Water-in-Salt Electrolytes: Advances and Chemistry for Sustainable Aqueous Monovalent-Metal-Ion Batteries
Abstract
1. Introduction
1.1. Monovalent-Metal-Ion Batteries
1.2. Electrolytes
1.2.1. Non-Aqueous Electrolytes
1.2.2. Aqueous Electrolytes
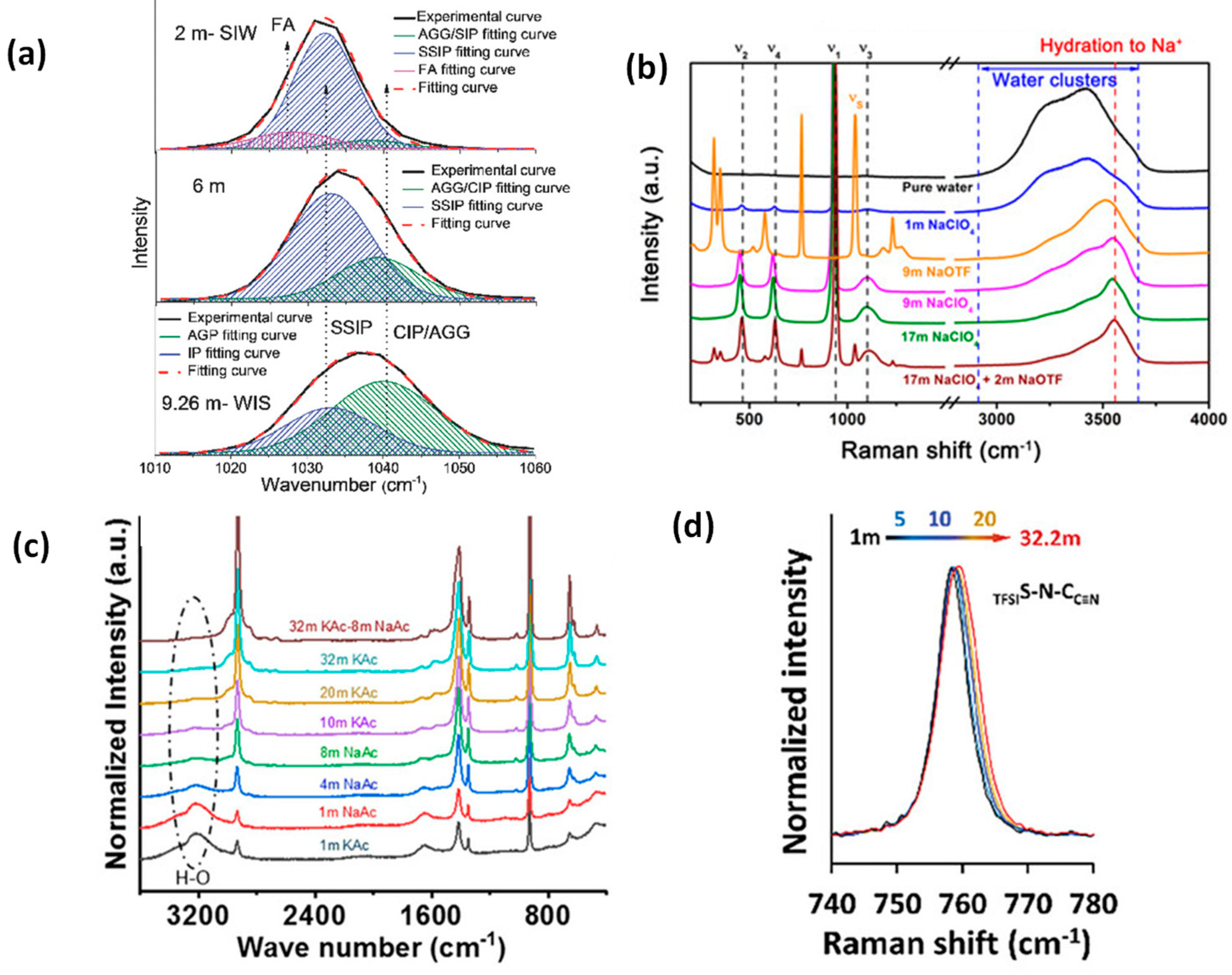
Water-in-Salt Electrolytes
2. AMIB Components and Compatibility with WiSEs
2.1. Development of WiSEs
2.1.1. Lithium-Ion Batteries (LIBs)
2.1.2. Sodium-Ion Batteries (SIBs)
2.1.3. Potassium-Ion Batteries (PIBs)
2.1.4. Trends in WiSEs
2.2. Electrode Materials
2.2.1. Lithium-Ion Batteries (LIBs)
2.2.2. Sodium-Ion Batteries (SIBs)
2.2.3. Potassium-Ion Batteries (PIBs)
| Battery | Cathode | Anode | Cell Voltage (V) | Stability (cycles/%retention/ c rate) | Energy Density (kWh kg−1) | Ref. |
|---|---|---|---|---|---|---|
| LIB | LiMn2O4 | Mo6S8 | 2.3 | 1000/68/4.5 | 84 | [34] |
| LiMn2O4 | TiO2 | 2.5 | 40/91.2 | 100 | [58] | |
| LiFePO4 | Mo6S8 | - | 1000/99/1 | 47 | [126] | |
| LiMn2O4 | c-TiO2 | 2.5 | 55/90 | [20] | ||
| LiNi0.5Mn1.5O4 | Mo6S8 | 2.9 | 0.075% decay per cycle (5 C) | 80 | [127] | |
| LiMn2O4 | Li4Ti5O12 | 2.5 | 150/100/0.2 | 145 | [106] | |
| LiCoO2 | Mo6S8 | 2.5 | 0.013% decay per cycle (1000) | 120 | [50] | |
| SIB | Na2VTi(PO4)3 | Na2VTi(PO4)3 | - | No decay (1000 cycles, 20 C) | [124] | |
| Na0.66[Mn0.66Ti0.34]O2 | NaTi2(PO4)3 | 1 | 0.006% decay per cycle (1200 cycles, 1 C) | 31 | [80] | |
| Na1.88Mn[Fe(CN)6]0.97·1.35H2O | NaTiOPO4 | 1.74 | 200/90/0.25 | 71 | [101] | |
| Na4Fe3(PO4)2(P2O7) | NaTi2(PO4)3 | 1.08 | 200/75/1 | 36 | [100] | |
| PIB | KxFeyMn1 y[Fe(CN)6]w·zH2O | 3,4,9,10-perylenetetracarboxylic diimide | 1.7 | 500/88/1 | 80 | [104] |
| δ-K0.5V2O5 | 3,4,9,10-perylenetetracarboxylic diimide | 20,000/77.3/10 | 77.3 | [128] |
2.3. Current Collectors
2.4. Binders
2.5. Separators
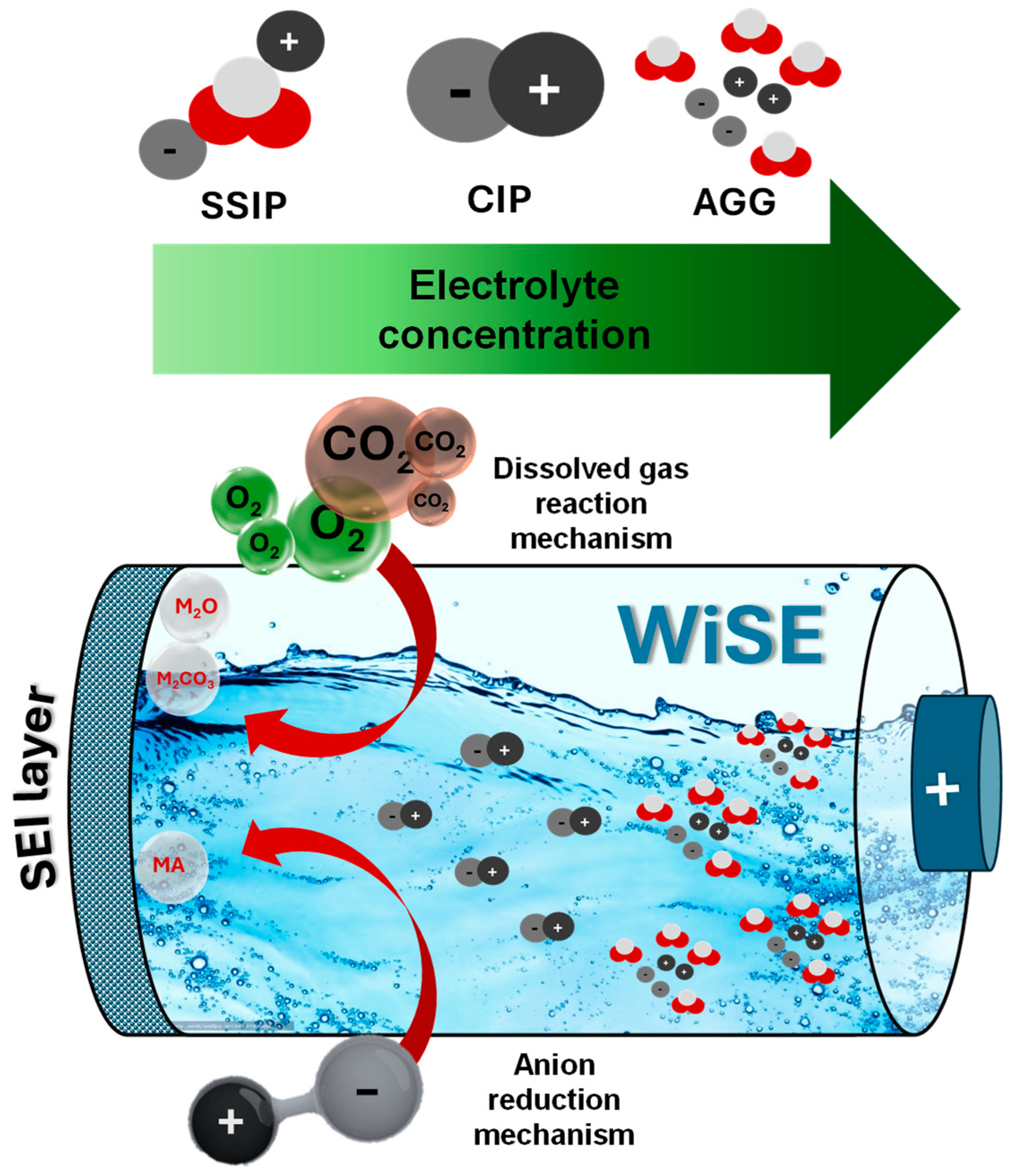
3. The Function of WiSEs in AMIBs
3.1. Anion Reduction Mechanism
3.2. Dissolved Gas Reaction Mechanism
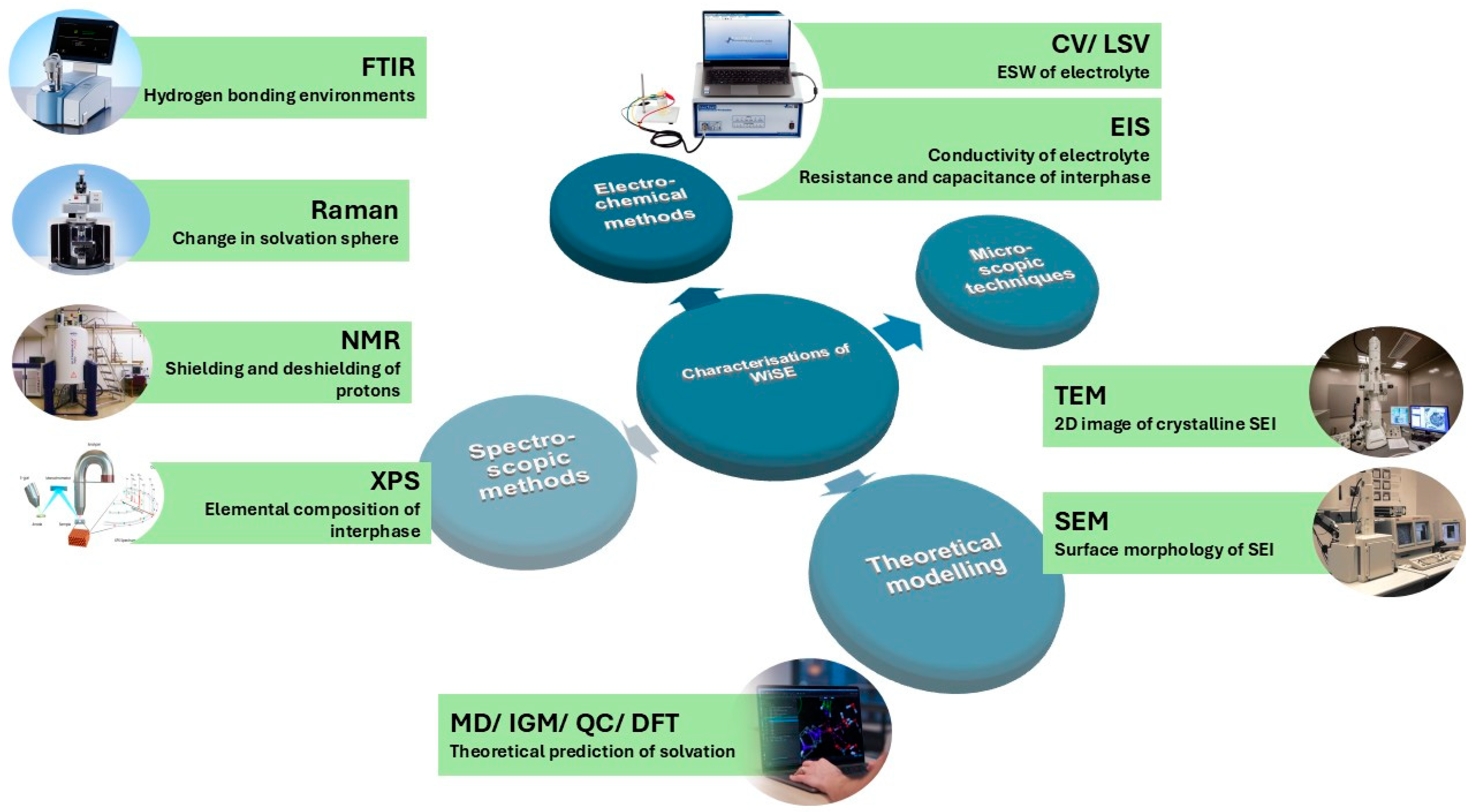
4. Characterization Techniques for WiSEs
4.1. Preliminary Testing
4.2. Characterization of Solvation
4.2.1. Theoretical Calculations
4.2.2. Spectroscopic Analysis of Solvation
4.3. SEI Composition and Properties
4.3.1. Spectroscopic Analysis of the SEI
4.3.2. Microscopic Studies of SEI Morphology
4.3.3. Electrochemical Techniques
5. Beyond WiSEs
6. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lux, S.F.; Terborg, L.; Hachmöller, O.; Placke, T.; Meyer, H.-W.; Passerini, S.; Winter, M.; Nowak, S. LiTFSI Stability in Water and Its Possible Use in Aqueous Lithium-Ion Batteries: pH Dependency, Electrochemical Window and Temperature Stability. J. Electrochem. Soc. 2013, 160, A1694–A1700. [Google Scholar] [CrossRef]
- Bogdanov, D.; Ram, M.; Aghahosseini, A.; Gulagi, A.; Oyewo, A.S.; Child, M.; Caldera, U.; Sadovskaia, K.; Farfan, J.; De Souza Noel Simas Barbosa, L.; et al. Low-Cost Renewable Electricity as the Key Driver of the Global Energy Transition towards Sustainability. Energy 2021, 227, 120467. [Google Scholar] [CrossRef]
- Child, M.; Koskinen, O.; Linnanen, L.; Breyer, C. Sustainability Guardrails for Energy Scenarios of the Global Energy Transition. Renew. Sustain. Energy Rev. 2018, 91, 321–334. [Google Scholar] [CrossRef]
- Solomon, B.D.; Krishna, K. The Coming Sustainable Energy Transition: History, Strategies, and Outlook. Asian Energy Secur. 2011, 39, 7422–7431. [Google Scholar] [CrossRef]
- Bhojane, P. Recent Advances and Fundamentals of Pseudocapacitors: Materials, Mechanism, and Its Understanding. J. Energy Storage 2022, 45, 103654. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Pham, H.D.; Nanjundan, A.K.; Fernando, J.F.S.; Jayaramulu, K.; Golberg, D.; Han, Y.-K.; Dubal, D.P. True Meaning of Pseudocapacitors and Their Performance Metrics: Asymmetric versus Hybrid Supercapacitors. Small 2020, 16, 2002806. [Google Scholar] [CrossRef]
- Wu, H.; Xie, G.; Jie, Z.; Hui, X.; Yang, D.; Du, C. Research Progress about Chemical Energy Storage of Solar Energy. IOP Conf. Ser. Earth Environ. Sci. 2018, 108, 052070. [Google Scholar] [CrossRef]
- Pan, Q.; Tong, Z.; Su, Y.; Qin, S.; Tang, Y. Energy Storage Mechanism, Challenge and Design Strategies of Metal Sulfides for Rechargeable Sodium/Potassium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2103912. [Google Scholar] [CrossRef]
- Zhu, W.H.; Zhu, Y.; Davis, Z.; Tatarchuk, B.J. Energy Efficiency and Capacity Retention of Ni–MH Batteries for Storage Applications. Appl. Energy 2013, 106, 307–313. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Li, Q.; Cartmell, S.; Ferrara, S.; Deng, Z.D.; Xiao, J. Lithium and Lithium Ion Batteries for Applications in Microelectronic Devices: A Review. J. Power Sources 2015, 286, 330–345. [Google Scholar] [CrossRef]
- Choi, S.; Wang, G. Advanced Lithium-Ion Batteries for Practical Applications: Technology, Development, and Future Perspectives. Adv. Mater. Technol. 2018, 3, 1700376. [Google Scholar] [CrossRef]
- Deng, J.; Bae, C.; Denlinger, A.; Miller, T. Electric Vehicles Batteries: Requirements and Challenges. Joule 2020, 4, 511–515. [Google Scholar] [CrossRef]
- Arbizzani, C.; Gabrielli, G.; Mastragostino, M. Thermal Stability and Flammability of Electrolytes for Lithium-Ion Batteries. J. Power Sources 2011, 196, 4801–4805. [Google Scholar] [CrossRef]
- Kawamura, T.; Kimura, A.; Egashira, M.; Okada, S.; Yamaki, J.-I. Thermal Stability of Alkyl Carbonate Mixed-Solvent Electrolytes for Lithium Ion Cells. J. Power Sources 2002, 104, 260–264. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-Ion Batteries: Outlook on Present, Future, and Hybridized Technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Wu, X.; Zhang, Y.; Danilov, D.L.; Eichel, R.-A.; Notten, P.H.L. Double-Shelled Co3O4/C Nanocages Enabling Polysulfides Adsorption for High-Performance Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2019, 2, 8153–8162. [Google Scholar] [CrossRef]
- Lithium Price Today|Lithium Spot Price Chart|Historical Price of Lithium—Shanghai Metal Market. Available online: https://www.metal.com/en/markets/40 (accessed on 10 March 2025).
- Swiderska-Mocek, A.; Jakobczyk, P.; Rudnicka, E.; Lewandowski, A. Flammability Parameters of Lithium-Ion Battery Electrolytes. J. Mol. Liq. 2020, 318, 113986. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Feldblyum, J.I.; Mackanic, D.G.; Lissel, F.; Michels, D.L.; Cui, Y.; Bao, Z. Concentrated Mixed Cation Acetate “Water-in-Salt” Solutions as Green and Low-Cost High Voltage Electrolytes for Aqueous Batteries. Energy Environ. Sci. 2018, 11, 2876–2883. [Google Scholar] [CrossRef]
- Luntz, A. Beyond Lithium Ion Batteries. J. Phys. Chem. Lett. 2015, 6, 300–301. [Google Scholar] [CrossRef]
- Gao, Y.; Pan, Z.; Sun, J.; Liu, Z.; Wang, J. High-Energy Batteries: Beyond Lithium-Ion and Their Long Road to Commercialisation. Nano-Micro Lett. 2022, 14, 94. [Google Scholar] [CrossRef]
- de Meatza, I.; Urdampilleta, I.; Boyano, I.; Castrillo, I.; Landa-Medrano, I.; Sananes-Israel, S.; Eguia-Barrio, A.; Palomares, V. From Lab to Manufacturing Line: Guidelines for the Development and Upscaling of Aqueous Processed NMC622 Electrodes. J. Electrochem. Soc. 2023, 170, 010527. [Google Scholar] [CrossRef]
- Molaiyan, P.; Bhattacharyya, S.; Dos Reis, G.S.; Sliz, R.; Paolella, A.; Lassi, U. Towards Greener Batteries: Sustainable Components and Materials for next-Generation Batteries. Green Chem. 2024, 26, 7508–7531. [Google Scholar] [CrossRef]
- Yang, F.; Wang, D.; Zhao, Y.; Tsui, K.-L.; Bae, S.J. A Study of the Relationship between Coulombic Efficiency and Capacity Degradation of Commercial Lithium-Ion Batteries. Energy 2018, 145, 486–495. [Google Scholar] [CrossRef]
- He, H.; Sun, D.; Tang, Y.; Wang, H.; Shao, M. Understanding and Improving the Initial Coulombic Efficiency of High-Capacity Anode Materials for Practical Sodium Ion Batteries. Energy Storage Mater. 2019, 23, 233–251. [Google Scholar] [CrossRef]
- Xu, T.; Yu, J.; Ma, J.; Ren, W.; Hu, M.; Li, X. The Critical Role of Water Molecules in the Development of Aqueous Electrolytes for Rechargeable Metal-Ion Batteries. J. Mater. Chem. A 2024, 12, 13551–13575. [Google Scholar] [CrossRef]
- Gao, H.; Tang, K.; Xiao, J.; Guo, X.; Chen, W.; Liu, H.; Wang, G. Recent Advances in “Water in Salt” Electrolytes for Aqueous Rechargeable Monovalent-Ion (Li+, Na+, K+) Batteries. J. Energy Chem. 2022, 69, 84–99. [Google Scholar] [CrossRef]
- Zuo, W.; Innocenti, A.; Zarrabeitia, M.; Bresser, D.; Yang, Y.; Passerini, S. Layered Oxide Cathodes for Sodium-Ion Batteries: Storage Mechanism, Electrochemistry, and Techno-Economics. Acc. Chem. Res. 2023, 56, 284–296. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, H.; Ran, F. Fast-Charging Cathode Materials for Lithium & Sodium Ion Batteries. Mater. Today 2023, 63, 360–379. [Google Scholar] [CrossRef]
- Slater, M.D.; Kim, D.; Lee, E.; Johnson, C.S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 947–958. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Vardhini, G.; Dilip, P.S.; Kumar, S.A.; Suriyakumar, S.; Hariharan, M.; Shaijumon, M.M. Polyimide-Based Aqueous Potassium Energy Storage Systems Using Concentrated WiSE Electrolyte. ACS Appl. Mater. Interfaces 2024, 16, 48782–48791. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-Salt” Electrolyte Enables High-Voltage Aqueous Lithium-Ion Chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.; Yang, M.; Chen, W. High-Safety Separators for Lithium-Ion Batteries and Sodium-Ion Batteries: Advances and Perspective. Energy Storage Mater. 2021, 41, 522–545. [Google Scholar] [CrossRef]
- Yang, M.; Luo, J.; Guo, X.; Chen, J.; Cao, Y.; Chen, W. Aqueous Rechargeable Sodium-Ion Batteries: From Liquid to Hydrogel. Batteries 2022, 8, 180. [Google Scholar] [CrossRef]
- Huang, W.; Yang, F.; Xu, G.; Chen, J.; Shi, W.; Yang, Y. Ba-Doped Na0.16MnO2 with Ultra-Long Cycling Life and Highly Reversible Insertion/Extraction Mechanism for Aqueous Rechargeable Sodium Ion Batteries. J. Energy Storage 2024, 98, 112983. [Google Scholar] [CrossRef]
- He, X.; Iqbal, N.; Ghani, U.; Li, T. Potassium Ion Batteries: Recent Advancements in Anodic, Cathodic, and Electrolytic Materials. J. Alloys Compd. 2024, 981, 173680. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Recent Progress in Rechargeable Potassium Batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar] [CrossRef]
- Leonard, D.P.; Wei, Z.; Chen, G.; Du, F.; Ji, X. Water-in-Salt Electrolyte for Potassium-Ion Batteries. ACS Energy Lett. 2018, 3, 373–374. [Google Scholar] [CrossRef]
- Xu, Y.; Ding, T.; Sun, D.; Ji, X.; Zhou, X. Recent Advances in Electrolytes for Potassium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2211290. [Google Scholar] [CrossRef]
- Suyama, H.; Sato, S.; Matsunaga, T.; Inoue, T.; Ikezawa, A.; Arai, H. Anticrystallization and Superionic Conduction of Highly Concentrated Potassium Pyrophosphate Aqueous Electrolytes. ACS Appl. Energy Mater. 2023, 6, 11897–11905. [Google Scholar] [CrossRef]
- Daily Sodium-Ion Battery Price, Lme Comex Shfe Price of Sodium-Ion Battery Live|SMM—Metal Market. Available online: https://www.metal.com/Sodium-ion%20Battery (accessed on 10 March 2025).
- Potassium Chloride (Muriate of Potash) Spot Price Monthly Insights: Commodity Markets Review|YCharts. Available online: https://ycharts.com/indicators/potassium_chloride_muriate_of_potash_spot_price (accessed on 11 March 2025).
- Kalhoff, J.; Eshetu, G.G.; Bresser, D.; Passerini, S. Safer Electrolytes for Lithium-Ion Batteries: State of the Art and Perspectives. ChemSusChem 2015, 8, 2154–2175. [Google Scholar] [CrossRef]
- Burton, T.F.; Jommongkol, R.; Zhu, Y.; Deebansok, S.; Chitbankluai, K.; Deng, J.; Fontaine, O. Water-in-Salt Electrolytes towards Sustainable and Cost-Effective Alternatives: Example for Zinc-Ion Batteries. Curr. Opin. Electrochem. 2022, 35, 101070. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Wei, Z.; Chen, G.; Yang, X.; Wang, C.; Du, F. Use of a Water-in-Salt Electrolyte to Avoid Organic Material Dissolution and Enhance the Kinetics of Aqueous Potassium Ion Batteries. Sustain. Energy Fuels 2020, 4, 128–131. [Google Scholar] [CrossRef]
- Liu, Y.-K.; Zhao, C.-Z.; Du, J.; Zhang, X.-Q.; Chen, A.-B.; Zhang, Q. Research Progresses of Liquid Electrolytes in Lithium-Ion Batteries. Small 2023, 19, 2205315. [Google Scholar] [CrossRef]
- Bommier, C.; Ji, X. Electrolytes, SEI Formation, and Binders: A Review of Nonelectrode Factors for Sodium-Ion Battery Anodes. Small 2018, 14, 1703576. [Google Scholar] [CrossRef]
- Wang, F.; Lin, Y.; Suo, L.; Fan, X.; Gao, T.; Yang, C.; Han, F.; Qi, Y.; Xu, K.; Wang, C. Stabilizing High Voltage LiCoO2 Cathode in Aqueous Electrolyte with Interphase-Forming Additive. Energy Environ. Sci. 2016, 9, 3666–3673. [Google Scholar] [CrossRef]
- Jommongkol, R.; Deebansok, S.; Deng, J.; Zhu, Y.; Bouchal, R.; Fontaine, O. Unveiling LiTFSI Precipitation as a Key Factor in Solid Electrolyte Interphase Formation in Li-Based Water-in-Salt Electrolytes. Small 2024, 20, 2303945. [Google Scholar] [CrossRef]
- Lin, Y.; Peng, Q.; Chen, L.; Zuo, Q.; Long, Q.; Lu, F.; Huang, S.; Chen, Y.; Meng, Y. Organic Liquid Electrolytes in Sodium-Based Batteries: Actualities and Perspectives. Energy Storage Mater. 2024, 67, 103211. [Google Scholar] [CrossRef]
- Mao, J.; Wang, C.; Lyu, Y.; Zhang, R.; Wang, Y.; Liu, S.; Wang, Z.; Zhang, S.; Guo, Z. Organic Electrolyte Design for Practical Potassium-Ion Batteries. J. Mater. Chem. A 2022, 10, 19090–19106. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, Z.; Wei, T.; Wang, W.; Wu, Q.; Fu, Y.; Yang, T.; Wang, J.-R.; Li, H.; Wang, B.; et al. Phosphonitrile Based Porous Organic Polymers as Effective Flame-Retardant Electrolytes for Lithium Battery. Polymer 2024, 311, 127504. [Google Scholar] [CrossRef]
- Ma, G.; Di, S.; Wang, Y.; Yuan, W.; Ji, X.; Qiu, K.; Liu, M.; Nie, X.; Zhang, N. Zn Metal Anodes Stabilized by an Intrinsically Safe, Dilute, and Hydrous Organic Electrolyte. Energy Storage Mater. 2023, 54, 276–283. [Google Scholar] [CrossRef]
- Shyma Sajeevan, A.; Bernard, L.; Tran-Van, P.; Brandell, D.; Renault, S.; Poizot, P. Combining Polyester-Based Solid Polymer Electrolytes with Lithiated Organic Cathodes for 3.5 V-Class Li-Organic Rechargeable Batteries. ACS Appl. Polym. Mater. 2024, 6, 10102–10112. [Google Scholar] [CrossRef]
- Zhu, K.; Li, Z.; Sun, Z.; Liu, P.; Jin, T.; Chen, X.; Li, H.; Lu, W.; Jiao, L. Inorganic Electrolyte for Low-Temperature Aqueous Sodium Ion Batteries. Small 2022, 18, 2107662. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Sun, W.; Fan, X.; Yang, C.; Wang, F.; Gao, T.; Ma, Z.; Schroeder, M.; von Cresce, A.; et al. Advanced High-Voltage Aqueous Lithium-Ion Battery Enabled by “Water-in-Bisalt” Electrolyte. Angew. Chem. Int. Ed. 2016, 55, 7136–7141. [Google Scholar] [CrossRef]
- Ji, D.; Kim, J. Trend of Developing Aqueous Liquid and Gel Electrolytes for Sustainable, Safe, and High-Performance Li-Ion Batteries. Nano-Micro Lett. 2024, 16, 2. [Google Scholar] [CrossRef]
- Tasaki, K.; Goldberg, A.; Winter, M. On the Difference in Cycling Behaviors of Lithium-Ion Battery Cell between the Ethylene Carbonate- and Propylene Carbonate-Based Electrolytes. Electrochim. Acta 2011, 56, 10424–10435. [Google Scholar] [CrossRef]
- Xing, L.; Li, W.; Wang, C.; Gu, F.; Xu, M.; Tan, C.; Yi, J. Theoretical Investigations on Oxidative Stability of Solvents and Oxidative Decomposition Mechanism of Ethylene Carbonate for Lithium Ion Battery Use. J. Phys. Chem. B 2009, 113, 16596–16602. [Google Scholar] [CrossRef]
- Huang, Y.; Luo, Y.; Wang, B.; Wang, H.; Zhang, L. Crucial Roles of Ethyl Methyl Carbonate in Lithium-Ion and Dual-Ion Batteries: A Review. Langmuir 2024, 40, 11353–11370. [Google Scholar] [CrossRef]
- Yoshida, H.; Fukunaga, T.; Hazama, T.; Terasaki, M.; Mizutani, M.; Yamachi, M. Degradation Mechanism of Alkyl Carbonate Solvents Used in Lithium-Ion Cells during Initial Charging. J. Power Sources 1997, 68, 311–315. [Google Scholar] [CrossRef]
- Seo, D.M.; Reininger, S.; Kutcher, M.; Redmond, K.; Euler, W.B.; Lucht, B.L. Role of Mixed Solvation and Ion Pairing in the Solution Structure of Lithium Ion Battery Electrolytes. J. Phys. Chem. C 2015, 119, 14038–14046. [Google Scholar] [CrossRef]
- Vignarooban, K.; Kushagra, R.; Elango, A.; Badami, P.; Mellander, B.-E.; Xu, X.; Tucker, T.G.; Nam, C.; Kannan, A.M. Current Trends and Future Challenges of Electrolytes for Sodium-Ion Batteries. Int. J. Hydrogen Energy 2016, 41, 2829–2846. [Google Scholar] [CrossRef]
- Karaseva, E.V.; Kuzmina, E.V.; Li, B.-Q.; Zhang, Q.; Kolosnitsyn, V.S. Effect of the Anionic Composition of Sulfolane Based Electrolytes on the Performances of Lithium-Sulfur Batteries. J. Energy Chem. 2024, 95, 231–240. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, X.; Misra, R.K.; Cai, Q.; Zhao, Y. Progress in Electrolytes for Beyond-Lithium-Ion Batteries. J. Mater. Sci. Technol. 2020, 44, 237–257. [Google Scholar] [CrossRef]
- Gallastegui, A.; Lingua, G.; Lopez-Larrea, N.; Carfora, R.; Pasini, D.; Mantione, D.; Mecerreyes, D. Piperazinium Poly(Ionic Liquid)s as Solid Electrolytes for Lithium Batteries. Macromol. Rapid Commun. 2024, 45, 2400184. [Google Scholar] [CrossRef]
- Qiu, B.; Lin, B.; Yan, F. Ionic Liquid/Poly(Ionic Liquid)-Based Electrolytes for Energy Devices. Polym. Int. 2013, 62, 335–337. [Google Scholar] [CrossRef]
- Tang, X.; Lv, S.; Jiang, K.; Zhou, G.; Liu, X. Recent Development of Ionic Liquid-Based Electrolytes in Lithium-Ion Batteries. J. Power Sources 2022, 542, 231792. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, D.; Lee, M.; Nam, K.W. Challenges and Possibilities for Aqueous Battery Systems. Commun. Mater. 2023, 4, 1–19. [Google Scholar] [CrossRef]
- Yang, X.; Fan, H.; Hu, F.; Chen, S.; Yan, K.; Ma, L. Aqueous Zinc Batteries with Ultra-Fast Redox Kinetics and High Iodine Utilization Enabled by Iron Single Atom Catalysts. Nano-Micro Lett. 2023, 15, 126. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, X.; Yang, Y.; Yin, H.; Lei, Y.; Liang, S.; Fang, G. Issues and Optimization Strategies of Binders for Aqueous Zinc Metal Batteries. Chem. Eng. J. 2024, 497, 154916. [Google Scholar] [CrossRef]
- Li, L.; Jia, S.; Cheng, Z.; Zhang, C. Improved Strategies for Separators in Zinc-Ion Batteries. ChemSusChem 2023, 16, e202202330. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yan, Y.; Zheng, Y.; Zhang, W.; He, X.; Wu, Z.; Yang, T.; Xia, X.; Huang, H.; Xia, Y.; et al. Recent Advances of Aqueous Rechargeable Lithium/Sodium Ion Batteries: Key Electrode Materials and Electrolyte Design Strategies. Mater. Today Energy 2023, 38, 101454. [Google Scholar] [CrossRef]
- Lv, C.; Bao, L.; Huo, Y.; Liu, Y.; Su, Z. Enhancing the Hydrogen Ion Adsorption Capacity to Improve the Performance of an Aqueous Zinc Ion Battery of V2O5. Inorg. Chem. Commun. 2024, 161, 112053. [Google Scholar] [CrossRef]
- Xie, J.; Lin, D.; Lei, H.; Wu, S.; Li, J.; Mai, W.; Wang, P.; Hong, G.; Zhang, W. Electrolyte and Interphase Engineering of Aqueous Batteries Beyond “Water-in-Salt” Strategy. Adv. Mater. 2024, 36, 2306508. [Google Scholar] [CrossRef]
- Olana, B.N.; Pan, S.-H.; Hwang, B.-J.; Althues, H.; Jiang, J.-C.; Lin, S.D. Understanding the Formation Chemistry of Native Solid Electrolyte Interphase over Lithium Anode and Its Implications Using a LiTFSI/TME-TTE Electrolyte and Polysulfide Additive. J. Mater. Chem. A 2024, 12, 3659–3670. [Google Scholar] [CrossRef]
- Jaumaux, P.; Yang, X.; Zhang, B.; Safaei, J.; Tang, X.; Zhou, D.; Wang, C.; Wang, G. Localized Water-In-Salt Electrolyte for Aqueous Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 19965–19973. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Wang, Y.; Rong, X.; Sun, W.; Fan, X.; Xu, S.; Schroeder, M.A.; Cresce, A.V.; Wang, F.; et al. “Water-in-Salt” Electrolyte Makes Aqueous Sodium-Ion Battery Safe, Green, and Long-Lasting. Adv. Energy Mater. 2017, 7, 1701189. [Google Scholar] [CrossRef]
- Nian, Q.; Zhu, W.; Zheng, S.; Chen, S.; Xiong, B.-Q.; Wang, Z.; Wu, X.; Tao, Z.; Ren, X. An Overcrowded Water-Ion Solvation Structure for a Robust Anode Interphase in Aqueous Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 51048–51056. [Google Scholar] [CrossRef]
- Kumar, M.; Nagaiah, T.C. High Energy Density Aqueous Rechargeable Sodium-Ion/Sulfur Batteries in ‘water in Salt” Electrolyte. Energy Storage Mater. 2022, 49, 390–400. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Liu, H.; Hu, X.; Zhang, Q.; Peng, W.; Li, Y.; Zhang, F.; Han, Y.; Fan, X. Suppressing Vanadium Dissolution in “Water-in-Salt” Electrolytes for 3.2 V Aqueous Sodium-Ion Pseudocapacitors. ACS Appl. Mater. Interfaces 2022, 14, 35485–35494. [Google Scholar] [CrossRef]
- Kumar, M.; Nagaiah, T.C. Tuning the Interfacial Chemistry for Stable and High Energy Density Aqueous Sodium-Ion/Sulfur Batteries. J. Mater. Chem. A 2022, 10, 12984–12996. [Google Scholar] [CrossRef]
- Rao, R.; Chen, L.; Su, J.; Cai, S.; Wang, S.; Chen, Z. Issues and Challenges Facing Aqueous Sodium-ion Batteries toward Practical Applications. Battery Energy 2024, 3, 20230036. [Google Scholar] [CrossRef]
- Droguet, L.; Grimaud, A.; Fontaine, O.; Tarascon, J. Water-in-Salt Electrolyte (WiSE) for Aqueous Batteries: A Long Way to Practicality. Adv. Energy Mater. 2020, 10, 2002440. [Google Scholar] [CrossRef]
- Kühnel, R.-S.; Reber, D.; Remhof, A.; Figi, R.; Bleiner, D.; Battaglia, C. “Water-in-Salt” Electrolytes Enable the Use of Cost-Effective Aluminum Current Collectors for Aqueous High-Voltage Batteries. Chem. Commun. 2016, 52, 10435–10438. [Google Scholar] [CrossRef]
- Kasprzak, D.; Wu, Z.; Tao, L.; Xu, J.; Zhang, Y.; Liu, J. Water-in-Salt Gel Biopolymer Electrolytes for Flexible and Wearable Zn/Alkali Metal Dual-Ion Batteries. ACS Appl. Mater. Interfaces 2024, 16, 36304–36314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Meng, X.; Sun, J.; Liu, Y.; Hou, L. Recent Progress in “Water-in-Salt” Electrolytes Toward Non-Lithium Based Rechargeable Batteries. Front. Chem. 2020, 8, 595. [Google Scholar] [CrossRef]
- Sun, W.; Suo, L.; Wang, F.; Eidson, N.; Yang, C.; Han, F.; Ma, Z.; Gao, T.; Zhu, M.; Wang, C. “Water-in-Salt” Electrolyte Enabled LiMn2O4/TiS2 Lithium-Ion Batteries. Electrochem. Commun. 2017, 82, 71–74. [Google Scholar] [CrossRef]
- Yamada, Y.; Usui, K.; Sodeyama, K.; Ko, S.; Tateyama, Y.; Yamada, A. Hydrate-Melt Electrolytes for High-Energy-Density Aqueous Batteries. Nat. Energy 2016, 1, 16129. [Google Scholar] [CrossRef]
- Ko, S.; Yamada, Y.; Miyazaki, K.; Shimada, T.; Watanabe, E.; Tateyama, Y.; Kamiya, T.; Honda, T.; Akikusa, J.; Yamada, A. Lithium-Salt Monohydrate Melt: A Stable Electrolyte for Aqueous Lithium-Ion Batteries. Electrochem. Commun. 2019, 104, 106488. [Google Scholar] [CrossRef]
- Becker, M.; Kühnel, R.-S.; Battaglia, C. Water-in-Salt Electrolytes for Aqueous Lithium-Ion Batteries with Liquidus Temperatures below −10 °C. Chem. Commun. 2019, 55, 12032–12035. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, R.; Yang, J.; Xin, Y.; Singh, P.; Wu, F.; Qian, Y.; Gao, H. The Safety Aspect of Sodium Ion Batteries for Practical Applications. J. Energy Chem. 2024, 95, 407–427. [Google Scholar] [CrossRef]
- Eshetu, G.G.; Grugeon, S.; Kim, H.; Jeong, S.; Wu, L.; Gachot, G.; Laruelle, S.; Armand, M.; Passerini, S. Comprehensive Insights into the Reactivity of Electrolytes Based on Sodium Ions. ChemSusChem 2016, 9, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yang, X.; Nai, J.; Wang, Y.; Liu, Y.; Liu, C.; Tao, X. Recent Development of Na Metal Anodes: Interphase Engineering Chemistries Determine the Electrochemical Performance. Chem. Eng. J. 2021, 409, 127943. [Google Scholar] [CrossRef]
- Suo, L.; Oh, D.; Lin, Y.; Zhuo, Z.; Borodin, O.; Gao, T.; Wang, F.; Kushima, A.; Wang, Z.; Kim, H.-C.; et al. How Solid-Electrolyte Interphase Forms in Aqueous Electrolytes. J. Am. Chem. Soc. 2017, 139, 18670–18680. [Google Scholar] [CrossRef] [PubMed]
- Kühnel, R.-S.; Reber, D.; Battaglia, C. A High-Voltage Aqueous Electrolyte for Sodium-Ion Batteries. ACS Energy Lett. 2017, 2, 2005–2006, Erratum in ACS Energy Lett. 2020, 5, 346. https://doi.org/10.1021/acsenergylett.9b02779. [Google Scholar] [CrossRef]
- Han, J.; Zarrabeitia, M.; Mariani, A.; Jusys, Z.; Hekmatfar, M.; Zhang, H.; Geiger, D.; Kaiser, U.; Behm, R.J.; Varzi, A.; et al. Halide-Free Water-in-Salt Electrolytes for Stable Aqueous Sodium-Ion Batteries. Nano Energy 2020, 77, 105176. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, S.J.; Chang, D.; Kim, J.; Moon, S.; Oh, K.; Park, K.-Y.; Seong, W.M.; Park, H.; Kwon, G.; et al. Toward a Low-Cost High-Voltage Sodium Aqueous Rechargeable Battery. Mater. Today 2019, 29, 26–36. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, L.; Yue, J.; Zhang, Q.; Zhou, A.; Borodin, O.; Suo, L.; Li, H.; Chen, L.; Xu, K.; et al. High-Voltage Aqueous Na-Ion Battery Enabled by Inert-Cation-Assisted Water-in-Salt Electrolyte. Adv. Mater. 2020, 32, 1904427. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Ji, X.; Wang, P.; Zhu, K.; Zhang, J.; Cao, L.; Chen, L.; Cui, C.; Deng, T.; Liu, S.; et al. High-Energy Aqueous Sodium-Ion Batteries. Angew. Chem. Int. Ed. 2021, 60, 11943–11948. [Google Scholar] [CrossRef]
- Han, J.; Mariani, A.; Zhang, H.; Zarrabeitia, M.; Gao, X.; Carvalho, D.V.; Varzi, A.; Passerini, S. Gelified Acetate-Based Water-in-Salt Electrolyte Stabilizing Hexacyanoferrate Cathode for Aqueous Potassium-Ion Batteries. Energy Storage Mater. 2020, 30, 196–205. [Google Scholar] [CrossRef]
- Kumaresan, T.K.; Ikhe, A.B.; Park, W.; Prabakar, S.J.R.; Noh, H.S.; Seo, J.Y.; Lee, Y.; Sohn, K.; Kwak, J.S.; Pyo, M. Highly Concentrated Asymmetric KTFSI for Aqueous Potassium Ion Batteries. Adv. Energy Mater. 2024, 14, 2402011. [Google Scholar] [CrossRef]
- Chen, N.; Gui, B.; Yang, B.; Deng, C.; Liang, Y.; Zhang, F.; Li, B.; Sun, W.; Wu, F.; Chen, R. LiPF6 Induces Phosphorization of Garnet-Type Solid-State Electrolyte for Stable Lithium Metal Batteries. Small 2024, 20, 2305576. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, J.; Li, Q.; Vatamanu, J.; Ji, X.; Pollard, T.P.; Cui, C.; Hou, S.; Chen, J.; Yang, C.; et al. A 63 m Superconcentrated Aqueous Electrolyte for High-Energy Li-Ion Batteries. ACS Energy Lett. 2020, 5, 968–974. [Google Scholar] [CrossRef]
- Akash Prabhu, S.; Kunhiraman, A.K.; Naveen, T.B.; Ajay Rakkesh, R.; Peeters, M. Recent Progress and Prospects in the Electrode Materials of Flexible Sodium-Ion Battery. Sustain. Chem. Pharm. 2022, 28, 100693. [Google Scholar] [CrossRef]
- Ye, C.; Zhang, L.; Guo, C.; Li, D.; Vasileff, A.; Wang, H.; Qiao, S. A 3D Hybrid of Chemically Coupled Nickel Sulfide and Hollow Carbon Spheres for High Performance Lithium–Sulfur Batteries. Adv. Funct. Mater. 2017, 27, 1702524. [Google Scholar] [CrossRef]
- Simanjuntak, E.K.; Danner, T.; Wang, P.; Buchmeiser, M.R.; Latz, A. A Novel Modeling Approach for Sulfurized Polyacrylonitrile (SPAN) Electrodes in Li Metal Batteries. Electrochim. Acta 2024, 497, 144571. [Google Scholar] [CrossRef]
- Han, J.; Zhang, H.; Varzi, A.; Passerini, S. Fluorine-Free Water-in-Salt Electrolyte for Green and Low-Cost Aqueous Sodium-Ion Batteries. ChemSusChem 2018, 11, 3704–3707. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, Y.; Wu, X.; Ji, X. Prussian Blue Analogues as Electrodes for Aqueous Monovalent Ion Batteries. Electrochem. Energy Rev. 2022, 5, 242–262. [Google Scholar] [CrossRef]
- Ahaliabadeh, Z.; Miikkulainen, V.; Mäntymäki, M.; Colalongo, M.; Mousavihashemi, S.; Yao, L.; Jiang, H.; Lahtinen, J.; Kankaanpää, T.; Kallio, T. Stabilized Nickel-Rich-Layered Oxide Electrodes for High-Performance Lithium-Ion Batteries. Energy Environ. Mater. 2024, 7, e12741. [Google Scholar] [CrossRef]
- Cui, M.; Liu, M.; Li, X.; Shi, W.; Yu, Y.; Li, J.; Liu, Y.; Zhang, F.; Wang, W.; Li, X.; et al. Order-Disorder Structural Engineering of Vanadium Oxide Anode: Balancing Ionic and Electronic Dynamic for Fast-Charging Aqueous Li-Ion Battery. Energy Storage Mater. 2024, 70, 103453. [Google Scholar] [CrossRef]
- Kim, M.; Lee, S.; Kang, B. High Energy Density Polyanion Electrode Material: LiVPO4O1–xFx (x ≈ 0.25) with Tavorite Structure. Chem. Mater. 2017, 29, 4690–4699. [Google Scholar] [CrossRef]
- Sun, X.; Tripathi, R.; Popov, G.; Balasubramanian, M.; Nazar, L.F. Stabilization of Lithium Transition Metal Silicates in the Olivine Structure. Inorg. Chem. 2017, 56, 9931–9937. [Google Scholar] [CrossRef]
- Cambaz, M.A.; Anji Reddy, M.; Vinayan, B.P.; Witte, R.; Pohl, A.; Mu, X.; Chakravadhanula, V.S.K.; Kübel, C.; Fichtner, M. Mechanical Milling Assisted Synthesis and Electrochemical Performance of High Capacity LiFeBO3 for Lithium Batteries. ACS Appl. Mater. Interfaces 2016, 8, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, X.; You, H.; Min, H.; Xu, X.; Hao, J.; Liu, X.; Yang, H. Template-Directed Prussian Blue Nanocubes Supported on Ni Foam as the Binder-Free Anode of Lithium-Ion Batteries. Appl. Surf. Sci. 2022, 571, 151194. [Google Scholar] [CrossRef]
- Zhang, Z.; Avdeev, M.; Chen, H.; Yin, W.; Kan, W.H.; He, G. Lithiated Prussian Blue Analogues as Positive Electrode Active Materials for Stable Non-Aqueous Lithium-Ion Batteries. Nat. Commun. 2022, 13, 7790. [Google Scholar] [CrossRef]
- Wi, T.-U.; Park, C.; Ko, S.; Kim, T.; Choi, A.; Muralidharan, V.; Choi, M.; Lee, H.-W. Cathode Electrolyte Interphase Engineering for Prussian Blue Analogues in Lithium-Ion Batteries. Nano Lett. 2024, 24, 7783–7791. [Google Scholar] [CrossRef]
- Yang, C.; Chen, J.; Qing, T.; Fan, X.; Sun, W.; Von Cresce, A.; Ding, M.S.; Borodin, O.; Vatamanu, J.; Schroeder, M.A.; et al. 4.0 V Aqueous Li-Ion Batteries. Joule 2017, 1, 122–132. [Google Scholar] [CrossRef]
- He, Z.; Huang, Y.; Liu, H.; Geng, Z.; Li, Y.; Li, S.; Deng, W.; Zou, G.; Hou, H.; Ji, X. Anode Materials for Fast Charging Sodium-Ion Batteries. Nano Energy 2024, 129, 109996. [Google Scholar] [CrossRef]
- Maddukuri, S.; Nimkar, A.; Chae, M.S.; Penki, T.R.; Luski, S.; Aurbach, D. Na0.44MnO2/Polyimide Aqueous Na-Ion Batteries for Large Energy Storage Applications. Front. Energy Res. 2021, 8, 615677. [Google Scholar] [CrossRef]
- Kumaresan, L.; Kirubakaran, K.P.; Priyadarshini, M.; Kasiviswanathan, K.; Senthil, C.; Lee, C.W.; Vediappan, K. Sustainable-Inspired Design of Efficient Organic Electrodes for Rechargeable Sodium-Ion Batteries: Conversion of P-Waste into E-Wealth Device. Sustain. Mater. Technol. 2021, 28, e00247. [Google Scholar] [CrossRef]
- Zhang, H.; Jeong, S.; Qin, B.; Vieira Carvalho, D.; Buchholz, D.; Passerini, S. Towards High-Performance Aqueous Sodium-Ion Batteries: Stabilizing the Solid/Liquid Interface for NASICON-Type Na2 VTi(PO4)3 Using Concentrated Electrolytes. ChemSusChem 2018, 11, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Sakamoto, R.; Nishimura, Y.; Xia, J.; Ito, M.; Okada, S. A Trifluoroacetate-Based Concentrated Electrolyte for Symmetrical Aqueous Sodium-Ion Battery with NASICON-Type Na2VTi(PO4)3 Electrodes. Electrochemistry 2021, 89, 415–419. [Google Scholar] [CrossRef]
- Suo, L.; Han, F.; Fan, X.; Liu, H.; Xu, K.; Wang, C. “Water-in-Salt” Electrolytes Enable Green and Safe Li-Ion Batteries for Large Scale Electric Energy Storage Applications. J. Mater. Chem. A 2016, 4, 6639–6644. [Google Scholar] [CrossRef]
- Wang, F.; Suo, L.; Liang, Y.; Yang, C.; Han, F.; Gao, T.; Sun, W.; Wang, C. Spinel LiNi0.5Mn1.5O4 Cathode for High-Energy Aqueous Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1600922. [Google Scholar] [CrossRef]
- Liang, G.; Gan, Z.; Wang, X.; Jin, X.; Xiong, B.; Zhang, X.; Chen, S.; Wang, Y.; He, H.; Zhi, C. Reconstructing Vanadium Oxide with Anisotropic Pathways for a Durable and Fast Aqueous K-Ion Battery. ACS Nano 2021, 15, 17717–17728. [Google Scholar] [CrossRef]
- Luo, W.; Hayden, J.; Jang, S.-H.; Wang, Y.; Zhang, Y.; Kuang, Y.; Wang, Y.; Zhou, Y.; Rubloff, G.W.; Lin, C.-F.; et al. Highly Conductive, Light Weight, Robust, Corrosion-Resistant, Scalable, All-Fiber Based Current Collectors for Aqueous Acidic Batteries. Adv. Energy Mater. 2018, 8, 1702615. [Google Scholar] [CrossRef]
- Levi, N.; Bergman, G.; Nimkar, A.; Tsubery, M.N.; Borenstein, A.; Adronov, A.; Aurbach, D.; Sharon, D.; Nessim, G.D.; Shpigel, N. Carbon Nanotubes as Efficient Anode Current Collectors for Stationary Aqueous Zn–Br2 Batteries. Carbon 2024, 228, 119407. [Google Scholar] [CrossRef]
- Li, S.; Church, B.C. Electrochemical Stability of Aluminum Current Collector in Aqueous Rechargeable Lithium-Ion Battery Electrolytes. J. Appl. Electrochem. 2017, 47, 839–853. [Google Scholar] [CrossRef]
- Hou, Z.; Zhang, X.; Ao, H.; Liu, M.; Zhu, Y.; Qian, Y. Passivation Effect for Current Collectors Enables High-Voltage Aqueous Sodium Ion Batteries. Mater. Today Energy 2019, 14, 100337. [Google Scholar] [CrossRef]
- Wen, Y.H.; Shao, L.; Zhao, P.C.; Wang, B.Y.; Cao, G.P.; Yang, Y.S. Carbon Coated Stainless Steel Mesh as a Low-Cost and Corrosion-Resistant Current Collector for Aqueous Rechargeable Batteries. J. Mater. Chem. A 2017, 5, 15752–15758. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, X.; Liu, Q.; Tang, W.; Liang, J.; Wu, W. Fully-Printed Flexible Aqueous Rechargeable Sodium-Ion Batteries. Small 2024, 20, 2312207. [Google Scholar] [CrossRef]
- Kucinskis, G.; Kruze, B.; Korde, P.; Sarakovskis, A.; Viksna, A.; Hodakovska, J.; Bajars, G. Enhanced Electrochemical Properties of Na0.67MnO2 Cathode for Na-Ion Batteries Prepared with Novel Tetrabutylammonium Alginate Binder. Batteries 2022, 8, 6. [Google Scholar] [CrossRef]
- Pace, G.T.; Wang, H.; Whitacre, J.F.; Wu, W. Comparative Study of Water-Processable Polymeric Binders in LiMn2O4 Cathode for Aqueous Electrolyte Batteries. Nano Sel. 2021, 2, 939–947. [Google Scholar] [CrossRef]
- Malchik, F.; Shpigel, N.; Levi, M.D.; Penki, T.R.; Gavriel, B.; Bergman, G.; Turgeman, M.; Aurbach, D.; Gogotsi, Y. MXene Conductive Binder for Improving Performance of Sodium-Ion Anodes in Water-in-Salt Electrolyte. Nano Energy 2021, 79, 105433. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, Z.; Xiao, M.; Wang, S.; Huang, S.; Han, D.; Meng, Y. Polymeric Binders Used in Lithium Ion Batteries: Actualities, Strategies and Trends. ChemElectroChem 2024, 11, e202300651. [Google Scholar] [CrossRef]
- Yi, H.; Lan, T.; Yang, Y.; Zeng, H.; Zhang, T.; Tang, T.; Wang, C.; Deng, Y. A Robust Aqueous-Processable Polymer Binder for Long-Life, High-Performance Lithium Sulfur Battery. Energy Storage Mater. 2019, 21, 61–68. [Google Scholar] [CrossRef]
- Ao, H.; Chen, C.; Hou, Z.; Cai, W.; Liu, M.; Jin, Y.; Zhang, X.; Zhu, Y.; Qian, Y. Electrolyte Solvation Structure Manipulation Enables Safe and Stable Aqueous Sodium Ion Batteries. J. Mater. Chem. A 2020, 8, 14190–14197. [Google Scholar] [CrossRef]
- He, B.; Man, P.; Zhang, Q.; Fu, H.; Zhou, Z.; Li, C.; Li, Q.; Wei, L.; Yao, Y. All Binder-Free Electrodes for High-Performance Wearable Aqueous Rechargeable Sodium-Ion Batteries. Nano-Micro Lett. 2019, 11, 101. [Google Scholar] [CrossRef]
- Kang, Y.; Deng, C.; Chen, Y.; Liu, X.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y. Binder-Free Electrodes and Their Application for Li-Ion Batteries. Nanoscale Res. Lett. 2020, 15, 112. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Jiang, Y.; Gui, Q.; Ba, D.; Li, Y.; Liu, J. Binder-Free Electrodes for Advanced Potassium-Ion Batteries: A Review. Chin. Chem. Lett. 2021, 32, 1299–1308. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Wen, K.; Chen, D.; Liu, Y.; Dong, Y.; Feng, C.; Han, Y.; Han, J.; Zhang, Y.; Xia, C.; et al. Composite Separators for Robust High Rate Lithium Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101420. [Google Scholar] [CrossRef]
- Lee, H.; Yanilmaz, M.; Toprakci, O.; Fu, K.; Zhang, X. A Review of Recent Developments in Membrane Separators for Rechargeable Lithium-Ion Batteries. Energy Environ. Sci. 2014, 7, 3857–3886. [Google Scholar] [CrossRef]
- Li, Y.; Yu, L.; Hu, W.; Hu, X. Thermotolerant Separators for Safe Lithium-Ion Batteries under Extreme Conditions. J. Mater. Chem. A 2020, 8, 20294–20317. [Google Scholar] [CrossRef]
- Deerattrakul, V.; Sakulaue, P.; Bunpheng, A.; Kraithong, W.; Pengsawang, A.; Chakthranont, P.; Iamprasertkun, P.; Itthibenchapong, V. Introducing Hydrophilic Cellulose Nanofiber as a Bio-Separator for “Water-in-Salt” Based Energy Storage Devices. Electrochim. Acta 2023, 453, 142355. [Google Scholar] [CrossRef]
- Peled, E. The Electrochemical Behavior of Alkali and Alkaline Earth Metals in Nonaqueous Battery Systems—The Solid Electrolyte Interphase Model. J. Electrochem. Soc. 1979, 126, 2047. [Google Scholar] [CrossRef]
- Yue, J.; Lin, L.; Jiang, L.; Zhang, Q.; Tong, Y.; Suo, L.; Hu, Y.; Li, H.; Huang, X.; Chen, L. Interface Concentrated-Confinement Suppressing Cathode Dissolution in Water-in-Salt Electrolyte. Adv. Energy Mater. 2020, 10, 2000665. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, R.; Zhao, C.; Wei, F.; Zhang, J.; Zhang, Q. A Review of Solid Electrolyte Interphases on Lithium Metal Anode. Adv. Sci. 2016, 3, 1500213. [Google Scholar] [CrossRef]
- Mense, M.; Bela, M.M.; Kühn, S.P.; Cekic-Laskovic, I.; Börner, M.; Wiemers-Meyer, S.; Winter, M.; Nowak, S. ToF-SIMS Sputter Depth Profiling of Interphases and Coatings on Lithium Metal Surfaces. Commun. Chem. 2025, 8, 31. [Google Scholar] [CrossRef]
- Sakamoto, R.; Yamashita, M.; Nakamoto, K.; Zhou, Y.; Yoshimoto, N.; Fujii, K.; Yamaguchi, T.; Kitajou, A.; Okada, S. Local Structure of a Highly Concentrated NaClO4 Aqueous Solution-Type Electrolyte for Sodium Ion Batteries. Phys. Chem. Chem. Phys. 2020, 22, 26452–26458. [Google Scholar] [CrossRef]
- Lazarenko, V.; Rublova, Y.; Meija, R.; Andzane, J.; Voikiva, V.; Kons, A.; Sarakovskis, A.; Viksna, A.; Erts, D. Bi2Se3 Nanostructured Thin Films as Perspective Anodes for Aqueous Rechargeable Lithium-Ion Batteries. Batteries 2022, 8, 144. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, C.; Wang, P.-F.; Li, Q.; Chen, L.; Han, F.; Jin, T.; Liu, S.; Choudhary, H.; Raghavan, S.R.; et al. “Water-in-Salt” Polymer Electrolyte for Li-Ion Batteries. Energy Environ. Sci. 2020, 13, 2878–2887. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Deng, W.; Li, C.; Yuan, X.; Hu, J.; Zhang, M.; Chen, H.; Li, R. A Superconcentrated Water-in-Salt Hydrogel Electrolyte for High-Voltage Aqueous Potassium-Ion Batteries. ChemElectroChem 2021, 8, 1451–1454. [Google Scholar] [CrossRef]

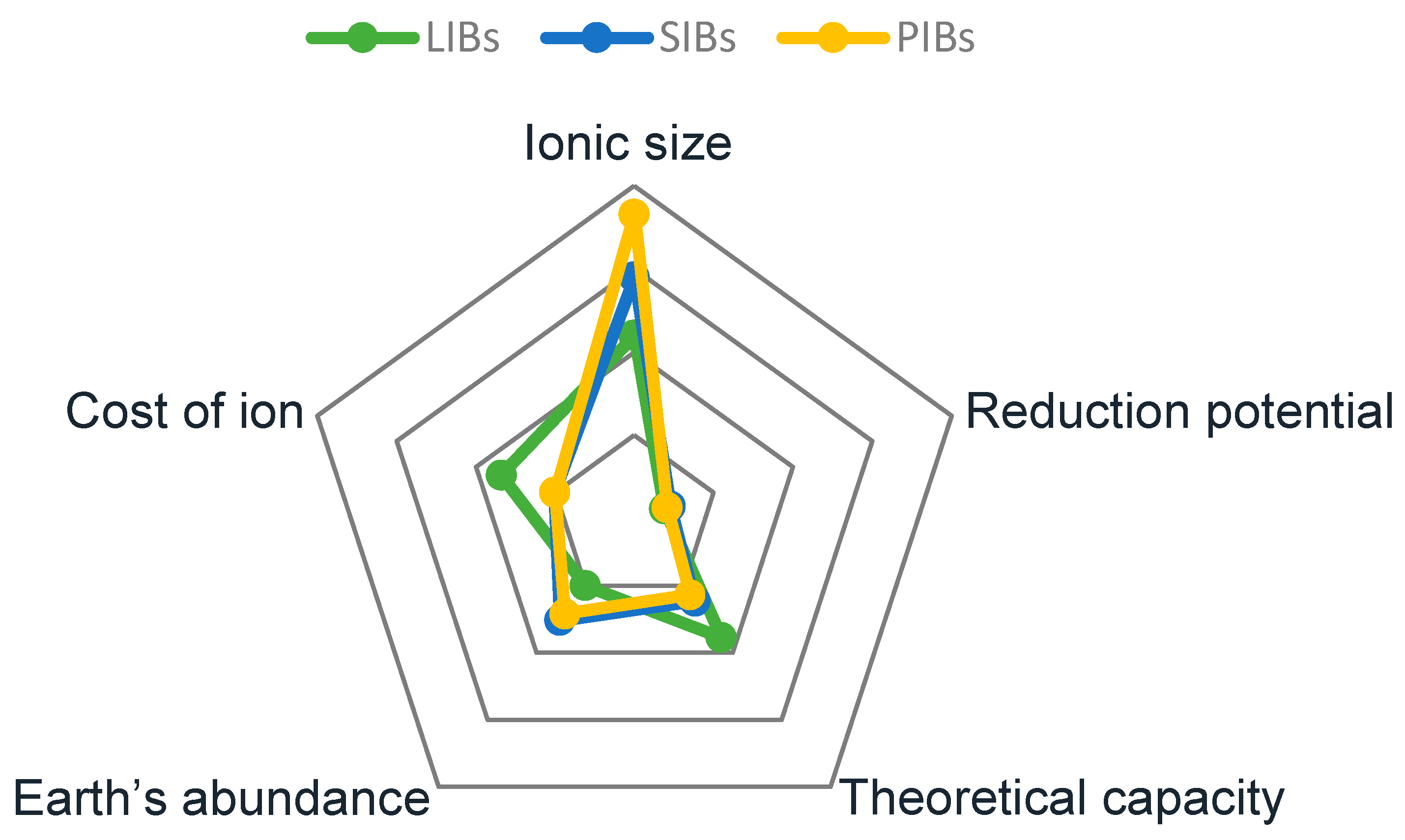




| LIBs | SIBs | PIBs | |
|---|---|---|---|
| Ionic size (nm) | 0.60 | 0.95 | 0.133 |
| Reduction potential (V) | −3.04 | −2.713 | −2.93 |
| Theoretical capacity (mAh g−1) | 3861 | 1166 | 685 |
| Earth’s abundance (% of earth’s crust) | 0.002 | 2.6 | 2.1 |
| Cost of ions (USD/ion) | 0.34 | 0.027 | 0.023 |
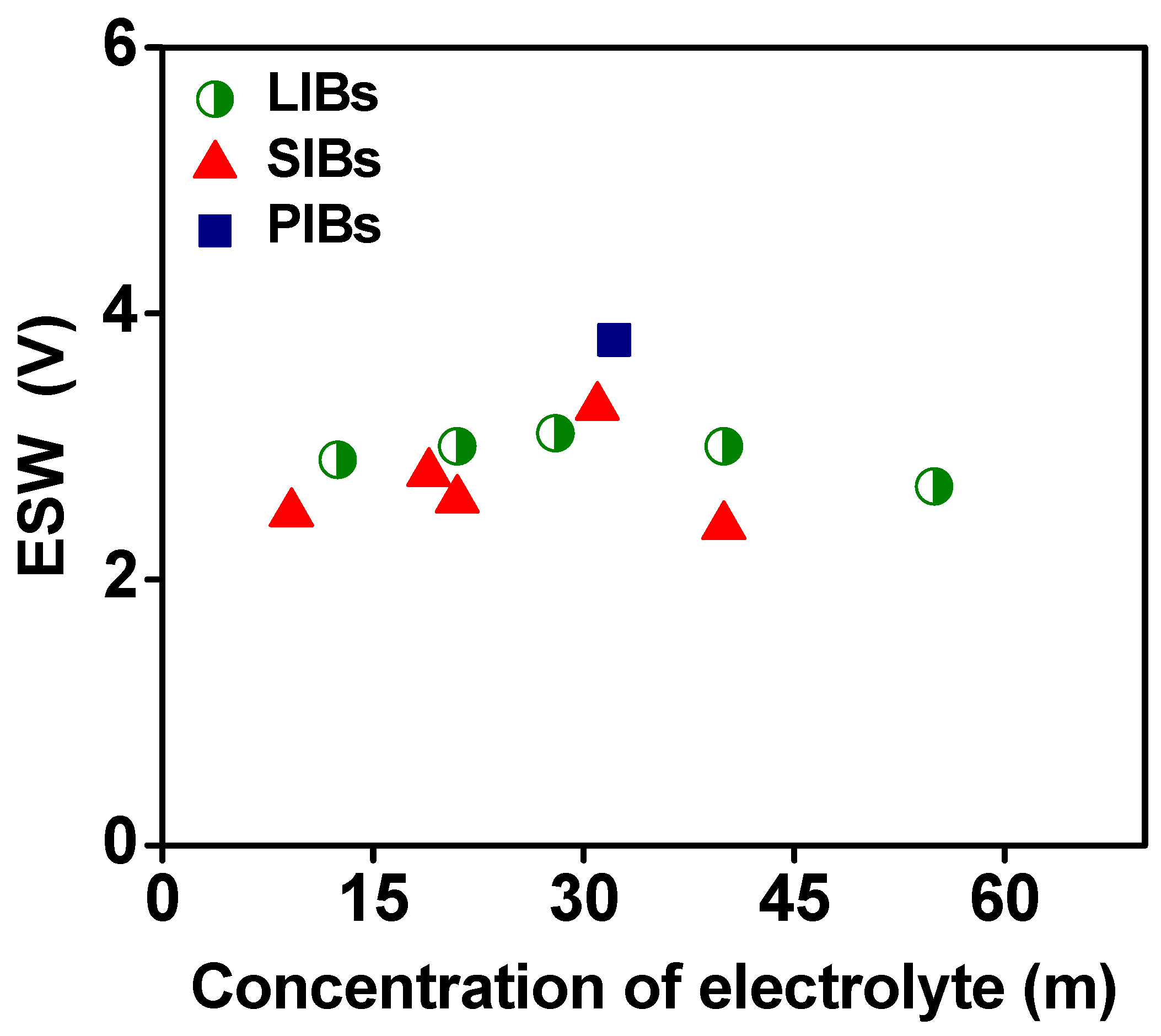
| Battery | Electrolyte Type | Composition | ESW (V) | Anodic-Cathodic Limits (V vs. Ag/AgCl) | Conductivity (mS cm−1) | Reference |
|---|---|---|---|---|---|---|
| LIB | WiSE | 21 m LiTFSI | 3 | −1.33 to 1.66 | 10 | [34] |
| WiBS | 21 m LiTFSI + 7 m LiOTf | 3.1 | −1.4 to 1.66 | 6.5 | [58] | |
| Mixed cation | 32 m KOAc + 8 m LiOAc | 3 | −1.3 to 1.7 | 5.3 | [20] | |
| Monohydrate melt | 55 m Li(PTFSI)0.6(TFSI)0.4 | 2.7 | −0.887 to 1.81 | 0.1 | [92] | |
| Inert diluent | 12.5 m LiNO3 + PD | 2.9 | −0.937 to 1.96 | 0.116 | [79] | |
| SIB | WiSE | 9.2 m NaOTf | 2.5 | −1.207 to 1.293 | 50 | [80] |
| WiSE | 21 m NaTFSI | 2.6 | −1.15 to 1.45 | 8 | [98] | |
| WiSE | 15 m NaClO4 | - | - | 70 | [110] | |
| Mixed anion | 17 m NaClO4 + 2 m NaOTf | 2.8 | −1.3 to 1.493 | 95.25 | [102] | |
| Mixed cation | 32 m Kac + 8 m NaAc | - | - | 12 | [99] | |
| Inert cation | 9 m NaOTf + 22 m TEAOTf | 3.3 | −1.7 to 1.6 | 11.2 | [101] | |
| PIB | WiSE | 32.2 m KCTFSI | 3.8 | −1.59 to 2.21 | 28 | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, R.N.; Madikere Raghunatha Reddy, A.K.; Goulet, M.-A.; Zaghib, K. Water-in-Salt Electrolytes: Advances and Chemistry for Sustainable Aqueous Monovalent-Metal-Ion Batteries. Batteries 2025, 11, 120. https://doi.org/10.3390/batteries11040120
Mishra RN, Madikere Raghunatha Reddy AK, Goulet M-A, Zaghib K. Water-in-Salt Electrolytes: Advances and Chemistry for Sustainable Aqueous Monovalent-Metal-Ion Batteries. Batteries. 2025; 11(4):120. https://doi.org/10.3390/batteries11040120
Chicago/Turabian StyleMishra, Rashmi Nidhi, Anil Kumar Madikere Raghunatha Reddy, Marc-Antoni Goulet, and Karim Zaghib. 2025. "Water-in-Salt Electrolytes: Advances and Chemistry for Sustainable Aqueous Monovalent-Metal-Ion Batteries" Batteries 11, no. 4: 120. https://doi.org/10.3390/batteries11040120
APA StyleMishra, R. N., Madikere Raghunatha Reddy, A. K., Goulet, M.-A., & Zaghib, K. (2025). Water-in-Salt Electrolytes: Advances and Chemistry for Sustainable Aqueous Monovalent-Metal-Ion Batteries. Batteries, 11(4), 120. https://doi.org/10.3390/batteries11040120







