Room-Temperature Liquid-Metal Coated Zn Electrode for Long Life Cycle Aqueous Rechargeable Zn-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Coating and Preparation of EGaIn Liquid-Metal Nanoparticles (LMNPs)
2.3. Materials Characterization
2.4. Preparation of Cathode Material and Electrochemical Measurements
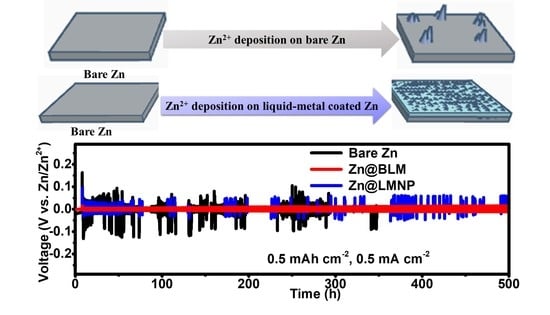
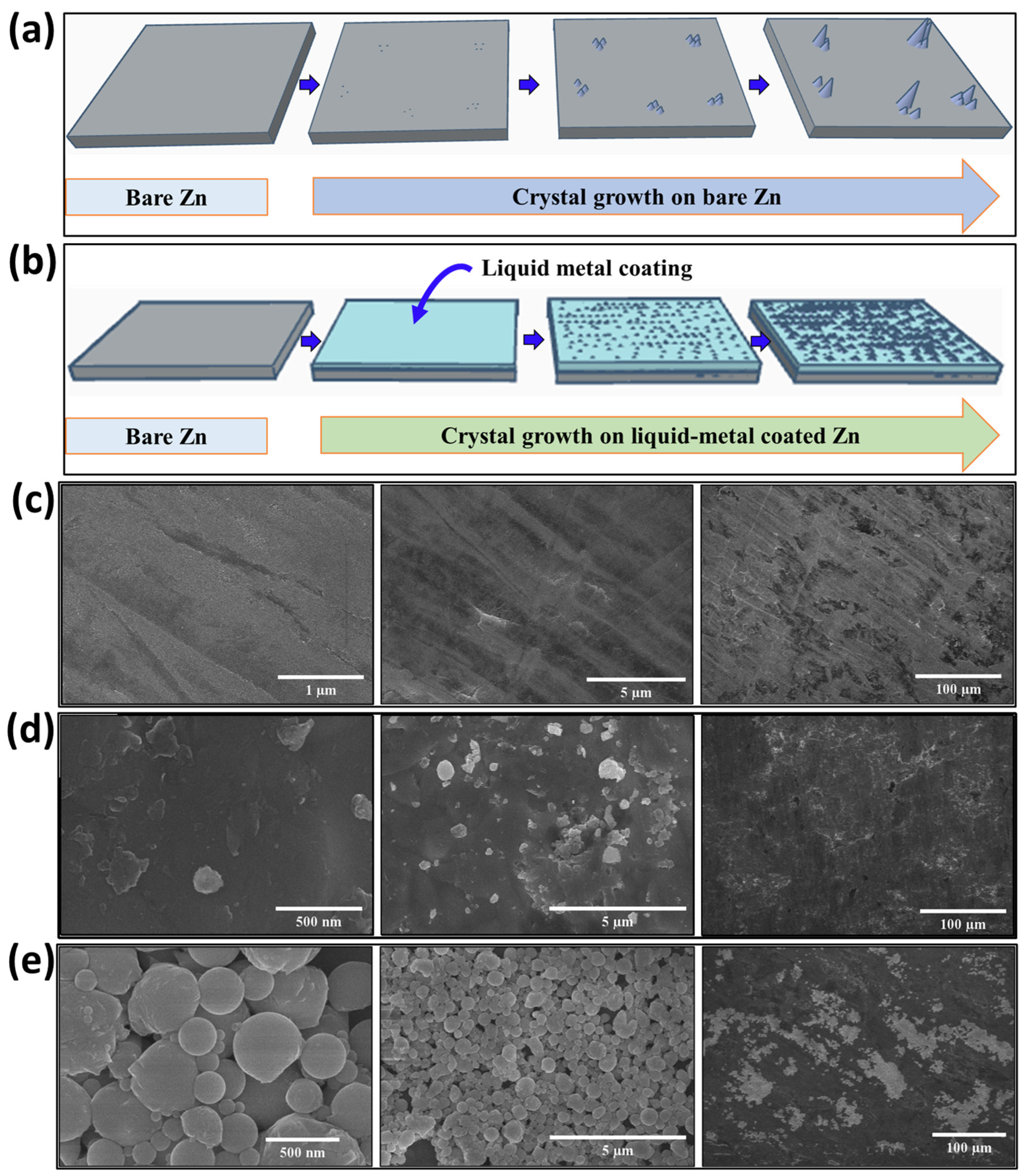
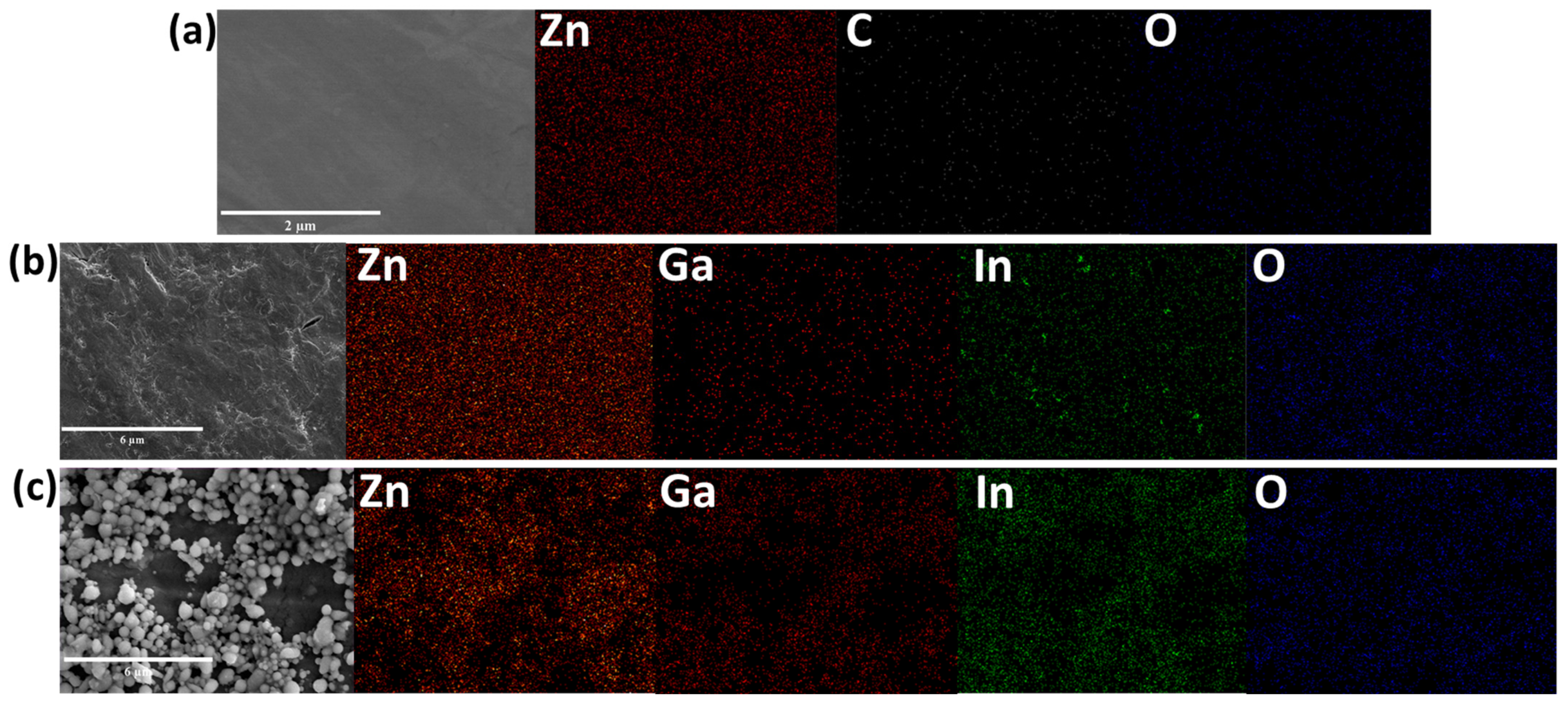
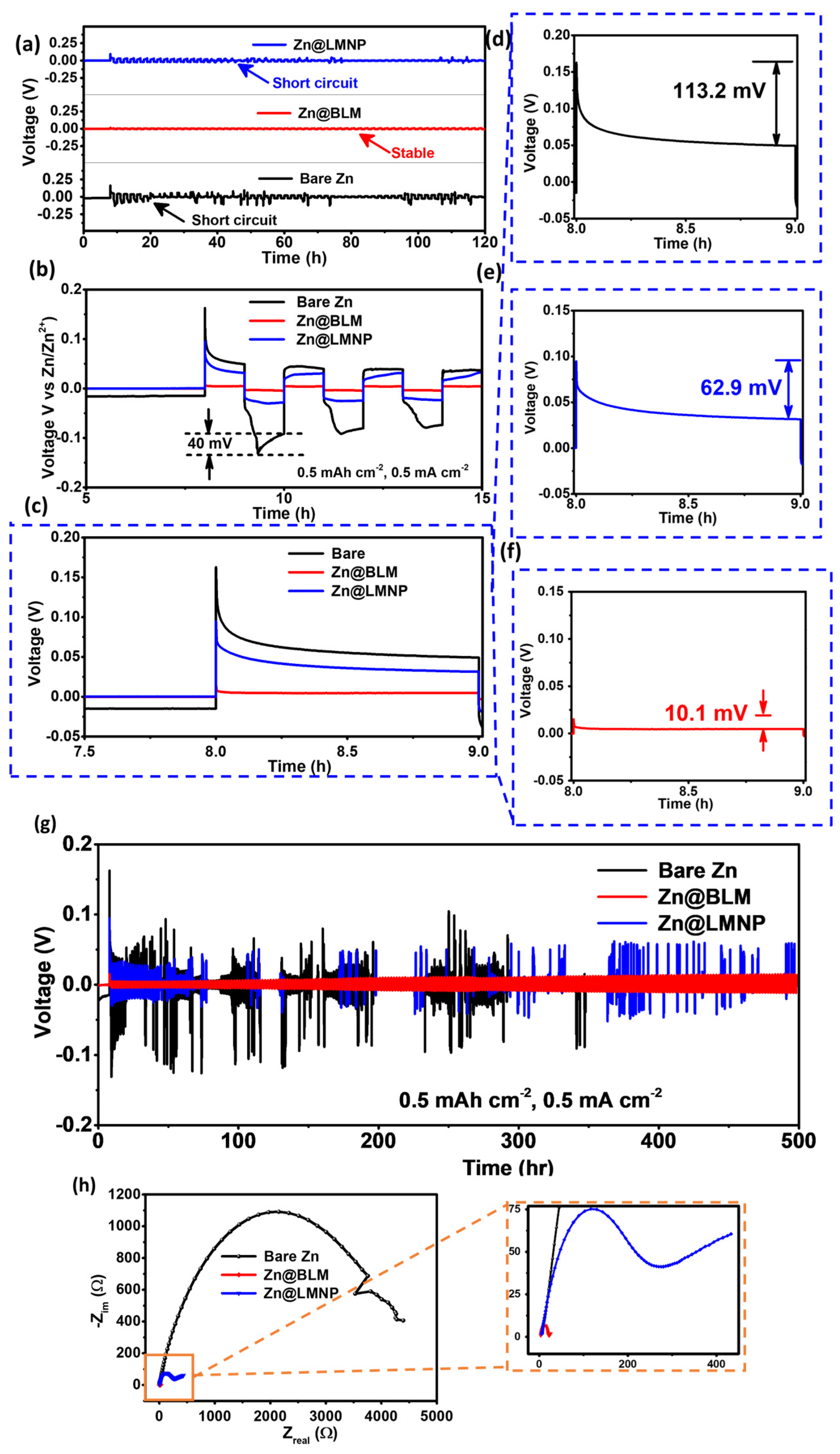
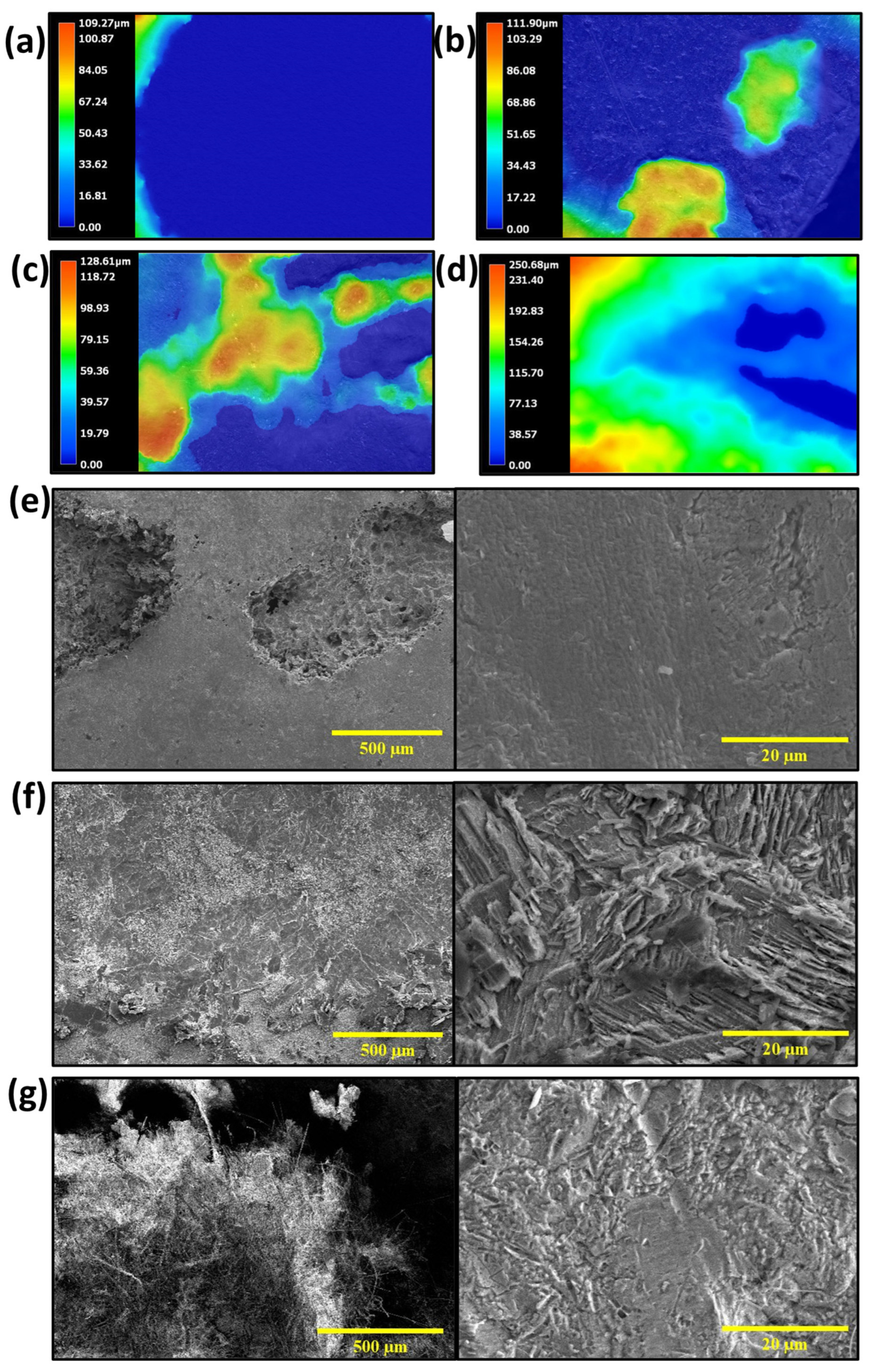
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, D.; Lu, M.; Cai, D.; Yang, H.; Han, W. Recent advances in energy storage mechanism of aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 712–726. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, A.; Sun, J. Promise and challenge of vanadium-based cathodes for aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 655–667. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, W.; Huang, A.; Chen, M.; Tian, Q.; Chen, J. Developing improved electrolytes for aqueous zinc-ion batteries to achieve excellent cyclability and antifreezing ability. J. Colloid Interface Sci. 2021, 586, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chen, X.; Yu, M.; Niu, Z.; Cheng, F.; Chen, J. Materials chemistry for rechargeable zinc-ion batteries. Chem. Soc. Rev. 2020, 49, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Jo, T.H.; Kim, J.S.; Hasan, F.; Yoo, H.D. A Chronocoulometric Method to Measure the Corrosion Rate on Zinc Metal Electrodes. ACS Appl. Mater. Interfaces 2020, 12, 42612–42621. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-life and deeply rechargeable aqueous Zn anodes enabled by a multifunctional brightener-inspired interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Zhang, Q.; Zhou, J.; Cai, Z.; Pan, A. Fabrication of an Inexpensive Hydrophilic Bridge on a Carbon Substrate and Loading Vanadium Sulfides for Flexible Aqueous Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 36676–36684. [Google Scholar] [CrossRef]
- Cheng, Y.; Luo, L.; Zhong, L.; Chen, J.; Li, B.; Wang, W.; Mao, S.X.; Wang, C.; Sprenkle, V.L.; Li, G.; et al. Highly Reversible Zinc-Ion Intercalation into Chevrel Phase Mo6S8 Nanocubes and Applications for Advanced Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 13673–13677. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, J.; Tian, J.; Niu, Z. Recent Progress in the Electrolytes of Aqueous Zinc-Ion Batteries. Chemistry 2019, 25, 14480–14494. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, Y.; Guo, S.; Cao, X.; Pan, A.; Fang, G.; Zhou, J.; Liang, S. Fundamentals and perspectives in developing zinc-ion battery electrolytes: A comprehensive review. Energy Environ. Sci. 2020, 13, 4625–4665. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Tawiah, B.; Wang, Y.; Chan, C.-Y.; Fei, B.; Pan, F. Recent advances in zinc anodes for high-performance aqueous Zn-ion batteries. Nano Energy 2020, 70, 104523. [Google Scholar] [CrossRef]
- Ghosh, M.; Vijayakumar, V.; Kurungot, S. Dendrite Growth Suppression by Zn2+-Integrated Nafion Ionomer Membranes: Beyond Porous Separators toward Aqueous Zn/V2O5 Batteries with Extended Cycle Life. Energy Technol. 2019, 7, 1900442. [Google Scholar] [CrossRef]
- Oberholzer, P.; Tervoort, E.; Bouzid, A.; Pasquarello, A.; Kundu, D. Oxide versus Nonoxide Cathode Materials for Aqueous Zn Batteries: An Insight into the Charge Storage Mechanism and Consequences Thereof. ACS Appl. Mater. Interfaces 2019, 11, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, W.; Liu, X.; Lu, X. Challenges and Strategies for Constructing Highly Reversible Zinc Anodes in Aqueous Zinc-Ion Batteries: Recent Progress and Future Perspectives. Adv. Sustain. Syst. 2020, 4, 2000082. [Google Scholar] [CrossRef]
- Li, C.; Xie, X.; Liang, S.; Zhou, J. Issues and Future Perspective on Zinc Metal Anode for Rechargeable Aqueous Zinc-ion Batteries. Energy Environ. Mater. 2020, 3, 146–159. [Google Scholar] [CrossRef]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Bayaguud, A.; Fu, Y.; Zhu, C. Interfacial parasitic reactions of zinc anodes in zinc ion batteries: Underestimated corrosion and hydrogen evolution reactions and their suppression strategies. J. Energy Chem. 2022, 64, 246–262. [Google Scholar] [CrossRef]
- Yang, G.; Li, Q.; Ma, K.; Hong, C.; Wang, C. The degradation mechanism of vanadium oxide-based aqueous zinc-ion batteries. J. Mater. Chem. A 2020, 8, 8084–8095. [Google Scholar] [CrossRef]
- Cao, H.; Huang, X.; Liu, Y.; Hu, Q.; Zheng, Q.; Huo, Y.; Xie, F.; Zhao, J.; Lin, D. An efficient electrolyte additive of tetramethylammonium sulfate hydrate for Dendritic-Free zinc anode for aqueous Zinc-ion batteries. J. Colloid Interface Sci. 2022, 627, 367–374. [Google Scholar] [CrossRef]
- Qiu, B.; Xie, L.; Zhang, G.; Cheng, K.; Lin, Z.; Liu, W.; He, C.; Zhang, P.; Mi, H. Toward reversible wide-temperature Zn storage by regulating the electrolyte solvation structure via trimethyl phosphate. Chem. Eng. J. 2022, 449, 137843. [Google Scholar] [CrossRef]
- Hieu, L.T.; So, S.; Kim, I.T.; Hur, J. Zn anode with flexible β-PVDF coating for aqueous Zn-ion batteries with long cycle life. Chem. Eng. J. 2021, 411, 128584. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, C.; Yu, Y.; Yan, M.; Wei, Q.; He, P.; Dong, Y.; Zhang, Z.; Wang, X.; Mai, L. Ultrathin Surface Coating Enables Stabilized Zinc Metal Anode. Adv. Mater. Interfaces 2018, 5, 1800848. [Google Scholar] [CrossRef]
- Guo, J.; Ming, J.; Lei, Y.; Zhang, W.; Xia, C.; Cui, Y.; Alshareef, H.N. Artificial Solid Electrolyte Interphase for Suppressing Surface Reactions and Cathode Dissolution in Aqueous Zinc Ion Batteries. ACS Energy Lett. 2019, 4, 2776–2781. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, Y.; Liu, G.; Zhu, R.; Huang, J.; Chen, Z.; Gao, Z.; Chen, X.; Qie, L. Redirected Zn Electrodeposition by an Anti-Corrosion Elastic Constraint for Highly Reversible Zn Anodes. Adv. Funct. Mater. 2020, 31, 2001867. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Zhou, M.; Zhan, H.; Cheng, S.; Jiang, K. Advanced Low-Cost, High-Voltage, Long-Life Aqueous Hybrid Sodium/Zinc Batteries Enabled by a Dendrite-Free Zinc Anode and Concentrated Electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 22059–22066. [Google Scholar] [CrossRef]
- Kang, Z.; Wu, C.; Dong, L.; Liu, W.; Mou, J.; Zhang, J.; Chang, Z.; Jiang, B.; Wang, G.; Kang, F.; et al. 3D Porous Copper Skeleton Supported Zinc Anode toward High Capacity and Long Cycle Life Zinc Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 3364–3371. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Liang, S.; Ma, X.; Han, M.; Wu, X.; Zhou, J. Spatially homogeneous copper foam as surface dendrite-free host for zinc metal anode. Chem. Eng. J. 2020, 379, 122248. [Google Scholar] [CrossRef]
- Ke, X.; Liang, Y.; Ou, L.; Liu, H.; Chen, Y.; Wu, W.; Cheng, Y.; Guo, Z.; Lai, Y.; Liu, P.; et al. Surface engineering of commercial Ni foams for stable Li metal anodes. Energy Storage Mater. 2019, 23, 547–555. [Google Scholar] [CrossRef]
- Yang, C.; Yao, Y.; He, S.; Xie, H.; Hitz, E.; Hu, L. Ultrafine Silver Nanoparticles for Seeded Lithium Deposition toward Stable Lithium Metal Anode. Adv. Mater. 2017, 29, 1702714. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Y.; Ke, X.; Zhang, Z.; Wu, W.; Lin, G.; Zhou, Z.; Shi, Z. Coupling of triporosity and strong Au–Li interaction to enable dendrite-free lithium plating/stripping for long-life lithium metal anodes. J. Mater. Chem. A 2020, 8, 18094–18105. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, L.; Huang, X.; Guo, X.; Liu, D.; Zheng, D.; Zhang, X.; Ren, R.; Qu, D.; Chen, J. A room-temperature liquid metal-based self-healing anode for lithium-ion batteries with an ultra-long cycle life. Energy Environ. Sci. 2017, 10, 1854–1861. [Google Scholar] [CrossRef]
- Wei, C.; Fei, H.; An, Y.; Tao, Y.; Feng, J.; Qian, Y. Uniform Li deposition by regulating the initial nucleation barrier via a simple liquid-metal coating for a dendrite-free Li–metal anode. J. Mater. Chem. A 2019, 7, 18861–18870. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, L.; Ding, Y.; Goodenough, J.B.; Yu, G. Room-temperature liquid metal and alloy systems for energy storage applications. Energy Environ. Sci. 2019, 12, 2605–2619. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Tabor, C.; Kalantar-Zadeh, K.; Dickey, M.D. Gallium Liquid Metal: The Devil’s Elixir. Annu. Rev. Mater. Res. 2021, 51, 381–408. [Google Scholar] [CrossRef]
- Wang, X.; Guo, R.; Liu, J. Liquid Metal Based Soft Robotics: Materials, Designs, and Applications. Adv. Mater. Technol. 2018, 4, 1800549. [Google Scholar] [CrossRef] [Green Version]
- Cochran, C.N.; Foster, L.M. Vapor Pressure of Gallium, Stability of Gallium Suboxide Vapor, and Equilibria of Some Reactions Producing Gallium Suboxide Vapor. J. Electrochem. Soc. 1962, 109, 144. [Google Scholar] [CrossRef]
- Kidanu, W.G.; Hur, J.; Kim, I.T. Gallium-Indium-Tin Eutectic as a Self-Healing Room-Temperature Liquid Metal Anode for High-Capacity Lithium-Ion Batteries. Materials 2021, 15, 168. [Google Scholar] [CrossRef]
- Khamsanga, S.; Uyama, H.; Nuanwat, W.; Pattananuwat, P. Polypyrrole/reduced graphene oxide composites coated zinc anode with dendrite suppression feature for boosting performances of zinc ion battery. Sci. Rep. 2022, 12, 8689. [Google Scholar] [CrossRef]
- Song, B.; Tang, M.; Hu, E.; Borkiewicz, O.J.; Wiaderek, K.M.; Zhang, Y.; Phillip, N.D.; Liu, X.; Shadike, Z.; Li, C.; et al. Understanding the Low-Voltage Hysteresis of Anionic Redox in Na2Mn3O7. Chem. Mater. 2019, 31, 3756–3765. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, H.; Hu, C.; Xu, H.; Huang, Y.; Wen, J.; Sun, H.; Luo, W.; Huang, Y. Toward a Stable Sodium Metal Anode in Carbonate Electrolyte: A Compact, Inorganic Alloy Interface. J. Phys. Chem. Lett. 2019, 10, 707–714. [Google Scholar] [CrossRef]
- Zhao, Y.; Goncharova, L.V.; Zhang, Q.; Kaghazchi, P.; Sun, Q.; Lushington, A.; Wang, B.; Li, R.; Sun, X. Inorganic-Organic Coating via Molecular Layer Deposition Enables Long Life Sodium Metal Anode. Nano Lett. 2017, 17, 5653–5659. [Google Scholar] [CrossRef]
- Liao, K.; Wu, S.; Mu, X.; Lu, Q.; Han, M.; He, P.; Shao, Z.; Zhou, H. Developing a “Water-Defendable” and “Dendrite-Free” Lithium-Metal Anode Using a Simple and Promising GeCl4 Pretreatment Method. Adv. Mater. 2018, 30, e1705711. [Google Scholar] [CrossRef]
- Pang, Q.; Liang, X.; Shyamsunder, A.; Nazar, L.F. An In Vivo Formed Solid Electrolyte Surface Layer Enables Stable Plating of Li Metal. Joule 2017, 1, 871–886. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yang, X.; Kuo, L.Y.; Kaghazchi, P.; Sun, Q.; Liang, J.; Wang, B.; Lushington, A.; Li, R.; Zhang, H.; et al. High Capacity, Dendrite-Free Growth, and Minimum Volume Change Na Metal Anode. Small 2018, 14, e1703717. [Google Scholar] [CrossRef]
- Baumgartner, J.; Dey, A.; Bomans, P.H.; Le Coadou, C.; Fratzl, P.; Sommerdijk, N.A.; Faivre, D. Nucleation and growth of magnetite from solution. Nat. Mater. 2013, 12, 310–314. [Google Scholar] [CrossRef]
- Yue, X.-Y.; Wang, W.-W.; Wang, Q.-C.; Meng, J.-K.; Zhang, Z.-Q.; Wu, X.-J.; Yang, X.-Q.; Zhou, Y.-N. CoO nanofiber decorated nickel foams as lithium dendrite suppressing host skeletons for high energy lithium metal batteries. Energy Storage Mater. 2018, 14, 335–344. [Google Scholar] [CrossRef]
- Yan, K.; Lu, Z.; Lee, H.-W.; Xiong, F.; Hsu, P.-C.; Li, Y.; Zhao, J.; Chu, S.; Cui, Y. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 2016, 1, 16010. [Google Scholar] [CrossRef]
- Guan, X.; Wang, A.; Liu, S.; Li, G.; Liang, F.; Yang, Y.W.; Liu, X.; Luo, J. Controlling Nucleation in Lithium Metal Anodes. Small 2018, 14, e1801423. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Y.X.; Li, J.Y.; Wang, S.H.; Guo, Y.G.; Wan, L.J. Uniform Lithium Nucleation/Growth Induced by Lightweight Nitrogen-Doped Graphitic Carbon Foams for High-Performance Lithium Metal Anodes. Adv. Mater. 2018, 30, 1706216. [Google Scholar] [CrossRef]
- Wu, W.; Wu, W.; Qiu, X.; Wang, S. Low-temperature reversible capacity loss and aging mechanism in lithium-ion batteries for different discharge profiles. Int. J. Energy Res. 2018, 43, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Jiang, Z.-J.; Yang, L.; Cheng, S.; Liu, M. A high-performance anode for lithium ion batteries: Fe3O4 microspheres encapsulated in hollow graphene shells. J. Mater. Chem. A 2015, 3, 11847–11856. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, W.; Zheng, W.; Cui, X.; Rojo, T.; Zhang, Q. Towards High-Safe Lithium Metal Anodes: Suppressing Lithium Dendrites via Tuning Surface Energy. Adv. Sci. 2017, 4, 1600168. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Genzer, J.; Dickey, M.D. Attributes, Fabrication, and Applications of Gallium-Based Liquid Metal Particles. Adv. Sci. 2020, 7, 2000192. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kidanu, W.G.; Yang, H.; Park, S.; Hur, J.; Kim, I.T. Room-Temperature Liquid-Metal Coated Zn Electrode for Long Life Cycle Aqueous Rechargeable Zn-Ion Batteries. Batteries 2022, 8, 208. https://doi.org/10.3390/batteries8110208
Kidanu WG, Yang H, Park S, Hur J, Kim IT. Room-Temperature Liquid-Metal Coated Zn Electrode for Long Life Cycle Aqueous Rechargeable Zn-Ion Batteries. Batteries. 2022; 8(11):208. https://doi.org/10.3390/batteries8110208
Chicago/Turabian StyleKidanu, Weldejewergis Gebrewahid, Hyewon Yang, Saemin Park, Jaehyun Hur, and Il Tae Kim. 2022. "Room-Temperature Liquid-Metal Coated Zn Electrode for Long Life Cycle Aqueous Rechargeable Zn-Ion Batteries" Batteries 8, no. 11: 208. https://doi.org/10.3390/batteries8110208
APA StyleKidanu, W. G., Yang, H., Park, S., Hur, J., & Kim, I. T. (2022). Room-Temperature Liquid-Metal Coated Zn Electrode for Long Life Cycle Aqueous Rechargeable Zn-Ion Batteries. Batteries, 8(11), 208. https://doi.org/10.3390/batteries8110208