The Use of Cognate Cosolvent to Mediate Localized High-Concentration Electrolytes for High-Voltage and Long-Cycling Lithium-Metal Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrolyte Preparation
2.2. Symmetric Li/Li Cell and Li/Cu Cell
2.3. Preparation of NMC811 Cathodes
3. Results and Discussion
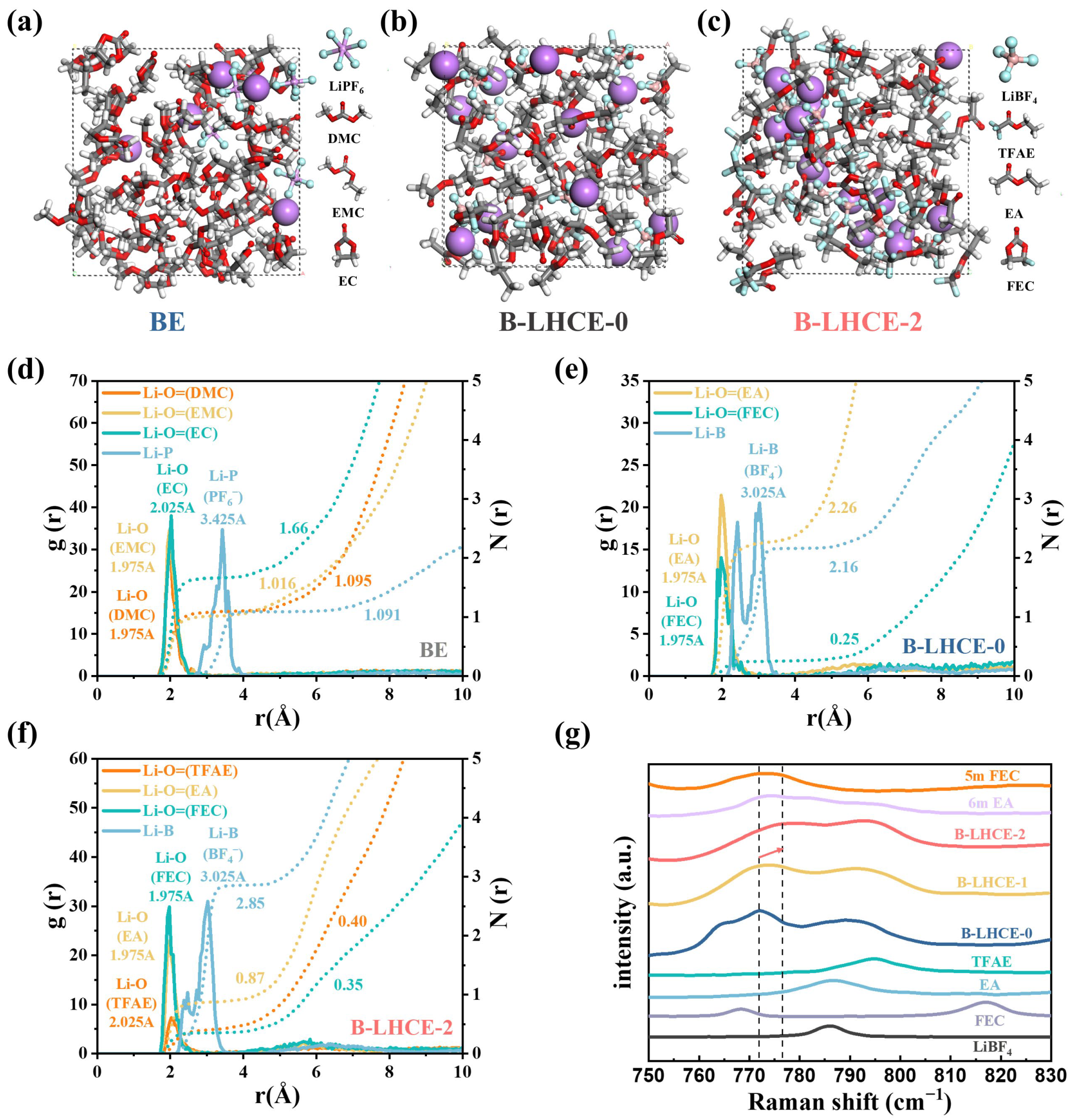
3.1. Physical Properties and Solvation Structures
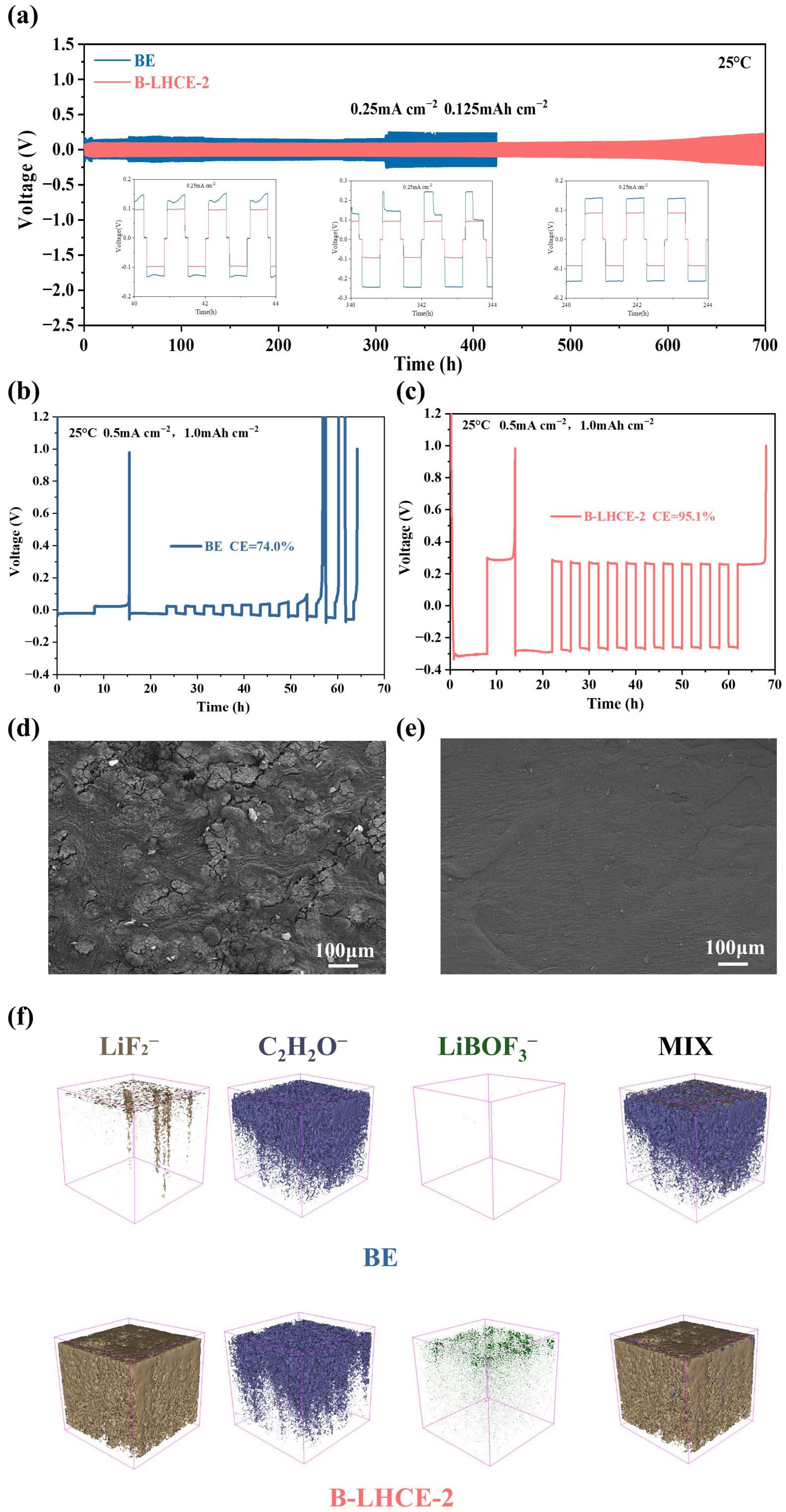
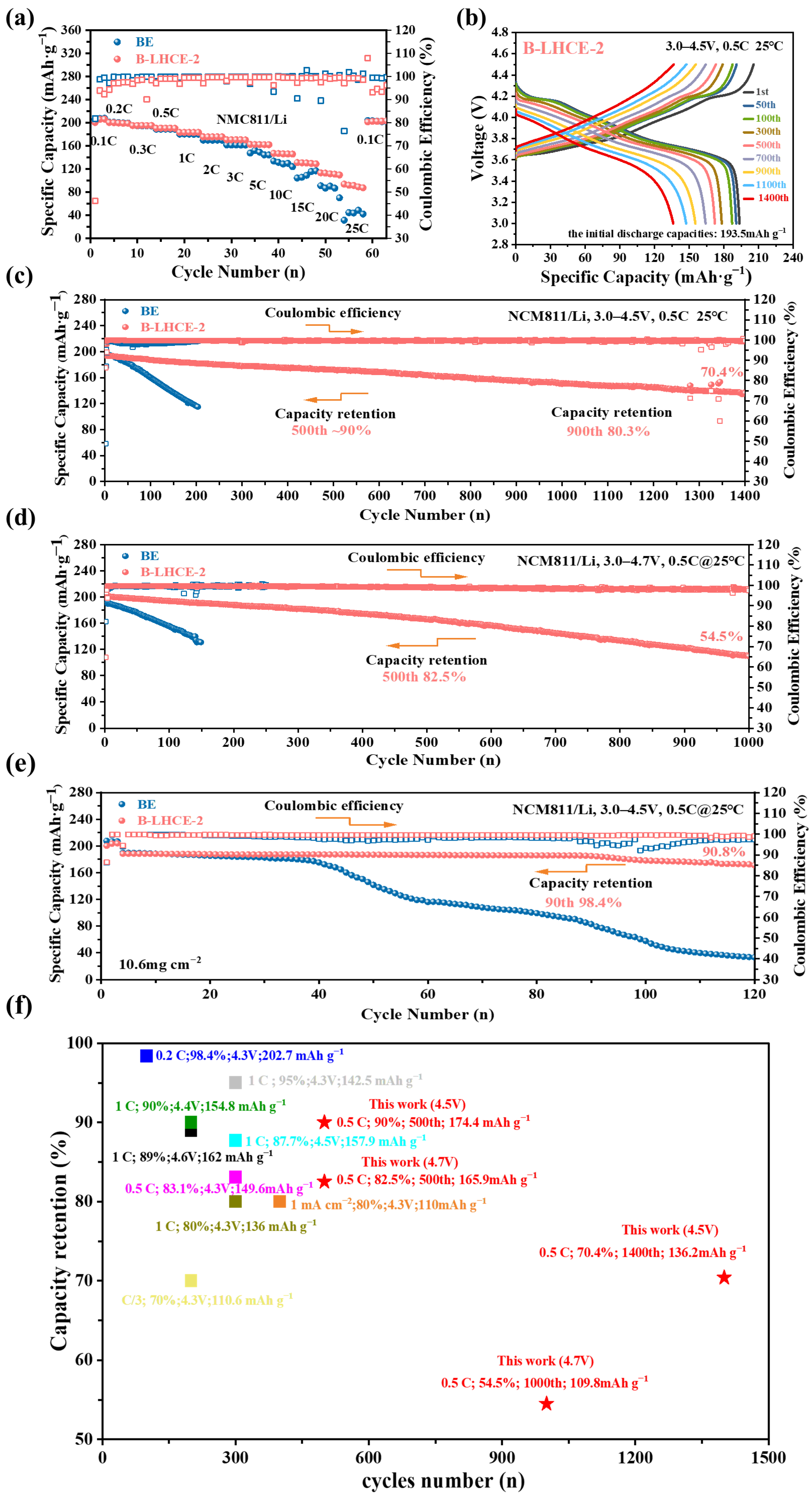
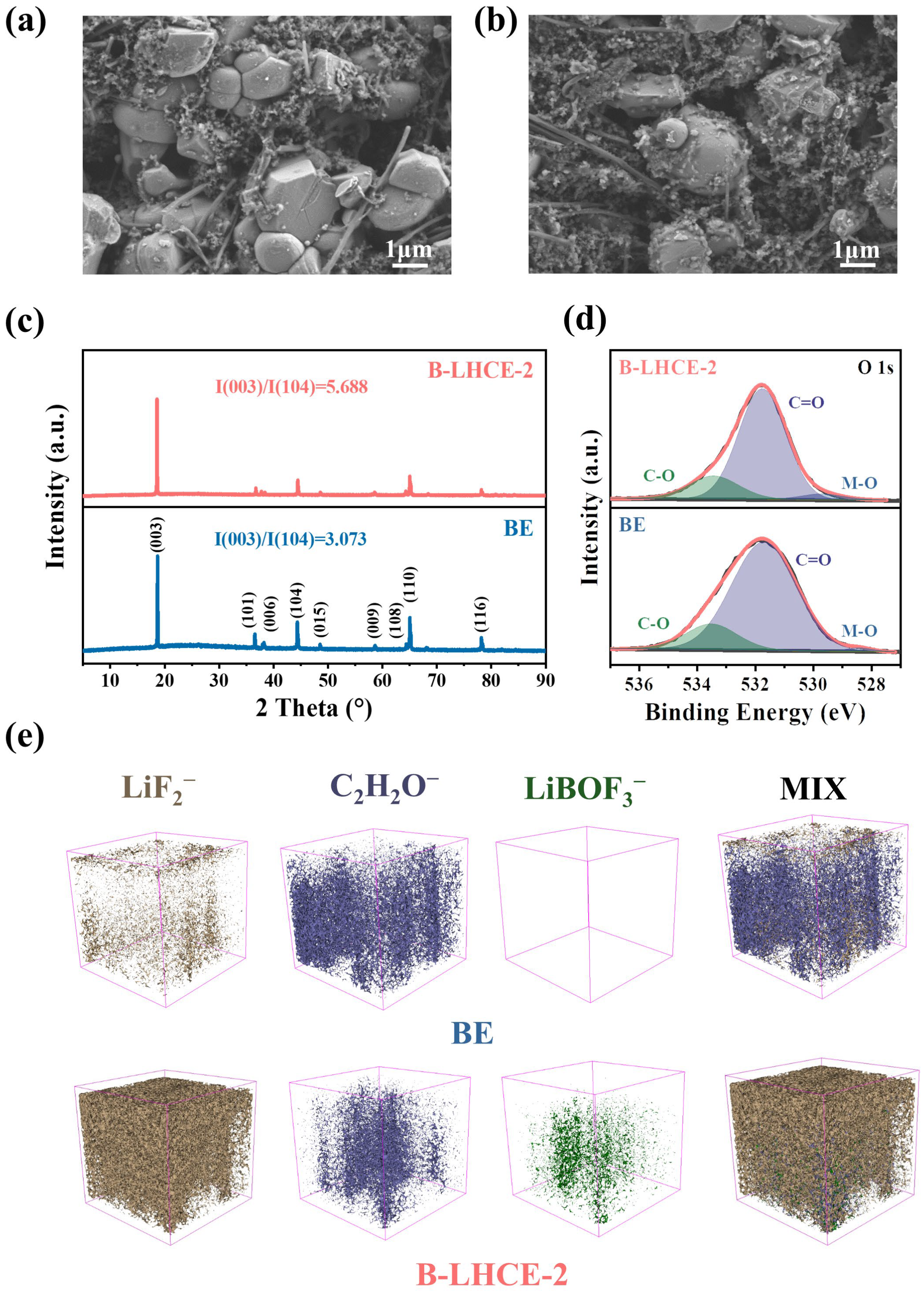
3.2. Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xue, W.; Huang, M.; Li, Y.; Zhu, Y.G.; Gao, R.; Xiao, X.; Zhang, W.; Li, S.; Xu, G.; Yu, Y.; et al. Ultra-high-voltage Ni-rich layered cathodes in practical Li metal batteries enabled by a sulfonamide-based electrolyte. Nat. Energy 2021, 6, 495–505. [Google Scholar] [CrossRef]
- He, R.; Deng, K.; Mo, D.; Guan, X.; Hu, Y.; Yang, K.; Yan, Z.; Xie, H. Active Diluent-Anion Synergy Strategy Regulating Nonflammable Electrolytes for High-Efficiency Li Metal Batteries. Angew. Chem. Int. Ed. 2024, 63, e202317176. [Google Scholar] [CrossRef] [PubMed]
- Doi, T.; Shimizu, Y.; Hashinokuchi, M.; Inaba, M. Dilution of Highly Concentrated LiBF4/Propylene Carbonate Electrolyte Solution with Fluoroalkyl Ethers for 5-V LiNi0.5Mn1.5O4 Positive Electrodes. J. Electrochem. Soc. 2017, 164, A6412–A6416. [Google Scholar] [CrossRef]
- Chen, S.R.; Zheng, J.M.; Mei, D.H.; Han, K.S.; Engelhard, M.H.; Zhao, W.G.; Xu, W.; Liu, J.; Zhang, J.G. High-Voltage Lithium-Metal Batteries Enabled by Localized High-Concentration Electrolytes. Adv. Mater. 2018, 30, 1706102. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shen, X.; Luo, L.; Chen, H.; Cao, R.; Feng, X.; Chen, W.; Fang, Y.; Cao, Y. Correlating the Solvating Power of Solvents with the Strength of Ion-Dipole Interaction in Electrolytes of Lithium-ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202312373. [Google Scholar] [CrossRef]
- Fan, X.; Ou, X.; Zhao, W.; Liu, Y.; Zhang, B.; Zhang, J.; Zou, L.; Seidl, L.; Li, Y.; Hu, G.; et al. In situ inorganic conductive network formation in high-voltage single-crystal Ni-rich cathodes. Nat. Commun. 2021, 12, 5320. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Xiao, B.; Liu, P.; Huang, T.; Li, Y.; Ren, X.; Zhang, Q.; Liu, J.; Ouyang, X.; et al. Challenges and approaches of single-crystal Ni-rich layered cathodes in lithium batteries. Natl. Sci. Rev. 2023, 10, nwad252. [Google Scholar] [CrossRef]
- Su, L.; Jarvis, K.; Charalambous, H.; Dolocan, A.; Manthiram, A. Stabilizing High-Nickel Cathodes with High-Voltage Electrolytes. Adv. Funct. Mater. 2023, 33, 2213675. [Google Scholar] [CrossRef]
- Zhao, W.A.; Wang, K.; Fan, X.M.; Ren, F.C.; Xu, X.Y.; Liu, Y.Y.; Xiong, S.Z.; Liu, X.S.; Zhang, Z.F.; Si, M.Y.; et al. Quantifying Degradation Parameters of Single-Crystalline Ni-Rich Cathodes in Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2023, 62, e202305281. [Google Scholar] [CrossRef]
- Doi, T.; Fujii, R.; Aoki, Y.; Nagashima, T.; Takehara, K.; Inaba, M. Physicochemical Features of Fluorinated Ethyl Acetate-Based Highly Concentrated Electrolyte Solutions and Their Effects on Electrochemical Properties of LiNi0.8Co0.1Mn0.1O2 Positive Electrodes. J. Phys. Chem. C 2021, 125, 12578–12584. [Google Scholar] [CrossRef]
- Zheng, J.; Lochala, J.A.; Kwok, A.; Deng, Z.D.; Xiao, J. Research Progress towards Understanding the Unique Interfaces Between Concentrated Electrolytes and Electrodes for Energy Storage Applications. Adv. Sci. 2017, 4, 1700032. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Fang, M.; Ke, C.; Liu, S.; Wang, J. Design of Localized High-Concentration Electrolytes via Donor Number. ACS Energy Lett. 2023, 8, 1723–1734. [Google Scholar] [CrossRef]
- Cao, X.; Jia, H.; Xu, W.; Zhang, J.-G. Review—Localized High-Concentration Electrolytes for Lithium Batteries. J. Electrochem. Soc. 2021, 168, 010522. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, W.; Chen, Y.; Mondonico, L.; Xiao, X.; Zheng, Y.; Liu, F.; Hung, S.T.; Cui, Y.; Bao, Z. Tuning Fluorination of Linear Carbonate for Lithium-Ion Batteries. J. Electrochem. Soc. 2022, 169, 040555. [Google Scholar] [CrossRef]
- Zheng, Q.; Yamada, Y.; Shang, R.; Ko, S.; Lee, Y.-Y.; Kim, K.; Nakamura, E.; Yamada, A. A cyclic phosphate-based battery electrolyte for high voltage and safe operation. Nat. Energy 2020, 5, 291–298. [Google Scholar] [CrossRef]
- Hou, W.; Zhu, D.; Ma, S.; Yang, W.; Yan, H.; Dai, Y. High-voltage nickel-rich layered cathodes in lithium metal batteries enabled by a sulfolane / fluorinated ether/ fluoroethylene carbonate-based electrolyte design. J. Power Sources 2022, 517, 230683. [Google Scholar] [CrossRef]
- Ren, X.; Chen, S.; Lee, H.; Mei, D.; Engelhard, M.H.; Burton, S.D.; Zhao, W.; Zheng, J.; Li, Q.; Ding, M.S.; et al. Localized High-Concentration Sulfone Electrolytes for High-Efficiency Lithium-Metal Batteries. Chem 2018, 4, 1877–1892. [Google Scholar] [CrossRef]
- Lee, S.; Park, K.; Koo, B.; Park, C.; Jang, M.; Lee, H.; Lee, H. Safe, Stable Cycling of Lithium Metal Batteries with Low-Viscosity, Fire-Retardant Locally Concentrated Ionic Liquid Electrolytes. Adv. Funct. Mater. 2020, 30, 2003132. [Google Scholar] [CrossRef]
- Takada, K.; Yamada, Y.; Yamada, A. Optimized Nonflammable Concentrated Electrolytes by Introducing a Low-Dielectric Diluent. ACS Appl. Mater. Interfaces 2019, 11, 35770–35776. [Google Scholar] [CrossRef]
- Cao, S.; Wen, F.; Ren, X.; Cao, Y.; Ai, X.; Xu, F. Nonflammable dual-salt localized high-concentration electrolyte for graphite/LiNi0.8Co0.1Mn0.1O2 lithium-ion batteries: Li+ solvation structure and interphase. J. Power Sources 2023, 555, 232392. [Google Scholar] [CrossRef]
- Yu, L.; Chen, S.; Lee, H.; Zhang, L.; Engelhard, M.H.; Li, Q.; Jiao, S.; Liu, J.; Xu, W.; Zhang, J.-G. A Localized High-Concentration Electrolyte with Optimized Solvents and Lithium Difluoro(oxalate)borate Additive for Stable Lithium Metal Batteries. ACS Energy Lett. 2018, 3, 2059–2067. [Google Scholar] [CrossRef]
- Jiang, Z.; Mo, J.; Li, C.; Li, H.; Zhang, Q.; Zeng, Z.; Xie, J.; Li, Y. Anion-Regulated Weakly Solvating Electrolytes for High-Voltage Lithium Metal Batteries. Energy Environ. Mater. 2022, 6, e12440. [Google Scholar] [CrossRef]
- Yoo, D.J.; Liu, Q.; Cohen, O.; Kim, M.; Persson, K.A.; Zhang, Z. Rational Design of Fluorinated Electrolytes for Low Temperature Lithium-Ion Batteries. Adv. Energy Mater. 2023, 13, 2204182. [Google Scholar] [CrossRef]
- Nagashima, T.; Aoki, Y.; Kimura, K.; Inaba, M.; Doi, T. High-Capacity LiNi0.8Co0.1Mn0.1O2 Positive-Electrodes in the Nearly Saturated and Fluorinated Acetate-Diluted Electrolyte Solutions. ACS Appl. Energy Mater. 2024, 7, 2707–2714. [Google Scholar] [CrossRef]
- Bedoch, A.M.; Koga, G.Y.; Nogueira, R.P.; Zepon, G. On the Anomalous Behavior of the Charge Transfer Resistance of the Hydrogen Evolution Reaction at Low Overpotentials and Its Relationship with Hydrogen Absorption in Metals. J. Phys. Chem. C 2023, 127, 12444–12453. [Google Scholar] [CrossRef]
- Cao, X.; Gao, P.; Ren, X.; Zou, L.; Engelhard, M.H.; Matthews, B.E.; Hu, J.; Niu, C.; Liu, D.; Arey, B.W.; et al. Effects of fluorinated solvents on electrolyte solvation structures and electrode/electrolyte interphases for lithium metal batteries. Proc. Natl. Acad. Sci. USA 2021, 118, e2020357118. [Google Scholar] [CrossRef]
- Borodin, O.; Olguin, M.; Ganesh, P.; Kent, P.R.; Allen, J.L.; Henderson, W.A. Competitive lithium solvation of linear and cyclic carbonates from quantum chemistry. Phys. Chem. Chem. Phys. 2016, 18, 164–175. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes, Interfaces and Interphases; The Royal Society of Chemistry: London, UK, 2023; p. 471. [Google Scholar]
- Su, C.-C.; He, M.; Amine, R.; Rojas, T.; Cheng, L.; Ngo, A.T.; Amine, K. Solvating power series of electrolyte solvents for lithium batteries. Energy Environ. Sci. 2019, 12, 1249–1254. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, C.; Wang, J.; Yao, Z.; Wang, S.; Kumar, S.G.H.; Ganapathy, S.; Eustace, S.; Bai, X.; Li, B.; et al. High entropy liquid electrolytes for lithium batteries. Nat. Commun. 2023, 14, 440. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Chen, M.; Pan, Z.; Ding, Y.; Song, Z.; Ai, X.; Cao, Y.; Chen, Z. “Dragging effect” induced fast desolvation kinetics and −50 °C workable high-safe lithium batteries. Energy Storage Mater. 2024, 65, 103098. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, W.; Xu, J.; Xu, Y.; Yang, Z.; Yoo, D.-J.; Pupek, K.Z.; Wang, C.; Liu, C.; Xu, K.; et al. A fluorinated cation introduces new interphasial chemistries to enable high-voltage lithium metal batteries. Nat. Commun. 2023, 14, 3678. [Google Scholar] [CrossRef]
- Dai, Y.; Zhuang, M.; Deng, Y.-X.; Liao, Y.; Gu, J.; Song, T.; Yan, H.; Zheng, J.-C. Stable Cycling of All-Solid-State Lithium Batteries Enabled by Cyano-Molecular Diamond Improved Polymer Electrolytes. Nano Micro Lett. 2024, 16, 217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Jiao, X.; Wang, W.; Chen, H.; Li, F. Localized high-concentration electrolyte enabled by a novel ester diluent for lithium metal batteries. Chem. Commun. 2023, 59, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, X.; Zhai, B.; Zeng, Z.; Hu, W.; Lei, S.; Zhang, H.; Cheng, S.; Xie, J. Diluted High-Concentration Electrolyte Based on Phosphate for High-Performance Lithium-Metal Batteries. Batter. Supercaps 2022, 5, e202100407. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Sun, Y.; Qian, Z.; Wang, R. New Insights on the Good Compatibility of Ether-Based Localized High-Concentration Electrolyte with Lithium Metal. ACS Mater. Lett. 2021, 3, 838–844. [Google Scholar] [CrossRef]
- Cao, Z.; Haruta, M.; Doi, T.; Inaba, M. Dilution Effects of Highly Concentrated LiBF4/DMC with Fluorinated Esters on Charge/Dishcharge Properties of Ni-rich LiNi0.8Co0.1Mn0.1O2 Positive Electrode. J. Electrochem. Soc. 2020, 167, 040508. [Google Scholar] [CrossRef]
- Holoubek, J.; Kim, K.; Yin, Y.; Wu, Z.; Liu, H.; Li, M.; Chen, A.; Gao, H.; Cai, G.; Pascal, T.A.; et al. Electrolyte design implications of ion-pairing in low-temperature Li metal batteries. Energy Environ. Sci. 2022, 15, 1647–1658. [Google Scholar] [CrossRef]
- Lin, S.; Hua, H.; Lai, P.; Zhao, J. A Multifunctional Dual-Salt Localized High-Concentration Electrolyte for Fast Dynamic High-Voltage Lithium Battery in Wide Temperature Range. Adv. Energy Mater. 2021, 11, 2101775. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; You, Y.; Manthiram, A. Extending the Service Life of High-Ni Layered Oxides by Tuning the Electrode–Electrolyte Interphase. Adv. Energy Mater. 2018, 8, 1801957. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, X.; Sun, X.; Zhang, J.N.; Yu, X.; Li, H. Investigations on the Fundamental Process of Cathode Electrolyte Interphase Formation and Evolution of High-Voltage Cathodes. ACS Appl. Mater. Interfaces 2020, 12, 2319–2326. [Google Scholar] [CrossRef]
- Xia, L.; Lee, S.; Jiang, Y.; Li, S.; Liu, Z.; Yu, L.; Hu, D.; Wang, S.; Liu, Y.; Chen, G.Z. Physicochemical and Electrochemical Properties of 1,1,2,2-Tetrafluoroethyl-2,2,3,3-Tetrafluoropropyl Ether as a Co-Solvent for High-Voltage Lithium-Ion Electrolytes. ChemElectroChem 2019, 6, 3747–3755. [Google Scholar] [CrossRef]
- Xu, Z.; Deng, K.; Zhou, S.; Mo, D. High-performance lithium metal batteries enabled by fluorinated aromatic diluent assisted nonflammable localized high-concentration electrolytes. J. Power Sources 2023, 559, 232631. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, Y.; Yang, Z.; Song, D.; Sun, X.; Wang, C.; Yang, L.; Zhang, Y.; Gao, J.; Ohsaka, T.; et al. A fluorinated electrolyte stabilizing high-voltage graphite/NCM811 batteries with an inorganic-rich electrode-electrolyte interface. Chem. Eng. J. 2022, 440, 135939. [Google Scholar] [CrossRef]
- Zheng, X.; Liao, Y.; Zhang, Z.; Zhu, J.; Ren, F.; He, H.; Xiang, Y.; Zheng, Y.; Yang, Y. Exploring high-voltage fluorinated carbonate electrolytes for LiNi0.5Mn1.5O4 cathode in Li-ion batteries. J. Energy Chem. 2020, 42, 62–70. [Google Scholar] [CrossRef]
- Flamme, B.; Światowska, J.; Haddad, M.; Phansavath, P.; Ratovelomanana-Vidal, V.; Chagnes, A. Sulfone Based-Electrolytes for Lithium-Ion Batteries: Cycling Performances and Passivation Layer Quality of Graphite and LiNi1/3Mn1/3Co1/3O2 Electrodes. J. Electrochem. Soc. 2020, 167, 070508. [Google Scholar] [CrossRef]
- Chen, L.; Lu, J.; Wang, Y.; He, P.; Huang, S.; Liu, Y.; Wu, Y.; Cao, G.; Wang, L.; He, X.; et al. Double-salt electrolyte for Li-ion batteries operated at elevated temperatures. Energy Storage Mater. 2022, 49, 493–501. [Google Scholar] [CrossRef]
- Lu, D.; Xu, G.; Hu, Z.; Cui, Z.; Wang, X.; Li, J.; Huang, L.; Du, X.; Wang, Y.; Ma, J.; et al. Deciphering the Interface of a High-Voltage (5 V-Class) Li-Ion Battery Containing Additive-Assisted Sulfolane-Based Electrolyte. Small Methods 2019, 3, 1900546. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Wang, D.; Yu, Q.; He, Z.; Deng, F.; Yan, H.; Song, T.; Zheng, J.-C.; Dai, Y. The Use of Cognate Cosolvent to Mediate Localized High-Concentration Electrolytes for High-Voltage and Long-Cycling Lithium-Metal Batteries. Batteries 2025, 11, 156. https://doi.org/10.3390/batteries11040156
Hu Y, Wang D, Yu Q, He Z, Deng F, Yan H, Song T, Zheng J-C, Dai Y. The Use of Cognate Cosolvent to Mediate Localized High-Concentration Electrolytes for High-Voltage and Long-Cycling Lithium-Metal Batteries. Batteries. 2025; 11(4):156. https://doi.org/10.3390/batteries11040156
Chicago/Turabian StyleHu, Ying, Dandan Wang, Qijie Yu, Ziyi He, Fengrui Deng, Hao Yan, Tinglu Song, Jin-Cheng Zheng, and Yang Dai. 2025. "The Use of Cognate Cosolvent to Mediate Localized High-Concentration Electrolytes for High-Voltage and Long-Cycling Lithium-Metal Batteries" Batteries 11, no. 4: 156. https://doi.org/10.3390/batteries11040156
APA StyleHu, Y., Wang, D., Yu, Q., He, Z., Deng, F., Yan, H., Song, T., Zheng, J.-C., & Dai, Y. (2025). The Use of Cognate Cosolvent to Mediate Localized High-Concentration Electrolytes for High-Voltage and Long-Cycling Lithium-Metal Batteries. Batteries, 11(4), 156. https://doi.org/10.3390/batteries11040156







