Comparison of Aqueous- and Non-Aqueous-Based Binder Polymers and the Mixing Ratios for Zn//MnO2 Batteries with Mildly Acidic Aqueous Electrolytes
Abstract
:1. Introduction
- Stability of the binder polymer in aqueous electrolyte (no peeling of the coating from the current collector due to dissolution or strong swelling of the binder polymer).
- Porosity of the coating for high specific surface area to provide a high (electrochemically active) surface area.
- Wetting of the coating by the aqueous electrolyte to enable deposition and intercalation processes of the dissolved components of the electrolyte.
- Advantageous transport characteristics of the porous electrode for the ions and electrons.
2. Results and Discussion
2.1. Binder Polymer Variation
2.1.1. Mechanical Stress Test (MST)
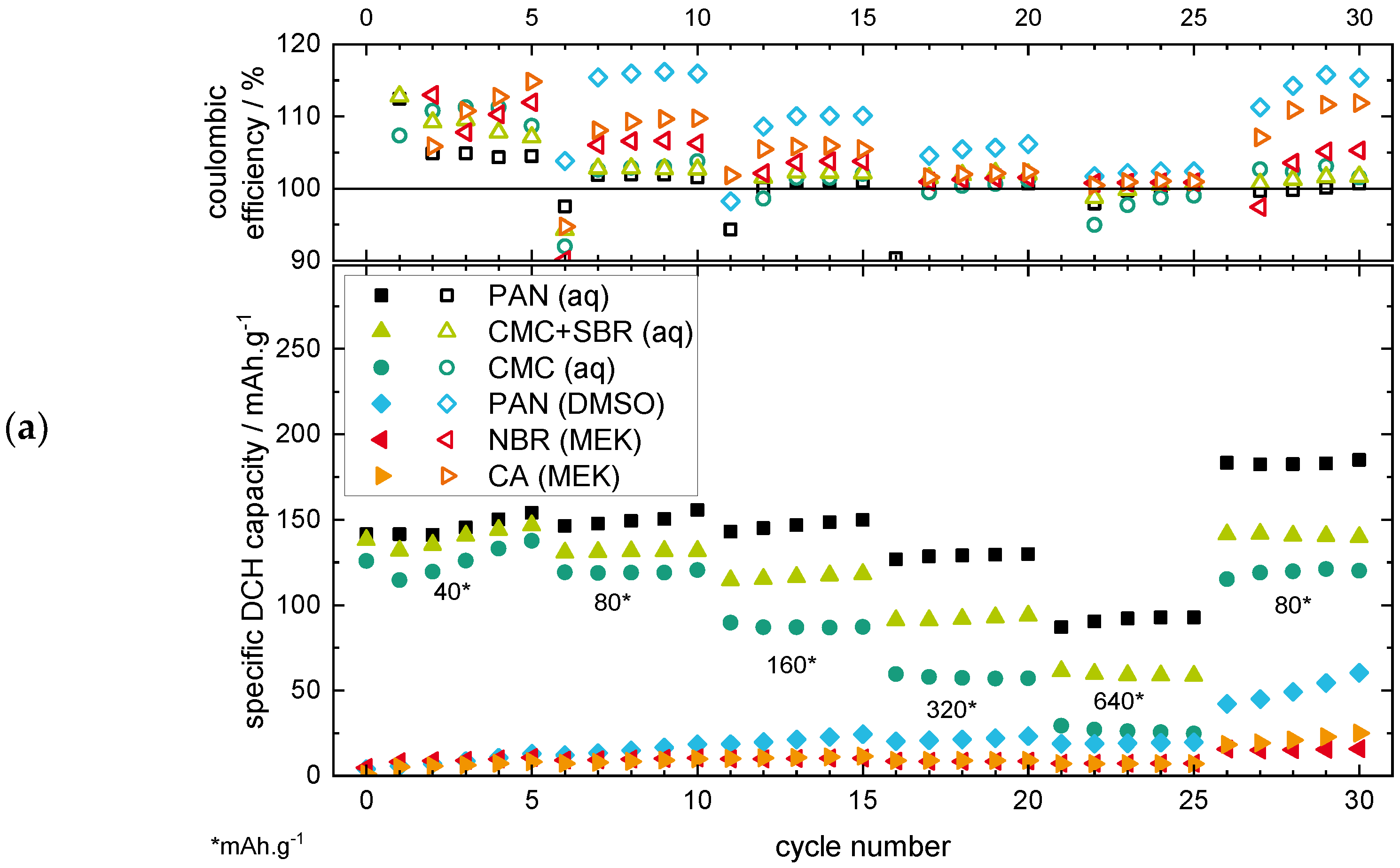
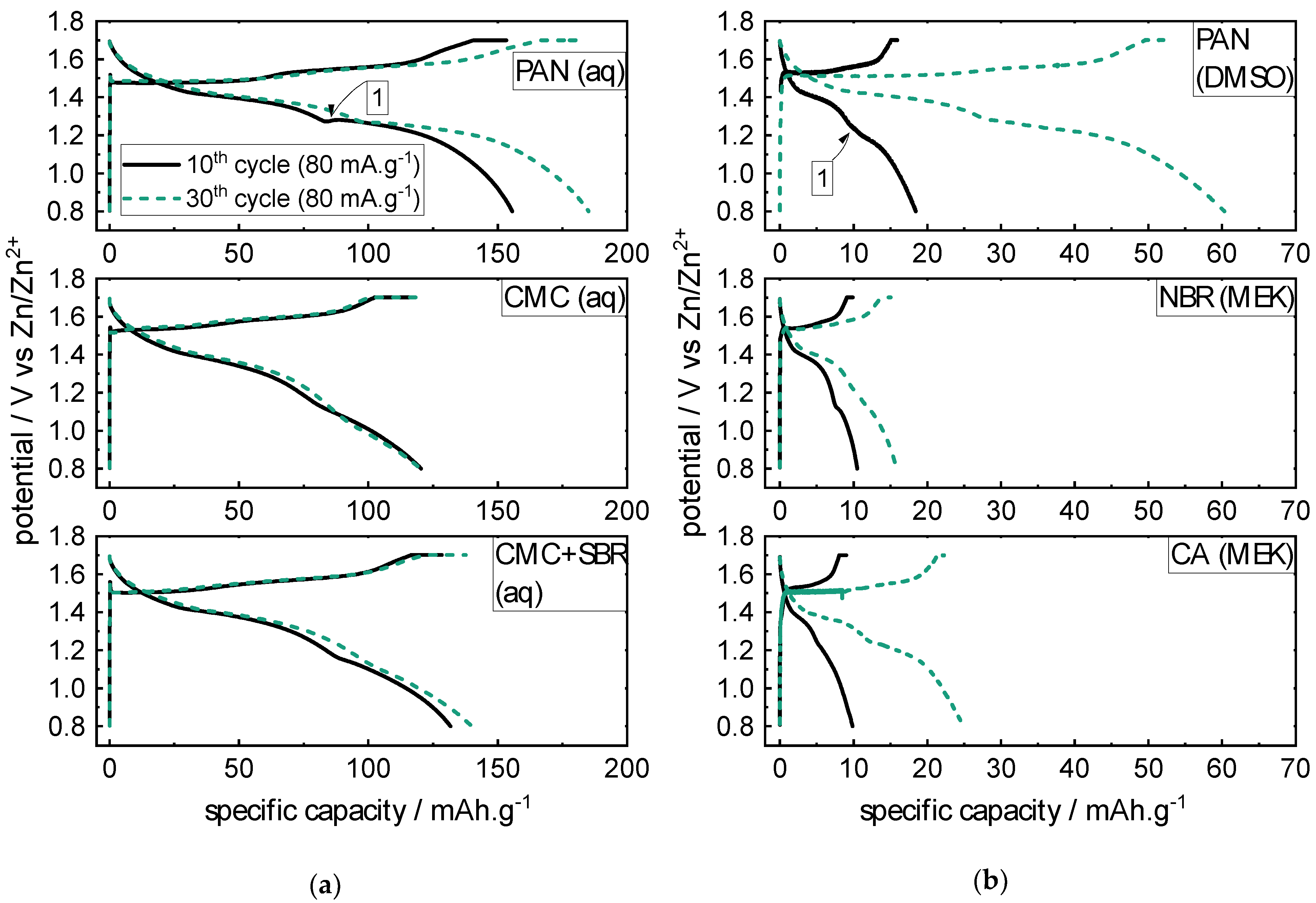
2.1.2. Rate Capability Test (RCT)
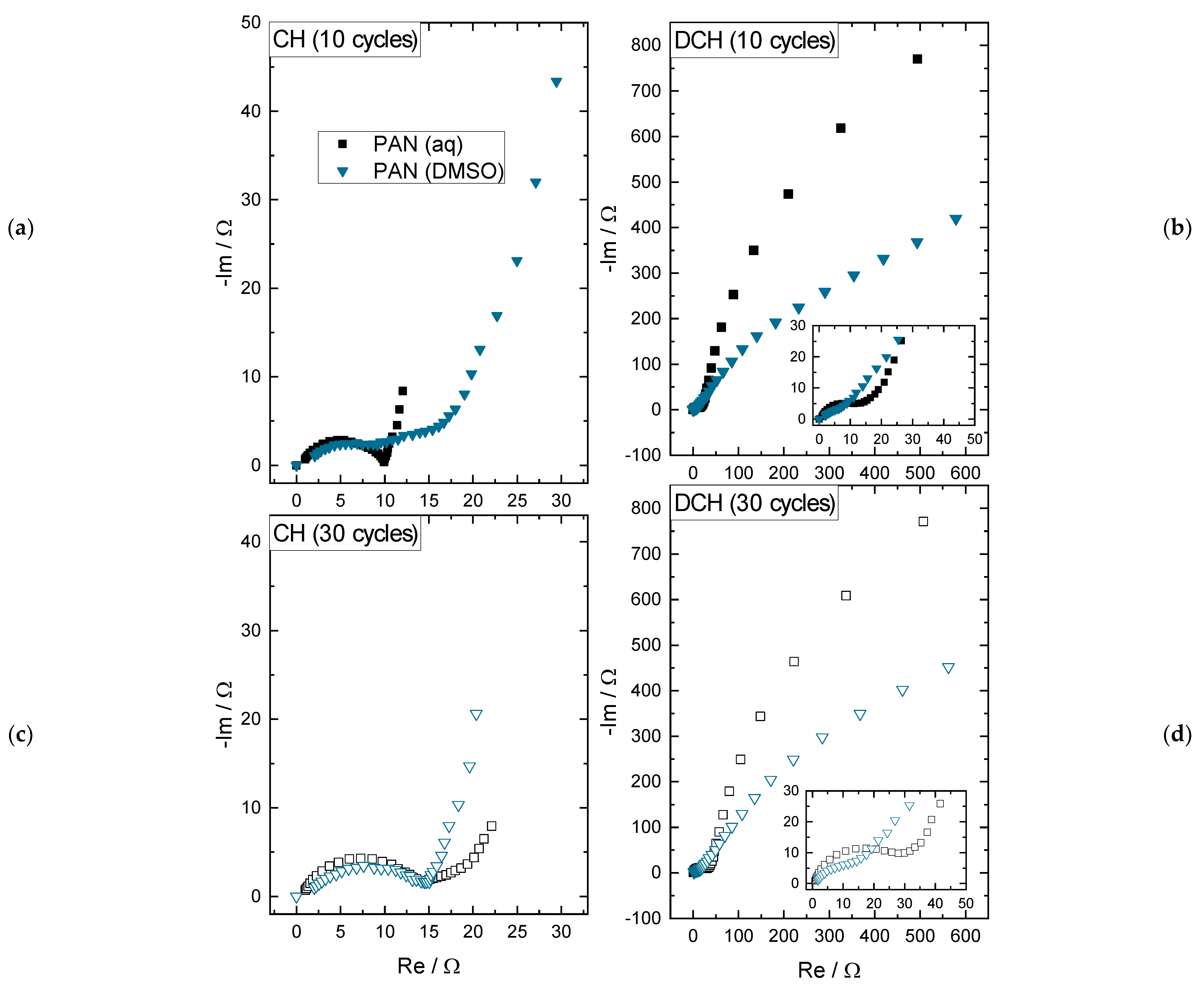
- In charged state after 10 cycles (see Figure 5a), there is a lower impedance for the PAN (aq) than for the PAN (DMSO) electrode: The double layer (DL) capacity and the charge transfer (CT) resistance of the PAN (aq) binder are both clearly visible as a semi-circle, whereas the DL capacity and CT resistance of the electrode PAN (DMSO) binder is stretched, which indicates a higher CT resistance and could refer to the inactive and isolated MnO2 active material particles in the electrode coating.
- In charged state after 30 cycles (see Figure 5c), the impedance spectra show quite comparable semi-circles, again representing the DL capacity and the CT resistance. This could be explained by the growing deposition layer of MnO2 on the positive electrode surface of the PAN (DMSO) coating. This assumption is supported by the capacity increase of the PAN (DMSO) electrode in Figure 2a.
- In discharged state after 10 and 30 cycles (see Figure 5b,d), the overall impedances increase by about two orders of magnitude for both PAN (aq) and PAN (DMSO). This can be explained by the precipitation of ZHS as a consequence of the MnO2 dissolution, as discussed in [19]. The precipitation results in a new DL capacity with a higher CT resistance, which is shown by the large low frequency half circle. Still, the impedance spectra for the lower impedance values are visible (see insets in Figure 5b,d), which were previously discussed for the charged state representing the MnO2 active material layer. Relating to the PAN (DMSO) binder, the capacitive part of the impedance spectra is still below the PAN (aq) spectra, which can be explained by the thinner MnO2 deposition layer and less ZHS precipitation (less wetting of the coating), resulting in lower specific capacity values (see Figure 2a).
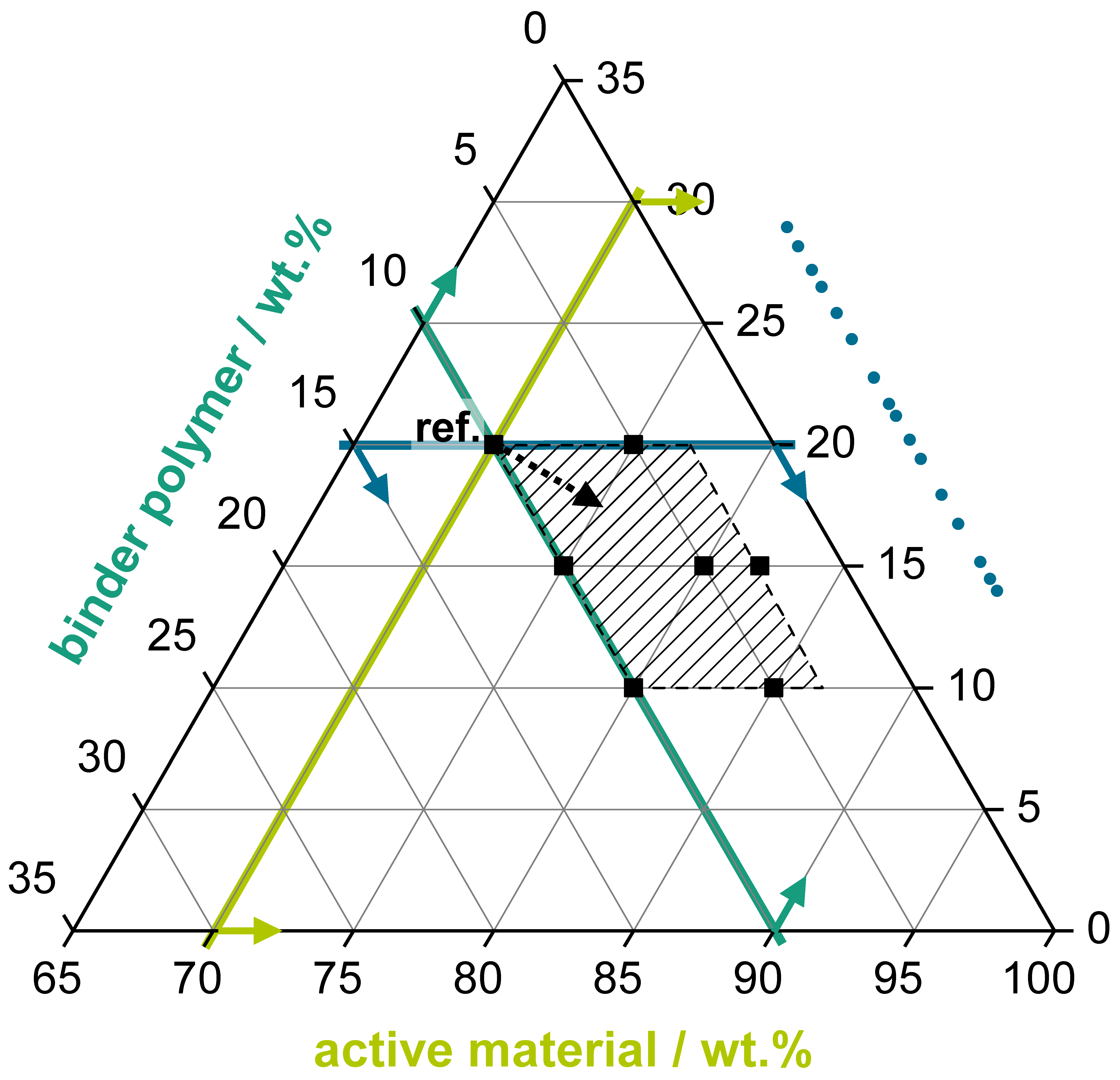
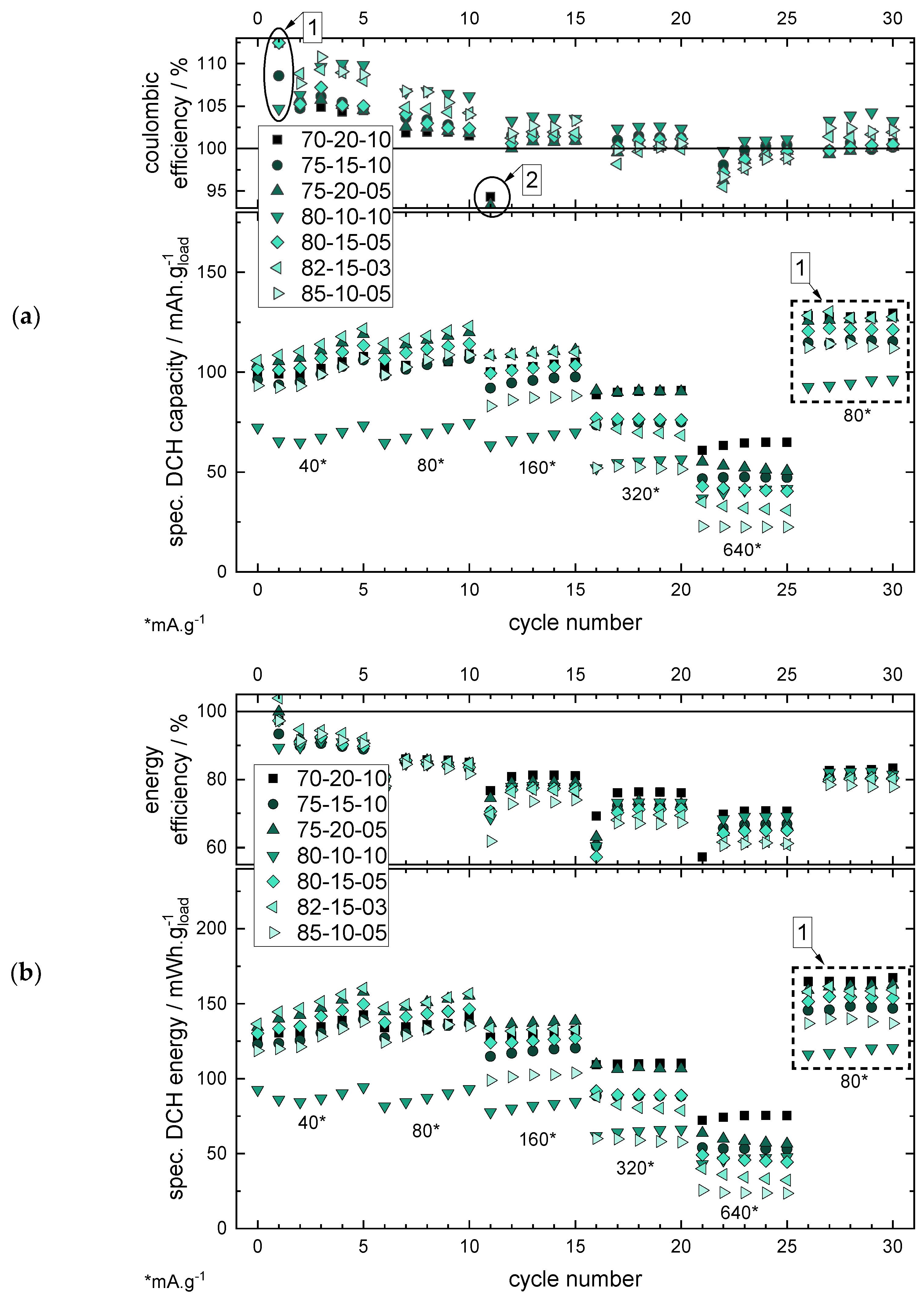
2.2. Mixing Ratio Variation
- An active material proportion as high as possible to reduce the share of the passive materials (carbon black, binder polymer) in the electrode coating.
- A binder polymer proportion as low as possible to reduce the share of a passive and electrically non-conducting coating component.
- A carbon black proportion as low as possible, while still enabling a sufficient electrical conductivity of the coating.
- Good cycling performance in the RCT considering different application fields such as high-power (current rates >1 C, here: >160 mA·g−1 based on an experimentally determined capacity of ~160 mAh·g−1 of MnO2) and high-energy (<1C, here: <160 mA·g−1) applications.
- Lowering the CB share allows reducing the BP share.
- Reducing the CB share without reducing the BP share results in poor cycling behaviour due to insufficient conductivity within the electrode.
- For HP applications, a CB share of >15 wt% is necessary to still enable a good RCT capacity also at higher current rates >320 mA·g−1. The CB/BP ratio should be >2 (see 70/20/10 or 75/20/05).
- For HE applications, an AM loading as high as possible is important for lower the specific costs of the battery, and therefore lower CB loadings are preferable. Considering this, a CB loading of 10 wt% and a CB/BP-ratio of ~2 (s. 85/10/05) still enables a good cycle performance at lower current rates <160 mA·g−1.
3. Conclusions
3.1. Binder Variation
3.2. Mixing Ratio Variation
4. Materials and Methods
4.1. Materials
- LA133 (polyacrylonitrile (PAN)-based aqueous binder dispersion, GELON LIB Group, Linyi, China)
- SBR (styrene-butadiene rubber, SSBR 100, aqueous suspension, Targray, Kirkland, QC, Canada)
- CMC (carboxymethyl cellulose, average MW ~250,000, degree of substitution 0.9, sodium salt, powder, Sigma-Aldrich, Merck KgaA, Darmstadt, Germany)
- PVP (polyvinylpyrrolidone, MW 1,300,000, powder, Alfa Aesar, Heysham, United Kingdom)
- CA (cellulose acetate, MW ~100,000, powder, Acros Organics, Thermo Fisher Scientific, New Brunswick, NJ, USA)
- NBR (nitrile butadiene rubber, acrylonitrile 37–39 wt%, chunk, Sigma-Aldrich, Merck KgaA, Darmstadt, Germany)
- PAN (polyacrylonitrile, copolymer 99.5% AN/0.5% MA, MW 230,000, powder, Goodfellow GmbH, Hamburg, Germany)
4.2. Cell Assembly
4.3. Characterization Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biswal, A.; Tripathy, B.C.; Subbaiah, T.; Meyrick, D.; Minakshi, M. Electrodeposition of manganese dioxide: Effect of quaternary amines. J. Solid State Electrochem. 2013, 17, 1349–1356. [Google Scholar] [CrossRef] [Green Version]
- Biswal, A.; Tripathy, B.C.; Subbaiah, T.; Meyrick, D.; Minakshi, M. Dual effect of anionic surfactants in the electrodeposited MnO2 trafficking redox ions for energy storage. J. Electrochem. Soc. 2015, 162, A30–A38. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent advances in Zn-ion batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Chang, H.J.; Rodríguez-Pérez, I.A.; Fayette, M.; Canfield, N.L.; Pan, H.; Choi, D.; Li, X.; Reed, D. Effects of water-based binders on electrochemical performance of manganese dioxide cathode in mild aqueous zinc batteries. Carbon Energy 2020. [Google Scholar] [CrossRef]
- Olbasa, B.W.; Fenta, F.W.; Chiu, S.-F.; Tsai, M.-C.; Huang, C.-J.; Jote, B.A.; Beyene, T.T.; Liao, Y.-F.; Wang, C.-H.; Su, W.-N.; et al. High-rate and long-cycle stability with a dendrite-free zinc anode in an aqueous Zn-Ion battery using concentrated electrolytes. ACS Appl. Energy Mater. 2020, 3, 4499–4508. [Google Scholar] [CrossRef]
- Pan, H.; Ellis, J.; Li, X.; Nie, Z.; Chang, H.J.; Reed, D. Electrolyte effect on the electrochemical performance of mild aqueous zinc-electrolytic manganese dioxide batteries. ACS Appl. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Palaniyandy, N.; Kebede, M.A.; Raju, K.; Ozoemena, K.I.; Le Roux, L.; Mathe, M.K.; Jayaprakasam, R. α-MnO2 nanorod/onion-like carbon composite cathode material for aqueous zinc-ion battery. Mater. Chem. Phys. 2019, 230, 258–266. [Google Scholar] [CrossRef]
- Guo, X.; Li, J.; Jin, X.; Han, Y.; Lin, Y.; Lei, Z.; Wang, S.; Qin, L.; Jiao, S.; Cao, R. A hollow-structured manganese oxide cathode for stable Zn-MnO2 batteries. Nanomaterials 2018, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Li, B.; Wei, C.; He, Y.-B.; Du, H.; Chu, X.; Qin, X.; Yang, Q.-H.; Kang, F. Preparation and characterization of MnO2/acid-treated CNT nanocomposites for energy storage with zinc ions. Electrochim. Acta 2014, 133, 254–261. [Google Scholar] [CrossRef]
- Bischoff, C.; Fitz, O.; Schiller, C.; Gentischer, H.; Biro, D.; Henning, H.-M. Investigating the impact of particle size on the performance and internal resistance of aqueous zinc ion batteries with a manganese sesquioxide cathode. Batteries 2018, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Chamoun, M.; Brant, W.R.; Tai, C.-W.; Karlsson, G.; Noréus, D. Rechargeability of aqueous sulfate Zn/MnO2 batteries enhanced by accessible Mn2+ ions. Energy Storage Mater. 2018, 15, 351–360. [Google Scholar] [CrossRef]
- Zhang, N.; Cheng, F.; Liu, J.; Wang, L.; Long, X.; Liu, X.; Li, F.; Chen, J. Rechargeable aqueous zinc-manganese dioxide batteries with high energy and power densities. Nat. Commun. 2017, 8, 405. [Google Scholar] [CrossRef] [Green Version]
- Jiang, B.; Xu, C.; Wu, C.; Dong, L.; Li, J.; Kang, F. Manganese sesquioxide as cathode material for multivalent zinc ion battery with high capacity and long cycle life. Electrochim. Acta 2017, 229, 422–428. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Islam, S.; Alfaruqi, M.H.; Mathew, V.; Song, J.; Kim, S.; Kim, S.; Jo, J.; Baboo, J.P.; Pham, D.T.; Putro, D.Y.; et al. Facile synthesis and the exploration of the zinc storage mechanism of β-MnO2 nanorods with exposed (101) planes as a novel cathode material for high performance eco-friendly zinc-ion batteries. J. Mater. Chem. A 2017, 5, 23299–23309. [Google Scholar] [CrossRef]
- Poyraz, A.S.; Laughlin, J.; Zec, Z. Improving the cycle life of cryptomelane type manganese dioxides in aqueous rechargeable zinc ion batteries: The effect of electrolyte concentration. Electrochim. Acta 2019, 305, 423–432. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Hou, M.; Dong, X.; Liu, Y.; Wang, Y.; Xia, Y. Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat. Commun. 2018, 9, 2906. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Liu, J.; Mao, J.; Hao, J.; Wang, Z.; Zhou, S.; Ling, C.D.; Guo, Z. Toward a reversible Mn4+/Mn2+ redox reaction and dendrite-free Zn anode in near-neutral aqueous Zn/MnO2 batteries via salt anion chemistry. Adv. Energy Mater. 2020, 10, 1904163. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, J.; Bai, C.; Li, X.; Fang, G.; Liang, S. Zn/MnO2 battery chemistry with dissolution-deposition mechanism. Mater. Today Energy 2020, 16, 100396. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Hou, S.; Yang, C.; Fan, X.; Ma, Z.; Gao, T.; Han, F.; Hu, R.; Zhu, M.; et al. Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J. Am. Chem. Soc. 2017, 139, 9775–9778. [Google Scholar] [CrossRef]
- Hou, Z.; Dong, M.; Xiong, Y.; Zhang, X.; Ao, H.; Liu, M.; Zhu, Y.; Qian, Y. A high-energy and long-life aqueous Zn/birnessite battery via reversible water and Zn2+ coinsertion. Small 2020, 16, e2001228. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Tawiah, B.; Wang, Y.; Chan, C.-Y.; Fei, B.; Pan, F. Recent advances in zinc anodes for high-performance aqueous Zn-ion batteries. Nano Energy 2020, 70, 104523. [Google Scholar] [CrossRef]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R Rep. 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Biswal, A.; Chandra Tripathy, B.; Sanjay, K.; Subbaiah, T.; Minakshi, M. Electrolytic manganese dioxide (EMD): A perspective on worldwide production, reserves and its role in electrochemistry. RSC Adv. 2015, 5, 58255–58283. [Google Scholar] [CrossRef]
- Xu, C.; Li, B.; Du, H.; Kang, F. Energetic zinc ion chemistry: The rechargeable zinc ion battery. Angew. Chem. Int. Ed. Engl. 2012, 51, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Yoon, C.S.; Lee, H.R.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Electrochemically-induced reversible transition from the tunneled to layered polymorphs of manganese dioxide. Sci. Rep. 2014, 4, 6066. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Lee, H.R.; Kim, H.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Elucidating the intercalation mechanism of zinc ions into α-MnO2 for rechargeable zinc batteries. Chem. Commun. 2015, 51, 9265–9268. [Google Scholar] [CrossRef]
- Han, S.-D.; Kim, S.; Li, D.; Petkov, V.; Yoo, H.D.; Phillips, P.J.; Wang, H.; Kim, J.J.; More, K.L.; Key, B.; et al. Mechanism of Zn insertion into nanostructured δ-MnO2: A nonaqueous rechargeable Zn metal battery. Chem. Mater. 2017, 29, 4874–4884. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Gim, J.; Kim, S.; Song, J.; Jo, J.; Kim, S.; Mathew, V.; Kim, J. Enhanced reversible divalent zinc storage in a structurally stable α-MnO2 nanorod electrode. J. Power Sources 2015, 288, 320–327. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Gim, J.; Song, J.; Kim, S.; Pham, D.T.; Jo, J.; Xiu, Z.; Mathew, V.; Kim, J. A high surface area tunnel-type α-MnO2 nanorod cathode by a simple solvent-free synthesis for rechargeable aqueous zinc-ion batteries. Chem. Phys. Lett. 2016, 650, 64–68. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Mathew, V.; Gim, J.; Kim, S.; Song, J.; Baboo, J.P.; Choi, S.H.; Kim, J. Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem. Mater. 2015, 27, 3609–3620. [Google Scholar] [CrossRef]
- Xu, C.; Chiang, S.W.; Ma, J.; Kang, F. Investigation on zinc ion storage in alpha manganese dioxide for zinc ion battery by electrochemical impedance spectrum. J. Electrochem. Soc. 2012, 160, A93–A97. [Google Scholar] [CrossRef]
- Qiu, C.; Zhu, X.; Xue, L.; Ni, M.; Zhao, Y.; Liu, B.; Xia, H. The function of Mn2+ additive in aqueous electrolyte for Zn/δ-MnO2 battery. Electrochim. Acta 2020, 351, 136445. [Google Scholar] [CrossRef]
- Khamsanga, S.; Pornprasertsuk, R.; Yonezawa, T.; Mohamad, A.A.; Kheawhom, S. δ-MnO2 nanoflower/graphite cathode for rechargeable aqueous zinc ion batteries. Sci. Rep. 2019, 9, 8441. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.S.; Sassin, M.B.; Parker, J.F.; Rolison, D.R.; Long, J.W. Combining battery-like and pseudocapacitive charge storage in 3D MnOx @carbon electrode architectures for zinc-ion cells. Sustain. Energy Fuels 2018, 2, 626–636. [Google Scholar] [CrossRef]
- Alfaruqi, M.H.; Islam, S.; Putro, D.Y.; Mathew, V.; Kim, S.; Jo, J.; Kim, S.; Sun, Y.-K.; Kim, K.; Kim, J. Structural transformation and electrochemical study of layered MnO2 in rechargeable aqueous zinc-ion battery. Electrochim. Acta 2018, 276, 1–11. [Google Scholar] [CrossRef]
- Huang, Y.; Mou, J.; Liu, W.; Wang, X.; Dong, L.; Kang, F.; Xu, C. Novel insights into energy storage mechanism of aqueous rechargeable Zn/MnO2 batteries with participation of Mn2+. Nano Micro Lett. 2019, 11, 860. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Chen, X.; Wang, Z.; Yang, L.; Qin, R.; Yang, J.; Song, Y.; Ding, S.; Weng, M.; Huang, W.; et al. Unravelling H+/Zn2+ synergistic intercalation in a novel phase of manganese oxide for high-performance aqueous rechargeable battery. Small 2019, 15, e1904545. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wu, H.; Li, W.; Tian, Y.; Zhang, Y.; Wu, H.; Yang, L.; Zou, G.; Hou, H.; Ji, X. H+-insertion boosted α-MnO2 for an aqueous Zn-ion battery. Small 2020, 16, e1905842. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hoang, T.K.A.; Zhi, J.; Han, M.; Li, S.; Chen, P. Functioning mechanism of the secondary aqueous Zn-β-MnO2 battery. ACS Appl. Mater. Interfaces 2020, 12, 12834–12846. [Google Scholar] [CrossRef]
- Mateos, M.; Makivic, N.; Kim, Y.-S.; Limoges, B.; Balland, V. Accessing the two-electron charge storage capacity of MnO2 in mild aqueous electrolytes. Adv. Energy Mater. 2020, 10, 2000332. [Google Scholar] [CrossRef]
- Lee, B.; Seo, H.R.; Lee, H.R.; Yoon, C.S.; Kim, J.H.; Chung, K.Y.; Cho, B.W.; Oh, S.H. Critical role of pH evolution of electrolyte in the reaction mechanism for rechargeable zinc batteries. ChemSusChem 2016, 9, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Salvador, J.R.; Wu, J.; Liu, B.; Yang, W.; Yang, J.; Zhang, W.; Liu, J.; Yang, J. Reaction mechanisms for long-life rechargeable Zn/MnO2 batteries. Chem. Mater. 2019. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, J.-G.; Liu, H.; Wei, C.; Kang, F. Zinc ion stabilized MnO2 nanospheres for high capacity and long lifespan aqueous zinc-ion batteries. J. Mater. Chem. A 2019, 7, 13727–13735. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Physical Chemistry, 9th ed.; W. H. Freeman and Company: New York, NY, USA, 2010; ISBN 9781429218122. [Google Scholar]
- Kim, S.H.; Oh, S.M. Degradation mechanism of layered MnO2 cathodes in Zn/ZnSO4/MnO2 rechargeable cells. J. Power Sources 1998, 72, 150–158. [Google Scholar] [CrossRef]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974; ISBN 0915567989. [Google Scholar]
- Minakshi, M.; Appadoo, D.; Martin, D.E. The anodic behavior of planar and porous zinc electrodes in alkaline electrolyte. Electrochem. Solid State Lett. 2010, 13, A77. [Google Scholar] [CrossRef]
- Lim, M.B.; Lambert, T.N.; Chalamala, B.R. Rechargeable alkaline zinc–manganese oxide batteries for grid storage: Mechanisms, challenges and developments. Mater. Sci. Eng. R Rep. 2021, 143, 100593. [Google Scholar] [CrossRef]
- Franks, F. Aqueous Solutions of Simple Electrolytes; Springer: Boston, MA, USA, 1973; ISBN 9781468429558. [Google Scholar]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z.; et al. Effect of different binders on the electrochemical performance of metal oxide anode for lithium-ion batteries. Nanoscale Res. Lett. 2017, 12, 575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2001; ISBN 0-471-04372-9. [Google Scholar]



| Binder (aq) | DI-Water | Electrolyte | Binder (non-aq) | DI-Water | Electrolyte |
|---|---|---|---|---|---|
|  |  |
|  |  |
|  |  |
|  |  |
|  |  |
|  |  |
|  |  |
| # | Ratio AM/CB/BP | Binder #1 | Binder #2 | Solvent | Comment |
|---|---|---|---|---|---|
| 1 | 70/20/10 | PAN (LA133) | DI-water | aqueous suspension | |
| 2 | 70/20/10 | CMC | DI-water | aqueous solution | |
| 3 | 70/20/10 | 50 wt% CMC | 50 wt% SBR | DI-water | aqueous solution/suspension |
| 4 | 70/20/10 | PAN | DMSO | solution | |
| 5 | 70/20/10 | NBR | MEK | solution | |
| 6 | 70/20/10 | CA | MEK | solution |
| # | Name | Active Material (AM)/wt% | Carbon Black (CB)/wt% | Binder Polymer (BP)/wt% | CB/BP Ratio |
|---|---|---|---|---|---|
| 1 | 70/20/10 | 70 | 20 | 10 | 2 |
| 2 | 75/20/05 | 75 | 20 | 5 | 4 |
| 3 | 75/15/10 | 75 | 15 | 10 | 1.5 |
| 4 | 80/15/05 | 80 | 15 | 5 | 3 |
| 5 | 80/10/10 | 80 | 10 | 10 | 1 |
| 6 | 82/15/03 | 82 | 15 | 3 | 5 |
| 7 | 85/10/05 | 85 | 10 | 5 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitz, O.; Ingenhoven, S.; Bischoff, C.; Gentischer, H.; Birke, K.P.; Saracsan, D.; Biro, D. Comparison of Aqueous- and Non-Aqueous-Based Binder Polymers and the Mixing Ratios for Zn//MnO2 Batteries with Mildly Acidic Aqueous Electrolytes. Batteries 2021, 7, 40. https://doi.org/10.3390/batteries7020040
Fitz O, Ingenhoven S, Bischoff C, Gentischer H, Birke KP, Saracsan D, Biro D. Comparison of Aqueous- and Non-Aqueous-Based Binder Polymers and the Mixing Ratios for Zn//MnO2 Batteries with Mildly Acidic Aqueous Electrolytes. Batteries. 2021; 7(2):40. https://doi.org/10.3390/batteries7020040
Chicago/Turabian StyleFitz, Oliver, Stefan Ingenhoven, Christian Bischoff, Harald Gentischer, Kai Peter Birke, Dragos Saracsan, and Daniel Biro. 2021. "Comparison of Aqueous- and Non-Aqueous-Based Binder Polymers and the Mixing Ratios for Zn//MnO2 Batteries with Mildly Acidic Aqueous Electrolytes" Batteries 7, no. 2: 40. https://doi.org/10.3390/batteries7020040
APA StyleFitz, O., Ingenhoven, S., Bischoff, C., Gentischer, H., Birke, K. P., Saracsan, D., & Biro, D. (2021). Comparison of Aqueous- and Non-Aqueous-Based Binder Polymers and the Mixing Ratios for Zn//MnO2 Batteries with Mildly Acidic Aqueous Electrolytes. Batteries, 7(2), 40. https://doi.org/10.3390/batteries7020040








