Repurposing Face Masks after Use: From Wastes to Anode Materials for Na-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Methods
2.3. Na-Metal and Na-Ion Cell Assembly and Electrochemical Testing
3. Results
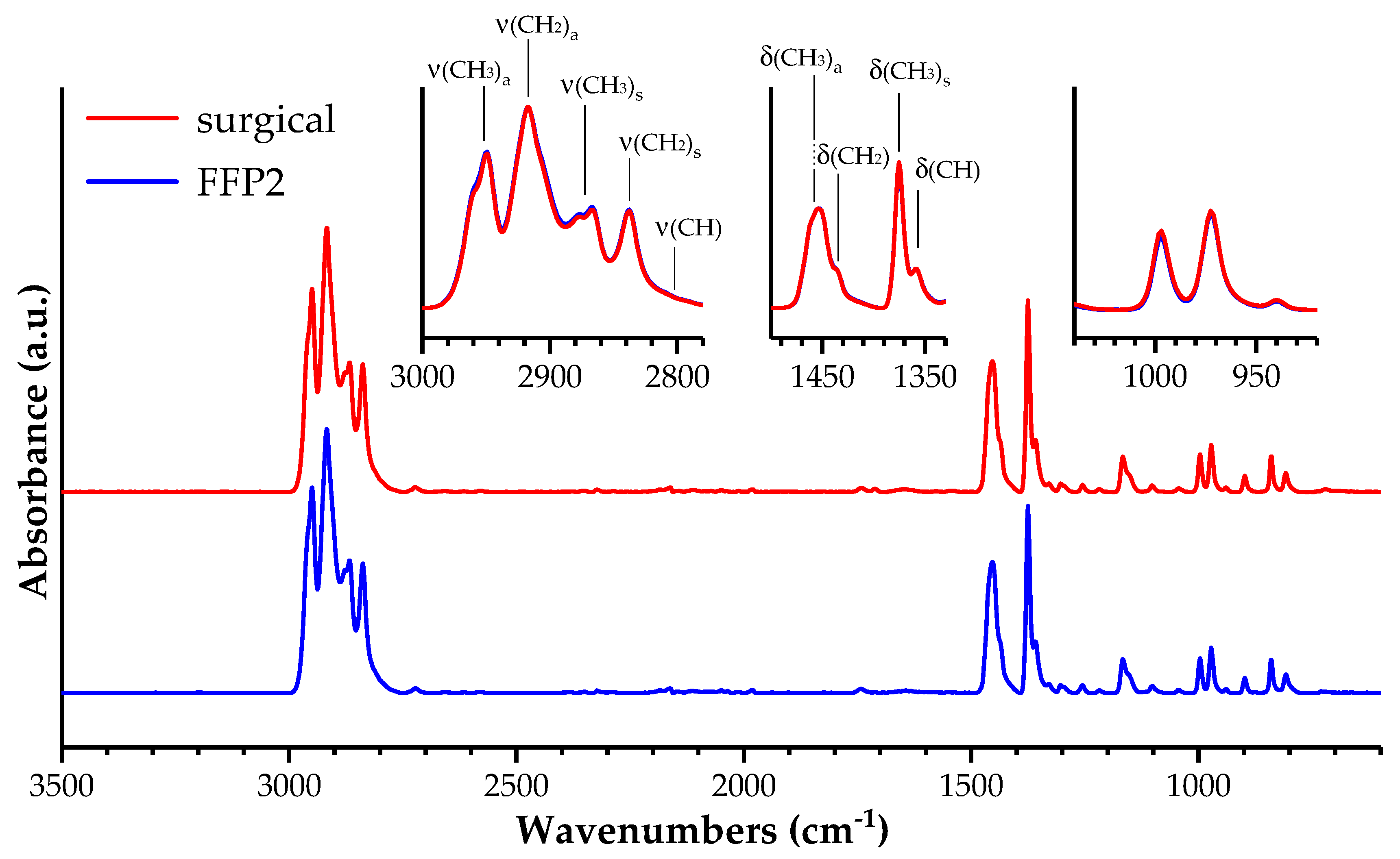
3.1. Materials Characterization
3.2. Electrochemical Behaviour in Lab-Scale Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33, e00028-20. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.O.; Okoffo, E.D. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraj, S.; Ashokkumar, V.; Hariharan, S.; Manibharathi, A.; Show, P.L.; Chong, C.T.; Ngamcharussrivichai, C. The COVID-19 pandemic face mask waste: A blooming threat to the marine environment. Chemosphere 2021, 272, 129601. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, T.; Teymoorian, T.; Kowsari, E.; Ramakrishna, S. Challenges, Strategies, and Recommendations for the Huge Surge in Plastic and Medical Waste during the Global COVID-19 Pandemic with Circular Economy Approach. Mater. Circ. Econ. 2021, 3, 6. [Google Scholar] [CrossRef]
- Battegazzore, D.; Cravero, F.; Frache, A. Is it Possible to Mechanical Recycle the Materials of the Disposable Filtering Masks? Polymers 2020, 12, 2726. [Google Scholar] [CrossRef] [PubMed]
- Sangkham, S. Face mask and medical waste disposal during the novel COVID-19 pandemic in Asia. Case Stud. Chem. Environ. Eng. 2020, 2, 100052. [Google Scholar] [CrossRef]
- Torres, F.G.; De-la-Torre, G.E. Face mask waste generation and management during the COVID-19 pandemic: An overview and the Peruvian case. Sci. Total Environ. 2021, 786, 147628. [Google Scholar] [CrossRef]
- Shanmugam, V.; Babu, K.; Garrison, T.F.; Capezza, A.J.; Olsson, R.T.; Ramakrishna, S.; Hedenqvist, M.S.; Singha, S.; Bartoli, M.; Giorcelli, M.; et al. Potential natural polymer-based nanofibres for the development of facemasks in countering viral outbreaks. J. Appl. Polym. Sci. 2021, 138, 50658. [Google Scholar] [CrossRef]
- Voudrias, E.A. Technology selection for infectious medical waste treatment using the analytic hierarchy process. J. Air Waste Manag. Assoc. 2016, 66, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Sadhukhan, J.; Ng, K.S.; Hernandez, E.M. Biorefineries and Chemical Processes: Design, Integration and Sustainability Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Park, C.; Choi, H.; Andrew Lin, K.-Y.; Kwon, E.E.; Lee, J. COVID-19 mask waste to energy via thermochemical pathway: Effect of Co-Feeding food waste. Energy 2021, 230, 120876. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Lee, J.; Tsang, Y.F.; Kim, Y.-M.; Jae, J.; Jung, S.-C.; Park, Y.-K. Production of value-added aromatics from wasted COVID-19 mask via catalytic pyrolysis. Environ. Pollut. 2021, 283, 117060. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gao, F.; Zhang, Z.; Zhuang, Q.; Yu, H.; Huang, Y.; Liu, Q.; Fu, M. Synthesis of the cathode and anode materials from discarded surgical masks for high-performance asymmetric supercapacitors. J. Colloid Interface Sci. 2021, 603, 157–164. [Google Scholar] [CrossRef]
- Yuwen, C.; Liu, B.; Rong, Q.; Zhang, L.; Guo, S. Self-activated pyrolytic synthesis of S, N and O co-doped porous carbon derived from discarded COVID-19 masks for lithium sulfur batteries. Renew. Energy 2022, 192, 58–66. [Google Scholar] [CrossRef]
- Graedel, T.E. On the Future Availability of the Energy Metals. Annu. Rev. Mater. Res. 2011, 41, 323–335. [Google Scholar] [CrossRef]
- Hirsh, H.S.; Li, Y.; Tan, D.H.S.; Zhang, M.; Zhao, E.; Meng, Y.S. Sodium-Ion Batteries Paving the Way for Grid Energy Storage. Adv. Energy Mater. 2020, 10, 2001274. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Usiskin, R.; Lu, Y.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.-S.; Maier, J. Fundamentals, status and promise of sodium-based batteries. Nat. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134. [Google Scholar] [CrossRef]
- You, Y.; Manthiram, A. Progress in High-Voltage Cathode Materials for Rechargeable Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1701785. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Chayambuka, K.; Mulder, G.; Danilov, D.L.; Notten, P.H.L. Sodium-Ion Battery Materials and Electrochemical Properties Reviewed. Adv. Energy Mater. 2018, 8, 1800079. [Google Scholar] [CrossRef]
- Yoon, G.; Kim, H.; Park, I.; Kang, K. Conditions for Reversible Na Intercalation in Graphite: Theoretical Studies on the Interplay Among Guest Ions, Solvent, and Graphite Host. Adv. Energy Mater. 2017, 7, 1601519. [Google Scholar] [CrossRef]
- Doeff, M.M.; Ma, Y.; Visco, S.J.; De Jonghe, L.C. Electrochemical Insertion of Sodium into Carbon. J. Electrochem. Soc. 1993, 140, L169–L170. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Zhao, L.-F.; Hu, Z.; Lai, W.-H.; Tao, Y.; Peng, J.; Miao, Z.-C.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Xie, L.; Tang, C.; Bi, Z.; Song, M.; Fan, Y.; Yan, C.; Li, X.; Su, F.; Zhang, Q.; Chen, C. Hard Carbon Anodes for Next-Generation Li-Ion Batteries: Review and Perspective. Adv. Energy Mater. 2021, 11, 2101650. [Google Scholar] [CrossRef]
- Piotrowska, A.; Kierzek, K.; Rutkowski, P.; Machnikowski, J. Properties and lithium insertion behavior of hard carbons produced by pyrolysis of various polymers at 1000 °C. J. Anal. Appl. Pyrolysis 2013, 102, 1–6. [Google Scholar] [CrossRef]
- Shao, W.; Shi, H.; Jian, X.; Wu, Z.-S.; Hu, F. Hard-Carbon Anodes for Sodium-Ion Batteries: Recent Status and Challenging Perspectives. Adv. Energy Sustain. Res. 2022, 3, 2200009. [Google Scholar] [CrossRef]
- Takenaka, N.; Bouibes, A.; Yamada, Y.; Nagaoka, M.; Yamada, A. Frontiers in Theoretical Analysis of Solid Electrolyte Interphase Formation Mechanism. Adv. Mater. 2021, 33, 2100574. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Eui Lee, M.; Kim, S.-S.; Joh, H.-I.; Lee, S. Efficient upcycling of polypropylene-based waste disposable masks into hard carbons for anodes in sodium ion batteries. J. Ind. Eng. Chem. 2022, 105, 268–277. [Google Scholar] [CrossRef]
- Pianta, N.; Locatelli, D.; Ruffo, R. Cycling properties of Na3V2(PO4)2F3 as positive material for sodium-ion batteries. Ionics 2021, 27, 1853–1860. [Google Scholar] [CrossRef]
- Tagliaferro, A.; Rovere, M.; Padovano, E.; Bartoli, M.; Giorcelli, M. Introducing the Novel Mixed Gaussian-Lorentzian Lineshape in the Analysis of the Raman Signal of Biochar. Nanomaterials 2020, 10, 1748. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Ji, X.; Wu, Z.; Yang, Y.; Zhou, Z.; Li, F.; Chen, Q.; Banks, C.E. Exploration of ion migration mechanism and diffusion capability for Na3V2(PO4)2F3 cathode utilized in rechargeable sodium-ion batteries. J. Power Sources 2014, 256, 258–263. [Google Scholar] [CrossRef]
- McDonald, M.P.; Ward, I.M. The assignment of the infra-red absorption bands and the measurement of tacticity in polypropylene. Polymer 1961, 2, 341–355. [Google Scholar] [CrossRef]
- Burfield, D.R.; Loi, P.S.T. The use of infrared spectroscopy for determination of polypropylene stereoregularity. J. Appl. Polym. Sci. 1988, 36, 279–293. [Google Scholar] [CrossRef]
- Hujuri, U.; Ghoshal, A.K.; Gumma, S. Temperature-dependent pyrolytic product evolution profile for polypropylene. J. Appl. Polym. Sci. 2011, 119, 2318–2325. [Google Scholar] [CrossRef]
- Chan, J.H.; Balke, S.T. The thermal degradation kinetics of polypropylene: Part III. Thermogravimetric analyses. Polym. Degrad. Stab. 1997, 57, 135–149. [Google Scholar] [CrossRef]
- Tuñón-Molina, A.; Takayama, K.; Redwan, E.M.; Uversky, V.N.; Andrés, J.; Serrano-Aroca, Á. Protective Face Masks: Current Status and Future Trends. ACS Appl. Mater. Interfaces 2021, 13, 56725–56751. [Google Scholar] [CrossRef]
- Aboulkas, A.; El harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Shimodaira, N.; Masui, A. Raman spectroscopic investigations of activated carbon materials. J. Appl. Phys. 2002, 92, 902–909. [Google Scholar] [CrossRef]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Bartoli, M.; Giorcelli, M.; Jagdale, P.; Rovere, M.; Tagliaferro, A.; Chae, M.; Bressler, D.C. Shape tunability of carbonized cellulose nanocrystals. SN Appl. Sci. 2019, 1, 1661. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, M.; Nasir, M.A.; Jagdale, P.; Passaglia, E.; Spiniello, R.; Rosso, C.; Giorcelli, M.; Rovere, M.; Tagliaferro, A. Influence of pyrolytic thermal history on olive pruning biochar and related epoxy composites mechanical properties. J. Compos. Mater. 2020, 54, 1863–1873. [Google Scholar] [CrossRef]
- Giorcelli, M.; Bartoli, M. Development of Coffee Biochar Filler for the Production of Electrical Conductive Reinforced Plastic. Polymers 2019, 11, 1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousef, S.; Eimontas, J.; Stasiulaitiene, I.; Zakarauskas, K.; Striūgas, N. Pyrolysis of all layers of surgical mask waste as a mixture and its life-cycle assessment. Sustain. Prod. Consum. 2022, 32, 519–531. [Google Scholar] [CrossRef]
- Carboni, M.; Manzi, J.; Armstrong, A.R.; Billaud, J.; Brutti, S.; Younesi, R. Analysis of the Solid Electrolyte Interphase on Hard Carbon Electrodes in Sodium-Ion Batteries. ChemElectroChem 2019, 6, 1745–1753. [Google Scholar] [CrossRef] [Green Version]
- Vadhva, P.; Hu, J.; Johnson, M.J.; Stocker, R.; Braglia, M.; Brett, D.J.L.; Rettie, A.J.E. Electrochemical Impedance Spectroscopy for All-Solid-State Batteries: Theory, Methods and Future Outlook. ChemElectroChem 2021, 8, 1930–1947. [Google Scholar] [CrossRef]
- Choi, W.; Shin, H.-C.; Kim, J.M.; Choi, J.-Y.; Yoon, W.-S. Modeling and Applications of Electrochemical Impedance Spectroscopy (EIS) for Lithium-ion Batteries. J. Electrochem. Sci. Technol. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Xia, B.; Li, B.; Cao, L.; Lai, Y.; Zheng, W.; Wang, H.; Wang, W.; Wang, M. A Study on the Open Circuit Voltage and State of Charge Characterization of High Capacity Lithium-Ion Battery Under Different Temperature. Energies 2018, 11, 2408. [Google Scholar] [CrossRef]







| Feedstock | Tonset (°C) | Tmax (°C) | Residue (wt.%) a |
|---|---|---|---|
| Surgical mask | 430 | 469 | 1.05 |
| FFP2 mask | 426 | 468 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porporato, S.; Bartoli, M.; Piovano, A.; Pianta, N.; Tagliaferro, A.; Elia, G.A.; Ruffo, R.; Gerbaldi, C. Repurposing Face Masks after Use: From Wastes to Anode Materials for Na-Ion Batteries. Batteries 2022, 8, 183. https://doi.org/10.3390/batteries8100183
Porporato S, Bartoli M, Piovano A, Pianta N, Tagliaferro A, Elia GA, Ruffo R, Gerbaldi C. Repurposing Face Masks after Use: From Wastes to Anode Materials for Na-Ion Batteries. Batteries. 2022; 8(10):183. https://doi.org/10.3390/batteries8100183
Chicago/Turabian StylePorporato, Silvia, Mattia Bartoli, Alessandro Piovano, Nicolò Pianta, Alberto Tagliaferro, Giuseppe Antonio Elia, Riccardo Ruffo, and Claudio Gerbaldi. 2022. "Repurposing Face Masks after Use: From Wastes to Anode Materials for Na-Ion Batteries" Batteries 8, no. 10: 183. https://doi.org/10.3390/batteries8100183
APA StylePorporato, S., Bartoli, M., Piovano, A., Pianta, N., Tagliaferro, A., Elia, G. A., Ruffo, R., & Gerbaldi, C. (2022). Repurposing Face Masks after Use: From Wastes to Anode Materials for Na-Ion Batteries. Batteries, 8(10), 183. https://doi.org/10.3390/batteries8100183














