3.1. Initial Performance—Comparison with Other Systems
Initially, the formation protocol used for the KIC system consists of five successive galvanostatic charge/discharge cycles at 0.82 mA·cm
−2 (5C, with C being the capacity of the cell) at room temperature. The performance of the KIC cells that undergo this initial formation protocol, and those of the conventional EDLC, are presented in
Figure 1 for a batch of three cells. A flow chart of cycling tests is presented in
Figure S3. The energy densities are expressed per kilo of electrochemical core, the detailed description of which is given in the
Supplementary Data.
Cycling performance of hybrid cells are characterized by a non-monotonous profile, with discharged capacities, which decrease drastically then increase in a few thousand cycles, as well as the non-reproducibility of the results between the identical cells. This initial formation protocol therefore allows obtaining energy densities higher than conventional supercapacitors; however, the performances are non-reproducible and unstable.
To improve the performance, a comparison with known systems such as lithium technologies has been made. In the case of the Li-ion battery, the formation protocol is well defined, with galvanostatic charge/discharge cycles at low current regime and high temperature [
21]. The current and temperature therefore seem to be two important parameters for the formation protocol, and their influence has been studied in order to define an adequate protocol for KIC cells. Different configurations were tested with two current regimes, 0.82 and 0.08 mA·cm
−2, respectively, 5C and C/2, and two temperatures, 20 and 40 °C. The capacities obtained during the five cycles of formation under different conditions are presented on
Figure 2a.
Higher capacities are obtained for a current of 0.08 mA·cm
−2 (blue and black curves) than of 0.82 mA·cm
−2 (red and green curves). In addition, a formation protocol at room temperature (blue and red curves) allows for better performance than at a higher temperature (black and green curves). A lower current regime is therefore beneficial, unlike a higher temperature. Based on these results, an intermediate formation protocol has been developed which consists of five galvanostatic charge/discharge cycles at 0.08 mA·cm
−2 (C/2) at room temperature. The performance of the cells that undergo this formation protocol are presented in
Figure 2b for a batch of three cells. The intermediate formation protocol allows to obtain reproducible results; however, the energy densities obtained are lower and the stability of the system remains low.
At this stage of the development of a formation protocol for the KICs, a problem of cell swelling was highlighted. The generation of gases within the cells is a well-known issue, whether for supercapacitors or Li-ion batteries, and is generally related to the decomposition of the electrolyte. In the case of the non-aqueous potassium-ion hybrid supercapacitor studied, it is therefore necessary to know the stability window of the electrolyte used, and more precisely, of the solvent, acetonitrile.
3.2. Hypothesis—Electrochemical Stability Window of Acetonitrile
Figure 3 shows the electrochemical stability window of acetonitrile measured with a three-electrode set-up, with glassy-carbon as the working, Ag/Ag
+ as the reference, and platinum as the counter electrode. The results are presented vs. K/K
+ in order to be placed in the case of our study.
The three-electrode set-up seems to indicate that the lower limit of the electrochemical stability is at 0.5 V vs. K/K+, although a small reduction current is observed at 1 V vs. K/K+.
In this study, the potassium-ion hybrid supercapacitor works over the voltage range of 0.5–3.5 V. However, the potentials of each electrode vs. K/K
+ are not known. While waiting to develop a reference electrode adapted to the KIC system studied, a hypothesis has been put forward to explain the generation of gas and continue the study. Studies have been able to show the compatibility of KPF
6 salt with graphite electrodes [
25] and acetonitrile [
26]. The hypothesis is therefore related to the negative graphite electrode and acetonitrile. Depending on the stage of intercalation reached, the negative electrode of the KIC system could operate outside the electrochemical stability window of acetonitrile, i.e., below 0.5 V vs. K/K
+. A schematic representation of the charge profile of the graphite electrode in the KIC system is therefore presented in
Figure S4a to illustrate this instability.
As can be seen, a potential at the negative electrode lower than expected can quickly lead to the reduction of the solvent. Consequently, the instability of acetonitrile was questioned over the operation voltage range of the system when the swelling phenomenon was first observed. The study therefore focused on the high voltage phenomena, i.e., low negative electrode potential, as framed in
Figure S4b on the charge/discharge profile of a KIC cell. However, as explained previously, the system is sized to reach a dilute intercalation stage to avoid volume expansion. The swelling phenomenon could therefore be caused by another phenomenon.
3.3. Identification of Voltage Ranges Responsible for Gas Production
To determine the instability voltage and understand the swelling of cells, constant voltage (CV) tests were carried out. CV is a well-known method for evaluating the aging of electrochemical systems, especially supercapacitors [
27]. These tests consist of charging KIC cells at a constant current (0.08 mA·cm
−2 corresponding to C/2 current regime) until various cut-off voltages, and then applying this voltage to the cells during 24 h. After that, the KIC cells are discharged at a constant current (0.08 mA·cm
−2) down to 0.5 V. The charging cut-off voltage of the KIC varies from 3.0 to 3.7 V. A flow chart of constant voltage tests is presented in
Figure S5. To ensure the reproducibility of the results, tests were carried out on a batch of three cells.
Figure 4a shows the currents of the KIC cells recorded at the constant-voltage steps. The residual current after 24 h at constant voltage, named the leakage current [
28], was determined to evaluate its evolution with the applied voltage, as can be seen in
Figure 4b. For the readability of the figure,
Figure 4a presents the curves for a single cell while
Figure 4b shows the results obtained for the batch of three cells.
At the beginning of the constant voltage step, a rapid decrease of the current was observed. Then, a stabilization occurred after approximatively 8 h. Two distinct behaviors were observed on the leakage current curve. First, low and stable values were measured between 3.0 and 3.4 V. Then, a significant increase was observed from 3.5 V.
The profiles of the leakage current during the CV step have been already observed in the case of the supercapacitors. As detailed in [
29], this can be explained by the double-layer structure at the capacitor-type electrode, composed of a diffusion layer and a compact layer. Initially, the ions of the bulk flow to the diffusion layer result in a drastic decay of current. Then, the ions of the diffusion layer are pushed to the compact layer until the structure of the electrical double-layer is ordered, reaching equilibrium. Regarding the residual leakage current, the low-leakage current values during the first tests (
Figure 4b) could reflect a range of stability for the KIC system that would be interesting to study. Indeed, these results suggest, as with [
30], that the introduction of a constant voltage step within the formation protocol of the system could be beneficial. The impact of a CV step during formation on the cycling performance of the KIC system have been investigated and will be discussed later. As for the results at a higher voltage, the leakage current increases dramatically between 3.5 and 3.7 V. This window is beyond the voltage range of the KIC system operation, which accentuates the aging phenomena [
31]. This increase can be related to the instability of acetonitrile. To obtain a higher cell voltage, the potential of the negative electrode must go down, and therefore potentially enter the instability window of acetonitrile, as illustrated in
Figure S3a. The decomposition of the electrolyte therefore explains the high leakage currents observed, as described in the case of the LICs [
30].
In parallel of the constant voltage tests, volume measurements using Archimedes’ principle were conducted.
Figure 5 shows the volume variations of the cells as a function of the applied voltage. The initial value corresponds to the volume of the cells before any test. Then, the measurements are realized at a discharged state after each step of the CV tests on the voltage window from 3.0 to 3.7 V.
The general trend of the curve is a gradual increase in the cell volume, and this seems to intensify at a high voltage. Regardless of the cell voltage, the reactions that take place within the cells result in the generation of gases, causing the cell swelling.
Based on these results, the swelling of the cells has its origin in two phenomena. The first is related to the formation of the solid electrolyte interphase (SEI). This phenomenon is well studied in the case of Li-ion batteries [
32] and starts to be referenced for K-ion batteries, but only in the case of carbonate solvents [
33]. The negative electrode of the KIC system studied here is a battery-type one, so it is assumed that it undergoes a similar reaction. A comparison with symmetrical supercapacitors could isolate this contribution. Moreover, the SEI formation is due to the electrochemical reduction of the electrolyte solvents and reductive gases such as H
2 dominate the gases released from the graphite anode. The composition of the gas generated within the cells would confirm this hypothesis. The second phenomenon is the decomposition of the electrolyte due to the extension of the operating voltage range up to 3.5 V, and therefore the operation of one or more electrodes of the system outside the stability window of the electrolyte. The SEI is therefore no longer sufficient to avoid the decomposition of the electrolyte at the negative electrode, or parasitic reactions take place at the positive electrode. The hypothesis put forward at the beginning of the swelling study must therefore be confirmed. It is then necessary to know the potential of each electrode, which requires the presence of a reference electrode in the system. The results in the three-electrode set-up are presented as well as additional tests in order to justify the phenomena described.
3.4. Hypotheses Evaluation
First, the hypothesis on the degradation of the electrolyte at a high cell voltage was evaluated. In order to do that, it is necessary to know the potential of the negative electrode to see if it drops below 0.5 V vs. K/K
+. The three-electrode set-up with an additional reference electrode were then assembled, as shown schematically in
Figure 6a. Considering the previous results, tests with this set-up were carried out for a voltage chosen in each of the following windows: 3.0–3.4 V and 3.5–3.7 V.
Figure 6 shows the cell voltage and potentials vs. K/K
+ of the positive and negative electrodes for the constant voltage tests, described previously, at 3.2 V (
Figure 6b) and 3.5 V (
Figure 6c). Each test was carried out on a batch of three cells in order to ensure the reproducibility of the results.
A rapid increase of cell voltage is observed between 0.5 and 2.2 V, followed by a neat change of slope for the two tests presented. The same behavior is observed for the potential of the negative electrode, while the potential of the AC positive electrode has a triangular profile highly characteristic of a purely capacitive behavior. For the test with a CV step at 3.2 V, the potential of the negative electrode drops to 0.66 V, whereas it decreases to 0.45 V for a CV step at 3.5 V. In addition, it can be seen that the potentials of both electrodes increase slightly during the constant voltage part. This phenomenon is still under study but is compatible with a reduction of the electrolyte at the graphite electrode.
These results show that the potential of the graphite electrode is lower with a CV step at 3.5 V, which could bring it below the stability limit of the acetonitrile (~0.5 V vs. K/K+) and explain the leakage current. If so, it can be seen from the enlargement of
Figure 6c that the negative electrode spends a lot of time below this limit during the CV. This could explain the important volume increase due to significant gas generation.
The origin of the phenomena observed at high voltage is now well defined. Although less important, the gas generation on the 3.0–3.4 V window still needs an explanation. For this purpose, we performed a degassing of the cells in order to know if this gas is linked to the passivation of the electrodes during the first use of the cells. Constant voltage tests as well as the volume measurements were performed again. Volume variations within cells and the evolution of the leakage current are shown in
Figure 7 for the three hybrid cells.
In addition, a comparison between the symmetrical and hybrid device was carried out to decorrelate the phenomena taking place at the negative electrode and at the positive electrode. Each electrode can have a passivation layer [
34], SEI at the negative side, and CEI at the positive side, giving rise to the generation of gas. Comparing the evolution of the cell volume in the symmetrical and hybrid configurations would therefore make it possible to identify at which electrode the degradation reactions take place. Symmetrical cells with two activated carbon electrodes were then assembled. To take into consideration the same degradation reactions, the same electrolyte is employed for both the hybrid and symmetrical devices. The operating voltage range of a KIC is up to 3.5 V, and the study was performed for voltages between 3.0 and 3.7 V. To keep the same logic and knowing that the operating voltage range of the EDLC studied is up to 2.7 V, constant voltage tests and volume measurements were performed on voltage ranges from 2.2 to 2.9 V for the symmetrical device. The evolutions of the leakage current and the volume variations of the cells with the applied voltage are shown on
Figure 7 for a batch of two symmetrical cells.
The evolution of the leakage current and volume variations after the degassing of the hybrid cells have a flat profile up to 3.4 V. An increase, which follows the same trend as in the hybrid cells before degassing, is then observed. As for the symmetrical cells, apart from large leakage current values outside the operating range of the KIC cells, the leakage currents are greater in the symmetrical configuration from the first test. However, no significant variation is observed despite an increasing trend. In terms of volume variation, no change in volume of the symmetrical cells was observed over the voltage range studied.
For the hybrid cells, the same trends are observed before and after degassing in terms of the leakage current. However, the initial volume increase after the first tests was no longer observed after degassing. This can confirm that this was related to the formation of the KIC cells when they were first used. More precisely, the gas formed can come from the formation of the solid electrolyte interphase (SEI) at the negative electrode. In addition, the fact that no significant volume variation is observed after the first use of the symmetrical cells tends to confirm this hypothesis. The results after the test at 3.5 V show the same increasing trend before and after degassing, so another phenomenon than the formation of the cells is involved, as detailed previously with tests in the three-electrode set-up. On the other hand, the large values of the leakage current in the symmetrical configuration can be explained by the analysis of the storage mechanism of the electric double-layer capacitors (EDLC). As explained earlier, when an EDLC is charged, an electrical double-layer is formed to store energy. However, electrons can move inside this layer via redox reactions on the surface of the electrodes. The effect of these reactions is a high leakage current, which is minimized in the hybrid configuration by replacing the negative electrode. The standard leakage current values for supercapacitors are generally lower. This must be due to the material used and could be minimized by using material that has undergone other chemical or thermal treatments [
35]. However, the same material is used throughout the study and therefore has no influence on the comparative observations made.
In a symmetrical configuration, this large leakage current is not accompanied by swelling, since no variation in volume is observed. A notable difference therefore exists between the behavior of the hybrid and symmetrical systems at a high voltage. Despite an increasing trend, the leakage current values of the symmetrical cells remain lower than those of the hybrid cells. This is explained by a difference in the working potential of the negative electrode, depending on the configuration. In the case of the KIC system, the negative graphite electrode drops to potentials below 0.5 V vs. K/K
+ (
Figure 6c). The reduction of acetonitrile gives rise to the generation of gas, explaining the high leakage currents and volume variations. For a symmetrical system working on a voltage window of 2.7 V, the negative electrode does not drop below 1.5 V vs. K/K
+ (i.e., 1.6 V vs. Li/Li
+), as detailed in [
36] and confirmed in this study with a three-electrode set-up (
Figure S6).
The swelling phenomenon therefore seems to be a problem specific to the hybrid configuration. These results are consistent with this study. The development of a formation protocol adapted to the hybrid system studied is necessary, whereas this is not the case for a symmetrical system. In addition, the hypothesis, according to which the phenomena observed during the first use are linked to the passivation of the negative electrode of the system, is confirmed by these additional tests. However, surface analyses to study the SEI layer and its evolution are still necessary.
3.5. Gas Analysis
By volume measurements, the extent of gassing is known, as well as the voltage ranges over which it occurs. To understand the origin of the swelling phenomenon, the constituents of the gas evolved need now to be identified. This is why the analysis by gas chromatography coupled with mass spectrometry were realized. Gas can be extracted from cells postmortem through the use of a syringe. Then, the resulting gas can be injected into a bag filled with argon, connected to the gas chromatography mass spectrometer for analysis to ascertain the species present. The cells used for this analysis did all constant voltage tests and started cycling at 16 mA·cm
−2 (100C/100D) during 20,000 cycles for the aging tests. The chromatogram obtained by making the ratio of our signal to that of argon is shown on
Figure S7.
Two contributions can be observed. The first one at 0.44 min is associated to hydrogen. Then, the second contribution is due to a pollution of the equipment by the ambient air. Thus, hydrogen is the only gas present in our cells, as it has been observed in LICs [
37].
It is important to note that all system components must be properly dried to minimize the swelling phenomenon. In fact, the quantity of gas generated is all the greater in the presence of water, since hydrogen can be produced by its reduction [
19]. The importance of the presence of water in the electrolyte could be observed. This is why Karl-Fischer analyses were carried out before the tests to rule out the presence of water (<2 ppm in the electrolyte) as a phenomenon responsible for the swelling.
The origin of hydrogen therefore comes from another reaction. As detailed in [
38], the decomposition of acetonitrile is likely to begin with the cleavage of a C-H bond because the C-N bond has a higher dissociation energy. H
+ atoms are therefore assumed to be formed through the reaction (4). However, the deprotonization could also be promoted by the oxidation of the salt component, as it is the case in conventional supercapacitors and described in [
39,
40].
As previously explained, hydrogen is the dominant gas produced during the electrochemical decomposition of electrolyte solvents resulting in the SEI formation in Li-ion batteries. These gas analyses confirm that this is also the case for the KIC system studied. In addition, the results presented above show that the lower limit of the stability window of acetonitrile is 0.5 V vs. K/K+. Below this limit, the decomposition reaction of acetonitrile takes place. To prevent additional hydrogen production, it is therefore essential that the negative electrode does not drop down to this potential. However, it has been proven in a three-electrode set-up that this is not the case when the system voltage rises up to 3.5 V. This explains the significant production of gas associated with high leakage currents from this voltage.
3.6. Definition of a New Formation Protocol—Performance Improvements
As explained previously, the performance of KIC cells could be improved by decreasing the current regime applied during the formation protocol. The results obtained are reproducible, but the energy densities are lower and the stability is still a problem. After the observation of a swelling of the cells, the study of this phenomenon made it possible to identify a possible origin. Additionally, this highlighted the need to further optimize the formation protocol to avoid gas generation and ensure long-term system operation.
During the swelling study, low leakage currents were observed over a given voltage range (
Figure 4b). As mentioned previously, this could reflect a range of stability for the KIC system that would be interesting to study. Indeed, these results suggest, as in [
30], that the introduction of a constant voltage step within the formation protocol of the system could be beneficial. Moreover, the results in the three-electrode set-up suggest that the potential of the negative electrode operates outside the electrochemical stability window of the electrolyte for a voltage of 3.5 V, whereas this is not the case for a voltage of 3.2 V.
Adding a constant voltage step at 3.2 V could help minimize gas generation by allowing the system to slowly develop an SEI while staying in the stability window of acetonitrile. The question is whether this SEI would be effective when operating over the full voltage range 0.5–3.5 V of the system. Additional tests in a three-electrode set-up were therefore carried out.
Figure S8 shows the cell voltage and potentials vs. K/K
+ of the positive and negative electrodes for constant voltage test at 3.2 V (charge at 0.08 mA·cm
−2 up to 3.2 V—24-h constant voltage step—discharge at 0.08 mA·cm
−2 up to 0.5 V), followed by a galvanostatic charge/discharge at 0.08 mA·cm
−2 mA (C/2). Tests were carried out on a batch of three cells in order to ensure the reproducibility of the results.
As detailed on
Figure 6, a rapid increase of cell voltage is observed between 0.5 and 2.2 V, followed by a neat change of slope. The potential of the negative electrode follows the same pattern, while the potential of the AC positive electrode has a triangular profile highly characteristic of a purely capacitive behavior. The same behaviors are observed during the following galvanostatic charge/discharge cycle. The potential of the negative electrode drops to 0.66 V during the CV step at 3.2 V and to 0.57 V during the cycle which follows.
The potential of each electrode, and in particular of the negative, is therefore within the stability window of acetonitrile during the constant voltage step, as well as during the cycle which follows. It can therefore be deduced that there is no decomposition of the electrolyte and therefore no gas.
Based on these results, a final formation protocol has been developed which consists of a charge at 0.08 mA·cm
−2 up to 3.2 V, a 24 h constant voltage step at 3.2 V, and a discharge at 0.08 mA·cm
−2 up to 0.5 V at room temperature. A degassing of the cell is then carried out before cycling to evacuate the gases generated during the formation of the SEI. The performance of the cells that undergo this formation protocol are presented in
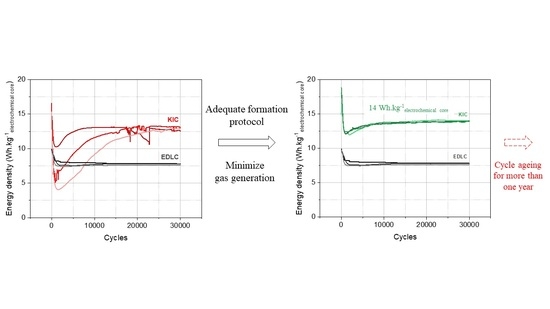
Figure 8 for a batch of three cells.
A significant drop in performance is observed at the beginning of cycling from approximately 19 to 12 Wh·kgelectrochemical core−1. This is followed by a rise and then a stabilization of the performance between 12.7 and 14 Wh·kgelectrochemical core−1. In addition, the results are reproducible on the batch of three cells.
The initial drop in performance is a behavior that has also been observed in the case of symmetrical supercapacitors [
9]. Regarding the rise, it is a phenomenon specific to M-ion hybrid supercapacitors (MICs) related to the SEI formation and a cycling-induced capacity gain, as detailed in [
2] for carbon-supported nanomaterials. After the stabilization of the performance, it can be concluded that the final formation protocol developed allows stable and reproducible results with high energy densities of up to 14 Wh·kg
electrochemical core−1.