EIS Study on the Electrode-Separator Interface Lamination
Abstract
:1. Introduction
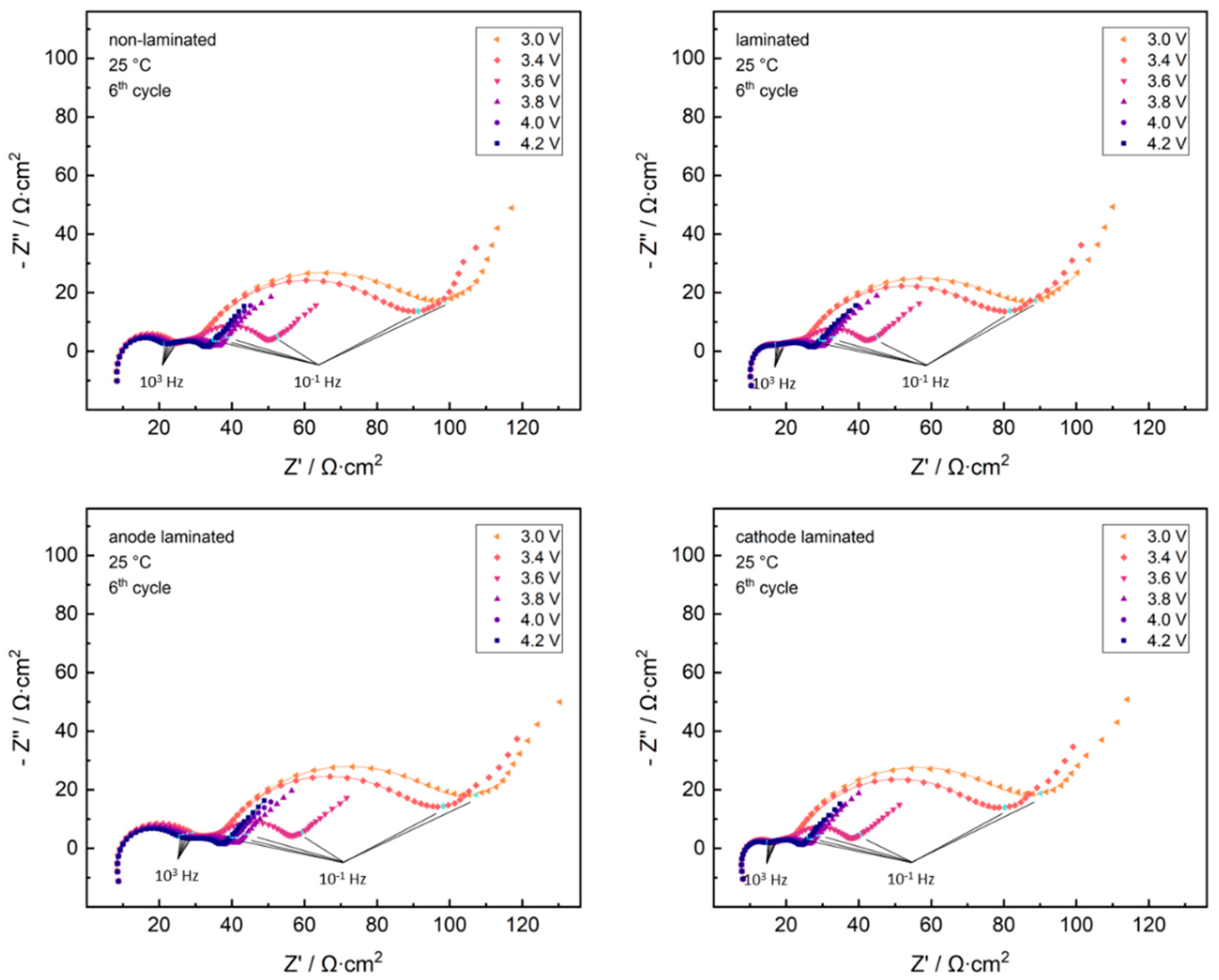
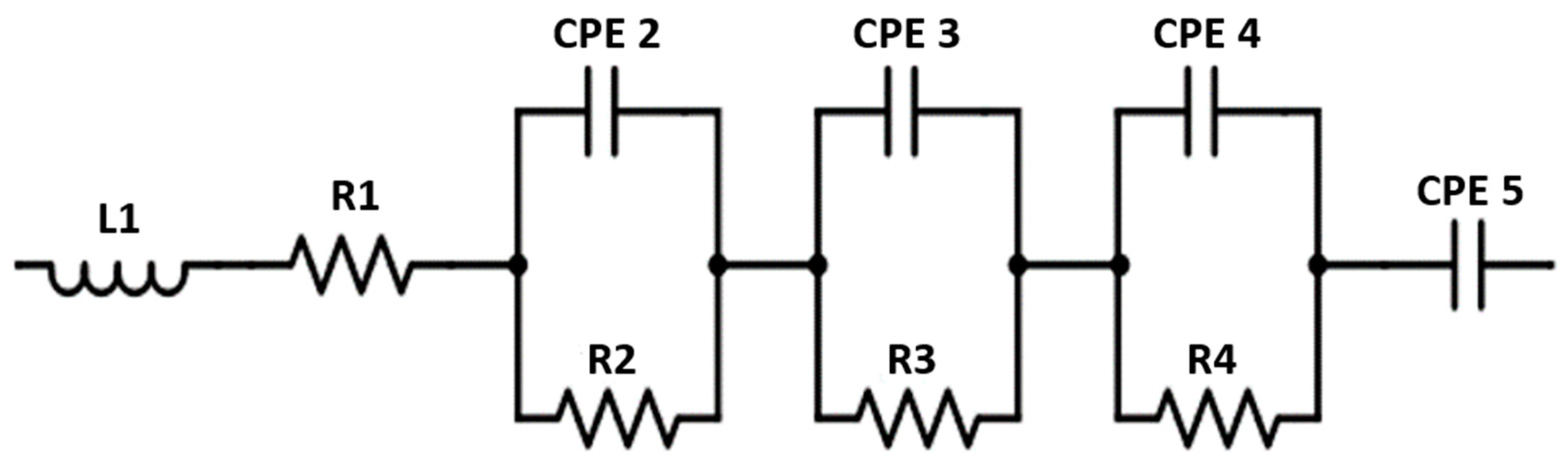
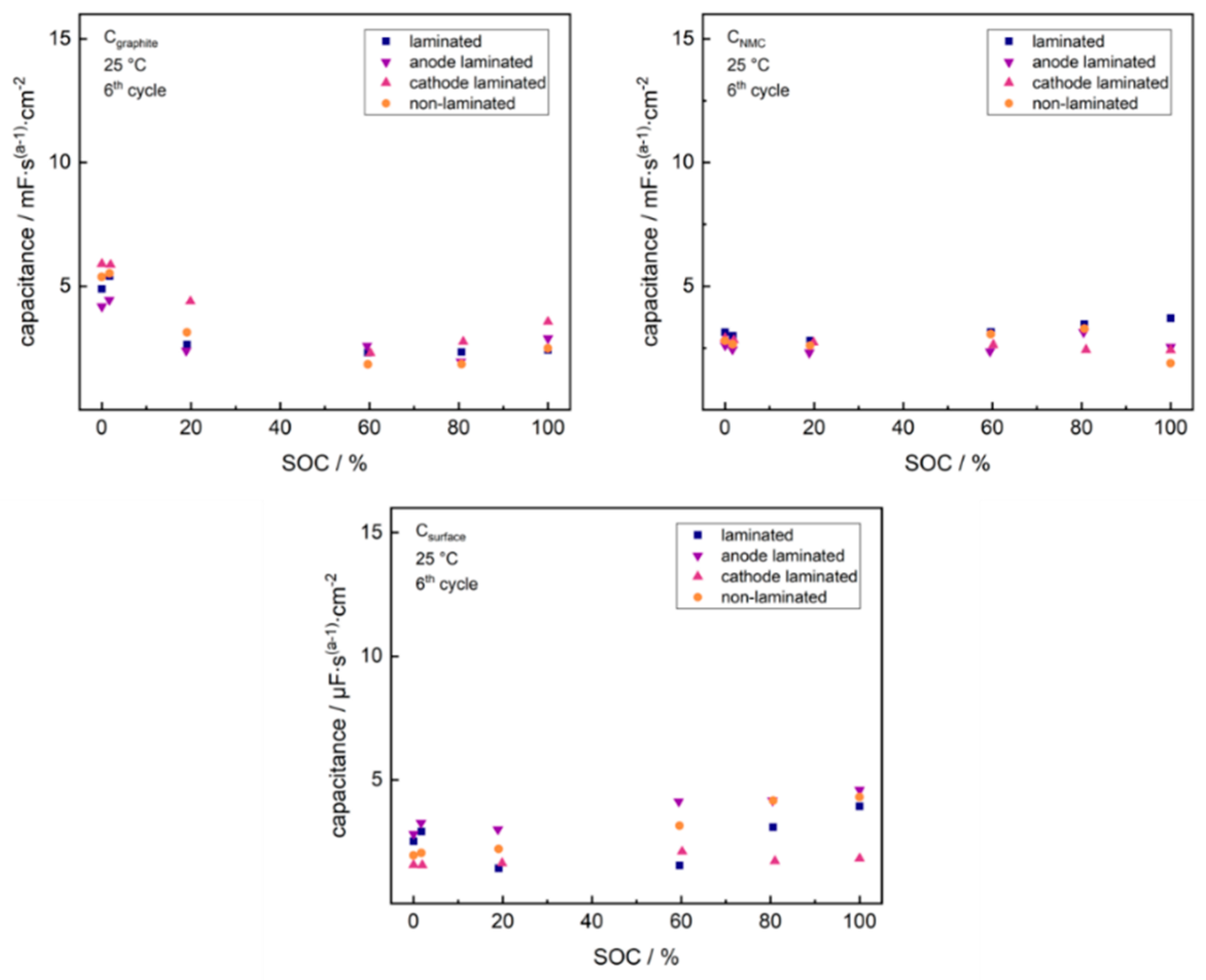
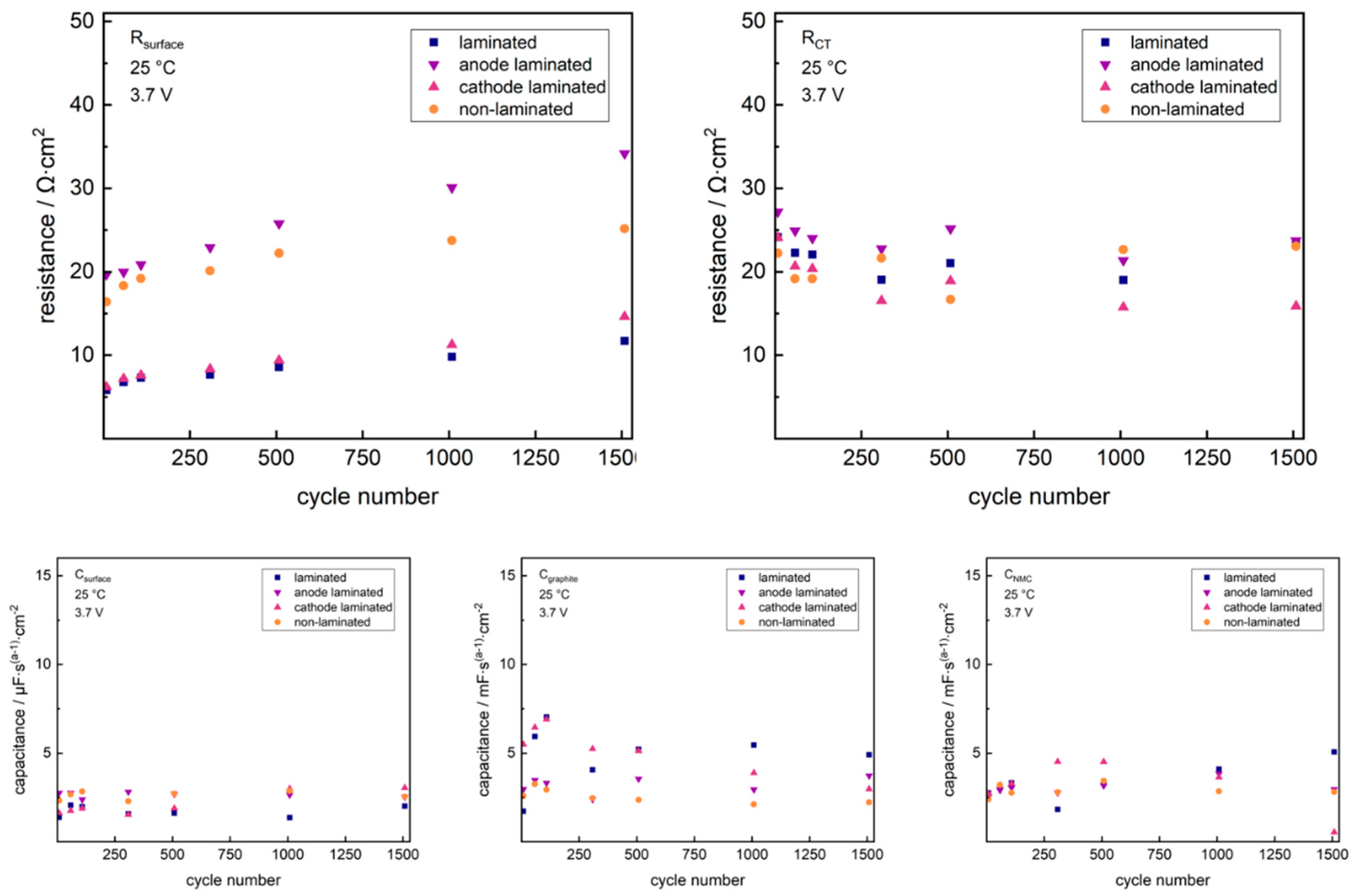
2. Results and Discussion
2.1. Cathode-Separator Lamination Effects
2.2. Anode-Separator Lamination Effects
3. Materials and Methods
3.1. Electrode Preparation
3.2. Pouch Cell Preparation
3.3. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geng, X.; Guo, H.; Wang, C.; Cheng, M.; Li, Y.; Zhang, H.; Huo, H. Surface modification of Li1.20Mn0.54Ni0.13Co0.13O2 cathode materials with SmF3 and the improved electrochemical properties. J. Mater. Sci. Mater. Electron. 2018, 29, 19207–19218. [Google Scholar] [CrossRef]
- Lee, M.-J.; Lho, E.; Oh, P.; Son, Y.; Cho, J. Simultaneous surface modification method for 0.4Li2MnO3-0.6LiNi1/3Co1/3Mn1/3O2 cathode material for lithium ion batteries: Acid treatment and LiCoPO4 coating. Nano Res. 2017, 10, 4210–4220. [Google Scholar] [CrossRef]
- Ming, J.; Li, M.; Kumar, P.; Lu, A.-Y.; Wahyudi, W.; Li, L.-J. Redox Species-Based Electrolytes for Advanced Rechargeable Lithium Ion Batteries. ACS Energy Lett. 2016, 1, 529–534. [Google Scholar] [CrossRef]
- Fukutsuka, T.; Koyamada, K.; Maruyama, S.; Miyazaki, K.; Abe, T. Ion Transport in Organic Electrolyte Solution through the Pore Channels of Anodic Nanoporous Alumina Membranes. Electrochim. Acta 2016, 199, 380–387. [Google Scholar] [CrossRef]
- Kannan, D.R.R.; Terala, P.K.; Moss, P.L.; Weatherspoon, M.H. Analysis of the Separator Thickness and Porosity on the Performance of Lithium-Ion Batteries. Int. J. Electrochem. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Xu, Q.; Kong, Q.; Liu, Z.; Zhang, J.; Wang, X.; Liu, R.; Yue, L.; Cui, G. Polydopamine-coated cellulose microfibrillated membrane as high performance lithium-ion battery separator. RSC Adv. 2014, 4, 7845–7850. [Google Scholar] [CrossRef]
- Zhang, J.; Yue, L.; Kong, Q.; Liu, Z.; Zhou, X.; Zhang, C.; Xu, Q.; Zhang, B.; Ding, G.; Qin, B.; et al. Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci. Rep. 2014, 4, 3935. [Google Scholar] [CrossRef]
- Danner, T.; Singh, M.; Hein, S.; Kaiser, J.; Hahn, H.; Latz, A. Thick electrodes for Li-ion batteries: A model based analysis. J. Power Sources 2016, 334, 191–201. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Thick Electrodes for High Energy Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A1196–A1201. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. A systematic study of thick electrodes for high energy lithium ion batteries. J. Electroanal. Chem. 2016, 782, 245–249. [Google Scholar] [CrossRef]
- Singh, M.; Kaiser, J.; Hahn, H. Effect of Porosity on the Thick Electrodes for High Energy Density Lithium Ion Batteries for Stationary Applications. Batteries 2016, 2, 35. [Google Scholar] [CrossRef]
- Kang, H.; Lim, C.; Li, T.; Fu, Y.; Yan, B.; Houston, N.; De Andrade, V.; De Carlo, F.; Zhu, L. Geometric and Electrochemical Characteristics of LiNi1/3Mn1/3Co1/3O2 Electrode with Different Calendering Conditions. Electrochim. Acta 2017, 232, 431–438. [Google Scholar] [CrossRef]
- Barai, A.; Guo, Y.; McGordon, A.; Jennings, P. A study of the effects of external pressure on the electrical performance of a lithium-ion pouch cell. In Proceedings of the 2013 International Conference on Connected Vehicles and Expo (ICCVE), Las Vegas, NV, USA, 2–6 December 2013; IEEE: Piscataway, NJ, USA, 2013; pp. 295–299, ISBN 978-1-4799-2491-2. [Google Scholar]
- Cannarella, J.; Arnold, C.B. Stress evolution and capacity fade in constrained lithium-ion pouch cells. J. Power Sources 2014, 245, 745–751. [Google Scholar] [CrossRef]
- Habedank, J.B.; Kraft, L.; Rheinfeld, A.; Krezdorn, C.; Jossen, A.; Zaeh, M.F. Increasing the Discharge Rate Capability of Lithium-Ion Cells with Laser-Structured Graphite Anodes: Modeling and Simulation. J. Electrochem. Soc. 2018, 165, A1563–A1573. [Google Scholar] [CrossRef]
- Kim, J.S.; Pfleging, W.; Kohler, R.; Seifert, H.J.; Kim, T.Y.; Byun, D.; Choi, W.; Lee, J.K.; Jung, H.-G.; Jung, H.-G.; et al. Three-dimensional silicon/carbon core–shell electrode as an anode material for lithium-ion batteries. J. Power Sources 2015, 279, 13–20. [Google Scholar] [CrossRef]
- Lim, D.G.; Chung, D.-W.; Kohler, R.; Pröll, J.; Scherr, C.; Pfleging, W.; Garcia, R.E. Designing 3D Conical-Shaped Lithium-Ion Microelectrodes. J. Electrochem. Soc. 2014, 161, A302–A307. [Google Scholar] [CrossRef]
- Mangang, M.; Seifert, H.; Pfleging, W. Influence of laser pulse duration on the electrochemical performance of laser structured LiFePO4 composite electrodes. J. Power Sources 2016, 304, 24–32. [Google Scholar] [CrossRef]
- Proll, J.; Kim, H.; Piqué, A.; Seifert, H.; Pfleging, W. Laser-printing and femtosecond-laser structuring of LiMn2O4 composite cathodes for Li-ion microbatteries. J. Power Sources 2014, 255, 116–124. [Google Scholar] [CrossRef]
- Smyrek, P.; Pröll, J.; Seifert, H.J.; Pfleging, W. Laser-Induced Breakdown Spectroscopy of Laser-Structured Li (NiMnCo) O2 Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A19–A26. [Google Scholar] [CrossRef]
- Frankenberger, M.; Singh, M.; Dinter, A.; Jankowksy, S.; Schmidt, A.; Pettinger, K.-H. Laminated Lithium Ion Batteries with improved fast charging capability. J. Electroanal. Chem. 2019, 837, 151–158. [Google Scholar] [CrossRef]
- Zheng, H.; Tan, L.; Liu, G.; Song, X.; Battaglia, V.S. Calendering effects on the physical and electrochemical properties of Li[Ni1/3Mn1/3Co1/3]O2 cathode. J. Power Sources 2012, 208, 52–57. [Google Scholar] [CrossRef]
- Pettinger, K.-H. Fertigungsprozesse von Lithium-Ionen-Zellen. In Handbuch Lithium-Ionen-Batterien; Korthauer, R., Ed.; Springer: Heidelberg/Berlin, Germany, 2013; ISBN 978-3-642-30652-5. [Google Scholar]
- Meyerson, M.L.; Sheavly, J.K.; Dolocan, A.; Griffin, M.P.; Pandit, A.H.; Rodriguez, R.; Stephens, R.M.; Bout, D.A.V.; Heller, A.; Mullins, C.B.; et al. The effect of local lithium surface chemistry and topography on solid electrolyte interphase composition and dendrite nucleation. J. Mater. Chem. A 2019, 7, 14882–14894. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, W.-W.; Li, Y.-J.; Wu, Q.-H.; Tang, S.; Yan, J.-W.; Zheng, M.-S.; Wu, D.-Y.; Fan, C.-H.; Hu, W.-Q.; et al. Designable ultra-smooth ultra-thin solid-electrolyte interphases of three alkali metal anodes. Nat. Commun. 2018, 9, 1339. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.; Eller, J.; Ebner, M.; Burns, C.; Dahn, J.; Wood, V. Quantifying Inhomogeneity of Lithium Ion Battery Electrodes and Its Influence on Electrochemical Performance. J. Electrochem. Soc. 2018, 165, A339–A344. [Google Scholar] [CrossRef]
- Song, J.; Lee, H.; Wang, Y.; Wan, C. Two- and three-electrode impedance spectroscopy of lithium-ion batteries. J. Power Sources 2002, 111, 255–267. [Google Scholar] [CrossRef]
- Solchenbach, S.; Pritzl, D.; Kong, E.J.Y.; Landesfeind, J.; Gasteiger, H.A. A Gold Micro-Reference Electrode for Impedance and Potential Measurements in Lithium Ion Batteries. J. Electrochem. Soc. 2016, 163, A2265–A2272. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Kim, S.; Deng, Y.; Minor, A.M.; Song, X.; Battaglia, V.S. Effects of Various Conductive Additive and Polymeric Binder Contents on the Performance of a Lithium-Ion Composite Cathode. J. Electrochem. Soc. 2008, 155, A887–A892. [Google Scholar] [CrossRef]
- Gordon, I.J.; Grugeon, S.; Takenouti, H.; Tribollet, B.; Armand, M.; Davoisne, C.; Débart, A.; Laruelle, S. Electrochemical Impedance Spectroscopy response study of a commercial graphite-based negative electrode for Li-ion batteries as function of the cell state of charge and ageing. Electrochim. Acta 2017, 223, 63–73. [Google Scholar] [CrossRef]
- Gordon, I.J.; Grugeon, S.; Debart, A.; Pascaly, G.; Laruelle, S. Electrode contributions to the impedance of a high-energy density Li-ion cell designed for EV applications. Solid State Ion. 2013, 237, 50–55. [Google Scholar] [CrossRef]
- Momma, T.; Matsunaga, M.; Mukoyama, D.; Osaka, T. Ac impedance analysis of lithium ion battery under temperature control. J. Power Sources 2012, 216, 304–307. [Google Scholar] [CrossRef]
- Stroe, D.I.; Swierczynski, M.; Stan, A.I.; Knap, V.; Teodorescu, R.; Andreasen, S.J. Diagnosis of lithium-ion batteries state-of-health based on electrochemical impedance spectroscopy technique. In Proceedings of the 2014 IEEE Energy Conversion Congress and Exposition (ECCE), Pittsburgh, PA, USA, 14–18 September 2014; Institute of Electrical and Electronics Engineers, Ed.; IEEE: Piscataway, NJ, USA, 2014; pp. 4576–4582, ISBN 978-1-4799-5776-7. [Google Scholar]
- Gaberšček, M.; Moskon, J.; Erjavec, B.; Dominko, R.; Jamnik, J. The Importance of Interphase Contacts in Li Ion Electrodes: The Meaning of the High-Frequency Impedance Arc. Electrochem. Solid-State Lett. 2008, 11, A170–A174. [Google Scholar] [CrossRef]
- Jossen, A.; Weydanz, W. Moderne Akkumulatoren, 2nd ed.; CUVILLIER VERLAG: Göttingen, Germany, 2019; ISBN 978-3-7369-9945-9. [Google Scholar]
- Dollé, M.; Orsini, F.; Gozdz, A.S.; Tarascon, J.-M. Development of Reliable Three-Electrode Impedance Measurements in Plastic Li-Ion Batteries. J. Electrochem. Soc. 2001, 148, A851–A857. [Google Scholar] [CrossRef]
- Waag, W.; Käbitz, S.; Sauer, D.U. Experimental investigation of the lithium-ion battery impedance characteristic at various conditions and aging states and its influence on the application. Appl. Energy 2013, 102, 885–897. [Google Scholar] [CrossRef]
- Levi, M.D.; Dargel, V.; Shilina, Y.; Aurbach, D.; Halalay, I.C. Impedance Spectra of Energy-Storage Electrodes Obtained with Commercial Three-Electrode Cells: Some Sources of Measurement Artefacts. Electrochim. Acta 2014, 149, 126–135. [Google Scholar] [CrossRef]
- Pritzl, D.; Landesfeind, J.; Solchenbach, S.; Gasteiger, H.A. An Analysis Protocol for Three-Electrode Li-Ion Battery Impedance Spectra: Part II. Analysis of a Graphite Anode Cycled vs. LNMO. J. Electrochem. Soc. 2018, 165, A2145–A2153. [Google Scholar] [CrossRef] [Green Version]
- Landesfeind, J.; Pritzl, D.; Gasteiger, H.A. An Analysis Protocol for Three-Electrode Li-Ion Battery Impedance Spectra: Part I. Analysis of a High-Voltage Positive Electrode. J. Electrochem. Soc. 2017, 164, A1773–A1783. [Google Scholar] [CrossRef]
- Ovejas, V.J.; Cuadras, A. Impedance Characterization of an LCO-NMC/Graphite Cell: Ohmic Conduction, SEI Transport and Charge-Transfer Phenomenon. Batteries 2018, 4, 43. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Li, Z.; Zhang, J.; Song, S.; Lou, Z.; Wu, N. An Analytical Three-Scale Impedance Model for Porous Electrode with Agglomerates in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A585–A595. [Google Scholar] [CrossRef] [Green Version]




| Cycle Number | Laminated | Anode-Laminated | Cathode-Laminated | Non-Laminated |
|---|---|---|---|---|
| 9 | 133.2 mAh∙g−1 | 129.6 mAh∙g−1 | 133.4 mAh∙g−1 | 133.0 mAh∙g−1 |
| 1508 | 112.5 mAh∙g−1 | 111.4 mAh∙g−1 | 99.3 mAh∙g−1 | 100.4 mAh∙g−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frankenberger, M.; Singh, M.; Dinter, A.; Pettinger, K.-H. EIS Study on the Electrode-Separator Interface Lamination. Batteries 2019, 5, 71. https://doi.org/10.3390/batteries5040071
Frankenberger M, Singh M, Dinter A, Pettinger K-H. EIS Study on the Electrode-Separator Interface Lamination. Batteries. 2019; 5(4):71. https://doi.org/10.3390/batteries5040071
Chicago/Turabian StyleFrankenberger, Martin, Madhav Singh, Alexander Dinter, and Karl-Heinz Pettinger. 2019. "EIS Study on the Electrode-Separator Interface Lamination" Batteries 5, no. 4: 71. https://doi.org/10.3390/batteries5040071





