Electrochemically Active Polymer Components in Next-Generation LiFePO4 Cathodes: Can Small Things Make a Big Difference?
Abstract
:1. Introduction
2. Electrochemically Active Electron-Conducting Polymers (EAECPs): Function-Enabling Properties
3. EAECP-Based Cathode Components
3.1. EAECPs as Lithium Iron Phosphate Coatings
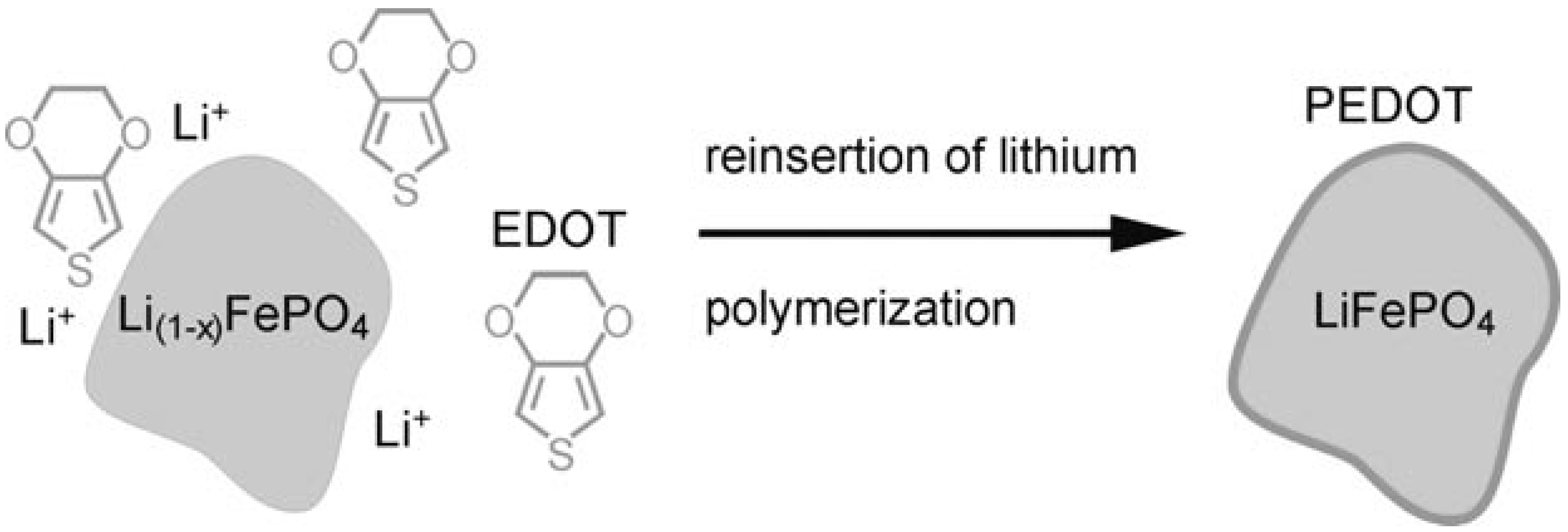
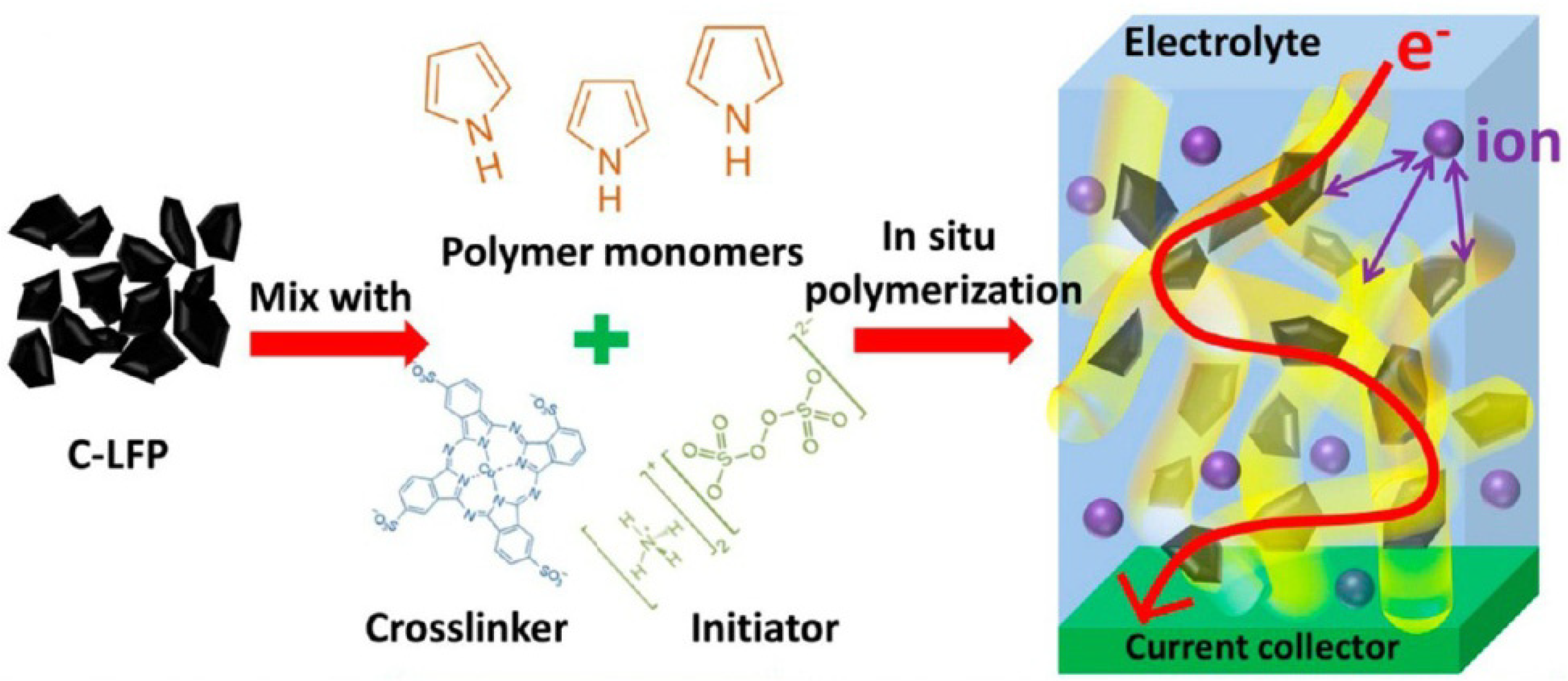
3.1.1. EAECP-Based Coatings by In Situ Chemical Polymerization over LiFePO4 Particles
3.1.2. EAECP-Based Coatings by Mixing LFP with Ex Situ Synthesized Polymers
| EAECP-LiFePO4 Material | Synthesis Route for EAECP-LiFePO4 Material | Cathode Active Layer Composition | Initial Discharge Capacity, mAh/g (Per EAECP-LiFePO4 Weight) | Capacity Retention, %/Number of Cycles (Discharge Current) | Reference | |
|---|---|---|---|---|---|---|
| At Low Discharge Current (Discharge Current) | At High Discharge Current (Discharge Current) | |||||
| PPy (7 wt%)-C-LFP | In situ chemical polymerization over LiFePO4 using an external oxidant in solution | PPy-C-LFP/CB/PTFE (75:20:5 wt%) | 150 (C/10) | 110 (10C) | 99.3%/20 cycles (C/10) | [40] |
| PPy (2.95 wt%)-LFP | PPy-LFP/CB/PVDF (85:7:8 wt%) | 153 (C/10) | 118 (5C) | 98.8%/20 cycles (C/10) | [46] | |
| PANI (7 wt%)-C-LFP | PPy-C-LFP/CB/PTFE (75:20:5 wt%) | 165 (C/5) | 123 (10C) | 97.4%/100 cycles (C/5) | [51] | |
| PPy-PEG (appr. 10 wt%; 33:1)-C-LFP | PPy-PEG-C-LFP/CB/PVDF (80:10:10 wt%) | 156 (C/5) | 97 (5C) | 100%/25 cycles (C/5) | [42] | |
| PANI-CRGO-LFP (0.15:0.005:1, by weight) | PANI-CRGO-LFP/CB/PTFE (80:10:10 wt%) | 165 (C/5) | 70 (25C) | 76.1%/1000 cycles (2C) | [57] | |
| PEDOT (7.1 wt%)-LFP | In situ chemical polymerization over delithiated LiFePO4 in solution | PEDOT-LFP/PVDF (8:84.5:7.5 wt%) | 163 (C/10) | 123 (10C) | close to 100%/30 cycles (C/2) | [38] |
| PPy (11.1 wt%)-C-LFP | CVD | PPy-C-LFP/CB/PVDF (75:15:10 wt%) | 148 (1C) at 20 °C | 80 (20C) at 20 °C; 135 (5C) at 55 °C | 82%/700 cycles (5C) at 55 °C | [37] |
| PEDOT:p-TSA (8 wt%)-LFP | Mixing of LiFePO4 and a polymer in solution | PEDOT:p-TSA-LFP/CB/PTFE (75:20:5 wt%) | 166 (C/15) | 120 (5C) | 97%/50 cycles (C/15) | [62] |
| PEDOT:PSS (10 wt%)-LFP | PEDOT:PSS-LFP/CB/PVDF (80:10:10 wt%) | 140.8 (C/10) | 98 (5C) | 92%/200 cycles (2C) | [66] | |
| PEDOT:PSS (5 wt%)-C-LFP | PEDOT:PSS-C-LFP/CB/PVDF (80:10:10 wt%) | 154.6 (C/10) | ca. 120 (5C) | 96%/200 cycles (2C) | [66] | |
| PANI:CSA (10 wt%)-C-LFP | PANI:CSA-C-LFP/CB/PVDF (70:20:10 wt%) | 165.3 (C/10) | 108.7 (5C) | ca. 97%/50 cycles (C/10) | [67] | |
| PTPA (10 wt%)-C-LFP | PTPA-C-LFP/CB/PTFE (70:20:10 wt%) | 154.5 (C/10) | 114.2 (10C) | ca. 97%/50 cycles (C/10) | [68] | |
| PANI (15 wt%)-C-LFP | Solvent-free mixing of LiFePO4 and a polymer | PANI-C-LFP/CB/B (75:15:10 wt%) | 164 (C/5) | 130 (2C) | close to 100%/150 cycles (C/5) | [69] |
3.2. EAECPs as Conductive Binders
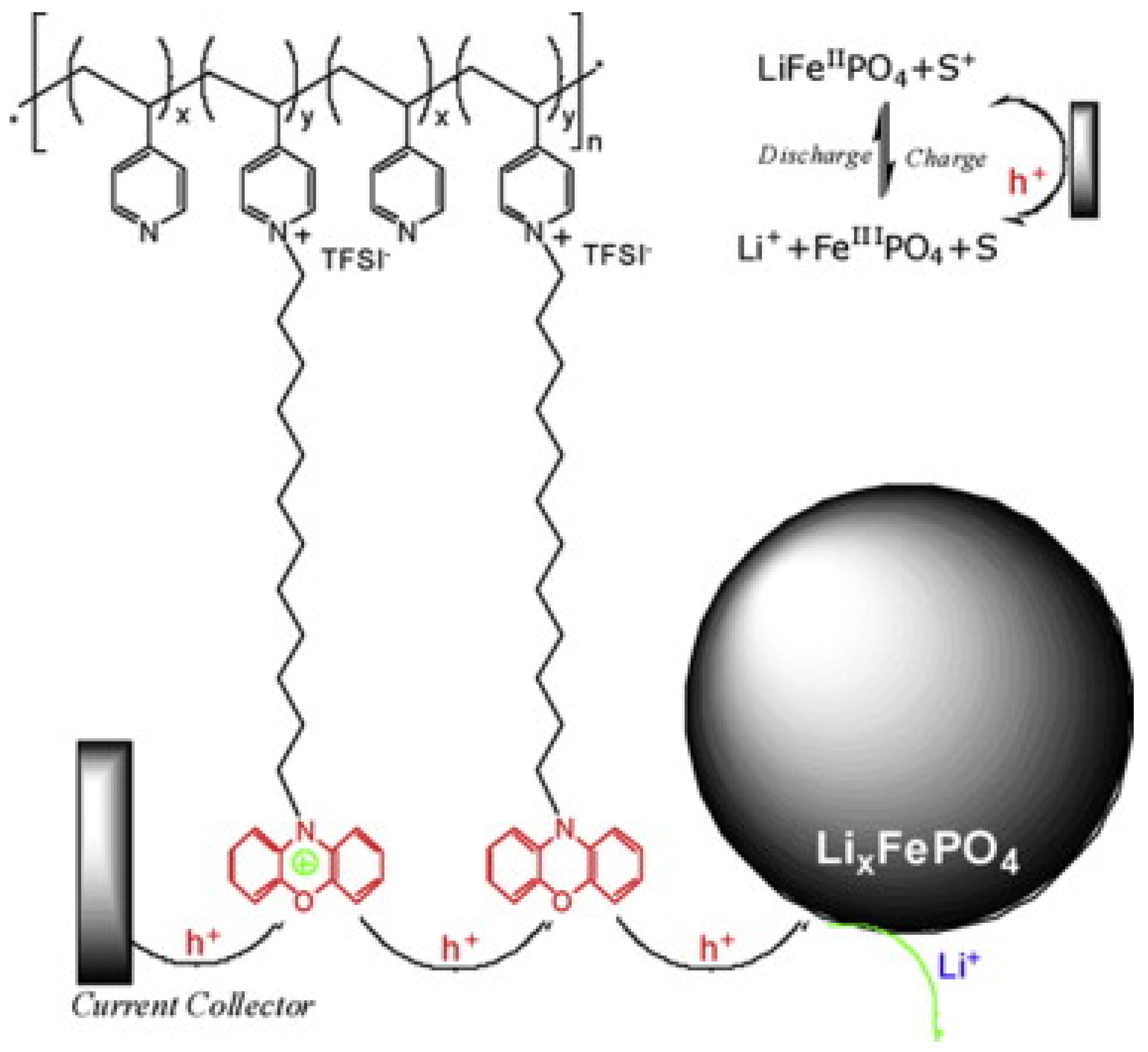
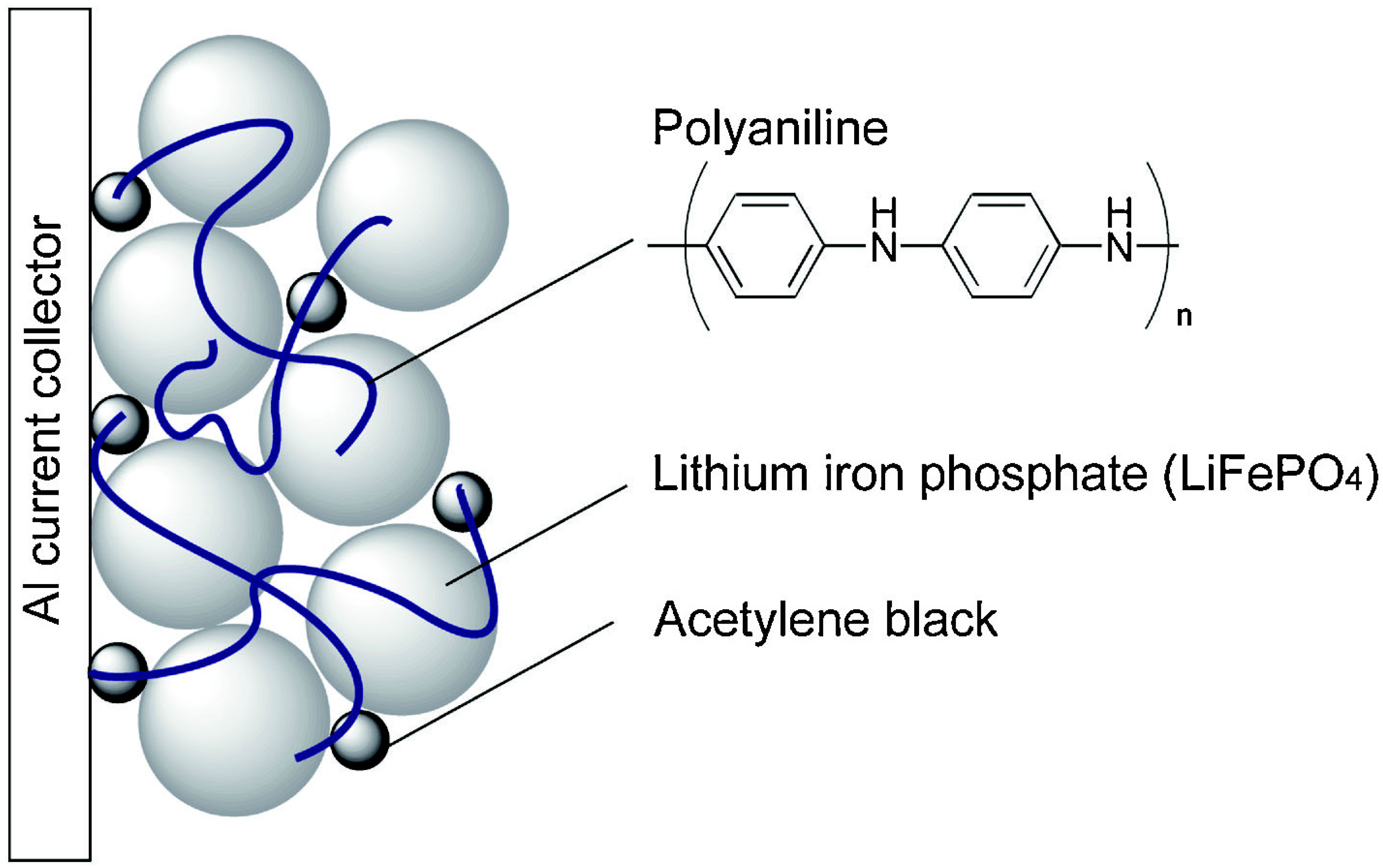
3.2.1. EAECPs as Single Conductive Binders
LiFePO4/EAECP Cathodes Prepared by Chemical Methods
LiFePO4/EAECP Cathodes Prepared by Electrochemical Methods
3.2.2. EAECPs as Components of Conductive Additive-Binder Networks
LiFePO4/CA/EAECP Cathodes
LiFePO4/EAECP/B Cathodes
LiFePO4/CA/EAECP/B Cathodes
| Electrode Composition | Synthesis Route | Initial Discharge Capacity, mAh/g (Per Active Materials) | Capacity Retention, %/Number of Cycles (Discharge Current) | Reference | |
|---|---|---|---|---|---|
| At Low Discharge Current (Discharge Current) | At High Discharge Current (Discharge Current) | ||||
| LiFePO4/EAECP Cathodes | |||||
| C-LFP/PEDOT:PSS (92:8 wt%) | Casting a slurry of LiFePO4 particles and a pre-synthesized polymer onto a current collector | 120 (C/5) | - | close to 100%/100 cycles (1C) | [76] |
| C-LFP/PEDOT:PSS (91:9 wt%) | 132 (C/10) | ca. 85 (1C) | close to 100%/50 cycles (C/2) | [80] | |
| C-LFP/PEDOT:PSS (99.5:0.5 wt%) | 147 (C/5) | 122 (5C) | 98%/100 cycles (1C) | [74] | |
| LFP/SA-PProDOT (80:20 wt%) | 137.6 (C/10) | 100 (2C) | 86.6% /400 cycles (1C) | [75] | |
| C-LFP/C-PPy (85:15 wt%) | Casting a slurry of LiFePO4 particles and a monomer onto a current collector followed by in situ chemical polymerization | 150 (C/2) | 60 (30C) | 75%/500 cycles (1C) | [83] |
| C-LFP/PPy (80:20 wt%) | Electrochemical co-deposition of LiFePO4 particles and a polymer matrix onto a current collector from LiFePO4/monomer solution | 154 (C/10) | ca. 110 (1C) | close to 100%/100 cycles (1C) | [86] |
| C-LFP/PEDOT (33.5:66.5 wt%), free-standing | Dynamic three phase interline electropolymerization (D3PIE) | 75 (C/10) | 52 (1C) | close to 100%/50 cycles (C/2) | [89] |
| LiFePO4/CA/EAECP cathodes | |||||
| C-LFP/ CB/PANI (85:9:6 wt%) | Casting a slurry of LiFePO4 particles, a conductive additive, and a pre-synthesized polymer onto a current collector | - | 132 (1C) 116 (5C) | close to 100%/100 cycles (1C) | [90] |
| C-LFP/ CB/PEDOT:PSS (92:4:4 wt%) | 165 (C/5) | 155 (1C) | 99+%/150 cycles (1C) | [92] | |
| C-LFP/ CB/PEDOT:PSS (82:9:9 wt%) | 148 (C/10) | ca. 100 (5C) 19 (20C) | 74%/500 cycles (5C) | [94] | |
| C-LFP/ MWCNT/PEDOT:PSS (82:9:9 wt%) | 160 (C/10) | ca. 115 (5C) 56 (20C) | 84%/500 cycles (5C) | [94] | |
| C-LFP/ SWCNT/PEDOT:PSS (82:9:9 wt%) | 166.6 (C/10) | ca. 120 (5C) | ca. 100 %/100 cycles (5C) 87.9%/2500 cycles (10C) | [80] | |
| LiFePO4/EAECP/B cathodes | |||||
| C-LFP/ PEDOT:PSS/CMC (96:2:2 wt%) | Casting a slurry of LiFePO4 particles, a binder, and a pre-synthesized polymer onto a current collector | 148 (C/5) | 126 (5C) | 99+%/100 cycles (1C) | [100] |
| C-LFP/ PEDOT:PSS/PEO (95:3:2 wt%) | 143 (C/10) | ca. 57 (10C) | close to 100%/27 cycles (C/3) in sulfolane | [101] | |
| C-LFP/ PEDOT:PSS/SPPO (95:2.5:2.5 wt%) | 146 (C/10) | ca. 85 (10C) | 99.4%/30 cycles (C/3) | [101] | |
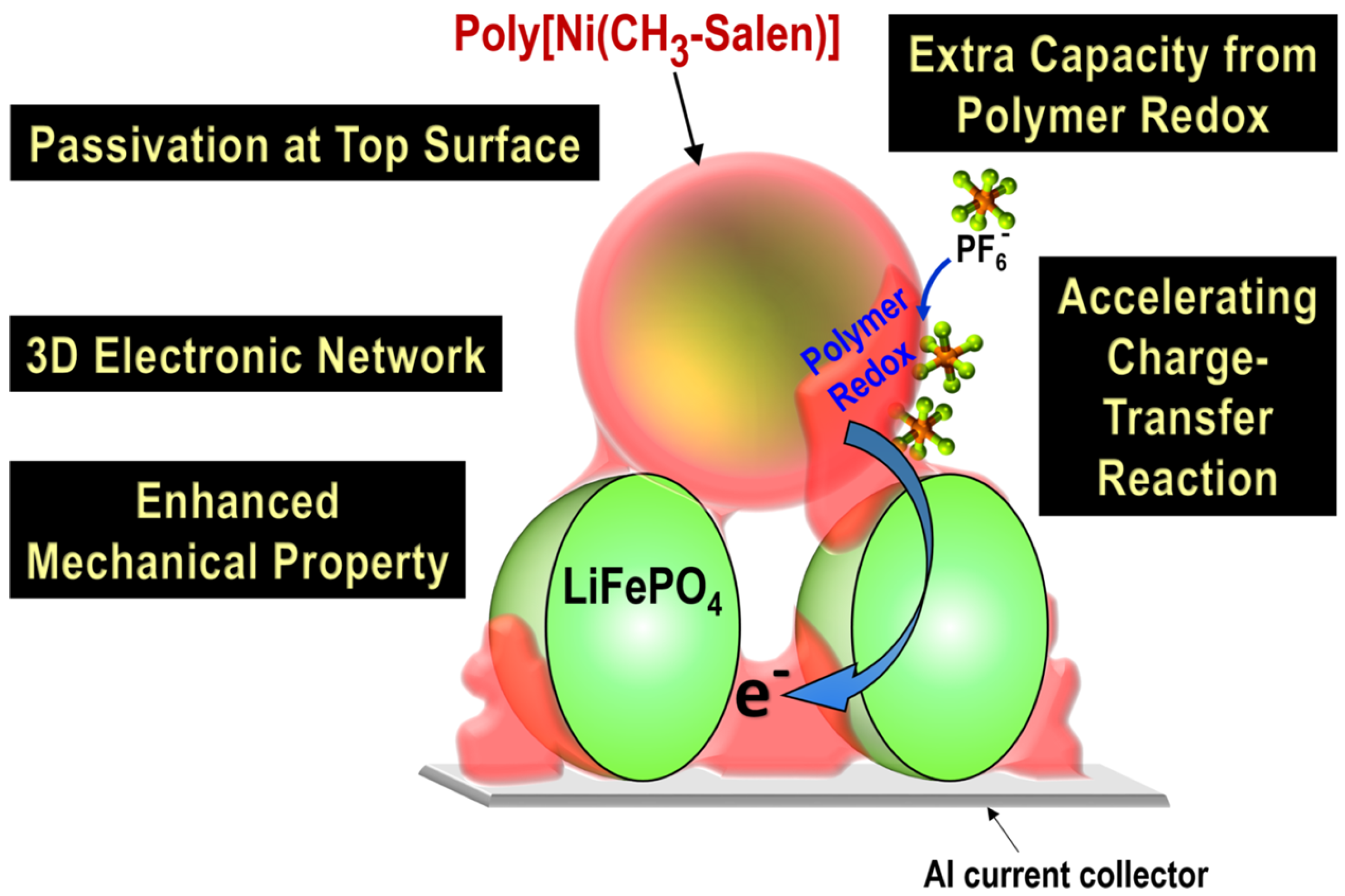
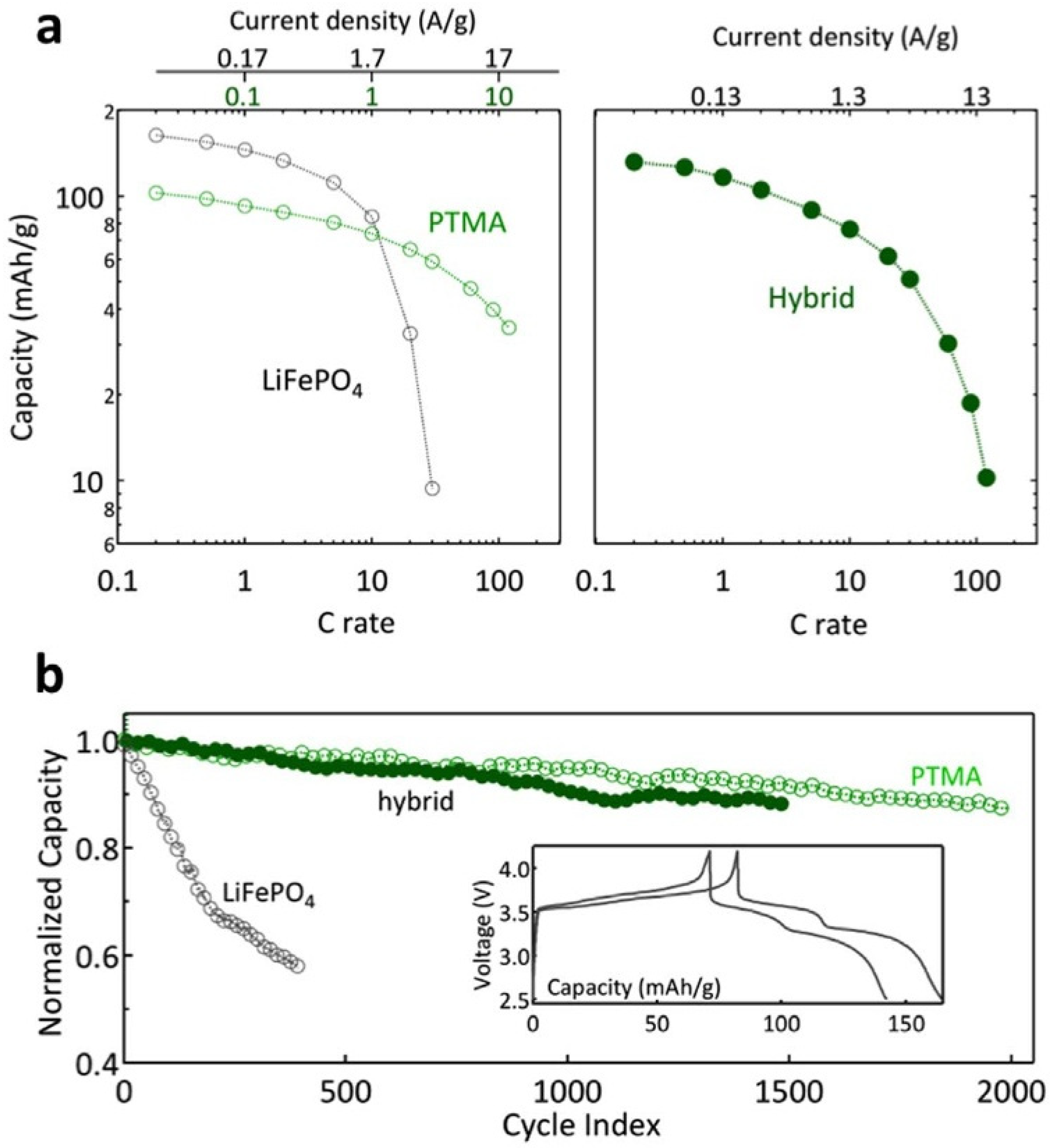
| C-LFP/poly-[Ni(CH3-salen)]/PVDF (93:5.5:1.5 wt%) | Casting a slurry of LiFePO4 particles, a binder, and a monomer onto a current collector, followed by in-battery electrochemical polymerization | 142 (C/10) | ca. 84 (5C) | 96%/150 cycles (C/2) | [99] |
| LiFePO4/CA/EAECP/B cathodes | |||||
| C-LFP/CB/ PEDOT:PSS/CMC (92:4:2:2 wt%) | Casting a slurry of LiFePO4 particles, a conductive additive, a binder, and a pre-synthesized polymer onto a current collector | 148 (C/5) | 128 (5C) | 99+%/100 cycles (1C) | [93] |
| LiFePO4/CB/ PEDOT:PSS/PVDF (84:8:1:7 wt%) | 128.5 (C/10) | ca. 111 (2C) | close to 100%/50 cycles (2C) | [102] | |
| C-LFP/CB/FP/PVDF (89.9:2:0.1:8 wt%) | 135 (C/2) | 59 (30C) | 78%/2000 cycles (5C) | [106] | |
| C-LFP/ CB/PEDOT/PVDF (82:7.7:3.6:6.7 wt%) | In-battery electrochemical polymerization of the monomer cast over a pre-formed and delithiated LiFePO4/CA/B electrode | ca. 135 (C/10) | ca. 107 (2C) | 96+%/50 cycles (C/2) | [111] |
3.3. EAECPs as Current Collector Coatings
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Grey, C.P.; Hall, D.S. Prospects for lithium-ion batteries and beyond—A 2030 vision. Nat. Commun. 2020, 11, 6279. [Google Scholar] [CrossRef]
- Voropaeva, D.Y.; Safronova, E.Y.; Novikova, S.A.; Yaroslavtsev, A.B. Recent progress in lithium-ion and lithium metal batteries. Mendeleev Commun. 2022, 32, 287–297. [Google Scholar] [CrossRef]
- Mohamed, N.; Allam, N.K. Recent advances in the design of cathode materials for Li-ion batteries. RSC Adv. 2020, 10, 21662–21685. [Google Scholar] [CrossRef]
- Noerochim, L.; Suwarno, S.; Idris, N.H.; Dipojono, H.K. Recent Development of Nickel-Rich and Cobalt-Free Cathode Materials for Lithium-Ion Batteries. Batteries 2021, 7, 84. [Google Scholar] [CrossRef]
- Kaur, G.; Gates, B. Review—Surface Coatings for Cathodes in Lithium Ion Batteries: From Crystal Structures to Electrochemical Performance. J. Electrochem. Soc. 2022, 169, 043504. [Google Scholar] [CrossRef]
- Akhilash, M.; Salini, P.; John, B.; Mercy, T. A journey through layered cathode materials for lithium ion cells—From lithium cobalt oxide to lithium-rich transition metal oxides. J. Alloys Compd. 2021, 869, 159239. [Google Scholar] [CrossRef]
- Jiang, M.; Danilov, D.L.; Eichel, R.; Notten, P.H.L. A Review of Degradation Mechanisms and Recent Achievements for Ni-Rich Cathode-Based Li-Ion Batteries. Adv. Energy Mater. 2021, 11, 2103005. [Google Scholar] [CrossRef]
- Dou, S. Review and prospects of Mn-based spinel compounds as cathode materials for lithium-ion batteries. Ionics 2015, 21, 3001–3030. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Zhang, W.-J. Structure and performance of LiFePO4 cathode materials: A review. J. Power Sources 2011, 196, 2962–2970. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Olivine LiFePO4: The remaining challenges for future energy storage. Energy Environ. Sci. 2015, 8, 1110–1138. [Google Scholar] [CrossRef]
- Hu, J.; Huang, W.; Yang, L.; Pan, F. Structure and performance of the LiFePO4 cathode material: From the bulk to the surface. Nanoscale 2020, 12, 15036–15044. [Google Scholar] [CrossRef]
- Yang, X.-G.; Liu, T.; Wang, C.-Y. Thermally modulated lithium iron phosphate batteries for mass-market electric vehicles. Nat. Energy 2021, 6, 176–185. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, L.; Liu, Y.; Li, L. Review—Recent Developments in the Doped LiFePO4 Cathode Materials for Power Lithium Ion Batteries. J. Electrochem. Soc. 2016, 163, A2600–A2610. [Google Scholar] [CrossRef]
- Ahsan, Z.; Ding, B.; Cai, Z.; Wen, C.; Yang, W.; Ma, Y.; Zhang, S.; Song, G.; Javed, M.S.; Bo, D. Recent progress in capacity enhancement of LiFePO4 cathode for Li-Ion batteries. J. Electrochem. Energy Convers. Storage 2020, 18, 010801. [Google Scholar] [CrossRef]
- Li, L.; Wu, L.; Wu, F.; Song, S.; Zhang, X.; Fu, C.; Yuan, D.; Xiang, Y. Review—Recent Research Progress in Surface Modification of LiFePO4 Cathode Materials. J. Electrochem. Soc. 2017, 164, A2138–A2150. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Z.; He, C.; Zhang, J.; Mei, P.; Han, X.; Wang, X.; Yang, Y. Binary carbon-based additives in LiFePO4 cathode with favorable lithium storage. Nanotechnol. Rev. 2020, 9, 934–944. [Google Scholar] [CrossRef]
- Huang, S.; Huang, X.; Huang, Y.; He, X.; Zhuo, H.; Chen, S. Rational Design of Effective Binders for LiFePO4 Cathodes. Polymers 2021, 13, 3146. [Google Scholar] [CrossRef]
- Arnot, D.J.; Mayilvahanan, K.S.; Hui, Z.; Takeuchi, K.J.; Marschilok, A.C.; Bock, D.C.; Wang, L.; West, A.C.; Takeuchi, E.S. Thick Electrode Design for Facile Electron and Ion Transport: Architectures, Advanced Characterization, and Modeling. Acc. Mater. Res. 2022, 3, 472–483. [Google Scholar] [CrossRef]
- Rohland, P.; Schröter, E.; Nolte, O.; Newkome, G.R.; Hager, M.D.; Schubert, U.S. Redox-active polymers: The magic key towards energy storage—A polymer design guideline progress in polymer science. Prog. Polym. Sci. 2022, 125, 101474. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Heinze, J.; Frontana-Uribe, B.A.; Ludwigs, S. Electrochemistry of Conducting Polymers—Persistent Models and New Concepts. Chem. Rev. 2010, 110, 4724–4771. [Google Scholar] [CrossRef] [PubMed]
- Apraksin, R.V.; Volkov, A.I.; Eliseeva, S.N.; Kondratiev, V.V. Influence of addition of lithium salt solution into PEDOT:PSS dispersion on the electrochemical and spectroscopic properties of film electrodes. J. Solid State Electrochem. 2017, 21, 3487–3494. [Google Scholar] [CrossRef]
- Hay, M.E.; Wong, S.H.; Mukherjee, S.; Boudouris, B.W. Controlling open-shell loading in norbornene-based radical polymers modulates the solid-state charge transport exponentially. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1516–1525. [Google Scholar] [CrossRef]
- Malacrida, C.; Lu, Y.; Dirnberger, K.; Gámez-Valenzuela, S.; Delgado, M.C.R.; Ludwigs, S. Towards highly conducting bicarbazole redox polymer films with plateau-like conductivities. J. Mater. Chem. C 2020, 8, 15393–15405. [Google Scholar] [CrossRef]
- Joo, Y.; Agarkar, V.; Sung, S.H.; Savoie, B.M.; Boudouris, B.W. A nonconjugated radical polymer glass with high electrical conductivity. Science 2018, 359, 1391–1395. [Google Scholar] [CrossRef] [Green Version]
- Gannett, C.N.; Peterson, B.M.; Shen, L.; Seok, J.; Fors, B.P.; Abruña, H.D. Cross-linking Effects on Performance Metrics of Phenazine-Based Polymer Cathodes. ChemSusChem 2020, 13, 2428–2435. [Google Scholar] [CrossRef]
- Jiménez, P.; Levillain, E.; Alévêque, O.; Guyomard, D.; Lestriez, B.; Gaubicher, J. Lithium n-Doped Polyaniline as a High-Performance Electroactive Material for Rechargeable Batteries. Angew. Chem. Int. Ed. 2017, 56, 1553–1556. [Google Scholar] [CrossRef]
- Thomas-Alyea, K.E.; Aryanpour, M. Design of Composite Electrodes with Anion-Absorbing Active Materials. J. Electrochem. Soc. 2017, 164, A6017–A6025. [Google Scholar] [CrossRef]
- Knaapila, M. Conjugated Polymers and Oligomers: Structural and Soft Matter Aspects; World Scientific: Singapore, 2018. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Li, C.; Liang, Y. High-strength conducting polymers prepared by electrochemical polymerization in boron trifluoride diethyl etherate solution. Adv. Mater. 1999, 11, 1145–1146. [Google Scholar] [CrossRef]
- Shoa, T.; Mirfakhrai, T.; Madden, J.D. Electro-stiffening in polypyrrole films: Dependence of Young’s modulus on oxidation state, load and frequency. Synth. Met. 2010, 160, 1280–1286. [Google Scholar] [CrossRef]
- Melling, D.; Martinez, J.G.; Jager, E.W.H. Conjugated Polymer Actuators and Devices: Progress and Opportunities. Adv. Mater. 2019, 31, e1808210. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Q.; Liang, Q. Carbon-Coatings Improve Performance of Li-Ion Battery. Nanomaterials 2022, 12, 1936. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X. Understanding and recent development of carbon coating on LiFePO4 cathode materials for lithium-ion batteries. Energy Environ. Sci. 2012, 5, 5163–5185. [Google Scholar] [CrossRef]
- Gong, Q.; He, Y.; Yang, Y.; Liao, X.-Z.; Ma, Z.-F. Synthesis and electrochemical characterization of LiFePO4/C-polypyrrole composite prepared by a simple chemical vapor deposition method. J. Solid State Electrochem. 2012, 16, 1383–1388. [Google Scholar] [CrossRef] [Green Version]
- Lepage, D.; Michot, C.; Liang, G.; Gauthier, M.; Schougaard, S.B. A Soft Chemistry Approach to Coating of LiFePO4 with a Conducting Polymer. Angew. Chem. Int. Ed. 2011, 50, 6884–6887. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Zhang, Y.; Wang, J.; Ma, L.; Ma, S.; Zhang, Y.; Wang, E.; Bi, Y.; Wang, D.; McKee, W.C.; et al. Unlocking the energy capabilities of micron-sized LiFePO4. Nat. Commun. 2015, 6, 7898. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-H.; Goodenough, J.B. High-Rate LiFePO4 Lithium Rechargeable Battery Promoted by Electrochemically Active Polymers. Chem. Mater. 2008, 20, 7237–7241. [Google Scholar] [CrossRef]
- Wang, G.; Yang, L.; Chen, Y.; Wang, J.; Bewlay, S.; Liu, H. An investigation of polypyrrole-LiFePO4 composite cathode materials for lithium-ion batteries. Electrochim. Acta 2005, 50, 4649–4654. [Google Scholar] [CrossRef]
- Fedorkova, A.S.; Wiemhöfer, H.-D.; Oriňáková, R.; Oriňák, A.; Stan, M.C.; Winter, M.; Kaniansky, D.; Alejos, A.V. Improved lithium exchange at LiFePO4 cathode particles by coating with composite polypyrrole–polyethylene glycol layers. J. Solid State Electrochem. 2009, 13, 1867–1872. [Google Scholar] [CrossRef]
- Fedorková, A.; Nacher-Alejos, A.; Gómez-Romero, P.; Oriňáková, R.; Kaniansky, D. Structural and electrochemical studies of PPy/PEG-LiFePO4 cathode material for Li-ion batteries. Electrochim. Acta 2010, 55, 943–947. [Google Scholar] [CrossRef]
- Fedorkova, A.S.; Oriňáková, R.; Oriňák, A.; Wiemhöfer, H.-D.; Kaniansky, D.; Winter, M. Surface treatment of LiFePO4 cathode material with PPy/PEG conductive layer. J. Solid State Electrochem. 2010, 14, 2173–2178. [Google Scholar] [CrossRef]
- Fedorková, A.; Oriňáková, R.; Oriňák, A.; Kupková, M.; Wiemhöfer, H.-D.; Audinot, J.; Guillot, J. Electrochemical and XPS study of LiFePO4 cathode nanocomposite with PPy/PEG conductive network. Solid State Sci. 2012, 14, 1238–1243. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, K.; Xu, H.; Zhu, B. Enhanced high-rate and low-temperature electrochemical properties of LiFePO4 /polypyrrole cathode materials for lithium-ion batteries. Int. J. Electrochem. Sci. 2019, 14, 3408–3417. [Google Scholar] [CrossRef]
- Yang, Y.; Liao, X.-Z.; Ma, Z.-F.; Wang, B.-F.; He, L.; He, Y.-S. Superior high-rate cycling performance of LiFePO4/C-PPy composite at 55 °C. Electrochem. Commun. 2009, 11, 1277–1280. [Google Scholar] [CrossRef]
- Kim, J.-K.; Manuel, J.; Lee, M.-H.; Scheers, J.; Lim, D.-H.; Johansson, P.; Ahn, J.-H.; Matic, A.; Jacobsson, P. Towards flexible secondary lithium batteries: Polypyrrole-LiFePO4 thin electrodes with polymer electrolytes. J. Mater. Chem. 2012, 22, 15045–15049. [Google Scholar] [CrossRef]
- Feng, S.; Shen, W.; Guo, S. Effects of polypyrrole and chemically reduced graphene oxide on electrochemical properties of lithium iron (II) phosphate. J. Solid State Electrochem. 2017, 21, 3021–3028. [Google Scholar] [CrossRef]
- Lei, G.; Yi, X.; Wang, L.; Li, Z.; Zhou, J. An investigation of the electrochemical performance of polyaniline coated LiFePO4 materials. Polym. Adv. Technol. 2009, 20, 576–580. [Google Scholar] [CrossRef]
- Chen, W.-M.; Qie, L.; Yuan, L.-X.; Xia, S.-A.; Hu, X.-L.; Zhang, W.-X.; Huang, Y.-H. Insight into the improvement of rate capability and cyclability in LiFePO4/polyaniline composite cathode. Electrochim. Acta 2011, 56, 2689–2695. [Google Scholar] [CrossRef]
- Chen, W.-M.; Huang, Y.-H.; Yuan, L.-X. Self-assembly LiFePO4/polyaniline composite cathode materials with inorganic acids as dopants for lithium-ion batteries. J. Electroanal. Chem. 2011, 660, 108–113. [Google Scholar] [CrossRef]
- Sehrawat, R.; Sil, A. Synthesis and characterization of LiFePO4-C/ PANI composite for cathode material of lithium ion battery. Adv. Mater. Res. 2012, 585, 240–244. [Google Scholar] [CrossRef]
- Gong, C.; Deng, F.; Tsui, C.-P.; Xue, Z.; Ye, Y.S.; Tang, C.-Y.; Zhou, X.; Xie, X. PANI–PEG copolymer modified LiFePO4 as a cathode material for high-performance lithium ion batteries. J. Mater. Chem. A 2014, 2, 19315–19323. [Google Scholar] [CrossRef]
- Lian, J.; Wang, X.; Zhang, W.; Huang, Y.; Xia, T.; Lian, Y. A ternary polyaniline/active carbon/lithium iron phosphate composite as cathode material for lithium ion battery. J. Nanosci. Nanotechnol. 2016, 16, 6494–6497. [Google Scholar] [CrossRef]
- Fagundes, W.S.; Xavier, F.F.S.; Santana, L.K.; Azevedo, M.E.; Canobre, S.C.; Amaral, F.A. PAni-coated LiFePO4 Synthesized by a Low Temperature Solvothermal Method. Mater. Res. 2018, 22, e20180566. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Y.; Yan, J.; Wu, H.; Guo, S. Enhanced electrochemical performance of lithium iron (II) phosphate modified cooperatively via chemically reduced graphene oxide and polyaniline. Electrochim. Acta 2015, 173, 310–315. [Google Scholar] [CrossRef]
- Bai, Y.-M.; Qiu, P.; Wen, Z.-L.; Han, S.-C. Improvement of electrochemical performances of LiFePO4 cathode materials by coating of polythiophene. J. Alloys Compd. 2010, 508, 1–4. [Google Scholar] [CrossRef]
- Shi, J.-Y.; Yi, C.-W.; Kim, K. An investigation of LiFePO4/poly(3,4-ethylenedioxythiophene) composite cathode materials for lithium-ion batteries. Bull. Korean Chem. Soc. 2010, 31, 2698–2700. [Google Scholar] [CrossRef] [Green Version]
- Ozerova, V.V.; Stenina, I.A.; Kuz’Mina, A.A.; Kulova, T.L.; Yaroslavtsev, A.B. Cathode Materials Based on Lithium Iron Phosphate/PEDOT Composites for Lithium-Ion Batteries. Inorg. Mater. 2020, 56, 648–656. [Google Scholar] [CrossRef]
- Fedorková, A.; Oriňáková, R.; Oriňák, A.; Talian, I.; Heile, A.; Wiemhöfer, H.-D.; Kaniansky, D.; Arlinghaus, H.F. PPy doped PEG conducting polymer films synthesized on LiFePO4 particles. J. Power Sources 2010, 195, 3907–3912. [Google Scholar] [CrossRef]
- Murugan, A.V.; Muraliganth, T.; Manthiram, A. Rapid microwave-solvothermal synthesis of phospho-olivine nanorods and their coating with a mixed conducting polymer for lithium ion batteries. Electrochem. Commun. 2008, 10, 903–906. [Google Scholar] [CrossRef]
- Dinh, H.-C.; Yeo, I.-H.; Cho, W.I.; Mho, S.-I. Characteristics of Conducting Polymer-Coated Nanosized LiFePO4 Cathode in the Li+ Batteries. ECS Meet. Abstr. 2010, 2010, 320. [Google Scholar] [CrossRef]
- Dinh, H.-C.; Mho, S.-I.; Yeo, I.-H. Electrochemical analysis of conductive polymer-coated LiFePO4 nanocrystalline cathodes with controlled morphology. Electroanalysis 2011, 23, 2079–2086. [Google Scholar] [CrossRef]
- Dinh, H.-C.; Lim, H.; Park, K.D.; Yeo, I.-H.; Kang, Y.; Mho, S.-I. Long-term cycle stability at a high current for nanocrystalline LiFePO4 coated with a conductive polymer. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 015011. [Google Scholar] [CrossRef]
- Raj, H.; Sil, A. PEDOT:PSS coating on pristine and carbon coated LiFePO4 by one-step process: The study of electrochemical performance. J. Mater. Sci. Mater. Electron. 2019, 30, 13604–13616. [Google Scholar] [CrossRef]
- Su, C.; Lu, G.; Xu, L.; Zhang, C. Preparation of LiFePO4/Carbon/PANI-CSA Composite and Its Properties as High-Capacity Cathodes for Lithium Ion Batteries. J. Electrochem. Soc. 2012, 159, A305–A309. [Google Scholar] [CrossRef]
- Chang, S.; Qi-Fei, H.; Li-Huan, X.; Cheng, Z. Preparation and performances of C-LiFePO4/polytriphenylamine composite as cathode material for lithium-ion batteries. Wuli Huaxue Xuebao/Acta Phys.-Chim. Sin. 2014, 30, 88–94. [Google Scholar] [CrossRef]
- Posudievsky, O.Y.; Kozarenko, O.A.; Dyadyun, V.S.; Koshechko, V.G.; Pokhodenko, V.D. Advanced electrochemical performance of hybrid nanocomposites based on LiFePO4 and lithium salt doped polyaniline. J. Solid State Electrochem. 2015, 19, 2733–2740. [Google Scholar] [CrossRef]
- Ajpi, C.; Leiva, N.; Vargas, M.; Lundblad, A.; Lindbergh, G.; Cabrera, S. Synthesis and Characterization of LiFePO4–PANI Hybrid Material as Cathode for Lithium-Ion Batteries. Materials 2020, 13, 2834. [Google Scholar] [CrossRef]
- Nguyen, V.A.; Kuss, C. Review—Conducting Polymer-Based Binders for Lithium-Ion Batteries and Beyond. J. Electrochem. Soc. 2020, 167, 065501. [Google Scholar] [CrossRef]
- Javier, A.E.; Patel, S.N.; Hallinan, D.T., Jr.; Srinivasan, V.; Balsara, N.P. Simultaneous electronic and ionic conduction in a block copolymer: Application in lithium battery electrodes. Angew. Chem. Int. Ed. 2011, 50, 9848–9851. [Google Scholar] [CrossRef] [PubMed]
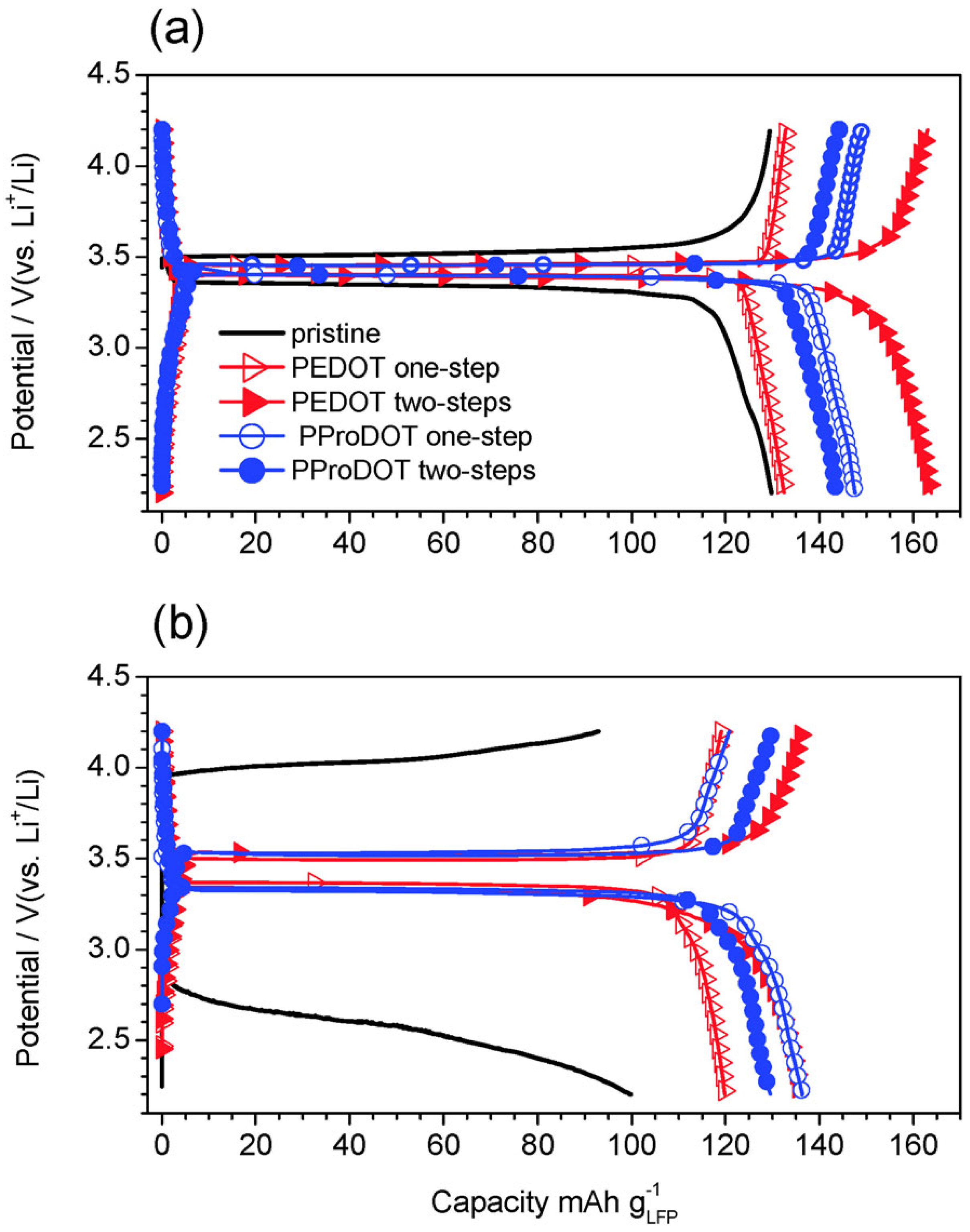
- Cíntora-Juárez, D.; Pérez-Vicente, C.; Ahmad, S.; Tirado, J.L. Improving the cycling performance of LiFePO4 cathode material by poly(3,4-ethylenedioxythiopene) coating. RSC Adv. 2014, 4, 26108–26114. [Google Scholar] [CrossRef]
- Levin, O.V.; Eliseeva, S.N.; Alekseeva, E.V.; Tolstopjatova, E.G.; Kondratiev, V.V. Composite LiFePO4/poly-3,4-ethylenedioxythiophene cathode for lithium-ion batteries with low content of non-electroactive components. Int. J. Electrochem. Sci. 2015, 10, 8175–8189. [Google Scholar]
- Ling, M.; Qiu, J.; Li, S.; Yan, C.; Kiefel, M.J.; Liu, G.; Zhang, S. Multifunctional SA-PProDOT Binder for Lithium Ion Batteries. Nano Lett. 2015, 15, 4440–4447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, P.R.; Komsiyska, L.; Osters, O.; Wittstock, G. PEDOT: PSS as a Functional Binder for Cathodes in Lithium Ion Batteries. J. Electrochem. Soc. 2015, 162, A674–A678. [Google Scholar] [CrossRef]
- Das, P.R.; Komsiyska, L.; Osters, O.; Wittstock, G. Effect of solid loading on the processing and behavior of PEDOT:PSS binder based composite cathodes for lithium ion batteries. Synth. Met. 2016, 215, 86–94. [Google Scholar] [CrossRef]
- Syrový, T.; Kazda, T.; Syrová, L.; Vondrák, J.; Kubáč, L.; Sedlaříková, M. Cathode material for lithium ion accumulators prepared by screen printing for Smart Textile applications. J. Power Sources 2016, 309, 192–201. [Google Scholar] [CrossRef]
- Sandu, G.; Ernould, B.; Rolland, J.; Cheminet, N.; Brassinne, J.; Das, P.R.; Filinchuk, Y.; Cheng, L.; Komsiyska, L.; Dubois, P.; et al. Mechanochemical Synthesis of PEDOT:PSS Hydrogels for Aqueous Formulation of Li-Ion Battery Electrodes. ACS Appl. Mater. Interfaces 2017, 9, 34865–34874. [Google Scholar] [CrossRef]
- Raj, H.; Sil, A. Energy and power densities of novel composite electrode driven by synergy of poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate) and single walled carbon nanotubes for lithium-ion battery. J. Power Sources 2020, 458, 228052. [Google Scholar] [CrossRef]
- Wang, D.; Ela, S.E.; Zakeeruddin, S.M.; Pechy, P.; Exnar, I.; Wang, Q.; Grätzel, M. Polymer wiring of insulating electrode materials: An approach to improve energy density of lithium-ion batteries. Electrochem. Commun. 2009, 11, 1350–1352. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer binders: Characterization and development toward aqueous electrode fabrication for sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Zhang, J.; Bruck, A.M.; Bond, A.C.; Marschilok, A.; Takeuchi, K.; Takeuchi, E.S.; Yu, G. Nanostructured Conductive Polymer Gels as a General Framework Material To Improve Electrochemical Performance of Cathode Materials in Li-Ion Batteries. Nano Lett. 2017, 17, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Schougaard, S.B.; Goodenough, J.B. Conducting-polymer/iron-redox-couple composite cathodes for lithium secondary batteries. Adv. Mater. 2007, 19, 848–851. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Park, K.-S.; Goodenough, J.B. Improving Lithium Batteries by Tethering Carbon-Coated LiFePO4 to Polypyrrole. J. Electrochem. Soc. 2006, 153, A2282–A2286. [Google Scholar] [CrossRef]
- Boyano, I.; Blazquez, J.A.; de Meatza, I.; Bengoechea, M.; Miguel, O.; Grande, H.; Huang, Y.; Goodenough, J.B. Preparation of C-LiFePO4/polypyrrole lithium rechargeable cathode by consecutive potential steps electrodeposition. J. Power Sources 2010, 195, 5351–5359. [Google Scholar] [CrossRef]
- Polozhentseva, Y.A.; Novozhilova, M.V.; Chepurnaya, I.A.; Karushev, M.P. Polymeric Complexes of Nickel with Salen-Type Ligands as Multifunctional Components of Lithium Ion Battery Cathodes. Tech. Phys. Lett. 2021, 47, 83–87. [Google Scholar] [CrossRef]
- Wang, J.-Z.; Chou, S.-L.; Chen, J.; Chew, S.-Y.; Wang, G.-X.; Konstantinov, K.; Wu, J.; Dou, S.-X.; Liu, H.K. Paper-like free-standing polypyrrole and polypyrrole–LiFePO4 composite films for flexible and bendable rechargeable battery. Electrochem. Commun. 2008, 10, 1781–1784. [Google Scholar] [CrossRef]
- Trinh, N.; Saulnier, M.; Lepage, D.; Schougaard, S. Conductive polymer film supporting LiFePO4 as composite cathode for lithium ion batteries. J. Power Sources 2013, 221, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Tamura, T.; Aoki, Y.; Ohsawa, T.; Dokko, K. Polyaniline as a Functional Binder for LiFePO4 Cathodes in Lithium Batteries. Chem. Lett. 2011, 40, 828–830. [Google Scholar] [CrossRef]
- Ranque, P.; George, C.; Dubey, R.K.; Van Der Jagt, R.; Flahaut, D.; Dedryvère, R.; Fehse, M.; Kassanos, P.; Jager, W.F.; Sudhölter, E.J.R.; et al. Scalable Route to Electroactive and Light Active Perylene Diimide Dye Polymer Binder for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2020, 3, 2271–2277. [Google Scholar] [CrossRef]
- Eliseeva, S.N.; Levin, O.V.; Tolstopyatova, E.G.; Alekseeva, E.V.; Kondratiev, V.V. Effect of addition of a conducting polymer on the properties of the LiFePO4-based cathode material for lithium-ion batteries. Russ. J. Appl. Chem. 2015, 88, 1146–1149. [Google Scholar] [CrossRef]
- Eliseeva, S.; Apraksin, R.; Tolstopjatova, E.; Kondratiev, V. Electrochemical impedance spectroscopy characterization of LiFePO4 cathode material with carboxymethylcellulose and poly-3,4-ethylendioxythiophene/polystyrene sulfonate. Electrochim. Acta 2016, 227, 357–366. [Google Scholar] [CrossRef]
- Raj, H.; Sil, A. Aqueous processing based novel composite electrode for Li-ion batteries using an environmentally benign binder. Ceram. Int. 2021, 47, 34639–34647. [Google Scholar] [CrossRef]
- Kubarkov, A.V.; Asharchuk, A.A.; Drozhzhin, O.A.; Karpushkin, E.A.; Stevenson, K.J.; Antipov, E.V.; Sergeyev, V.G. Effect of Polymer Binders with Single-Walled Carbon Nanotubes on the Electrochemical and Physicochemical Properties of the LiFePO4 Cathode. ACS Appl. Energy Mater. 2021, 4, 12310–12318. [Google Scholar] [CrossRef]
- Hatakeyama-Sato, K.; Masui, T.; Serikawa, T.; Sasaki, Y.; Choi, W.; Doo, S.-G.; Nishide, H.; Oyaizu, K. Nonconjugated Redox-Active Polymer Mediators for Rapid Electrocatalytic Charging of Lithium Metal Oxides. ACS Appl. Energy Mater. 2019, 2, 6375–6382. [Google Scholar] [CrossRef]
- Wijayati, A.; Susanti, S.; Rahayu, I.; Hidayat, S. Effect of polyaniline to enhance lithium iron phosphate conductivity. AIP Conf. Proc. 2016, 1712, 050021. [Google Scholar] [CrossRef] [Green Version]
- Rahayu, I.; Wijayati, A.; Noviyanti, A.R.; Hidayat, S. Risdiana Electrode capacity and voltage performance of lithium iron phosphate–polyaniline coin cell battery. J. Phys. Conf. Ser. 2018, 1080, 012039. [Google Scholar] [CrossRef]
- O’Meara, C.; Karushev, M.P.; Polozhentceva, I.A.; Dharmasena, S.; Cho, H.; Yurkovich, B.J.; Kogan, S.; Kim, J.-H. Nickel–Salen-Type Polymer as Conducting Agent and Binder for Carbon-Free Cathodes in Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.; Levin, O.; Tolstopjatova, E.; Alekseeva, E.; Apraksin, R.; Kondratiev, V. New functional conducting poly-3,4-ethylenedioxythiopene:polystyrene sulfonate/carboxymethylcellulose binder for improvement of capacity of LiFePO4-based cathode materials. Mater. Lett. 2015, 161, 117–119. [Google Scholar] [CrossRef]
- Kubarkov, A.V.; Drozhzhin, O.A.; Karpushkin, E.A.; Stevenson, K.J.; Antipov, E.V.; Sergeyev, V.G. Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonic acid)–polymer composites as functional cathode binders for high power LiFePO4 batteries. Colloid Polym. Sci. 2019, 297, 475–484. [Google Scholar] [CrossRef]
- Cíntora-Juárez, D.; Pérez-Vicente, C.; Kazim, S.; Ahmad, S.; Tirado, J.L. Judicious design of lithium iron phosphate electrodes using poly(3,4-ethylenedioxythiophene) for high performance batteries. J. Mater. Chem. A 2015, 3, 14254–14262. [Google Scholar] [CrossRef]
- Vicente, N.; Haro, M.; Cíntora-Juárez, D.; Pérez-Vicente, C.; Tirado, J.L.; Ahmad, S.; Garcia-Belmonte, G. LiFePO4 particle conductive composite strategies for improving cathode rate capability. Electrochimica Acta 2015, 163, 323–329. [Google Scholar] [CrossRef]
- Das, P.R.; Gräfenstein, A.; Ledwoch, D.; Osters, O.; Komsiyska, L.; Wittstock, G. Conducting Polymers as Binder Additives for Cathodes in Li Ion Battery. ECS Trans. 2014, 63, 31–43. [Google Scholar] [CrossRef]
- Puthirath, A.B.; John, B.; Gouri, C.; Jayalekshmi, S. Lithium doped polyaniline and its composites with LiFePO4 and LiMn2O4-prospective cathode active materials for environment friendly and flexible Li-ion battery applications. RSC Adv. 2015, 5, 69220–69228. [Google Scholar] [CrossRef]
- Hatakeyama-Sato, K.; Akahane, T.; Go, C.; Kaseyama, T.; Yoshimoto, T.; Oyaizu, K. Ultrafast Charge/Discharge by a 99.9% Conventional Lithium Iron Phosphate Electrode Containing 0.1% Redox-Active Fluoflavin Polymer. ACS Energy Lett. 2020, 5, 1712–1717. [Google Scholar] [CrossRef]
- Vlad, A.; Singh, N.; Rolland, J.; Melinte, S.; Ajayan, P.M.; Gohy, J.-F. Hybrid supercapacitor-battery materials for fast electrochemical charge storage. Sci. Rep. 2014, 4, 4315. [Google Scholar] [CrossRef] [Green Version]
- Zhong, H.; He, A.; Lu, J.; Sun, M.; He, J.; Zhang, L. Carboxymethyl chitosan/conducting polymer as water-soluble composite binder for LiFePO4 cathode in lithium ion batteries. J. Power Sources 2016, 336, 107–114. [Google Scholar] [CrossRef]
- Han, J.-J.; Guo, A.-R.; Wang, Y.-F. Synthesis of PANI and its application in LiFePO4 cathode material. Ionics 2022, 28, 1073–1080. [Google Scholar] [CrossRef]
- Yamamoto, K.; Suemasa, D.; Masuda, K.; Aita, K.; Endo, T. Hyperbranched Triphenylamine Polymer for UltraFast Battery Cathode. ACS Appl. Mater. Interfaces 2018, 10, 6346–6353. [Google Scholar] [CrossRef]
- Cíntora-Juárez, D.; Pérez-Vicente, C.; Ahmad, S.; Tirado, J.L. Electrochemical in battery polymerization of poly(alkylenedioxythiophene) over lithium iron phosphate for high-performance cathodes. Phys. Chem. Chem. Phys. 2014, 16, 20724–20730. [Google Scholar] [CrossRef]
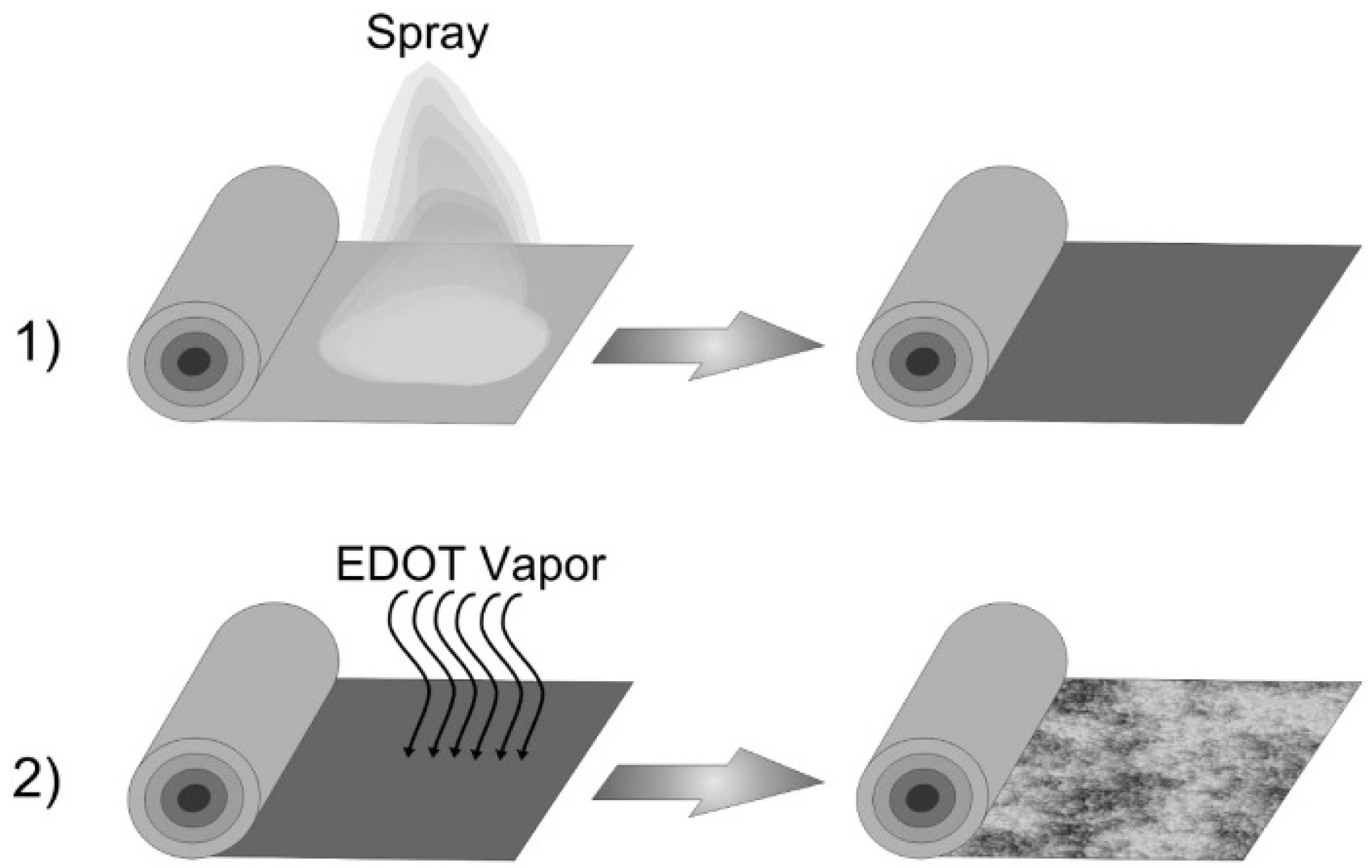
- Jeong, H.; Jang, J.; Jo, C. A review on current collector coating methods for next-generation batteries. Chem. Eng. J. 2022, 446, 136860. [Google Scholar] [CrossRef]
- Lepage, D.; Savignac, L.; Saulnier, M.; Gervais, S.; Schougaard, S. Modification of aluminum current collectors with a conductive polymer for application in lithium batteries. Electrochem. Commun. 2019, 102, 1–4. [Google Scholar] [CrossRef]
- Ding, K.; Chen, J.; Zhang, D.; Shi, F.; Li, B.; Tian, W.; He, X.; Wang, L.; Wang, H. Electrochemical deposition of leaf stalk-shaped polyaniline doped with sodium dodecyl sulfate on aluminum and its use as a novel type of current collector in lithium ion batteries. Synth. Met. 2021, 278, 116837. [Google Scholar] [CrossRef]
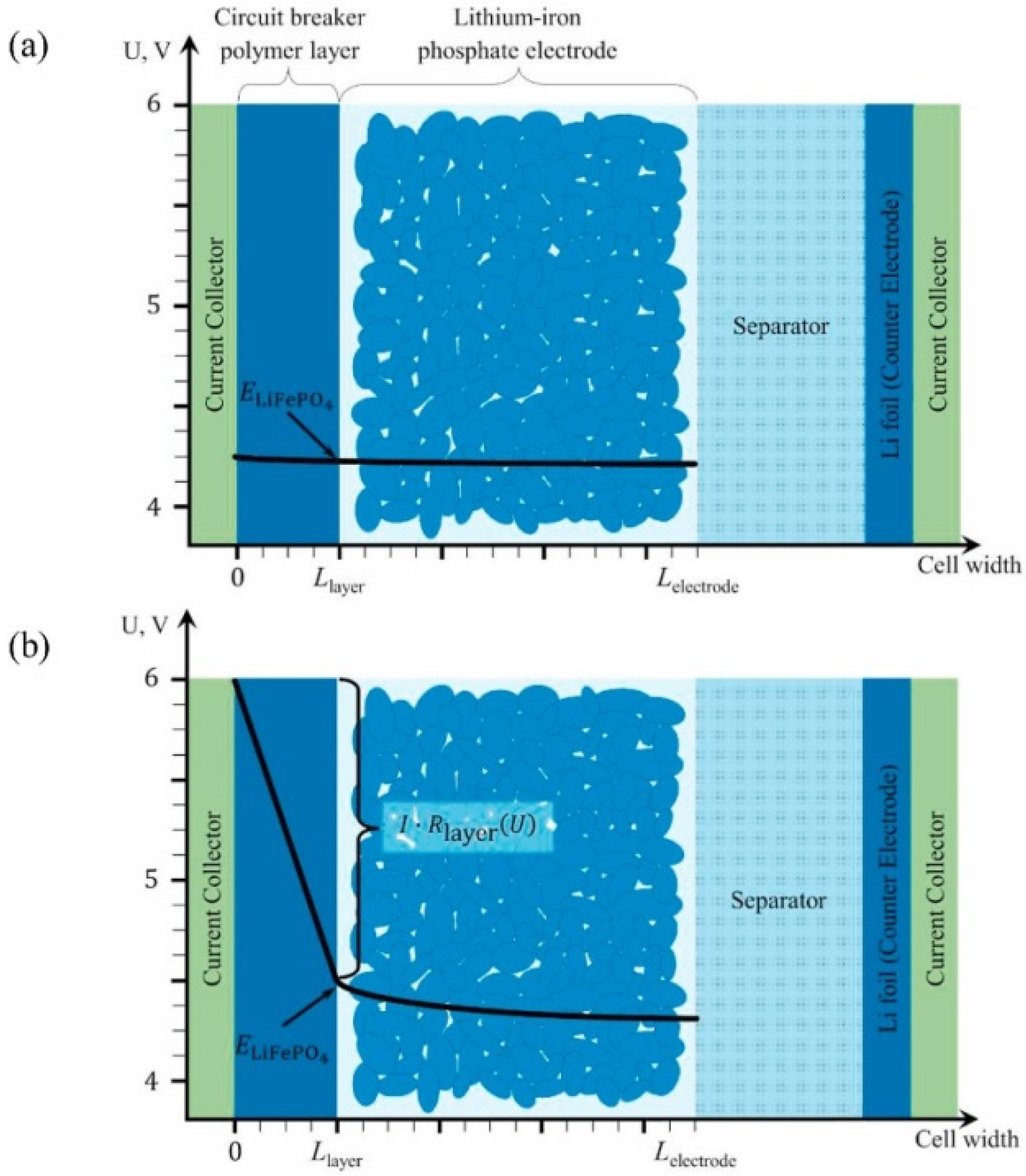
- Beletskii, E.V.; Kal’Nin, A.Y.; Luk’Yanov, D.A.; Kamenskii, M.A.; Anishchenko, D.V.; Levin, O.V. A Polymer Layer of Switchable Resistance for the Overcharge Protection of Lithium-Ion Batteries. Russ. J. Electrochem. 2021, 57, 1028–1036. [Google Scholar] [CrossRef]
- Beletskii, E.; Fedorova, A.; Lukyanov, D.; Kalnin, A.; Ershov, V.; Danilov, S.; Spiridonova, D.; Alekseeva, E.; Levin, O. Switchable resistance conducting-polymer layer for Li-ion battery overcharge protection. J. Power Sources 2021, 490, 229548. [Google Scholar] [CrossRef]




| Abbreviation | Definition |
|---|---|
| ANI | Aniline |
| B | Binder |
| CA | Conductive additive |
| CB | Carbon black |
| CCTS | Carboxylmethyl chitosan |
| C-LFP | Lithium iron phosphate, carbon-coated |
| CMC | Carboxymethyl cellulose |
| C-PPy | Cross-linked polypyrrole |
| CRGO | Chemically reduced graphene oxide |
| D3PIE | Dynamic three phase interline electropolymerization |
| EAECP | Electrochemically active electron-conducting polymer |
| EAECP-C-LFP | Carbon-coated lithium iron phosphate particles additionally coated with electrochemically active electron-conducting polymer |
| EAECP-LFP | Pristine lithium iron phosphate particles coated with electrochemically active electron-conducting polymer |
| EAECP-LiFePO4 | Lithium iron phosphate particles coated with electrochemically active electron-conducting polymer |
| EDOT | 3,4-ethylenedioxythiophene |
| FP | Polynorbornene polymer with fluoflavin pendant groups |
| LFP | Lithium iron phosphate, pristine (without carbon coating) |
| LIB | Lithium-ion battery |
| LiCoO2 | Lithium cobalt oxide |
| LiFePO4 | Lithium iron phosphate |
| Li-PANI | Polyaniline lithiated by treating with n-butyllithium |
| LiTFSI | Lithium bis(trifluoromethanesulfonyl)imide |
| LMO | Layered lithium transition metal oxide |
| MWCNTs | Multi-wall carbon nanotubes |
| NCA | Lithium nickel cobalt aluminum oxide |
| [Ni(CH3-salen)] | N,N′-bis(3-methylsalicylideneiminate) nickel(II) |
| NMC | Lithium nickel manganese cobalt oxide |
| NMP | N-methyl pyrrolidone |
| P2b | Ethylene oxide-functionalized poly(TEMPO-substituted glycidyl ether) |
| PANI | Polyaniline |
| PANI:CSA | Camphorsulfonic acid-doped polyaniline |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene) polystyrene sulfonate |
| PEDOT:p-Tos | p-toluene sulfonate-doped poly(3,4-ethylenedioxythiophene) |
| PEDOT:p-TSA | p-toluene sulfonic acid-doped poly(3,4-ethylenedioxythiophene) |
| PEG | Polyethylene glycol |
| PEO | Poly(ethylene oxide) |
| PHTPA | Hyperbranched poly(triphenylamine) |
| POMA | Poly-o-methoxyaniline |
| PPDI | Perylene diimide-functionalized polyacrylate |
| PProDOT | Poly(3,4-propylenedioxythiophene-2,5-dicarboxylic acid) |
| PPy | Polypyrrole |
| ProDOT | 3,4-propylenedioxythiophene-2,5-dicarboxylic acid |
| PTFE | Polytetrafluorethylene |
| PTh | Polythiophene |
| PTMA | Poly(2,2,6,6-tetramethyl-1-piperinidyloxy-4-yl methacrylate) |
| PTPA | Poly(triphenylamine) |
| PVDF | Polyvinylidene fluoride |
| r-PANI | Reduced polyaniline |
| SA | Sodium algenate |
| SBR | Styrene-butadiene rubber |
| SDS | Sodium dodecyl sulfate |
| SPPO | Sulfonated poly(2,6-dimethyl-1,4-phenylene oxide) |
| SWCNTs | Single wall carbon nanotubes |
| TEMPO | (2,2,6,6-Tetramethylpiperidin-1-yl)oxyl |
| Tr | Triton X-100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chepurnaya, I.; Smirnova, E.; Karushev, M. Electrochemically Active Polymer Components in Next-Generation LiFePO4 Cathodes: Can Small Things Make a Big Difference? Batteries 2022, 8, 185. https://doi.org/10.3390/batteries8100185
Chepurnaya I, Smirnova E, Karushev M. Electrochemically Active Polymer Components in Next-Generation LiFePO4 Cathodes: Can Small Things Make a Big Difference? Batteries. 2022; 8(10):185. https://doi.org/10.3390/batteries8100185
Chicago/Turabian StyleChepurnaya, Irina, Evgenia Smirnova, and Mikhail Karushev. 2022. "Electrochemically Active Polymer Components in Next-Generation LiFePO4 Cathodes: Can Small Things Make a Big Difference?" Batteries 8, no. 10: 185. https://doi.org/10.3390/batteries8100185
APA StyleChepurnaya, I., Smirnova, E., & Karushev, M. (2022). Electrochemically Active Polymer Components in Next-Generation LiFePO4 Cathodes: Can Small Things Make a Big Difference? Batteries, 8(10), 185. https://doi.org/10.3390/batteries8100185







