Improved Discharge Capacity of Zinc Particles by Applying Bismuth-Doped Silica Coating for Zinc-Based Batteries
Abstract
:1. Introduction
2. Experimental Setup
2.1. Synthesis of Zinc Composite Material
2.2. Analytical Methods
2.3. Electrochemical Characterization
3. Results and Discussion
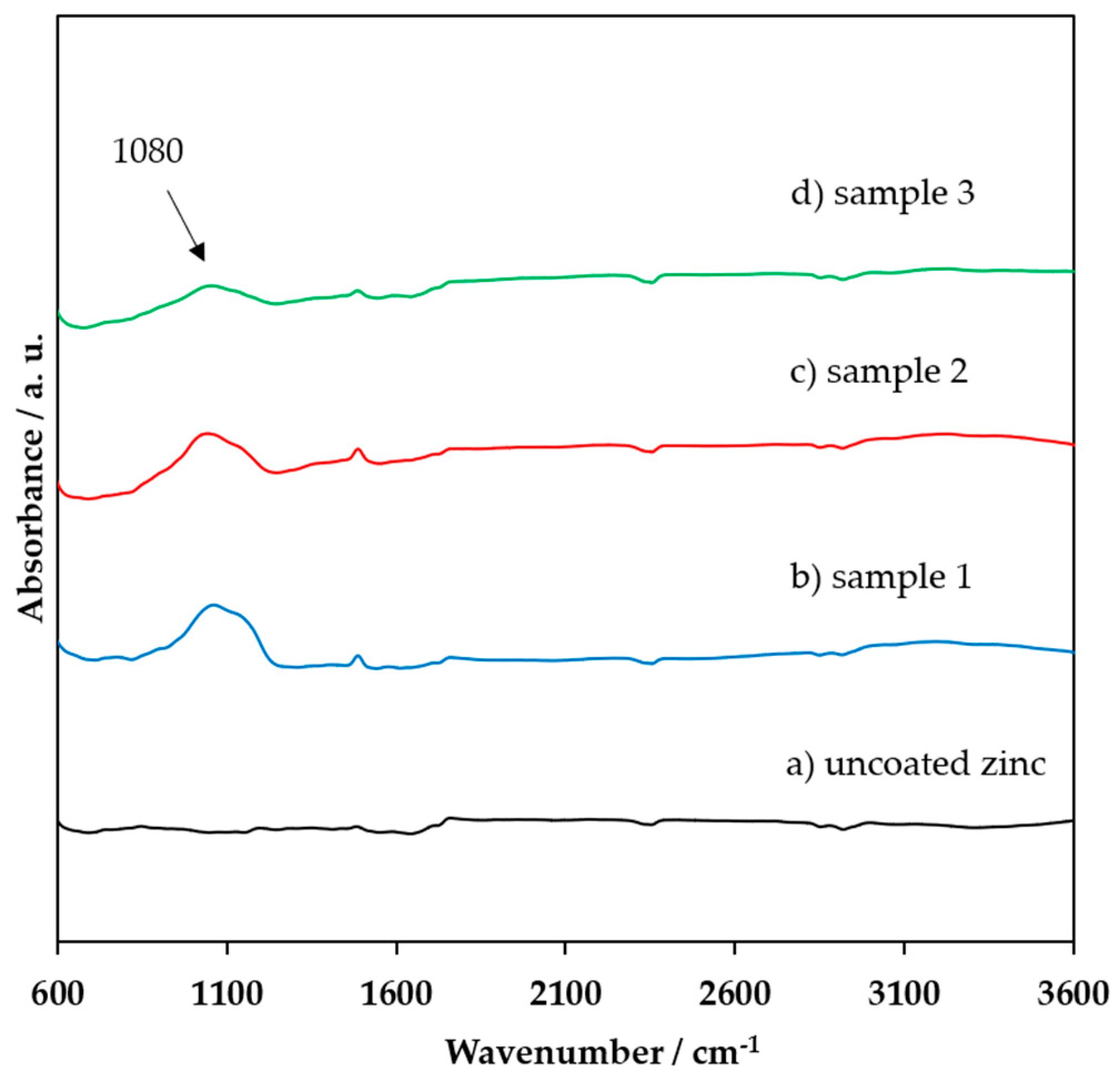
3.1. FTIR, XRD, and XPS Characterization of the Coating
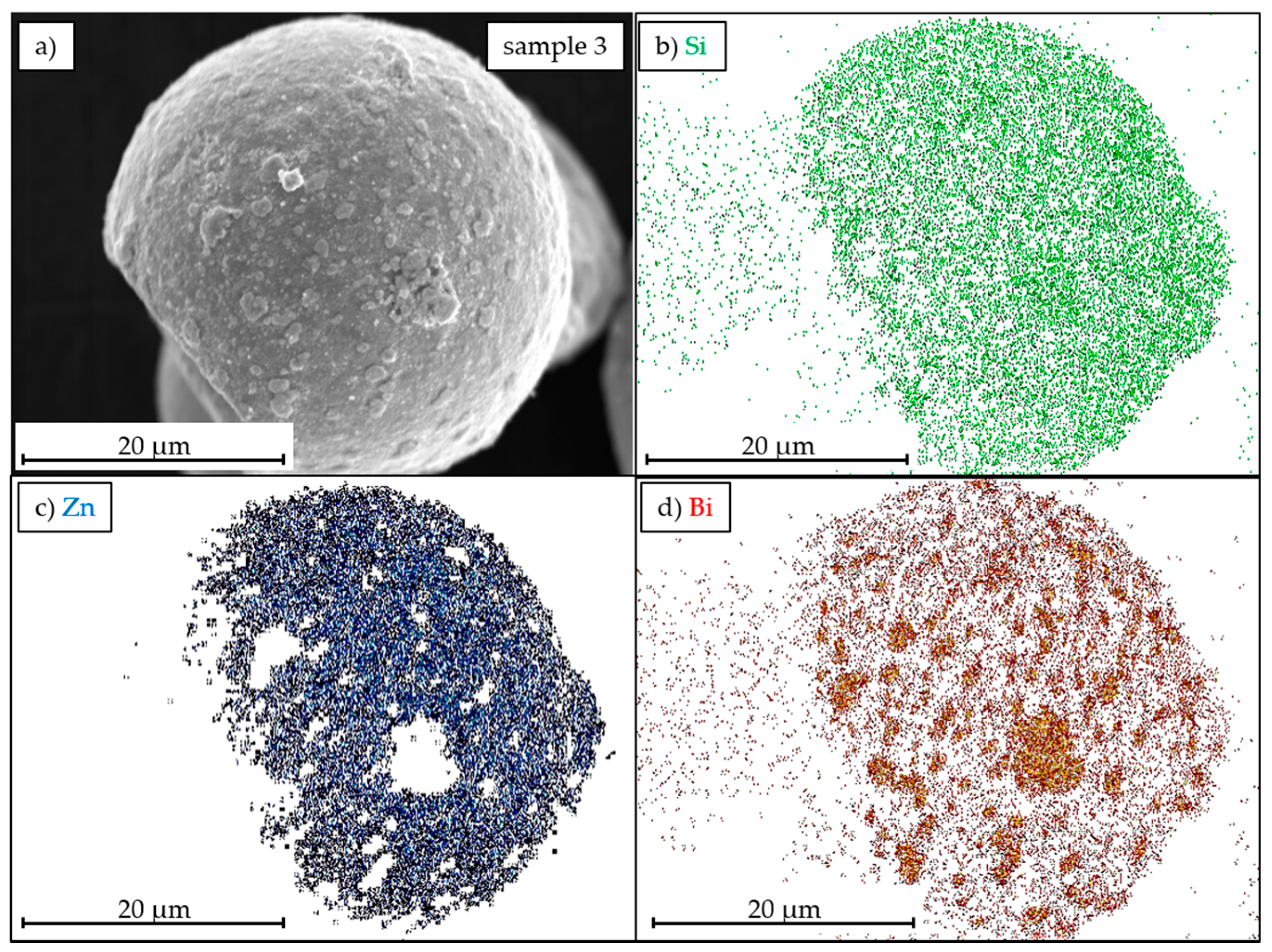
3.2. Morphology Characterization
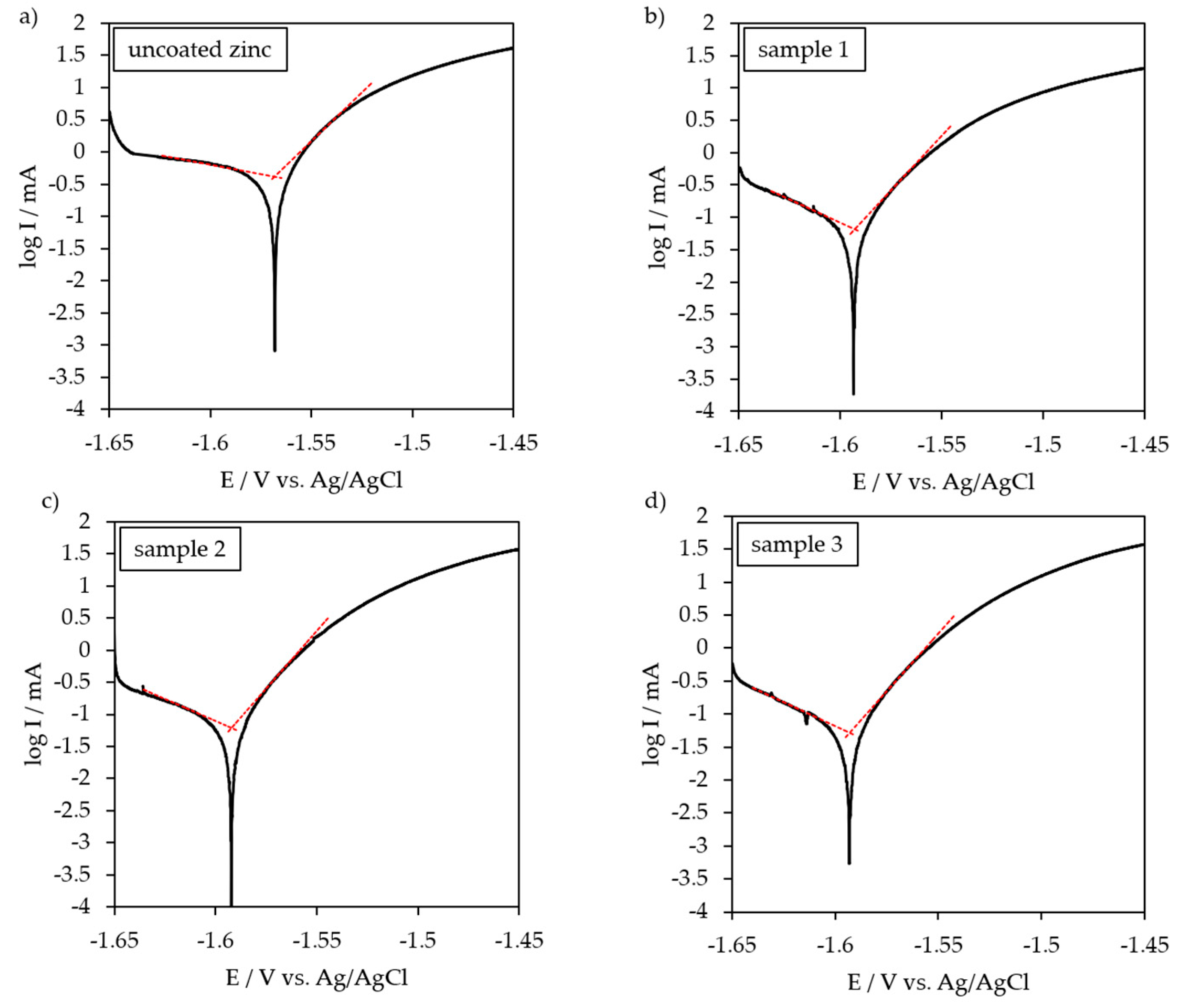
3.3. Potentio-Dynamic Polarization Behavior
3.4. Electrochemical Discharge Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, W.; Xie, C.; Zhang, H.; Li, X. Inhibition of Zinc Dendrite Growth in Zinc-Based Batteries. ChemSusChem 2018, 11, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Liang, P.; Liu, X.; Wu, K.; Liu, Y.; Wang, Y.; Xia, Y.; Zhang, J. Challenges, mitigation strategies and perspectives in development of zinc-electrode materials and fabrication for rechargeable zinc-air batteries. Energy Environ. Sci. 2018, 11, 3075–3095. [Google Scholar] [CrossRef]
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable nickel-3D zinc batteries: An energy-dense, safer alternative to lithium-ion. Science 2017, 356, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Wang, K.; Ma, Z. Technologies for extending zinc-air battery’s cyclelife: A review. Appl. Energy 2014, 128, 315–324. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically rechargeable zinc-air batteries: Progress, challenges, and perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ivey, D.G.; Xie, Z.; Qu, W. Rechargeable Zn-air batteries: Progress in electrolyte development and cell configuration advancement. J. Power Sources 2015, 283, 358–371. [Google Scholar] [CrossRef]
- Larsson, F.; Rytinki, A.; Ahmed, I.; Albinsson, I.; Mellander, B.-E. Overcurrent Abuse of Primary Prismatic Zinc-air Battery Cells Studying Air Supply Effects on Performance and Safety Shut-Down. Batteries 2017, 3, 1. [Google Scholar] [CrossRef]
- Garcia, G.; Schuhmann, W.; Ventosa, E. A Three-Electrode, Battery-Type Swagelok Cell for the Evaluation of Secondary Alkaline Batteries: The Case of the Ni–Zn Battery. ChemElectroChem 2016, 3, 592–597. [Google Scholar] [CrossRef]
- Michlik, T.; Schmid, M.; Rosin, A.; Gerdes, T.; Moos, R. Mechanical Coating of Zinc Particles with Bi2O3-Li2O-ZnO Glasses as Anode Material for Rechargeable Zinc-Based Batteries. Batteries 2018, 4, 12. [Google Scholar] [CrossRef]
- Ma, H.; Wang, B.; Fan, Y.; Hong, W. Development and Characterization of an Electrically Rechargeable Zinc-Air Battery Stack. Energies 2014, 7, 6549–6557. [Google Scholar] [CrossRef] [Green Version]
- Franke-Lang, R.; Arlt, T.; Manke, I.; Kowal, J. X-ray tomography as a powerful method for zinc-air battery research. J. Power Sources 2017, 370, 45–51. [Google Scholar] [CrossRef]
- Stamm, J.; Varzi, A.; Latz, A.; Horstmann, B. Modeling nucleation and growth of zinc oxide during discharge of primary zinc-air batteries. J. Power Sources 2017, 360, 136–149. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Tai Kim, S.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal-air batteries with high energy density: Li–air versus Zn–air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Garcia, G.; Ventosa, E.; Schuhmann, W. Complete prevention of dendrite formation in Zn metal anodes by means of pulsed charging protocols. ACS Appl. Mater. Interfaces 2017, 9, 18691–18698. [Google Scholar] [CrossRef]
- Mainar, A.R.; Colmenares, L.C.; Grande, H.-J.; Blázquez, J.A. Enhancing the Cycle Life of a Zinc-air Battery by Means of Electrolyte Additives and Zinc Surface Protection. Batteries 2018, 4, 46. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, T.; Pulletikurthi, G.; Lahiri, A.; Carstens, T.; Olschewski, M.; Endres, F. Dendrite-Free Nanocrystalline Zinc Electrodeposition from an Ionic Liquid Containing Nickel Triflate for Rechargeable Zn-Based Batteries. Angew. Chem. Int. Ed. 2016, 55, 2889–2893. [Google Scholar] [CrossRef]
- McBreen, J.; Gannon, E. Bismuth oxide as an additive in pasted zinc electrodes. J. Power Sources 1985, 15, 169–177. [Google Scholar] [CrossRef]
- Huang, H.; Gu, S.J.; Gan, Y.P.; Tao, X.Y.; Zhang, W.K. ZnO/ZnO-Bi2O3 Nanocomposite as an Anode Material for Ni-Zn Rechargeable Battery. Adv. Mater. Res. 2012, 396, 1725–1729. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Yu, L.Q.; Wu, H.M.; Yang, J.L.; Chen, Y.B.; Guo, S.Y.; Tu, J.P. Electrochemical performances of Bi based compound film-coated ZnO as anodic materials of Ni-Zn secondary batteries. Electrochim. Acta 2011, 56, 4378–4383. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Miyazaki, K.; Abe, T.; Janek, J.; Schröder, D. Towards zinc-oxygen batteries with enhanced cycling stability: The benefit of anion-exchange ionomer for zinc sponge anodes. J. Power Sources 2018, 395, 195–204. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Walther, F.; Sann, J.; Janek, J.; Schroder, D. Homogeneous Coating with an Anion-Exchange Ionomer Improves the Cycling Stability of Secondary Batteries with Zinc Anodes. ACS Appl. Mater. Interfaces 2018, 10, 8640–8648. [Google Scholar] [CrossRef]
- Clark, S.; Latz, A.; Horstmann, B. Rational Development of Neutral Aqueous Electrolytes for Zinc-Air Batteries. ChemSusChem 2017, 10, 4735–4747. [Google Scholar] [CrossRef]
- Ingale, P.; Sakthivel, M.; Drillet, J.F. Test of Diethylmethylammonium Trifluoromethanesulfonate Ionic Liquid as Electrolyte in Electrically Rechargeable Zn/Air Battery. J. Electrochem. Soc. 2017, 164, 5224–5229. [Google Scholar] [CrossRef]
- Lee, C.W.; Sathiyanarayanan, K.; Eom, S.W.; Yun, M.S. Novel alloys to improve the electrochemical behavior of zinc anodes for zinc/air battery. J. Power Sources 2006, 160, 1436–1441. [Google Scholar] [CrossRef]
- Huot, J.Y.; Boubour, E. Electrochemical performance of gelled zinc alloy powders in alkaline solutions. J. Power Sources 1997, 65, 81–85. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.M.; Zhang, L.; Zhang, J.Q.; Cao, C.N. Study of the performance of secondary alkaline pasted zinc electrodes. J. Appl. Electrochem. 2001, 31, 1049–1054. [Google Scholar] [CrossRef]
- Schmid, M.; Willert-Porada, M. Electrochemical behavior of zinc particles with silica based coatings as anode material for zinc air batteries with improved discharge capacity. J. Power Sources 2017, 351, 115–122. [Google Scholar] [CrossRef]
- Cho, Y.D.; Fey, G.T.K. Surface treatment of zinc anodes to improve discharge capacity and suppress hydrogen gas evolution. J. Power Sources 2008, 184, 610–616. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, Y.J.; Eom, S.W.; Choi, N.S.; Kim, K.W.; Cho, S.B. Improvement in self-discharge of Zn anode by applying surface modification for Zn-air batteries with high energy density. J. Power Sources 2013, 227, 177–184. [Google Scholar] [CrossRef]
- Lucovsky, G.; Yang, J.; Chao, S.S.; Tyler, J.E.; Czubatyj, W. Oxygen-bonding environments in glow-discharge-deposited amorphous silicon-hydrogen alloy films. Phys. Rev. B 1983, 28, 3225. [Google Scholar] [CrossRef]
- Chastain, J.; Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; pp. 190–191. [Google Scholar]






| Parameter | Value | ||
|---|---|---|---|
| Sample Name | Sample 1 | Sample 2 | Sample 3 |
| Schematic illustration |  |  |  |
| Input mass: | |||
| Zinc powder/g | 10 | 10 | 10 |
| Si Precursor/g | 2.08 ( | 2.08 ( | 2.08 ( |
| Bi Precursor/g | - | 0.10 ( | 0.21 ( |
| BET surface area/m2 g−1 | 0.257 ± 0.003 | 0.327 ± 0.003 | 0.448 ± 0.015 |
| Sample Name | Parameter | ||
|---|---|---|---|
| Ecorr in V vs. Ag/AgCl | Icorr in µA | η in % | |
| Uncoated zinc | −1.568 | 419.8 | - |
| Sample 1 | −1.593 | 65.7 | 84.3 |
| Sample 2 | −1.592 | 61.2 | 85.4 |
| Sample 3 | −1.593 | 50.9 | 87.9 |
| Sample Name | Parameter | ||||
|---|---|---|---|---|---|
| c (mAh g−1) 1 h Dwell Time | DoD in % 1 h Dwell Time | c (mAh g−1) 48 h Dwell Time | DoD in % 48 h Dwell Time | Capacity Retention in % | |
| Uncoated zinc | 633 | 77.2 | 461 | 56.2 | 72.8 |
| Sample 1 | 677 | 82.6 | 540 | 65.9 | 79.8 |
| Sample 2 | 713 | 87.0 | 603 | 73.5 | 84.6 |
| Sample 3 | 737 | 89.9 | 670 | 81.7 | 90.9 |
| Parameter | Sample Name | |||
|---|---|---|---|---|
| Uncoated Zinc | Sample 1 | Sample 2 | Sample 3 | |
| Ri/Ω | 1.09 ± 0.07 | 1.10 ± 0.05 | 0.98 ± 0.05 | 0.97 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michlik, T.; Rosin, A.; Gerdes, T.; Moos, R. Improved Discharge Capacity of Zinc Particles by Applying Bismuth-Doped Silica Coating for Zinc-Based Batteries. Batteries 2019, 5, 32. https://doi.org/10.3390/batteries5010032
Michlik T, Rosin A, Gerdes T, Moos R. Improved Discharge Capacity of Zinc Particles by Applying Bismuth-Doped Silica Coating for Zinc-Based Batteries. Batteries. 2019; 5(1):32. https://doi.org/10.3390/batteries5010032
Chicago/Turabian StyleMichlik, Tobias, Andreas Rosin, Thorsten Gerdes, and Ralf Moos. 2019. "Improved Discharge Capacity of Zinc Particles by Applying Bismuth-Doped Silica Coating for Zinc-Based Batteries" Batteries 5, no. 1: 32. https://doi.org/10.3390/batteries5010032
APA StyleMichlik, T., Rosin, A., Gerdes, T., & Moos, R. (2019). Improved Discharge Capacity of Zinc Particles by Applying Bismuth-Doped Silica Coating for Zinc-Based Batteries. Batteries, 5(1), 32. https://doi.org/10.3390/batteries5010032





