered overall water splitting. Appl. Catal. B-Environ. 2021, 283, 119643. [Google Scholar] [CrossRef]
- Tian, W.-W.; Ren, J.-T.; Yuan, Z.-Y. In-situ cobalt-nickel alloy catalyzed nitrogen-doped carbon nanotube arrays as superior freestanding air electrodes for flexible zinc-air and aluminum-air batteries. Appl. Catal. B-Environ. 2022, 317, 121764. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Ma, L.; Xue, Y.; Liu, Z.-H.; Cui, H.; Zhang, N.; Jiang, R. Atomically Dispersed Fe–N4 Sites and NiFe-LDH Sub-Nanoclusters as an Excellent Air Cathode for Rechargeable Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2023, 15, 16732–16743. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Cui, Z.; Li, G.; Han, B.; Zhang, Q.; Sui, J.; Dong, H.; Yu, J.; Yu, L.; et al. Dual-active-site hierarchical architecture containing NiFe-LDH and ZIF-derived carbon-based framework composite as efficient bifunctional oxygen electrocatalysts for durable rechargeable Zn-air batteries. Chem. Eng. J. 2020, 399, 125718. [Google Scholar] [CrossRef]
- Chang, F.; Du, H.; Su, P.; Sun, Y.; Ye, R.; Tian, Q.; Zhang, G.; Li, H.; Liu, J. Facile Synthesis of Vertical Layered Double Hydroxides Nanosheets on Co@Carbon Nanoframes as Robust Bifunctional Oxygen Electrocatalysts for Rechargeable Zn–Air Batteries. Small Struct. 2024, 5, 2300111. [Google Scholar] [CrossRef]
- Wang, W.-H.; Han, C.-H.; Hong, W.-X.; Chiu, Y.-C.; Tseng, I.H.; Chang, Y.-H.; Pourzolfaghar, H.; Li, Y.-Y. NiFe layered double hydroxide (LDH) anchored, Fe single atom and nanoparticle embedded on nitrogen-doped carbon-CNT (carbon nanotube) framework as a bifunctional catalyst for rechargeable zinc-air batteries. J. Energy Storage 2024, 85, 111058. [Google Scholar] [CrossRef]
- Allwyn, N.; Gokulnath, S.; Sathish, M. In-Situ Nanoarchitectonics of Fe/Co LDH over Cobalt-Enriched N-Doped Carbon Cookies as Facile Oxygen Redox Electrocatalysts for High-Rate Rechargeable Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2024, 16, 20360–20374. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Luo, D.; Xie, L.; Yu, F.; Bao, J.; Li, Y.; Yu, Y.; et al. Hierarchical Cu@CoFe layered double hydroxide core-shell nanoarchitectures as bifunctional electrocatalysts for efficient overall water splitting. Nano Energy 2017, 41, 327–336. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Li, M.; Xiong, Y.; Liu, X.; Han, C.; Zhang, Y.; Bo, X.; Guo, L. Iron and nitrogen co-doped carbon nanotube@hollow carbon fibers derived from plant biomass as efficient catalysts for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9658–9667. [Google Scholar] [CrossRef]
- Park, Y.-E.; Park, S.-H.; Ahn, S.H. Tissue-derived highly compressible anisotropic carbon aerogels with aligned fibrous matrices for solid-state rechargeable zinc-cobalt-air hybrid batteries. EcoMat 2024, 6, e12431. [Google Scholar] [CrossRef]
- Son, H.J.; Cho, Y.R.; Park, Y.-E.; Ahn, S.H. Flexible, compressible, versatile biomass-derived freestanding carbon monoliths as binder- and substrate-free tri-functional electrodes for solid-state zinc-air batteries and overall water splitting. Appl. Catal. B-Environ. 2022, 304, 120977. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Li, Z.; Chen, W.; Xu, Q.; He, D.; Xi, D.; Zhang, Q.; Yuan, T.; Qu, Y.; et al. Solid-Diffusion Synthesis of Single-Atom Catalysts Directly from Bulk Metal for Efficient CO2 Reduction. Joule 2019, 3, 584–594. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, C.; Qu, Y.; Zhou, H.; Zhou, F.; Wang, J.; Wu, Y.; Li, Y. Trifunctional Self-Supporting Cobalt-Embedded Carbon Nanotube Films for ORR, OER, and HER Triggered by Solid Diffusion from Bulk Metal. Adv. Mater. 2019, 31, 1808043. [Google Scholar] [CrossRef]
- Zhou, Z.; Lu, J.; Zhang, R.; Cao, Y.; Li, H. Construction and differential growth mechanism of uniform and controllable CNTs and CNWs by ZIF-67 precursor. Appl. Surf. Sci. 2023, 640, 158342. [Google Scholar] [CrossRef]
- Milikić, J.; Nastasić, A.; Knežević, S.; Rakočević, L.; Stojadinović, S.; Stanković, D.; Šljukić, B. Efficient nano-size ZnM/rGO (M = Ni, Cu, and Fe) electrocatalysts for oxygen electrode reactions in alkaline media. Int. J. Hydrogen Energy 2025, 97, 247–258. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Z.; Zou, Y.; Kuang, J.; Ye, D.; Zhang, S.; Gao, Q.; Yang, S.; Cai, X.; Fang, Y. Interface reinforced 2D/2D heterostructure of Cu-Co oxides/FeCo hydroxides as monolithic multifunctional catalysts for rechargeable/flexible zinc-air batteries and self-powered water splitting. Appl. Catal. B-Environ. 2023, 325, 122332. [Google Scholar] [CrossRef]
- Moloudi, M.; Noori, A.; Rahmanifar, M.S.; Shabangoli, Y.; El-Kady, M.F.; Mohamed, N.B.; Kaner, R.B.; Mousavi, M.F. Layered Double Hydroxide Templated Synthesis of Amorphous NiCoFeB as a Multifunctional Electrocatalyst for Overall Water Splitting and Rechargeable Zinc–Air Batteries. Adv. Energy Mater. 2023, 13, 2203002. [Google Scholar] [CrossRef]
- Li, Q.; Sun, Z.; Yin, C.; Chen, Y.; Pan, D.; Yu, B.; Zhang, Y.; He, T.; Chen, S. Template-assisted synthesis of ultrathin graphene aerogels as bifunctional oxygen electrocatalysts for water splitting and alkaline/neutral zinc-air batteries. Chem. Eng. J. 2023, 458, 141492. [Google Scholar] [CrossRef]
- Sun, J.; Leng, P.; Xie, Y.; Yu, X.; Qu, K.; Feng, L.; Bao, H.; Luo, F.; Yang, Z. Co single atoms and Co nanoparticle relay electrocatalyst for rechargeable zinc air batteries. Appl. Catal. B-Environ. 2022, 319, 121905. [Google Scholar] [CrossRef]
- Cai, X.; Jiang, T.; Wu, M. Confined growth of NiFe LDH with hierarchical structures on copper nanowires for long-term stable rechargeable Zn-air batteries. Appl. Surf. Sci. 2022, 577, 151911. [Google Scholar] [CrossRef]
- Zhang, G.W.; Zeng, J.R.; Yin, J.; Zuo, C.Y.; Wen, P.; Chen, H.T.; Qiu, Y.J. Iron-facilitated surface reconstruction to in-situ generate nickel-iron oxyhydroxide on self-supported FeNi alloy fiber paper for efficient oxygen evolution reaction. Appl. Catal. B-Environ. 2021, 286, 119902. [Google Scholar] [CrossRef]
- Wang, Q.; Feng, Q.; Lei, Y.; Tang, S.; Xu, L.; Xiong, Y.; Fang, G.; Wang, Y.; Yang, P.; Liu, J.; et al. Quasi-solid-state Zn-air batteries with an atomically dispersed cobalt electrocatalyst and organohydrogel electrolyte. Nat. Commnun. 2022, 13, 3689. [Google Scholar] [CrossRef]
- Yang, H.; Gao, S.; Rao, D.; Yan, X. Designing superior bifunctional electrocatalyst with high-purity pyrrole-type CoN4 and adjacent metallic cobalt sites for rechargeable Zn-air batteries. Energy Storage Mater. 2022, 46, 553–562. [Google Scholar] [CrossRef]
- Xu, N.N.; Wilson, J.A.; Wang, Y.D.; Su, T.S.; Wei, Y.N.; Qiao, J.L.; Zhou, X.D.; Zhang, Y.X.; Sun, S.H. Flexible self-supported bi-metal electrode as a highly stable carbon- and binder-free cathode for large-scale solid-state zinc-air batteries. Appl. Catal. B-Environ. 2020, 272, 118953. [Google Scholar] [CrossRef]
- Liu, T.; Mou, J.R.; Wu, Z.P.; Lv, C.; Huang, J.L.; Liu, M.L. A Facile and Scalable Strategy for Fabrication of Superior Bifunctional Freestanding Air Electrodes for Flexible Zinc-Air Batteries. Adv. Funct. Mater. 2020, 30, 2003407. [Google Scholar] [CrossRef]
- Yang, L.; Shi, L.; Wang, D.; Lv, Y.; Cao, D. Single-atom cobalt electrocatalysts for foldable solid-state Zn-air battery. Nano Energy 2018, 50, 691–698. [Google Scholar] [CrossRef]
- Ma, L.T.; Chen, S.M.; Pei, Z.X.; Huang, Y.; Liang, G.J.; Mo, F.N.; Yang, Q.; Su, J.; Gao, Y.H.; Zapien, J.A.; et al. Single-Site Active Iron-Based Bifunctional Oxygen Catalyst for a Compressible and Rechargeable Zinc-Air Battery. ACS Nano 2018, 12, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, L.; Yuan, Y.; Zhong, L.; Chen, Z.; Chi, X.; Lu, H.; Chen, Z.; Zou, R.; Li, T.; et al. An Iron-Decorated Carbon Aerogel for Rechargeable Flow and Flexible Zn-Air Batteries. Adv. Mater. 2020, 32, 2002292. [Google Scholar] [CrossRef]
- Zhong, X.; Zheng, Z.; Xu, J.; Xiao, X.; Sun, C.; Zhang, M.; Ma, J.; Xu, B.; Yu, K.; Zhang, X.; et al. Flexible Zinc–Air Batteries with Ampere-Hour Capacities and Wide-Temperature Adaptabilities. Adv. Mater. 2023, 35, 2209980. [Google Scholar] [CrossRef]
- Ge, B.; Hu, L.; Yu, X.; Wang, L.; Fernandez, C.; Yang, N.; Liang, Q.; Yang, Q.-H. Engineering Triple-Phase Interfaces around the Anode toward Practical Alkali Metal–Air Batteries. Adv. Mater. 2024, 36, 2400937. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-W.; Lee, A.; Ahn, S.H. Tailoring Two-Dimensional NiFeCo-Layered Double Hydroxide onto One-Dimensional N-Doped CNTs for High-Performance Bifunctional Air Electrodes in Flexible Zinc–Air Batteries. Batteries 2025, 11, 155. https://doi.org/10.3390/batteries11040155
Kim Y-W, Lee A, Ahn SH. Tailoring Two-Dimensional NiFeCo-Layered Double Hydroxide onto One-Dimensional N-Doped CNTs for High-Performance Bifunctional Air Electrodes in Flexible Zinc–Air Batteries. Batteries. 2025; 11(4):155. https://doi.org/10.3390/batteries11040155
Chicago/Turabian StyleKim, Yeon-Woo, Ayeon Lee, and Sung Hoon Ahn. 2025. "Tailoring Two-Dimensional NiFeCo-Layered Double Hydroxide onto One-Dimensional N-Doped CNTs for High-Performance Bifunctional Air Electrodes in Flexible Zinc–Air Batteries" Batteries 11, no. 4: 155. https://doi.org/10.3390/batteries11040155
APA StyleKim, Y.-W., Lee, A., & Ahn, S. H. (2025). Tailoring Two-Dimensional NiFeCo-Layered Double Hydroxide onto One-Dimensional N-Doped CNTs for High-Performance Bifunctional Air Electrodes in Flexible Zinc–Air Batteries. Batteries, 11(4), 155. https://doi.org/10.3390/batteries11040155







